FXIII-A increases FXIII-B levels in plasma, but FXIII-A does not induce de novo FXIII-B synthesis or release from hepatocytes.
The FXIII-A and FXIII-B subunits exhibit reciprocal stabilization in plasma.
Visual Abstract
Transglutaminase factor XIII (FXIII) is essential for hemostasis, wound healing, and pregnancy maintenance. Plasma FXIII is composed of A and B subunit dimers synthesized in cells of hematopoietic origin and hepatocytes, respectively. The subunits associate tightly in circulation as FXIII-A2B2. FXIII-B2 stabilizes the (pro)active site-containing FXIII-A subunits. Interestingly, people with genetic FXIII-A deficiency have decreased FXIII-B2, and therapeutic infusion of recombinant FXIII-A2 (rFXIII-A2) increases FXIII-B2, suggesting FXIII-A regulates FXIII-B secretion, production, and/or clearance. We analyzed humans and mice with genetic FXIII-A deficiency and developed a mouse model of rFXIII-A2 infusion to define mechanisms mediating plasma FXIII-B levels. Like humans with FXIII-A deficiency, mice with genetic FXIII-A deficiency had reduced circulating FXIII-B2, and infusion of FXIII-A2 increased FXIII-B2. FXIII-A-deficient mice had normal hepatic function and did not store FXIII-B in liver, indicating FXIII-A does not mediate FXIII-B secretion. Transcriptional analysis and polysome profiling indicated similar F13b levels and ribosome occupancy in FXIII-A-sufficient and -deficient mice and in FXIII-A-deficient mice infused with rFXIII-A2, indicating FXIII-A does not induce de novo FXIII-B synthesis. Unexpectedly, pharmacokinetic/pharmacodynamic modeling of FXIII-B antigen after rFXIII-A2 infusion in humans and mice suggested FXIII-A2 slows FXIII-B2 loss from plasma. Accordingly, comparison of free FXIII-B2 vs FXIII-A2-complexed FXIII-B2 (FXIII-A2B2) infused into mice revealed faster clearance of free FXIII-B2. These data show FXIII-A2 prevents FXIII-B2 loss from circulation and establish the mechanism underlying FXIII-B2 behavior in FXIII-A deficiency and during rFXIII-A2 therapy. Our findings reveal a unique, reciprocal relationship between independently synthesized subunits that mediate an essential hemostatic protein in circulation. This trial was registered at www.ClinicalTrials.com as #NCT00978380.
Introduction
Plasma coagulation factor XIII (FXIII) circulates as a noncovalent heterotetramer (FXIII-A2B2).1,2 Each FXIII-B subunit consists of 10 sushi domains and has homology to complement factor H (20 sushi domains).3 The FXIII-A subunits, which contain the (pro)active sites, are members of a widespread transglutaminase superfamily that has roles in and beyond coagulation, including skin barrier maintenance, pregnancy, and osteogenesis.4 Circulating FXIII-A2B2 heterotetramer is essential for hemostasis, and individuals lacking FXIII-A2B2 exhibit bruising, poor wound healing, delayed bleeding after surgery, and increased risk of intracerebral hemorrhage (reviewed in5).
The FXIII-A and -B subunits are synthesized in different tissues. FXIII-A polymorphisms track with bone marrow transplantation,6 and transcriptional analysis and murine Cre/lox studies suggest plasma FXIII-A is produced by tissue macrophages thought to reside in the aorta.7 In contrast, FXIII-B polymorphisms track with liver transplantation,6 and F13B expression in humans is detected exclusively in hepatocytes.8 Both FXIII-A and FXIII-B are secreted as dimers. FXIII-A2B2 assembly occurs in plasma. Essentially all (∼99%) plasma FXIII-A2 (∼43-86 nM) circulates tightly bound to FXIII-B22,9; however, an approximately twofold excess of FXIII-B2 circulates in plasma, so that ∼50% of FXIII-B2 is not bound to FXIII-A2 (“free” FXIII-B2).2
Compared with FXIII-A2B2, isolated FXIII-A2 has a short half-life in plasma (∼3 hours vs ∼9-10 days),10-16 and in vivo genetic and pharmacologic studies have established that FXIII-B2 stabilizes FXIII-A2 in plasma.17-20 Interestingly, several lines of evidence suggest FXIII-A2, in turn, increases FXIII-B2 levels in plasma. First, studies in humans and mice show a F13A1/F13a1 gene-dose–dependent effect on plasma FXIII-B2 concentrations; humans with FXIII-A deficiency have only 50% of normal FXIII-B antigen,9 and F13a1−/− mice have <20% of normal FXIII-B2 in circulation.20,21 Second, recombinant FXIII-A2 (rFXIII-A2) infusion increases plasma FXIII-B2 in both humans11 and monkeys.22 Collectively, these observations imply a reciprocal relationship between these subunits that determines the concentration of FXIII-A2B2 in circulation.
