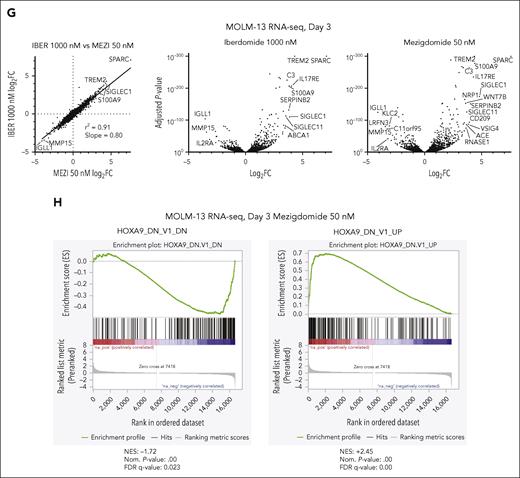
Mezigdomide potently degrades IKAROS, conferring broader activity in KMT2A-r and NPM1c AML models compared with lenalidomide and iberdomide.
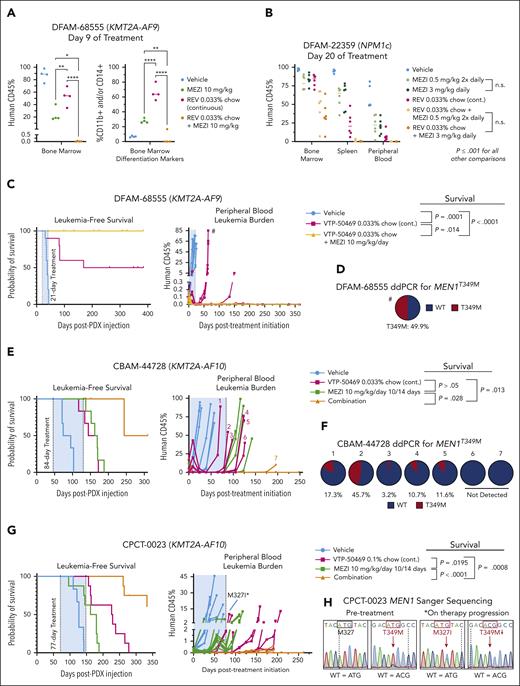
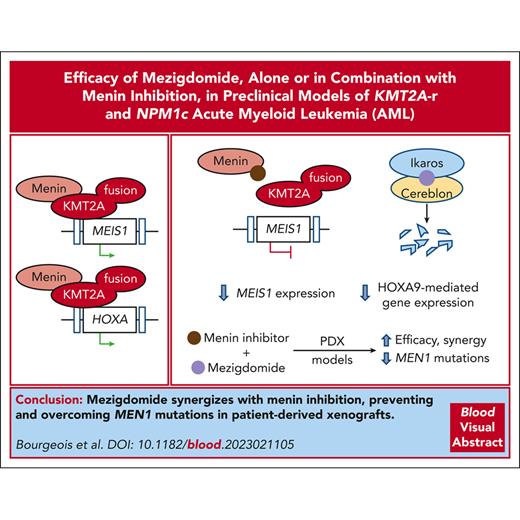
Mezigdomide synergizes with menin inhibition and prevents and overcomes MEN1 mutations in patient derived xenografts.
Visual Abstract
Small molecules that target the menin-KMT2A protein-protein interaction (menin inhibitors) have recently entered clinical trials in lysine methyltransferase 2A (KMT2A or MLL1)–rearranged (KMT2A-r) and nucleophosmin-mutant (NPM1c) acute myeloid leukemia (AML) and are demonstrating encouraging results. However, rationally chosen combination therapy is needed to improve responses and prevent resistance. We have previously identified IKZF1/IKAROS as a target in KMT2A-r AML and shown in preclinical models that IKAROS protein degradation with lenalidomide or iberdomide has modest single-agent activity yet can synergize with menin inhibitors. Recently, the novel IKAROS degrader mezigdomide was developed with greatly enhanced IKAROS protein degradation. In this study, we show that mezigdomide has increased preclinical activity in vitro as a single-agent in KMT2A-r and NPM1c AML cell lines, including sensitivity in cell lines resistant to lenalidomide and iberdomide. Further, we demonstrate that mezigdomide has the greatest capacity to synergize with and induce apoptosis in combination with menin inhibitors, including in MEN1 mutant models. We show that the superior activity of mezigdomide compared with lenalidomide or iberdomide is due to its increased depth, rate, and duration of IKAROS protein degradation. Single-agent mezigdomide was efficacious in 5 patient-derived xenograft models of KMT2A-r and 1 NPM1c AML. The combination of mezigdomide with the menin inhibitor VTP-50469 increased survival and prevented and overcame MEN1 mutations that mediate resistance in patients receiving menin inhibitor monotherapy. These results support prioritization of mezigdomide for early phase clinical trials in KMT2A-r and NPM1c AML, either as a single agent or in combination with menin inhibitors.
Introduction
Acute myeloid leukemia (AML) with lysine methyltransferase 2A (KMT2A and MLL1) gene rearrangements (KMT2A-r) constitute 15% to 20% of pediatric and 3% to 7% of adult cases.1-4,KMT2A-r AML is an aggressive disease with poor outcomes and high treatment-related morbidity and characterized by aberrant, increased expression of HOXA cluster genes and MEIS1.5,6 A portion of the KMT2A-fusion driven gene expression program is dependent on menin, which binds the N-terminus of KMT2A and chromatin.7-9 The menin-KMT2A protein-protein interaction is also essential in AML with nucleophosmin mutations (NPM1c), which constitutes about one-third of primary AML in adults and 8% of pediatric AML cases, and is similarly characterized by overexpression of HOXA cluster genes and MEIS1, in addition to HOXB cluster genes.1,10-12
Recently developed small molecule inhibitors of the menin-KMT2A interaction, menin inhibitors, have shown strong preclinical activity in KMT2A-r and NPM1c AML.13-15 In the phase 1 AUGMENT-101 trial, 30% of patients with relapsed/refractory KMT2A-r or NPM1c acute leukemias who received revumenib monotherapy had a complete remission or complete remission with partial hematologic recovery.16 However, nearly 40% of patients on the trial developed mutations in MEN1, which encodes menin.17 These mutations affect menin inhibitor binding to menin without displacing KMT2A, thereby markedly diminishing menin inhibitor responses. Consequently, rationally chosen combination therapy that increases responses to menin inhibitors while also preventing and overcoming MEN1 mutations, is paramount.
We have previously shown that IKZF1 (IKAROS) is an essential transcription factor in KMT2A-r AML that has functional cooperativity and extensive chromatin co-occupancy with KMT2A-MENIN and MEIS1.18 IMiDs (immunomodulatory imide drugs) and CELMoDs (cereblon E3 ligase modulators) are thalidomide analogs that function as molecular glues and recruit neosubstrate proteins such as IKAROS to the cereblon receptor component of an E3 ubiquitin ligase complex, resulting in neosubstrate ubiquitylation and proteasomal degradation.19-21 We showed that the IMiD lenalidomide and the CELMoD iberdomide (CC-220) had modest single-agent activity but were effective in combination with menin inhibitors in models of KMT2A-r AML due to their dual targeting of a HOXA/MEIS1 transcriptional program.
