In this issue of Blood, Byrnes and colleagues discuss an elegant series of experiments, in both mice and humans, to explore how the subunits of factor XIII (FXIII) reciprocally stabilize each other in circulation.1
First identified in 1944 as an essential protein for fibrin stabilization, coagulation factor XIII serves a unique enzymatic function in the hemostatic process, functioning as a transglutaminase that forms bonds between the glutamine and lysine side chains of fibrin monomers.2,3 With an estimated prevalence of 1 in ≈2 million people, inherited factor XIII deficiency is rare and is clinically characterized by delayed bleeding; however, factor XIII is also essential for wound healing and maintenance of pregnancy.4-6 Treatment of factor XIII deficiency consists of infusions of factor XIII–containing products, such as cryoprecipitate or either plasma-derived or recombinant factor XIII concentrate.
In plasma, factor XIII circulates as a heterodimer (FXIII-A2B2) composed of 2 A subunits (FXIII-A) and 2 B subunits (FXIII-B), synthesized in hematopoietic cells and hepatocytes, respectively. Thrombin mediates the cleavage of factor XIII activation peptides leading to factor XIIIa. Although the factor XIII heterodimer has a half-life of 9 to 10 days, isolated factor XIII-A2 has a half-life of only ≈3 hours, with prior studies showing that FXIII-B2 stabilizes factor XIII-A2 in plasma.7
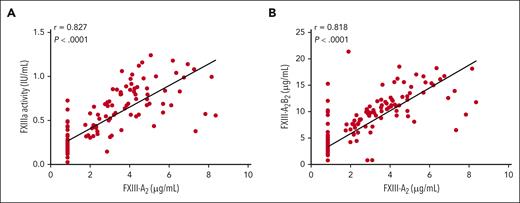
Using both an in vivo experimental model, in which C57BL/6 F13a1−/− mice were infused with human recombinant FXIII-A2 (rFXIII-A2), and pharmacokinetic data from the mentor2 trial in human subjects receiving prophylactic recombinant FXIII-A2 for treatment of factor XIII-A deficiency, the authors investigated the mechanisms by which FXIII-A2 regulates plasma FXIII-B2 levels.8 They found that although FXIII-A2 increased plasma FXIII-B2 levels in both humans (see figure) and mice, they also discovered that FXIII-A2 did not induce de novo FXIII-B2 synthesis or enhance FXIII-B2 secretion from the liver. Instead, FXIII-A2 prevented the loss of FXIII-B2 from plasma. The authors demonstrate that FXIII-A does not induce FXIII-B synthesis or release from hepatocytes, suggesting rather that it decreased clearance of FXIII-B by stabilizing the subunit. Free FXIII-B2 has a shorter half-life in plasma than FXIII-A2B2, and when FXIII-A2 is infused into humans or mice, free FXIII-B2 levels initially decline as FXIII-B2 becomes incorporated into FXIII-A2B2 complexes. Free FXIII-B2 levels rebounded to baseline within a few days, suggesting that FXIII-A2 prevents the clearance of free FXIII-B2 from plasma.
FXIII-A2 level correlates with FXIIIa activity and FXIII-A2B2 level after rFXIII-A2 infusion in humans. Spearman correlations between FXIII-A2 antigen and (A) FXIIIa activity or (B) FXIII-A2B2 antigen in human plasma after infusion of 35 IU/kg rFXIII-A2 (mentor2 trial). Each dot represents 1 measurement; the lower limit of detection for FXIII-A2 antigen was 0.845 μg/mL.
FXIII-A2 level correlates with FXIIIa activity and FXIII-A2B2 level after rFXIII-A2 infusion in humans. Spearman correlations between FXIII-A2 antigen and (A) FXIIIa activity or (B) FXIII-A2B2 antigen in human plasma after infusion of 35 IU/kg rFXIII-A2 (mentor2 trial). Each dot represents 1 measurement; the lower limit of detection for FXIII-A2 antigen was 0.845 μg/mL.
Although interactions of 2 subunits of protein are common throughout normal physiology, including cell cycle kinases, phosphatases, and ubiquitin-regulated protein degradation, reciprocal regulation of the subunits has not been demonstrated before in this manner. For instance, von Willebrand factor stabilizes coagulation factor VIII in circulation, leading to a longer half-life, but both proteins are synthesized in vascular endothelial cells and factor VIII is not believed to stabilize von Willebrand factor in a reciprocal relationship. Further research is needed to understand whether the interactions between FXIII-A and FXIII-B subunits obscure motifs on each other that modify clearance from circulation. The authors note that high-resolution structural studies of FXIII-A2B2 are needed to fully characterize the mechanism by which reciprocal stabilization occurs. In addition, the authors theorize that FXIII-B2 circulates in a 2-fold excess relative to FXIII-A2 to ensure that newly released FXIII-A2 is rapidly stabilized to avoid premature clearance.
These data also have important implications for the clinical management of people with factor XIII deficiency. First, they provide data on which to base novel therapeutics based on factor XIII replacement or stabilization. Such therapies could help to maintain higher levels of FXIII-B2 in plasma and improve hemostasis in people with FXIII deficiency. Second, they provide physiological insights about whether the use of plasma-derived or recombinant factor XIII concentrate would be appropriate depending on the clinical situation. For instance, in a trauma situation, in which a rapid increase in factor XIII activity is needed, infusing plasma-derived factor XIII containing FXIII-A2B2 would be appropriate, as the heterodimer is fully formed and does not require time for stabilization. The available recombinant factor XIII product only contains FXIII-A2, so it is only approved for people with A-subunit mutations. Furthermore, these experiments provide an understanding as to why certain mutations in factor XIII lead to decreased stability of the circulating heterodimer. Several FXIII-B variants produce normal antigen levels and stability, but with reduced binding to FXIII-A2, leading to an unstable heterodimer and mild FXIII deficiency.
The authors' findings will support the development of new therapeutic strategies for other bleeding disorders. For example, other proteins that form heterotetramers in plasma may be regulated in a similar way to FXIII. By understanding these regulatory mechanisms, researchers may be able to develop new therapies to stabilize other hemostatic proteins and improve hemostasis in people with bleeding disorders.
Conflict-of-interest disclosure: N.T.C. reports serving as a consultant for Takeda, participating in advisory boards for Takeda, Genentech, and Medzown, and receiving honoraria/travel support from Octapharma AG.