Instances of reciprocal protein regulation are common in intracellular feedback loops.23,24 Natural killer and autoreactive T-cell populations also exhibit systemic, cellular reciprocal regulation.25 However, an intertissue, reciprocal regulatory mechanism in which 2 protein subunits regulate their respective levels in systemic circulation has, to our knowledge, not previously been described. Herein, we studied humans and mice with genetic FXIII-A deficiency and used a novel model of therapeutic FXIII-A2 administration to define a newly recognized reciprocal regulatory relationship between FXIII-A2 and FXIII-B2 subunits in circulation.
Materials and methods
Pharmacokinetic (PK) data for rFXIII-A2 dosing in humans were from the mentor2 trial, a multicenter, international, open-label, single-arm, multiple-dosing, nonrandomized trial to investigate the long-term safety of rFXIII-A2.26 Details regarding patient demographics and trial design were previously reported.26 FXIII activity and antigen in human plasmas were measured by chromogenic assay and enzyme-linked immunosorbent assays. FXIII-A and -B in F13a1+/+ and F13a1–/– mice, as well as F13a1–/– mice treated with rFXIII-A2, were measured by immunoblotting. F13b transcription and translation were measured by reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR) and polysome profiling,27 respectively. FXIII pharmacologic behavior was modeled by nonlinear mixed effects modeling, and pharmacodynamic (PD) estimates were derived according to Sharma and Jusko.28 Details for materials and methods are in the online supplement.
Results
FXIII-A2 increases FXIII-B levels in human plasma
To characterize the effect of rFXIII-A2 administration on circulating FXIII-B2, we first reexamined data collected in the mentor2 trial of prophylactic rFXIII-A2 administration for treatment of FXIII-A deficiency.26 Kerlin et al26 previously published an immediate 4.6-fold increase in available FXIIIa activity upon rFXIII-A2 infusion (reprised here in Figure 1A). We now show that total FXIII-A2 increased 5.7-fold in parallel with FXIIIa activity (Figure 1B), and total FXIII-A2 and FXIIIa activity correlated significantly (Spearman r = 0.827; P < .0001; supplemental Figure 1A, available on the Blood website). The circulating concentration of FXIII-A2B2 heterotetramer increased 12.1-fold upon rFXIII-A2 infusion (Figure 1C), and FXIII-A2B2 and FXIII-A2 correlated significantly (Spearman r = 0.818; P < .0001; supplemental Figure 1B), consistent with complex formation between infused rFXIII-A2 and endogenous FXIII-B2 (molecular weight of FXIII-A2B2 is twice that of FXIII-B2 [∼320 vs 166 kDa, respectively]). FXIIIa activity, FXIII-A2, and FXIII-A2B2, each declined to baseline within 28 days after rFXIII-A2 dosing (Figure 1A-C), consistent with the ∼9 to 10–day half-life of the FXIII-A2B2 heterotetramer.10 After rFXIII-A2 administration, free FXIII-B antigen (not in complex with rFXIII-A2) levels first rapidly declined, reaching a nadir within 1 hour after infusion because available FXIII-B2 became incorporated into heterotetrameric complexes with administered rFXIII-A2 (Figure 1D). Interestingly, free FXIII-B2 subsequently rebounded to baseline (pre–rFXIII-A2 administration) levels within 3 days (Figure 1D), such that the total circulating FXIII-B2 (FXIII-A2B2 + free FXIII-B2) increased ∼1.7-fold compared with levels before rFXIII-A2 administration (Figure 1E). Together with previous findings on the pharmacologic behavior of FXIII-B2 in patients infused with rFXIII-A2,11,12 these data indicate FXIII-A2 enhances circulating FXIII-B2 levels in humans.
FXIII-A2 increases circulating FXIII-B in humans. (A) Time course of FXIII(a) activity measured by chromogenic assay after infusion of 35 IU/kg recombinant FXIII-A2 in 23 patients with FXIII deficiency (mentor2 trial, adapted from Kerlin et al26). (B-D) Plasma concentrations of FXIII-A2 (B), FXIII-A2B2 heterotetramer (C), and free FXIII-B (lower detection limit, 0.845 μg/mL) (D) after FXIII-A2 infusion in these patients, determined by ELISA. (E) Estimated total FXIII-B was determined by calculating the sum of FXIII-B in heterotetrameric FXIII-A2B2 and free FXIII-B at each timepoint. Curves from individual subjects are indicated in gray, and the geometric mean is shown with a colored line in each graph. The x-axis represents nominal time. Predose, baseline measurements (“Pre”) are indicated in each graph. ELISA, enzyme-linked immunosorbent assay; IU, international units.