Subsequently, the novel CELMoD mezigdomide (CC-92480) was developed as a selective molecular glue with enhanced IKAROS protein degradation.22 Mezigdomide has shown encouraging activity with acceptable toxicity in a phase 1/2 study enrolling patients with heavily pretreated, IMiD-refractory multiple myeloma.23 Given our prior work establishing IKAROS as a target, we hypothesized that mezigdomide would have enhanced activity in preclinical models of KMT2A-r and NPM1c AML, including in models that have or develop MEN1 mutations. Herein, we identify mezigdomide as the most promising IKAROS-degrading IMiD/CELMoD for early phase clinical trials in KMT2A-r and NPM1c AML as a single-agent or in combination with menin inhibitors.
Methods
Animals and PDX experiments
For experiments with patient-derived xenograft (PDX) models, female NOD.Cg-PrkdcscidIl2rgtm1Sug/JicTac mice were purchased from Taconic. Animal experiments were approved by Dana-Farber Cancer Institute’s Institutional Animal Care and Use Committee (protocol number 16-021). PDX samples were originally obtained from the Public Repository of Xenografts or developed by the Center for Pediatric Cancer Therapeutics at Dana-Farber Cancer Institute.24 Mice were injected with 50 000 to 3 000 000 leukemia cells based on prior patterns of time-to-engraftment. Upon detection of human CD45+ cells in the peripheral blood, mice were randomized to treatment with lenalidomide, mezigdomide, VTP-50469 or revumenib, or combinations thereof. Lenalidomide and mezigdomide were purchased from MedChemExpress and dissolved in 0.5% carboxymethylcellulose and 0.25% Tween-80 in water before dosing by oral gavage. VTP-50469 or revumenib chow was provided by Syndax Pharmaceuticals. Mice were euthanized when humane end points were reached. Peripheral blood leukemia burden was monitored by human CD45+ cell percentage. At the time of death, leukemia burden was confirmed and quantified in the bone marrow, spleen, and peripheral blood.
Drug experiments
For proliferation, cell viability/CellTiter-Glo, and apoptosis assessments, cells were plated on day 0 with cells split, drug replenished, and readings assessed every 3 to 4 days. For proliferation assessments, cells were stained with DAPI (4′,6-diamidino-2-phenylindole) for nonviable cell exclusion then counted on an LSR Fortessa flow cytometer (Becton Dickinson). For cell viability/CellTiter-Glo assessments, 1:1 ratio of cells and CellTiter-Glo reagent (Promega) were mixed and specimens analyzed. For apoptosis assessments, cells were stained with annexin V and 4′,6-diamidino-2-phenylindole and/or propidium iodide using the Affymetrix eBioscience annexin V staining protocol. Samples were then analyzed on an LSR Fortessa flow cytometer (Becton Dickinson). Dose-response curves and statistical analyses were calculated with GraphPad Prism Version 9.4.1. Synergy scores were calculated with SynergyFinder 3.0.25
Cell lines, drugs, vectors, Western blots, MEN1 sequencing, and antibodies
Please refer to the supplemental Data, available on the Blood website, for extended methods.
Results
Mezigdomide has enhanced IKAROS degradation and antiproliferative activity in KMT2A-r and NPM1c cell lines
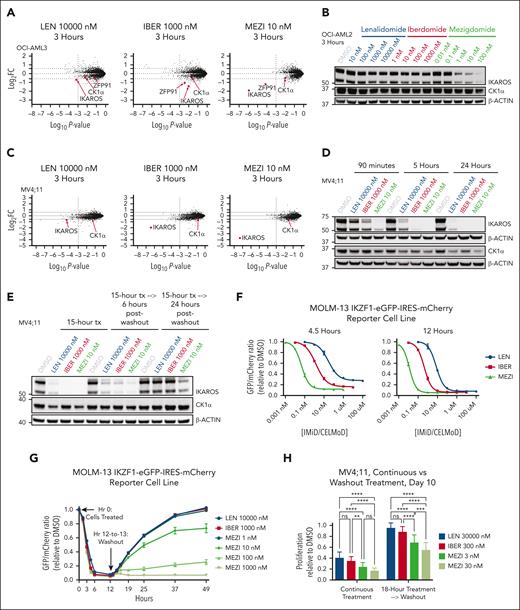
To assess whether increased potency of IKAROS degradation confers greater antiproliferative activity, we compared the IMiD lenalidomide and the CELMoDs iberdomide and mezigdomide. Lenalidomide was chosen because it holds US Food and Drug Administration approvals for several hematologic malignancies21 and degrades IKAROS and casein kinase 1α (CK1α).26 Iberdomide was selected because it was, before mezigdomide, the most potent IKAROS-degrading IMiD/CELMoD to enter clinical trials.27-29 Upon treating the MOLM-13 (KMT2A-AF9) and MV4;11 (KMT2A-AF4) cell lines with equal concentrations of drug (100 nM), mezigdomide had the greatest antiproliferative effect. Lenalidomide at a 1000× and iberdomide at a 100× higher dose were required to generate similar antiproliferative effects to mezigdomide 10 nM. This correlated with each compound’s capacity for IKAROS protein degradation (Figure 1A-B; supplemental Figure 1A-G). We compared antiproliferative effects in 5 KMT2A-r and 2 NPM1c AML cell lines, defining sensitivity as an absolute 50% inhibitory concentration (IC50) of ≤1000 nM after 9 days or an absolute IC85 ≤1000 nM after 18 days of treatment. All 7 cell lines were sensitive to mezigdomide, 4 of 7 were sensitive to iberdomide, and only 2 of 7 were sensitive to lenalidomide. Conversely, in 5 myeloid leukemia cell lines without KMT2A-r or NPM1c, only 2 were sensitive to mezigdomide and iberdomide and none to lenalidomide (Figure 1C-D; supplemental Figure 1H-K). Sensitivity in the cell lines tested largely corroborated our prior work demonstrated IKZF1 is a dependency in KMT2A-r and NPM1c cell lines.18 To assess for a potential therapeutic window, we treated CD34+-selected human cord blood cells with and without KMT2A-AF9 transformation30 and found that the therapeutic window for mezigdomide was greater than iberdomide or lenalidomide, but smaller than the menin inhibitor VTP-50469 (preclinical analog of revumenib, Figure 1E). These experiments show that mezigdomide has enhanced single-agent activity in KMT2A-r and NPM1c AML cell lines and a potential therapeutic window.
Mezigdomide has enhanced IKAROS degradation and antiproliferative activity in KMT2A-r and NPM1c cell lines. (A) Proliferation (cell counts) relative to dimethyl sulfoxide (DMSO) control in the MOLM-13 cell line treated for 18 days with 100 nM of lenalidomide (LEN), iberdomide (IBER), or mezigdomide (MEZI), (data pooled from 5 independent experiments; n = 15 per data point; data points represent mean ± standard deviation [SD]). Two-way analysis of variance (ANOVA) with Tukey multiple comparisons test: MEZI vs LEN or IBER: P < .005 for all days from 3 to 18; LEN vs IBER: no significant difference at day 3; P < .005 for all days from 6 to 18. (B) Western blot in MOLM-13 cell line treated for 12 hours. (C) Proliferation relative to DMSO in a panel of KMT2A-r and NPM1c AML cell lines treated for 9 days with 8-point dose curves of LEN, IBER, or MEZI. (n ≥ 3 per data point; data points represent mean ± SD; each curve representative of 3 independent experiments). (D) Absolute IC50 at day 9 for panel C (left) and 5 non–KMT2A-r, non-NPM1c myeloid leukemia cell lines (right) assessed by CellTiter-Glo or cell counts. Bolded and italicized values represent cell lines sensitive to drug (absolute IC50 ≤ 1000 nM). Absolute IC50 calculated with data pooled from 3 independent experiments per cell line for proliferation; 2 independent experiments for CellTiter-Glo assays, with each experiment having at least a 5-point dose titration per IMiD/CELMoD. See supplemental Figure 1I-K for 95% confidence intervals. (E) Proliferation relative to DMSO in CD34+-selected human cord blood cells that were KMT2A-AF9 transformed (left) vs untransformed (middle), assessed at day 6. (Left and middle) Data pooled from 3 independent experiments; n = 9 per data point; data points represent mean ± SD. (Right) For the top concentration of each drug tested in the graphs on the left and middle, proliferation relative to DMSO in KMT2A-AF9 transformed vs untransformed human cord blood cells. Statistical comparison with 1-way ANOVA with Tukey multiple comparisons test; ∗∗∗∗P < .0001.