FXIII-A2 increases circulating FXIII-B in humans. (A) Time course of FXIII(a) activity measured by chromogenic assay after infusion of 35 IU/kg recombinant FXIII-A2 in 23 patients with FXIII deficiency (mentor2 trial, adapted from Kerlin et al26). (B-D) Plasma concentrations of FXIII-A2 (B), FXIII-A2B2 heterotetramer (C), and free FXIII-B (lower detection limit, 0.845 μg/mL) (D) after FXIII-A2 infusion in these patients, determined by ELISA. (E) Estimated total FXIII-B was determined by calculating the sum of FXIII-B in heterotetrameric FXIII-A2B2 and free FXIII-B at each timepoint. Curves from individual subjects are indicated in gray, and the geometric mean is shown with a colored line in each graph. The x-axis represents nominal time. Predose, baseline measurements (“Pre”) are indicated in each graph. ELISA, enzyme-linked immunosorbent assay; IU, international units.
FXIII-A increases FXIII-B levels in mouse plasma
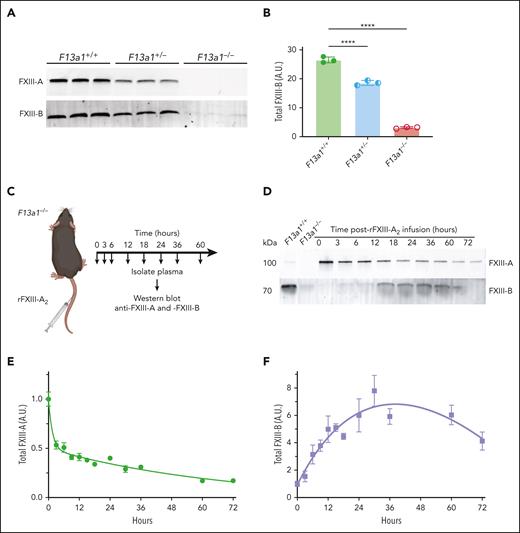
To investigate the mechanism(s) mediating the effect of FXIII-A2 on plasma FXIII-B levels, we first characterized FXIII-A and FXIII-B levels in mice with genetic deletion of F13a1. F13a1+/– mice have ∼50% of normal plasma FXIII-A antigen, and F13a1–/–mice are completely deficient in FXIII-A (Figure 2A and Souri et al20 and Kattula et al29). Similar to humans, F13a1+/− and F13a1–/– mice have reduced FXIII-B antigen (∼70% and ∼10% of normal FXIII-B levels, respectively; Figure 2B and Souri et al20) that did not vary with sex (supplemental Figure 2). Variability in FXIII-B in F13a1–/– mice (median, 3.7 μg/mL; interquartile range [IQR], 2.2-6.9) was somewhat higher than that seen in humans (estimated total FXIII-B predose values in Figure 1E; median, 5.0 μg/mL; IQR, 4.4-6.7), likely reflecting the detection limit of this relatively low abundance protein in addition to the dynamic fluctuations that occur in homeostasis.
FXIII-A2 increases circulating FXIII-B in mice. (A) FXIII-A and -B in F13a1+/+, F13a1+/−, and F13a1–/– mouse plasma were visualized using immunoblotting with anti-FXIII-A and -B antibodies, respectively. Each lane shows plasma from 1 mouse. (B) FXIII-B band intensity was quantified using densitometry. Each dot represents 1 mouse, and lines show mean ± standard deviation. Groups were compared by 1-way ANOVA with Dunnett post hoc testing, ∗∗∗∗ P < .0001. (C) Schematic of in vivo rFXIII-A2 infusion model. F13a1–/– mice (aged 8-12 weeks) were infused with rFXIII-A2 (4 mg/kg) via the tail vein, and blood was collected at various times after infusion. (D) At the indicated times after infusion, plasma FXIII-A and -B antigen was visualized by immunoblotting with anti-FXIII-A and anti-FXIII-B antibodies, respectively. Representative blots are shown. (E) FXIII-A and (F) FXIII-B were quantified using densitometry and normalized to subunit levels immediately after infusion (determined from samples collected within 1 minute of infusion, indicated as the 0-hour timepoint). Dots represent means ± standard error, N = 3-18 per time point. ANOVA, analysis of variance; A.U., arbitrary units.
FXIII-A2 increases circulating FXIII-B in mice. (A) FXIII-A and -B in F13a1+/+, F13a1+/−, and F13a1–/– mouse plasma were visualized using immunoblotting with anti-FXIII-A and -B antibodies, respectively. Each lane shows plasma from 1 mouse. (B) FXIII-B band intensity was quantified using densitometry. Each dot represents 1 mouse, and lines show mean ± standard deviation. Groups were compared by 1-way ANOVA with Dunnett post hoc testing, ∗∗∗∗ P < .0001. (C) Schematic of in vivo rFXIII-A2 infusion model. F13a1–/– mice (aged 8-12 weeks) were infused with rFXIII-A2 (4 mg/kg) via the tail vein, and blood was collected at various times after infusion. (D) At the indicated times after infusion, plasma FXIII-A and -B antigen was visualized by immunoblotting with anti-FXIII-A and anti-FXIII-B antibodies, respectively. Representative blots are shown. (E) FXIII-A and (F) FXIII-B were quantified using densitometry and normalized to subunit levels immediately after infusion (determined from samples collected within 1 minute of infusion, indicated as the 0-hour timepoint). Dots represent means ± standard error, N = 3-18 per time point. ANOVA, analysis of variance; A.U., arbitrary units.
We then developed an in vivo experimental model of rFXIII-A2 infusion, in which we infused C57BL/6 F13a1–/– mice with human rFXIII-A2 (4 mg/kg, IV) and measured plasma rFXIII-A2 and total FXIII-B antigen over time (Figure 2C). Human and mouse FXIII-A have 86.9% sequence identity, and human rFXIII-A2 is functional in mice.30 Similar to that observed in humans (Figure 1B), rFXIII-A2 exhibited biphasic clearance (Figure 2D-E), representing initial, rapid clearance of uncomplexed rFXIII-A2, followed by slower clearance of rFXIII-A2 complexed with FXIII-B2 (FXIII-A2B2). Also similar to humans (Figure 1E), total FXIII-B antigen levels rose quickly after rFXIII-A2 treatment and peaked ∼30 hours after infusion (7.8-fold increase over baseline; Figure 2D,F) before dropping ∼50% 72 hours after infusion. We observed a similar effect of rFXIII-A2 on total FXIII-B antigen in a separate line of FXIII-A-deficient mice on a mixed 129Ola/CBACa background (supplemental Figure 3).31 Together, these data show that, similar to what is observed in humans, both endogenous FXIII-A2 expression and exogenous rFXIII-A2 infusion increase plasma FXIII-B2 in mice and establish both genetic and pharmacologic models as tools to interrogate the regulatory relationship between FXIII-A2 and -B2.
FXIII-A is not required for FXIII-B secretion from liver
The mechanism(s) underlying the regulatory role of FXIII-A2 on FXIII-B2 levels is unknown. FXIII-A2 could induce de novo FXIII-B2 synthesis, enhance FXIII-B2 secretion or stability, or a combination of these mechanisms. We first tested the hypothesis that FXIII-A2 is required for FXIII-B2 secretion, in which the loss of FXIII-A causes FXIII-B retention in the liver. Characterization of F13a1+/+, F13a1+/−, and F13a1−/− mice showed similar serum alkaline phosphatase, alanine transaminase, and aspartate transaminase activities, suggesting no obvious hepatic insufficiency in FXIII-A–deficient mice (Figure 3A-C). Livers from F13a1+/+, F13a1+/−, and F13a1–/– mice also did not differ in mass, and histological analysis of hematoxylin and eosin–stained sections revealed normal liver architecture (Figure 3D-E). The Human Protein Atlas indicates little-to-no staining for FXIII-B in healthy liver sections of humans.32 Accordingly, immunoblotting of liver lysates from F13a1+/+ and F13a1−/− mice, as well as F13a1–/– mice treated with rFXIII-A2, showed no evidence of FXIII-B accretion or intracellular stores (Figure 3F). Unlike humans, mice also express F13b in kidney20; however, F13a1+/+, F13a1–/–, and rFXIII-A2–treated F13a1–/– mice had similar serum blood urea nitrogen, creatine, kidney weight, and kidney histology, and blotting of kidney lysates did not reveal retained FXIII-B (supplemental Figure 4). Together, these data suggest FXIII-B2 secretion does not require FXIII-A2, and therefore, the FXIII-A2–dependent increase in circulating FXIII-B2 is not because of enhanced FXIII-B2 secretion into circulation.
FXIII-A2 is not required for FXIII-B2 secretion from mouse liver. Serum alkaline phosphatase (ALP) (A), alanine transaminase (ALT) (B), and aspartate transaminase (AST) (C) levels were determined in F13a1+/+, F13a1+/−, and F13a1–/– mice; liver weight (D) in F13a1+/+, F13a1+/−, and F13a1–/– mice, reported as a percentage of body weight. Dots represent individual mice, bars show mean ± standard deviation. Groups were compared by 1-way ANOVA with Dunnett post hoc testing (A-D). (E) Histology of liver tissue from F13a1+/+, F13a1+/−, and F13a1–/– mice. Sections were stained with hematoxylin and eosin. Representative low (top) and high (bottom) magnification images are shown. Scale bar indicates 100 μm (top) and 20 μm (bottom). (F) FXIII-B antigen in liver tissue lysates from F13a1+/+, F13a1–/–, and rFXIII-A2–treated F13a1–/– was visualized by immunoblotting with anti–FXIII-B antibody. Lysates were normalized by protein concentration and the same amount of total protein was loaded for each sample. Each lane shows 1 mouse. Recombinant FXIII-B (rFXIII-B) was included as a standard. ANOVA, analysis of variance; ns, nonsignificant; U/L, units/liter.