Mezigdomide has enhanced IKAROS degradation and antiproliferative activity in KMT2A-r and NPM1c cell lines. (A) Proliferation (cell counts) relative to dimethyl sulfoxide (DMSO) control in the MOLM-13 cell line treated for 18 days with 100 nM of lenalidomide (LEN), iberdomide (IBER), or mezigdomide (MEZI), (data pooled from 5 independent experiments; n = 15 per data point; data points represent mean ± standard deviation [SD]). Two-way analysis of variance (ANOVA) with Tukey multiple comparisons test: MEZI vs LEN or IBER: P < .005 for all days from 3 to 18; LEN vs IBER: no significant difference at day 3; P < .005 for all days from 6 to 18. (B) Western blot in MOLM-13 cell line treated for 12 hours. (C) Proliferation relative to DMSO in a panel of KMT2A-r and NPM1c AML cell lines treated for 9 days with 8-point dose curves of LEN, IBER, or MEZI. (n ≥ 3 per data point; data points represent mean ± SD; each curve representative of 3 independent experiments). (D) Absolute IC50 at day 9 for panel C (left) and 5 non–KMT2A-r, non-NPM1c myeloid leukemia cell lines (right) assessed by CellTiter-Glo or cell counts. Bolded and italicized values represent cell lines sensitive to drug (absolute IC50 ≤ 1000 nM). Absolute IC50 calculated with data pooled from 3 independent experiments per cell line for proliferation; 2 independent experiments for CellTiter-Glo assays, with each experiment having at least a 5-point dose titration per IMiD/CELMoD. See supplemental Figure 1I-K for 95% confidence intervals. (E) Proliferation relative to DMSO in CD34+-selected human cord blood cells that were KMT2A-AF9 transformed (left) vs untransformed (middle), assessed at day 6. (Left and middle) Data pooled from 3 independent experiments; n = 9 per data point; data points represent mean ± SD. (Right) For the top concentration of each drug tested in the graphs on the left and middle, proliferation relative to DMSO in KMT2A-AF9 transformed vs untransformed human cord blood cells. Statistical comparison with 1-way ANOVA with Tukey multiple comparisons test; ∗∗∗∗P < .0001.
Mezigdomide in combination with VTP-50469 demonstrates superior synergy and increased induction of apoptosis in MEN1 wild-type and mutant models
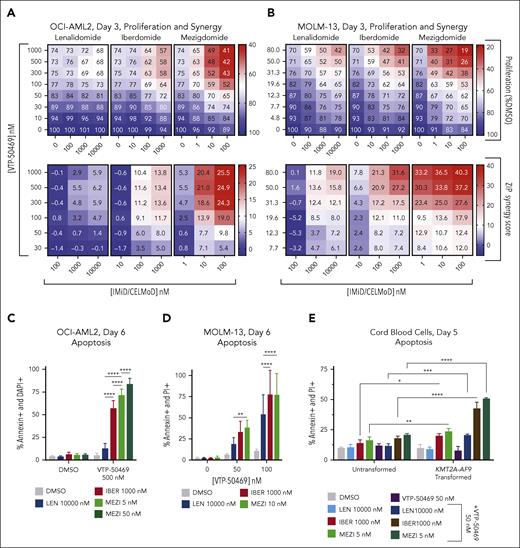
Given our previous work describing that IKAROS degradation and menin inhibition are synergistic and can cause apoptosis in KMT2A-r AML,18 we hypothesized that greater IKAROS degradation with mezigdomide would confer greater effects. We treated cells with VTP-50469 and IMiD/CELMoD and calculated zero interaction potency model (ZIP) synergy scores.25 In 4 KMT2A-r and 1 NPM1c cell lines, there were greater antiproliferative effects and higher synergy scores when a 100-fold dosing range of mezigdomide (1-100 nM) was combined with VTP-50469 compared with the combination with lenalidomide (100-10 000 nM) or iberdomide (10-1000 nM) (Figure 2A-B; supplemental Figure 2A-E). In each cell line, mezigdomide synergized with VTP-50469, often at lower doses and earlier time points than either lenalidomide or iberdomide.
To assess whether greater IKAROS degradation induced more apoptosis, we evaluated apoptotic markers by flow cytometry after treatment with VTP-50469 + IMiDs/CELMoDs. After 6 days of treatment in the OCI-AML2 (KMT2A-AF6), MOLM-13, and MV4;11 cell lines, mezigdomide + VTP-50469 caused either equivalent or increased apoptosis compared with lenalidomide or iberdomide at doses and time points in which there was minimal single-agent apoptosis with any single agent (Figure 2C-D; supplemental Figure 2F-G). In contrast, cord blood cells with KMT2A-AF9 transformation had large increases in apoptosis with iberdomide or mezigdomide + VTP-50469 treatment but untransformed cells did not (Figure 2E).
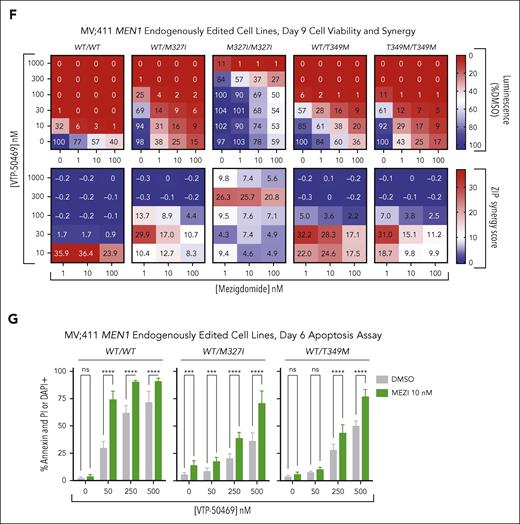
Cell lines with MEN1 hotspot mutations (M327I, T349M) introduced into the endogenous MEN1-coding sequence have been established and characterized.17 Although MEN1 mutations markedly shift the IC50 of menin inhibitor dose-response curves, activity is nevertheless retained at higher doses. We thus hypothesized that at higher doses of menin inhibitor, combination therapy could recapitulate the synergy and apoptosis we observed in wild-type cell lines. We observed strong synergy with mezigdomide + VTP-50469 in MV4;11 cell lines with MEN1 mutations (WT/M327I, M327I/M327I, WT/T349M, and T349M/T349M). The VTP-50469 concentrations in which synergy was observed were shifted proportionally to the IC50 shift induced by the MEN1 mutation (Figure 2F). We evaluated apoptotic markers in MV4;11WT/M327I and MV4;11WT/T349M cell lines and found that apoptosis was induced with mezigdomide + VTP-50469 to a far greater extent than either monotherapy treatment, again at VTP-50469 doses that were shifted proportionally to the IC50 shift induced by MEN1 mutations (Figure 2G). These data show that mezigdomide synergizes and causes apoptosis in combination with VTP-50469, including in cell lines with MEN1 binding pocket mutations that confer menin inhibitor resistance, and there is a potential therapeutic window.