FXIII-A2 is not required for FXIII-B2 secretion from mouse liver. Serum alkaline phosphatase (ALP) (A), alanine transaminase (ALT) (B), and aspartate transaminase (AST) (C) levels were determined in F13a1+/+, F13a1+/−, and F13a1–/– mice; liver weight (D) in F13a1+/+, F13a1+/−, and F13a1–/– mice, reported as a percentage of body weight. Dots represent individual mice, bars show mean ± standard deviation. Groups were compared by 1-way ANOVA with Dunnett post hoc testing (A-D). (E) Histology of liver tissue from F13a1+/+, F13a1+/−, and F13a1–/– mice. Sections were stained with hematoxylin and eosin. Representative low (top) and high (bottom) magnification images are shown. Scale bar indicates 100 μm (top) and 20 μm (bottom). (F) FXIII-B antigen in liver tissue lysates from F13a1+/+, F13a1–/–, and rFXIII-A2–treated F13a1–/– was visualized by immunoblotting with anti–FXIII-B antibody. Lysates were normalized by protein concentration and the same amount of total protein was loaded for each sample. Each lane shows 1 mouse. Recombinant FXIII-B (rFXIII-B) was included as a standard. ANOVA, analysis of variance; ns, nonsignificant; U/L, units/liter.
FXIII-A2 does not increase F13b transcription or translation
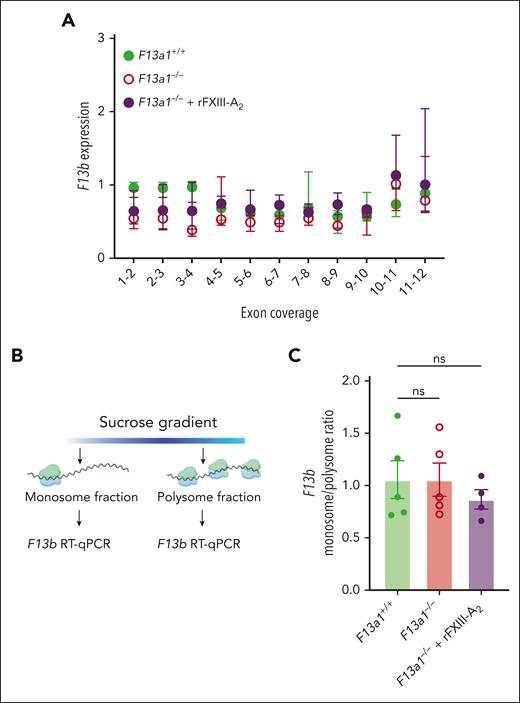
A second potential mechanism underlying the effect of FXIII-A2 on circulating FXIII-B2 is that FXIII-A2 enhances FXIII-B synthesis. To determine whether FXIII-A2 increases F13b transcription, we performed RT-qPCR using tiled primers sampling all 11 F13b exons to compare F13b messenger RNA (mRNA) in liver from F13a1+/+ mice with F13a1–/– and rFXIII-A2–treated F13a1–/– mice. Neither comparison showed significant differences in the expression of any F13b exons (Figure 4A). F13b transcripts also did not differ in kidneys from F13a1+/+, F13a1–/–, and rFXIII-A2–treated F13a1–/– mice (supplemental Figure 5).
FXIII-A2 does not alter F13b transcription or translation in mouse liver. (A) RNA was isolated from F13a1+/+, F13a1–/–, and rFXIII-A2–treated F13a1–/– mouse livers and F13b transcript level was determined by RT-qPCR using primers spanning each indicated exon junction. Dots show median fold change ± IQR, N = 4-5 per group. No significant differences in transcript levels relative to F13a1+/+ mice were detected by Kruskal-Wallis test with Dunn post hoc testing. (B) Schematic of polysome profiling protocol. (C) F13b transcript abundance in the monosome and polysome fractions isolated from F13a1+/+, F13a1–/–, and rFXIII-A2–treated F13a1–/– mouse livers, represented as a monosome/polysome ratio. Dots represent individual mice, bars show mean ± standard deviation. Groups were compared with 1-way ANOVA with Dunnett post hoc testing. ANOVA, analysis of variance; RT-qPCR, reverse transcriptase-quantitative polymerase chain reaction; ns, nonsignificant.