Mezigdomide mediates its effects through potent IKAROS degradation
To evaluate whether the enhanced activity of mezigdomide is due to degradation of a neosubstrate other than IKAROS, we performed mass spectrometry in 2 KMT2A-r AML cell lines. The only common substrate among lenalidomide, iberdomide, and mezigdomide in the OCI-AML2 and MV4;11 cell lines was IKAROS (Figure 3A-B). Surprisingly, we found that mezigdomide and lenalidomide had comparable CK1α fold changes in these cell lines. We confirmed CK1α degradation with mezigdomide in vitro and in vivo by Western blot (Figure 3C; supplemental Figures 3A-C and 5D). We further demonstrated that micromolar concentrations of CK1α can displace IKAROS from preformed complexes of IKAROS-mezigdomide-cereblon-DDB1ΔB, suggesting substrate competition between IKAROS and CK1α (supplemental Figure 3D). Thus, mezigdomide degrades CK1α to a much lesser extent than IKAROS and at concentrations in which IKAROS is nearly already maximally degraded.
To further evaluate whether IKAROS is the critical target of mezigdomide, we used MOLM-13 cells with overexpressed wild-type IKAROS or IKAROSQ146H, which has a single amino acid substitution that confers resistance to degradation.20 Notably, wild-type IKAROS overexpression was sufficient to rescue lenalidomide’s single-agent activity and apoptosis induction with VTP-50469. IKAROSQ146H overexpression rescued these effects with iberdomide and all but a small fraction of apoptosis induction with mezigdomide combined with VTP-50469 (Figure 3D-F; supplemental Figure 3E). These data strongly suggest that IKAROS degradation is the main target responsible for mezigdomide’s single-agent activity and is essential to induce apoptosis in combination with menin inhibitors.
We hypothesized that if IKAROS is the primary target of these IMiDs/CELMoDs, iberdomide and mezigdomide should have highly correlated gene expression changes (as previously shown with iberdomide and lenalidomide).18 RNA-sequencing after 3-day treatment with iberdomide or mezigdomide demonstrated highly correlated gene expression changes (r2 = 0.91) that were of greater magnitude with mezigdomide, as seen in the slope of the linear regression line and in examining the number of genes with greater/less than log2FC of ±2 (Figure 3G; supplemental Figure 3F). To validate this, we performed quantitative polymerase chain reaction (PCR) for 2 of the most upregulated genes from the RNA-sequencing data (SPARC and TREM2) in addition to BCL2, which we have previously shown to be downregulated consistently with VTP-50469 and VTP-50469 + IMiDs/CELMoDs.18 There was either equivalent or greater gene expression changes with mezigdomide with or without VTP-50469 compared with lenalidomide or iberdomide (supplemental Figure 3G). We found that mezigdomide and iberdomide affect a HOXA9 gene expression program, as evidenced by the high degree of correlation with previously published HOXA9 responsive genes (Figure 3H; supplemental Figure 3H-I).31 These data suggest that the enhanced activity of mezigdomide is not due to degradation of a unique neosubstrate but rather increased potency of IKAROS degradation.
Potency of mezigdomide is related to increased depth, rate, and duration of IKAROS degradation
To assess factors other than the depth of IKAROS degradation that contribute to enhanced mezigdomide activity, we examined the rate and duration of degradation. Mass spectrometry experiments in the OCI-AML2 and MV4;11 cell lines, corroborated by Western blots, showed much greater IKAROS degradation at earlier time points with mezigdomide (Figure 4A-D).
Potency of mezigdomide is related to increased depth, rate, and duration of IKAROS degradation. (A,C) Mass spectrometry in the OCI-AML2 and MV4;11 cell lines after 3 hours of treatment. The y-axis depicts log2 FC in abundance for each protein relative to DMSO (dotted line at threshold of ± 1.5-FC), and the x-axis depicts log10P value (dotted line at significance threshold of 10−3). Proteins with log2FC ≤ −1.5-fold that were shared in common after 3- or 12-hour treatment with any of the 3 IMiDs/CELMoDs in each cell line, in addition to CK1α, are labeled. (B,D) Western blot in the OCI-AML2 cell line after 3 hours of treatment and in the MV4;11 cell line after 90 minutes, 5 hours, and 24 hours of treatment. (E) Western blot in the MV4;11 cell line after treatment (tx) for 15 hours, followed by large volume washing of cells (3 washes), and culturing cells for 6 and 24 more hours before cell pellet collection. (F-G) Experiments performed in the MOLM-13 cell line transduced with IKZF1-eGFP-IRES-mCherry degradation reporter vector. (F) Cells treated for 4.5 and 12 hours and eGFP/mCherry ratio relative to DMSO control calculated. Cells plated in triplicate with each data point representing mean ± SD. (G) Cells treated for 12 hours followed by extensive washing (7 washes that lasted from hour 12-13 in duration) and then assessing eGFP recovery thereafter. Representative of 3 experiments, with each data point representing mean of technical triplicates ± SD. (H) Proliferation (cell count) relative to DMSO after 10 days from initial treatment comparing continuous treatment (left) with 18-hour treatment followed by washout (right) in MV4;11 cell line. Data depicted pooled from 3 independent experiments, each of which had at least 5 technical replicates for n > 15 per bar; bars represent mean + SD. Statistical analysis with 2-way ANOVA with Tukey multiple comparisons test. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
Potency of mezigdomide is related to increased depth, rate, and duration of IKAROS degradation. (A,C) Mass spectrometry in the OCI-AML2 and MV4;11 cell lines after 3 hours of treatment. The y-axis depicts log2 FC in abundance for each protein relative to DMSO (dotted line at threshold of ± 1.5-FC), and the x-axis depicts log10P value (dotted line at significance threshold of 10−3). Proteins with log2FC ≤ −1.5-fold that were shared in common after 3- or 12-hour treatment with any of the 3 IMiDs/CELMoDs in each cell line, in addition to CK1α, are labeled. (B,D) Western blot in the OCI-AML2 cell line after 3 hours of treatment and in the MV4;11 cell line after 90 minutes, 5 hours, and 24 hours of treatment. (E) Western blot in the MV4;11 cell line after treatment (tx) for 15 hours, followed by large volume washing of cells (3 washes), and culturing cells for 6 and 24 more hours before cell pellet collection. (F-G) Experiments performed in the MOLM-13 cell line transduced with IKZF1-eGFP-IRES-mCherry degradation reporter vector. (F) Cells treated for 4.5 and 12 hours and eGFP/mCherry ratio relative to DMSO control calculated. Cells plated in triplicate with each data point representing mean ± SD. (G) Cells treated for 12 hours followed by extensive washing (7 washes that lasted from hour 12-13 in duration) and then assessing eGFP recovery thereafter. Representative of 3 experiments, with each data point representing mean of technical triplicates ± SD. (H) Proliferation (cell count) relative to DMSO after 10 days from initial treatment comparing continuous treatment (left) with 18-hour treatment followed by washout (right) in MV4;11 cell line. Data depicted pooled from 3 independent experiments, each of which had at least 5 technical replicates for n > 15 per bar; bars represent mean + SD. Statistical analysis with 2-way ANOVA with Tukey multiple comparisons test. ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001.