FXIII-A2 does not alter F13b transcription or translation in mouse liver. (A) RNA was isolated from F13a1+/+, F13a1–/–, and rFXIII-A2–treated F13a1–/– mouse livers and F13b transcript level was determined by RT-qPCR using primers spanning each indicated exon junction. Dots show median fold change ± IQR, N = 4-5 per group. No significant differences in transcript levels relative to F13a1+/+ mice were detected by Kruskal-Wallis test with Dunn post hoc testing. (B) Schematic of polysome profiling protocol. (C) F13b transcript abundance in the monosome and polysome fractions isolated from F13a1+/+, F13a1–/–, and rFXIII-A2–treated F13a1–/– mouse livers, represented as a monosome/polysome ratio. Dots represent individual mice, bars show mean ± standard deviation. Groups were compared with 1-way ANOVA with Dunnett post hoc testing. ANOVA, analysis of variance; RT-qPCR, reverse transcriptase-quantitative polymerase chain reaction; ns, nonsignificant.
To determine whether FXIII-A2 enhances translation of F13b transcripts, we performed polysome profiling of mRNA isolated from livers of F13a1+/+, F13a1–/–, and rFXIII-A2–treated F13a1−/− mice (Figure 4B). F13a1+/+, F13a1–/–, and rFXIII-A2–treated F13a1−/− mice showed similar F13b transcript levels in monosome and polysome fractions (Figure 4C, reported as monosome/polysome ratio), suggesting the presence of FXIII-A2, either genetically or pharmacologically, does not enhance F13b translation. Collectively, these data indicate that the FXIII-A2–dependent increase in circulating FXIII-B2 arises through nonsynthetic mechanisms.
PK/PD modeling of rFXIII-A2 and -B levels in mice and humans suggests FXIII-A2 stabilizes FXIII-B2 in circulation
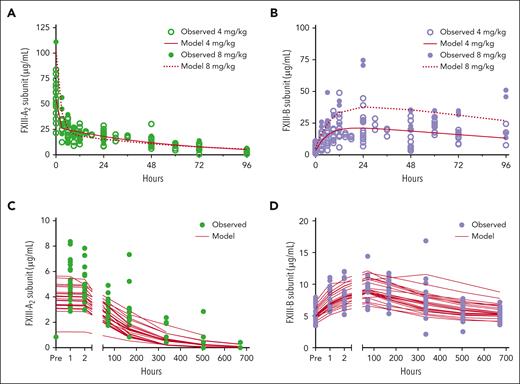
The surprising, but consistent, lack of evidence to support a synthetic mechanism for FXIII-A–dependent enhancement of FXIII-B led us to speculate that FXIII-A2 stabilizes FXIII-B2 in circulation. To test this hypothesis, we first used data from F13a1–/– mice to generate PK/PD (input/response) models of FXIII-A and -B levels after rFXIII-A2 infusion. As expected, models using measurements taken after infusion of either 4 or 8 mg/kg rFXIII-A2 were consistent with the observation that FXIII-A2 increases FXIII-B levels in plasma (Figure 5A-B). Because FXIII-A behavior appeared nonlinear (4 mg/kg AUC0-inf = 1465 μg × h/mL; 8 mg/kg AUC0-inf = 1590 μg × h/mL), we modeled these doses separately. Exploratory analysis of the 4 mg/mL dosing regimen indicated a biphasic distribution mechanism for FXIII-A, in which a 2-compartment model best described FXIII-A behavior (Akaike Information Criterion [AIC] values were 844.1, 783.3, and 787.3 for 1-, 2-, and 3-compartmental models, respectively). In this 2-compartment model, the first compartment can be conceptualized as free rFXIII-A2 immediately after infusion, and the second compartment represents the FXIII-A2B2 complex. AIC values for data from the 8 mg/kg dosing regimen showed a similar pattern (361.52, 289.33, and 293.33 for 1-, 2-, and 3-compartment models, respectively). We then used the estimates from the 2-compartment model to compare fits with 2 indirect response models28: model 2, in which FXIII-A inhibits the loss of FXIII-B, and model 3, in which FXIII-A2 stimulates FXIII-B production, both of which could explain the observed increase in FXIII-B antigen after FXIII-A2 administration. We also explored a combination of models 2 and 3, in which both potential effects of FXIII-A2 occur simultaneously. This analysis suggested indirect response model 2 better predicted rFXIII-A2 and FXIII-B behavior (AIC = 1544.3 vs AIC of indirect response model 3 = 1546.3 or AIC of the combination of models 2 and 3 = 1552.5). Diagnostic plots for the 3 indirect response models were similar; observed concentrations vs predicted concentrations, weighted residuals vs time, and weighted residuals vs predicted concentrations for rFXIII-A2 and FXIII-B2 are shown in supplemental Figure 6.
Pharmacologic modeling defines FXIII-A2 and -B behavior in mice and humans. (A-B) Observed concentrations of FXIII-A2 (A) and FXIII-B (B) antigen after the administration of 4 mg/kg (open symbols) or 8 mg/kg (closed symbols) rFXIII-A2 to F13a1–/– mice; model-predicted concentrations are overlaid (red lines). (C-D) Observed concentrations of FXIII-A2 (C) and FXIII-B (D) (lower detection limit 0.845 μg/mL) per nominal time after the administration of 35 IU/kg rFXIII-A2 to humans with FXIII-A deficiency; model-predicted concentrations are overlaid (red lines).
Pharmacologic modeling defines FXIII-A2 and -B behavior in mice and humans. (A-B) Observed concentrations of FXIII-A2 (A) and FXIII-B (B) antigen after the administration of 4 mg/kg (open symbols) or 8 mg/kg (closed symbols) rFXIII-A2 to F13a1–/– mice; model-predicted concentrations are overlaid (red lines). (C-D) Observed concentrations of FXIII-A2 (C) and FXIII-B (D) (lower detection limit 0.845 μg/mL) per nominal time after the administration of 35 IU/kg rFXIII-A2 to humans with FXIII-A deficiency; model-predicted concentrations are overlaid (red lines).
We next used a similar approach to model FXIII-A and -B levels measured after rFXIII-A2 infusion in humans with FXIII-A deficiency (Figure 1). Given the sparsity of data points and some misfits in the highest FXIII-A concentrations early in the concentration-time curve (Figure 5C-D), we were not able to fit models incorporating potential nonstationary clearance or a “burst” effect of rFXIII-A2 administration. Thus, FXIII-A behavior in this data set best fit a 1-compartment model with bolus input and first-order elimination (AIC = 435.19 vs 443.19 and 451.18 for 2- and 3-compartmental models, respectively). We then used estimates from the 1-compartment model to compare indirect response models using inverse parameterization without incorporating a gamma exponent. Most parameters were well-estimated (low coefficient of variation, CV%) with the exception of the 50% inhibitory concentration (CV% = 60.5%). Diagnostic plots demonstrated adequate fits, with the exception of the misfits in FXIII-A2 noted above (supplemental Figure 7). Consistent with that seen in mice, this analysis showed that the combined rFXIII-A2 and FXIII-B2 behavior in humans was best described by indirect response model 2 (FXIII-A2 inhibits the loss of FXIII-B2, AIC = 1067.28), rather than indirect response model 3 (FXIII-A2 stimulates FXIII-B2 production, AIC = 1080.86). Given the sparsity of human data at single-dose level, we did not fit the combined model 2/model 3 effect model.
Thus, PK/PD modeling in both mice and humans was consistent with our experimental analysis suggesting that FXIII-A2 enhances FXIII-B2 levels in plasma by nonsynthetic mechanisms that reduce its removal from circulation.
FXIII-A2 enhances FXIII-B2 stability in circulation
Finally, we used in vitro and in vivo models to directly test the hypothesis that FXIII-A2 preserves FXIII-B2 in circulation. We first determined whether rFXIII-A2 protects FXIII-B2 from proteolytic degradation in plasma by incubating rFXIII-B2 in human plasma in the absence or presence of rFXIII-A2 for 24 hours at 37°C in vitro. Immunoblotting showed rFXIII-B antigen did not degrade, even in the absence of rFXIII-A2 (Figure 6A), suggesting there is not a constitutively active protease in plasma that degrades FXIII-B. Because both FXIII-A2B2 and “free” FXIII-B2 circulate bound to fibrinogen in vivo and binding is mediated by the FXIII-B subunits,21 we characterized the contribution of fibrinogen to circulating FXIII-B2 levels in vivo. Consistent with observations in afibrinogenemic humans,33 immunoblotting of plasma from mice with either disrupted binding of FXIII-A2B2 to fibrinogen (Fibγ390-396A)34 or afibrinogenemia (Fga–/–)35 showed normal levels of both FXIII-A and -B antigen (Figure 6B). Thus, binding to fibrinogen is not required to maintain FXIII-B2 levels in circulation.
FXIII-A2 stabilizes FXIII-B2 in circulation. (A) rFXIII-B2 was incubated in normal, pooled human plasma for 24 hours at 37°C in the absence or presence of rFXIII-A2 and visualized by immunoblotting with anti-FXIII-B antibody. Representative blot of N = 2 experiments. (B) Fibrinogen, FXIII-A, and FXIII-B antigen were visualized in plasma from wild-type, Fibγ390-396A, or afibrinogenemic (Fga–/–) mice by immunoblotting using anti-fibrinogen, anti–FXIII-A, and anti–FXIII-B antibodies. (C) Schematic of in vitro FXIII-A2B2 activation with thrombin (IIa) and CaCl2 to separate FXIII-A and -B subunits before infusion. Reactions were performed in the presence of the transglutaminase inhibitor T101 to inhibit activated FXIII-A2∗ (FXIII-A2∗-I [inhibited dimeric or monomeric FXIIIa]), and thrombin was quenched with hirudin before infusion into F13a1–/– mice via the tail vein. (D) Representative immunoblot of FXIII-A and -B antigen in mice infused with complexed (FXIII-A2B2) or uncomplexed FXIII-B2 at the indicated times after infusion. Subunits were visualized with anti–FXIII-A and -B antibodies. (E) FXIII-B antigen over time, after infusion, was visualized for each condition (solid line, complexed; dashed line, uncomplexed) using immunoblot with anti–FXIII-B antibody and quantified by densitometry. Data were quantified relative to FXIII-B antigen at the 0 hour timepoint immediately after infusion. Dots show mean ± standard deviation, N = 1-11/time point. (F) Relative FXIII-B antigen for each condition at the 6 hour timepoint. Dots represent individual mice, bars show mean ± standard deviation. Groups were compared using a 2-tailed, unpaired t test, ∗∗ P < .01. A.U., arbitrary units.