To evaluate the differences in duration of degradation, we used drug washout experiments. We performed Western blots in cells treated for 15 hours followed by drug washout. Only mezigdomide retained significant IKAROS degradation 24 hours thereafter (Figure 4E). To further characterize this, we transduced MOLM-13 and MV4;11 cells with an IKAROS protein degradation reporter vector (IKZF1-eGFP-IRES-mCherry).32,33 After IMiD/CELMoD treatment, IKAROS-eGFP is degraded but mCherry is not, which permits quantification by measuring the eGFP/mCherry ratio (Figure 4F; supplemental Figure 4A). After a 12-hour treatment followed by washout, cells treated with lenalidomide and iberdomide exhibited linear substate recovery of IKAROS-eGFP, whereas cells treated with mezigdomide had a greatly prolonged, dose-dependent duration of IKAROS degradation (Figure 4G). Mezigdomide’s unique capacity for prolonged substrate degradation conferred prolonged antiproliferative activity as well. We treated MV4;11 cells with IMiDs/CELMoDs for 18 hours followed by washout. After passaging cells on days 4 and 7, by day 10, nearly all of the antiproliferative effect was attenuated for lenalidomide and iberdomide, but antiproliferative activity was retained for mezigdomide (Figure 4H; supplemental Figure 4B-C). These experiments show that mezigdomide’s increased rate and duration of activity after drug washout contribute to its enhanced potency.
Mezigdomide monotherapy is efficacious in PDX models of KMT2A-r and NPM1c AML in vivo
When assessing the single-agent activity of mezigdomide in vivo in PDX models, we treated mice with 10 mg/kg per day based on published data,22 which conferred peak concentrations (>1000 nM) that did not fully clear from the plasma after 24 hours (supplemental Figure 5A-B). In recently published data with mezigdomide (combined with dexamethasone) in multiple myeloma, the peak concentration was far lower but also did not clear from the plasma within 24 hours.23 When treating with 0.5 mg/kg twice daily and 3 mg/kg per day, we found markedly lower peak concentrations than 10 mg/kg per day that entirely cleared after 12 and 24 hours respectively. Substrate degradation was similar among 0.5 mg/kg twice daily, 3 mg/kg per day, and 10 mg/kg per day treatments (supplemental Figure 5C-E). These data suggest that the main advantage of 10 mg/kg in mouse models is the prolonged plasma concentration of mezigdomide (similar to what is observed in patients), as opposed to the elevated peak concentration.
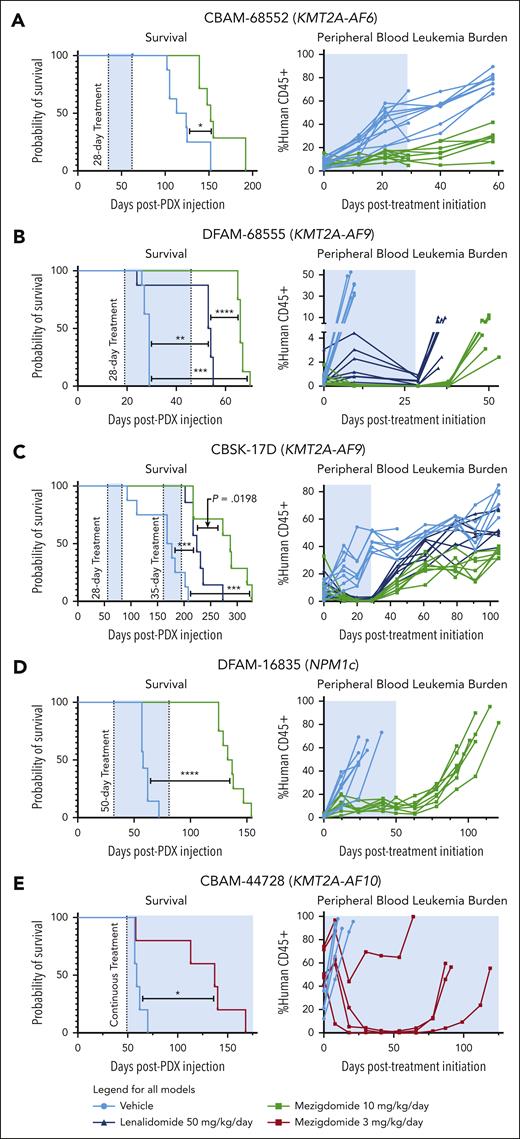
We first treated the CBAM-68552 (KMT2A-AF6) PDX for 28 days, euthanizing 3 mice in the vehicle control and mezigdomide cohorts at the end of the treatment period and following the rest for survival analysis (Figure 5A; supplemental Figure 5F). We found decreased burden of disease in the bone marrow, spleen, and peripheral blood and mezigdomide extended survival by 1 month. We next tested vehicle control vs lenalidomide 50 mg/kg per day vs mezigdomide 10 mg/kg per day (poor bioavailability of iberdomide precluded testing) in the DFAM-68555 (KMT2A-AF9) and CBSK-17D (KMT2A-AF9) PDX models (Figure 5B-C; supplemental Figure 5G). In both models, lenalidomide and mezigdomide decreased peripheral blood leukemia burden compared with vehicle control, with mezigdomide conferring greater reductions. Both increased survival compared with vehicle control, with mezigdomide exhibiting prolonged survival in DFAM-68555 model and trending toward significance in the CBSK-17D model. In the DFAM-16835 (NPM1c) PDX, we treated continuously with mezigdomide 10 mg/kg for 50 days (Figure 5D). Although the leukemia never fully cleared from the peripheral blood, it remained at a stable level with increased differentiation markers (supplemental Figure 5H), resulting in a nearly 3-month survival advantage. Lastly, in the CBAM-44728 (KMT2A-AF10) model, we treated continuously with a lower dose of mezigdomide (3 mg/kg per day) and found this lower dose nevertheless conferred a survival advantage and decreased peripheral blood leukemia burden (Figure 5E). Despite several mice acquiring resistance after a prolonged response, we did not find decreased CRBN/cereblon or increased IKZF1/IKAROS gene expression/protein levels to explain this resistance (data not shown). These PDX data suggest broad antileukemic activity of mezigdomide in vivo as a single agent, although notably without cures.
Mezigdomide monotherapy is efficacious in PDX models of KMT2A-r and NPM1c AML in vivo. (A) DFAM-68552 (KMT2A-AF6) PDX. Median survival: vehicle, 118.5 days (n = 8); MEZI, 152 days (n = 7); P = .016. Peripheral blood leukemia burden: P < .05 for all time points after day 0. (B) DFAM-68555 (KMT2A-AF9) PDX. Median survival: vehicle, 29 days (n = 8); lenalidomide (LEN), 53.5 days (n = 8); MEZI, 66 days (n = 8); vehicle vs LEN, P = .0032; vehicle vs MEZI, P = .0002; LEN vs MEZI, P < .0001. Peripheral blood leukemia burden: vehicle vs LEN or MEZI (P < .05 for day 10 time point); LEN vs MEZI (P < .05 for all time points assessed). (C) MSK-017D (KMT2A-AF9) PDX. Median survival: vehicle, 172.5 days (n = 8); LEN, 224 days (n = 7); MEZI, 287 days (n = 7); vehicle vs LEN, P = .0004; vehicle vs MEZI, P = .0001; LEN vs MEZI, P = .0198 (threshold for statistical significance P = .0167 with Bonferroni correction for multiple comparisons). Peripheral blood leukemia burden depicted from beginning of first treatment period to beginning of second treatment period: vehicle vs LEN (P < .05 for days 12, 20, 29, 44, 61, and 105); vehicle vs MEZI (P < .05 for all time points shown after day 0); and LEN vs MEZI (P < .05 for days 44 and beyond). (D) DFAM-16835 (NPM1c) PDX. Median survival: vehicle, 58 days (n = 7); MEZI, 135 days (n = 8); P < .0001. Peripheral blood leukemia burden: P < .05 for all time points after day 0. (E) CBAM-44728 (KMT2A-AF10) PDX. Median survival: vehicle, 59 days (n = 5); MEZI, 137 days (n = 5); P = .0198. (A-E) Survival analyses by log-rank (Mantel-Cox) test for survival curves. Peripheral blood leukemia burden quantified as percentage of CD45+ cells (mouse + human) that were human CD45+ and analyzed by 2-way ANOVA with Tukey multiple comparisons test or multiple t tests depending on number of treatment groups. The shaded blue area represents the treatment period.
Mezigdomide monotherapy is efficacious in PDX models of KMT2A-r and NPM1c AML in vivo. (A) DFAM-68552 (KMT2A-AF6) PDX. Median survival: vehicle, 118.5 days (n = 8); MEZI, 152 days (n = 7); P = .016. Peripheral blood leukemia burden: P < .05 for all time points after day 0. (B) DFAM-68555 (KMT2A-AF9) PDX. Median survival: vehicle, 29 days (n = 8); lenalidomide (LEN), 53.5 days (n = 8); MEZI, 66 days (n = 8); vehicle vs LEN, P = .0032; vehicle vs MEZI, P = .0002; LEN vs MEZI, P < .0001. Peripheral blood leukemia burden: vehicle vs LEN or MEZI (P < .05 for day 10 time point); LEN vs MEZI (P < .05 for all time points assessed). (C) MSK-017D (KMT2A-AF9) PDX. Median survival: vehicle, 172.5 days (n = 8); LEN, 224 days (n = 7); MEZI, 287 days (n = 7); vehicle vs LEN, P = .0004; vehicle vs MEZI, P = .0001; LEN vs MEZI, P = .0198 (threshold for statistical significance P = .0167 with Bonferroni correction for multiple comparisons). Peripheral blood leukemia burden depicted from beginning of first treatment period to beginning of second treatment period: vehicle vs LEN (P < .05 for days 12, 20, 29, 44, 61, and 105); vehicle vs MEZI (P < .05 for all time points shown after day 0); and LEN vs MEZI (P < .05 for days 44 and beyond). (D) DFAM-16835 (NPM1c) PDX. Median survival: vehicle, 58 days (n = 7); MEZI, 135 days (n = 8); P < .0001. Peripheral blood leukemia burden: P < .05 for all time points after day 0. (E) CBAM-44728 (KMT2A-AF10) PDX. Median survival: vehicle, 59 days (n = 5); MEZI, 137 days (n = 5); P = .0198. (A-E) Survival analyses by log-rank (Mantel-Cox) test for survival curves. Peripheral blood leukemia burden quantified as percentage of CD45+ cells (mouse + human) that were human CD45+ and analyzed by 2-way ANOVA with Tukey multiple comparisons test or multiple t tests depending on number of treatment groups. The shaded blue area represents the treatment period.
Mezigdomide in combination with menin inhibition is efficacious and can prevent and overcome MEN1 mutations in vivo
To evaluate the efficacy of mezigdomide in combination with menin inhibitor therapy, we first assessed burden of disease after short treatment periods in a KMT2A-r and an NPM1c PDX model. In the DFAM-68555 PDX, after 9 days of treatment both mezigdomide and revumenib monotherapy cohorts had significant bone marrow disease burden with upregulated differentiation markers. Remarkably, mezigdomide in combination with revumenib nearly entirely cleared the leukemia (Figure 6A; supplemental Figure 6A-B). In the DFAM-22359 PDX (NPM1c), mezigdomide at 0.5 mg/kg twice daily and 3 mg/kg per day had equally efficacious effects on disease burden after 20 days of treatment. Single-agent mezigdomide was slightly less efficacious compared with revumenib. Combination therapy outperformed either monotherapy (Figure 6B; supplemental Figure 6C). In this model, mezigdomide monotherapy increased differentiation markers (CD11b, CD14, and CD13) to a greater extent than revumenib monotherapy, yet the combination therapy mediated its greater antileukemic effect with less induction of differentiation markers compared with mezigdomide monotherapy. Thus, in both burden of disease models, augmenting differentiation is not the primary means by which combination therapy mediates its effects, despite both mezigdomide and revumenib doing so as single agents.
In a survival experiment in the DFAM-68555 PDX comparing a 21-day treatment course of vehicle, VTP-50469, and VTP-50469 + mezigdomide, half the VTP-50469 monotherapy mice died of leukemia, whereas no VTP-50469–mezigdomide mice relapsed (Figure 6C). We assessed MEN1 mutation status by extracting genomic DNA from the bone marrow of mice at the time of death and performing Sanger sequencing (5/5 VTP-50469 mice and 8/10 vehicle mice assessed) at 4 hotspot residues in which mutations are most commonly identified in patients receiving revumenib on the AUGMENT-101 trial (S160, M327, G331, and T349).17 One VTP-50469 monotherapy mouse had a MEN1 mutation (MEN1T349M), which was confirmed by droplet digital PCR (ddPCR; Figure 6D; supplemental Figure 6D). ddPCR did not detect a T349M mutation in vehicle mice (n = 3), suggesting that mutations can arise and be selected for rapidly.
To further model the prevention of MEN1 mutations, we assessed combination therapy in the CBAM-44728 PDX. Mice were treated for 84 days with either vehicle control, mezigdomide 10 of every 14 days, VTP-50469, or the combination. Menin inhibitor monotherapy nearly entirely cleared the disease from the peripheral blood after 6 weeks, however, 5 of these 6 mice then began to progress on treatment (Figure 6E; supplemental Figure 6E). Combination therapy led to a more rapid and sustained clearance of peripheral blood leukemia. At the time of leukemia relapse, Sanger sequencing found and ddPCR confirmed MEN1T349M in 5 of 6 relapsed VTP-50469 monotherapy mice. Notably MEN1T349M was absent in the only relapsed VTP-50469 + mezigdomide mouse (Figure 6F; supplemental Figure 6F). In vehicle and mezigdomide-monotherapy mice, we did not detect MEN1T349M mutations by ddPCR, nor in any of the hotspot residues by Sanger sequencing.
To examine whether we could overcome MEN1 mutations, we established a PDX model from a patient who acquired a MEN1T349M mutation while receiving revumenib (Figure 6G-H; supplemental Figure 6G). We used a threefold higher dose of chow (0.1%) in this experiment, which confers similar peak plasma concentrations to patients on clinical trials but more sustained levels over a 24-hour period.13,16 Mezigdomide monotherapy resulted in a modest antileukemic response. VTP-50469 reduced leukemia burden in all mice for about 2 months, however, 2 mice then began to progress on treatment. One of these mice was found to have a new MEN1M327I mutation. Given that MEN1WT/M327I shifts the dose-response curve for VTP-50469 to a greater extent than MEN1T349M/T349M, this suggests that in models with a preexisting MEN1 mutation, a more deleterious MEN1 mutation may arise as a form of acquired resistance. The other mouse progressing on therapy did not have a new MEN1 hotspot mutation by Sanger sequencing, demonstrating that non-MEN1 mutation–driven mechanisms of acquired resistance can arise. Combination therapy greatly extended survival. Two mice receiving combination therapy relapsed several months after the treatment period ended, neither of which had a new MEN1 hotspot mutation detected. Thus, despite a preexisting MEN1 mutation, more than half the mice treated with combination therapy were effectively cured. These PDX models show that combination therapy more rapidly clears disease burden, prevents or delays the development of MEN1 mutations, and can leverage the partially retained activity of menin inhibitors at higher doses to overcome a preexisting MEN1 mutation.
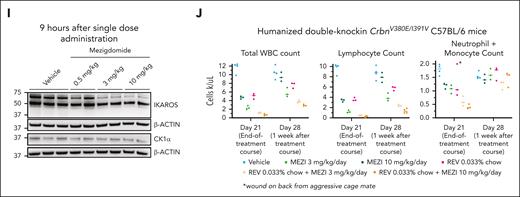
Lastly, we sought to assess toxicity of mezigdomide with and without menin inhibition in vivo, in part because, in the CBAM-44728 PDX, 4 of 6 mice treated with mezigdomide + VTP-50469 had to be euthanized due to acute weight loss (4, 8, 11, and 25 days after the last dose of mezigdomide). At the time of death, these mice had no evidence of leukemia by flow cytometry; no signs of hematologic toxicity; and had elevated murine white blood cell and neutrophil counts suggestive of infection (supplemental Figure 6H-K). Mouse cereblon has critical amino acid residues that are different compared with humans, rendering IMiDs/CELMoDs ineffective in murine cells.26 “Humanized” mouse models, wherein key amino acid residues have been changed to the human form, allow for neosubstrate degradation with lenalidomide (CrbnI391V) and the GSPT1-degrading CELMoD eragidomide (CrbnV380E/I391V).34,35 Using CrbnV380E/I391V C57BL/6 mice, we confirmed mezigdomide-induced IKAROS degradation in vivo, finding comparable substrate degradation to what we observed after a single dose in PDX models (Figure 6I; supplemental Figure 5E). We further demonstrated substrate degradation and antiproliferative activity ex vivo in c-KIT+, CrbnV380E/I391V mouse cells transduced with KMT2A-AF9 but not in CrbnWT/WT mouse cells (supplemental Figure 6L-M). We then treated CRBNV380E/I391V C57BL/6 mice for 21 days with mezigdomide at 3 mg/kg per day and 10 mg/kg per day with and without revumenib 0.033% chow. The mice gained weight throughout the treatment course with no deaths observed during the treatment course or in the 3 months following treatment cessation. Consistent with prior experience with IMiDs/CELMoDs, there was leukopenia that was predominantly driven by lymphopenia and, to a lesser extent, by neutrophils/monocytes (Figure 6J; supplemental Figure 6N-O). This effect was similar between single-agent mezigdomide and revumenib and more pronounced with combination therapy. The neutrophil/monocyte count recovered fully in all treatment groups 1 week after therapy cessation. Further evaluation of the impact of combination therapy in colony forming assays performed in CD34+–selected cord blood samples and adult bone marrow revealed more pronounced effects with combination therapy than with either agent alone, however not substantially so (supplemental Figure 6P). These data suggest that combination therapy is tolerated in mice with humanized cereblon and that there is reversible neutropenia/monocytopenia.
Discussion
The development of a highly potent IKAROS degrader, mezigdomide, has enabled further demonstration that IKAROS is an important target in KMT2A-r and NPM1c AML. We have found that, compared with the less potent IKAROS degraders lenalidomide and iberdomide, mezigdomide has superior single-agent activity as well as greater synergy and ability to increase apoptosis in combination with menin inhibitors. Furthermore, synergistic and apoptotic activity of mezigdomide and menin inhibition is retained in MEN1-mutant models at higher doses of menin inhibitor. We have shown that mezigdomide affects a HOXA9 gene expression program that is highly correlated with but more potent than iberdomide-induced changes. Thus, the greater efficacy seen with mezigdomide is possibly due to larger impacts on a HOXA9 gene expression program. The greater synergy seen with VTP-50469, which markedly decreases MEIS1 gene expression,13 could be explained by enhanced dual-targeting of HOXA/MEIS1-driven gene expression programs. This greater potency of mezigdomide is due to the rate, depth, and duration of IKAROS degradation after washout. This phenomenon is being observed in multiple myeloma clinical trials, in which patients with 4-day breaks in mezigdomide dosing did not exhibit full substrate recovery, whereas those receiving 7-day breaks did.23,36
We have shown that mezigdomide has potent single-agent activity in vivo, as demonstrated by extended survival compared with vehicle control in 6 PDX models, although without cure of any mice. Mezigdomide greatly enhances the activity of menin inhibitors in vivo, including in 3 PDX models in which mice receiving menin inhibitor monotherapy developed a new MEN1 mutation. One of these PDXs was established from a patient who acquired a MEN1T349M mutation while receiving revumenib, and half the mice in this experiment were effectively cured with combination therapy. This latter PDX model corroborates our in vitro findings in MEN1-mutant models that mezigdomide in combination with revumenib can still synergize and cause apoptosis with higher doses of menin inhibition. These findings strongly support targeted combination therapy with menin inhibitors moving into clinical trials and suggests that patients with select MEN1 mutations may continue to benefit from further therapy containing menin inhibitors.
Lenalidomide has been tested in numerous clinical trials in AML due to its CK1α degradation and efficacy in del(5q) myelodysplastic syndrome26 and exhibited mixed results. Nevertheless, a 2011 phase 2 study in de novo AML found that lenalidomide had a 30% complete remission (CR)/CR with incomplete blood count recovery (CRi) rate, and, of the 4 patients in CR/CRi at the time of publication (>16 months), 1 patient had a FAB M5b with “MLL abnormal” and another an NPM1c AML.37 Given this and our data here presented, we believe clinical trials using a much more potent IKAROS degrader (mezigdomide) in rationally chosen molecular subtypes of AML (KMT2A-r and NPM1c) are warranted, with or without menin inhibitors. Encouragingly, menin inhibitors and mezigdomide are in phase 2 and 3 clinical trials, respectively,38-40 suggesting the ability for rapid clinical translation. Although we have demonstrated in mice with humanized Crbn that mezigdomide as a single agent and in combination with menin inhibition is well tolerated apart from reversible neutropenia, we did observe nonleukemic deaths in some mouse models with prolonged survival. We hypothesize this toxicity is a result of the frailty of immunodeficient mice. However, toxicity (hematopoietic and otherwise) should be carefully monitored if human clinical trials are initiated.
Acknowledgments
The authors thank Michael Cameron for performing LC-MS to infer plasma concentrations of mezigdomide for in vivo experiments.
This study was supported by the following sources: National Institutes of Health (NIH), National Heart Lung and Blood Institute training grant 5T32HL007574-40, Wong Family Award in Translational Oncology, and The Damon Runyon Physician-Scientist Training Award (W.B.); Ruth L. Kirschstein Postdoctoral Individual National Research Service Award (NIH, National Cancer Institute (NCI) F32CA250240-02; J.A.C.) and a K99/R00: Pathway to Independence Award (NIH/NCI) 1K99CA279888-01; J.A.C.); Career Development fellowship from the Leukemia and Lymphoma Society, USA (B.J.A.); Walter Benjamin Fellowship by the German Research Foundation (DFG, WE 7304/1-1; D.V.W.); German Research Foundation (DFG, PE 3217/1-1) and a research grant from the “Else Kröner-Fresenius-Stiftung” (2021-EKEA.111; F.P.); Fonds de recherche du Québec- Santé (FRQS) Postdoctoral Fellowship (M.B.); the Claudia Adams Barr Program for Innovative Cancer Research (S.N.O.); St. Baldrick’s Foundation Consortium grant and Hannah’s Heroes (Y.P.); NIH/NCI K08CA252174 (A.S.S.); and NIH/NCI grants CA176745, CA206963, CA204639 and CA066996; a Curing Kids Cancer grant; and an Alex’s Lemonade Stand Foundation Crazy 8 grant (S.A.A.).
Authorship
Contribution: W.B., J.A.C, B.J.A., D.V.W., F.P., K.K., R.P.N., K.A.D., M.B. B.D.C, A.S.S., E.S.F., and S.A.A designed experiments; W.B., J.A.C, B.J.A., D.V.W., F.P., C.M., J.A.H., K.K., R.P.N., K.A.D, M.B. A.A.A., S.N.O., and N.K. performed the experiments; W.B., B.J.A., F.P., M.B. S.N.O., Y.P, J.A. Perry, A.S.S., and B.L.E. developed models used throughout the manuscript; Y.W. and C.H. performed bioinformatics analysis; W.B, J.A.C, B.J.A., D.V.W., F.P., K.K., R.P.N., K.A.D., C.W., C.H., Y.P. J. A. Pollard, J. A. Perry, A.S.S., B.L.E., G.M.M., E.S.F., and S.A.A. analyzed and interpreted the data; W.A.B., J.A.C., J. A. Perry, and S.A.A. wrote and edited the manuscript; and all authors read and approved the manuscript.
Conflict-of-interest disclosure: B.J.A. is a former employee of the Walter and Eliza Hall Institute of Medical Research, Melbourne, Australia, and receives proceeds from royalties and milestone payments related to the BCL2 inhibitor ABT-199 (venetoclax). K.A.D is a consultant to Kronos Bio and Neomorph Inc. F.P. received travel funding from Syndax Pharmaceuticals. J.A. Pollard receives clinical trial funding from AbbVie and Servier Pharmaceuticals. A.S.S. is a consultant for Novartis and Roche. B.L.E. has received research funding from Celgene, Deerfield, Novartis, and Calico; and is a member of the scientific advisory board of and a shareholder in Neomorph Inc, TenSixteen Bio, Skyhawk Therapeutics, and Exo Therapeutics. E.S.F is a founder, scientific advisory board member, and equity holder of Civetta Therapeutics, Lighthorse Therapeutics, Proximity Therapeutics, and Neomorph, Inc (board of directors); is an equity holder and scientific advisory board member in Avilar Therapeutics and Photys Therapeutics; and a consultant to Novartis, Sanofi, EcoR1 Capital, Odyssey, and Deerfield. The Fischer laboratory receives or has received research funding from Deerfield, Novartis, Ajax, Interline, and Astellas. S.A.A. has been a consultant and/or shareholder for Neomorph, Inc, Cyteir Therapeutics, C4 Therapeutics, Nimbus Therapeutics, and Accent Therapeutics; has received research support from Janssen, and Syndax; and is an inventor on a patent related to menin Inhibition WO/2017/132398A1. The remaining authors declare no competing financial interests.
Correspondence: Scott A. Armstrong, Dana-Farber Cancer Institute, Pediatric Oncology, 450 Brookline Avenue, Boston, MA 02215; email: scott_armstrong@dfci.harvard.edu.
References
Author notes
The RNA-sequencing and proteomics data have been deposited in public repositories listed below.
RNA-sequencing data are available at Gene Expression Omnibus database (accession number GSE232007).
Proteomics data are available at PRIDE archives: PXD041735 (wp-esf_280) and PXD041737 (wp-esf_281).
Original data are available upon request from the corresponding author, Scott A. Armstrong (scott_armstrong@dfci.harvard.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Mezigdomide has enhanced IKAROS degradation and antiproliferative activity in KMT2A-r and NPM1c cell lines. (A) Proliferation (cell counts) relative to dimethyl sulfoxide (DMSO) control in the MOLM-13 cell line treated for 18 days with 100 nM of lenalidomide (LEN), iberdomide (IBER), or mezigdomide (MEZI), (data pooled from 5 independent experiments; n = 15 per data point; data points represent mean ± standard deviation [SD]). Two-way analysis of variance (ANOVA) with Tukey multiple comparisons test: MEZI vs LEN or IBER: P < .005 for all days from 3 to 18; LEN vs IBER: no significant difference at day 3; P < .005 for all days from 6 to 18. (B) Western blot in MOLM-13 cell line treated for 12 hours. (C) Proliferation relative to DMSO in a panel of KMT2A-r and NPM1c AML cell lines treated for 9 days with 8-point dose curves of LEN, IBER, or MEZI. (n ≥ 3 per data point; data points represent mean ± SD; each curve representative of 3 independent experiments). (D) Absolute IC50 at day 9 for panel C (left) and 5 non–KMT2A-r, non-NPM1c myeloid leukemia cell lines (right) assessed by CellTiter-Glo or cell counts. Bolded and italicized values represent cell lines sensitive to drug (absolute IC50 ≤ 1000 nM). Absolute IC50 calculated with data pooled from 3 independent experiments per cell line for proliferation; 2 independent experiments for CellTiter-Glo assays, with each experiment having at least a 5-point dose titration per IMiD/CELMoD. See supplemental Figure 1I-K for 95% confidence intervals. (E) Proliferation relative to DMSO in CD34+-selected human cord blood cells that were KMT2A-AF9 transformed (left) vs untransformed (middle), assessed at day 6. (Left and middle) Data pooled from 3 independent experiments; n = 9 per data point; data points represent mean ± SD. (Right) For the top concentration of each drug tested in the graphs on the left and middle, proliferation relative to DMSO in KMT2A-AF9 transformed vs untransformed human cord blood cells. Statistical comparison with 1-way ANOVA with Tukey multiple comparisons test; ∗∗∗∗P < .0001.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/15/10.1182_blood.2023021105/3/m_blood_bld-2023-021105-gr1.jpeg?Expires=1765319722&Signature=iP4x5d3jPeM8GHXDvw7v2CUjUrmPJ3WwU4~2~DuJCufDUwrIZfL8wsA0L35cEIQ9y3vAw8cIa5gWuUuSrNQFBgt9qpvUFq-lgeUnCsdXHy9i6xMa1C6AVKIxu4We2941~2oRqKxOIHTgBuWVnnooZv5Gml3oom88wj7L40q-yv0AmcWRGak9WeQNB2reQ4QeypafpYKYO5MPoSGjudyvlP-b7RP1ylm0bDOKAiSMTHbS3ZTraPnzKL5jZz0XulgW4J7r59Nv3oE7h5dZ9Gp4ZQ1w3OK968HTrHQu78r2AJWVqiTG8B4yahtDxkb9w2UtHUcDOMhFo-gt~Xsv-NvYGw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)