FXIII-A2 stabilizes FXIII-B2 in circulation. (A) rFXIII-B2 was incubated in normal, pooled human plasma for 24 hours at 37°C in the absence or presence of rFXIII-A2 and visualized by immunoblotting with anti-FXIII-B antibody. Representative blot of N = 2 experiments. (B) Fibrinogen, FXIII-A, and FXIII-B antigen were visualized in plasma from wild-type, Fibγ390-396A, or afibrinogenemic (Fga–/–) mice by immunoblotting using anti-fibrinogen, anti–FXIII-A, and anti–FXIII-B antibodies. (C) Schematic of in vitro FXIII-A2B2 activation with thrombin (IIa) and CaCl2 to separate FXIII-A and -B subunits before infusion. Reactions were performed in the presence of the transglutaminase inhibitor T101 to inhibit activated FXIII-A2∗ (FXIII-A2∗-I [inhibited dimeric or monomeric FXIIIa]), and thrombin was quenched with hirudin before infusion into F13a1–/– mice via the tail vein. (D) Representative immunoblot of FXIII-A and -B antigen in mice infused with complexed (FXIII-A2B2) or uncomplexed FXIII-B2 at the indicated times after infusion. Subunits were visualized with anti–FXIII-A and -B antibodies. (E) FXIII-B antigen over time, after infusion, was visualized for each condition (solid line, complexed; dashed line, uncomplexed) using immunoblot with anti–FXIII-B antibody and quantified by densitometry. Data were quantified relative to FXIII-B antigen at the 0 hour timepoint immediately after infusion. Dots show mean ± standard deviation, N = 1-11/time point. (F) Relative FXIII-B antigen for each condition at the 6 hour timepoint. Dots represent individual mice, bars show mean ± standard deviation. Groups were compared using a 2-tailed, unpaired t test, ∗∗ P < .01. A.U., arbitrary units.
We then infused FXIII-A2B2 and FXIII-B2 into mice to directly compare their time in circulation. We were unable to use rFXIII-B2 for this experiment because this material tended to precipitate at concentrations needed for in vivo administration and detection, as we and others have previously observed.21,36 Instead, we incubated plasma-derived FXIII-A2B2 with thrombin and calcium in the presence of the FXIIIa inhibitor T101 to uncouple the B subunits from the A subunits and then inhibited the thrombin with hirudin (Figure 6C). In control reactions, we pretreated the activation mixture of thrombin/calcium/T101 with hirudin before incubating with FXIII-A2B2 to maintain the FXIII-A2B2 complex. We then infused F13a1–/– mice with FXIII-B2 in its FXIII-A2–complexed (FXIII-A2B2) or –uncomplexed (FXIII-B2) form and sampled plasma at the indicated time points (Figure 6D-E). Immunoblotting of plasmas harvested after infusion revealed that 6 hours after infusion, approximately twice as much complexed FXIII-B antigen remained relative to uncomplexed FXIII-B antigen (P = .0014; Figure 6F). This observation is consistent with estimated half-lives of 11.2 h (95% confidence interval [CI], 7.5-21.6) for complexed FXIII-B vs 3.8 h (95% CI, 3.1-4.8) for uncomplexed FXIII-B (Figure 6E). The rapid clearance of uncomplexed “free” FXIII-B2 suggests FXIII-A2 prolongs FXIII-B2 in circulation and exposes a newly recognized relationship involving reciprocal stabilization between the FXIII-A and -B subunits.
Discussion
The role of FXIII-B2 in stabilizing FXIII-A2 is well established11,20,37; however, mechanisms mediating the clinical observation that FXIII-A2 increases circulating FXIII-B2 levels were unknown. Our study combined the analysis of biological, biochemical, and pharmacologic data from humans and mouse models of FXIII-A deficiency and therapeutic rFXIII-A2 administration to identify a unique relationship between these subunits. We showed that FXIII-A2 is not required for FXIII-B2 secretion from hepatocytes, and FXIII-A2 does not enhance FXIII-B2 transcription or translation. These observations indicate that FXIII-B2 synthesis is independent of FXIII-A2 levels, and the regulatory effect of FXIII-A2 on FXIII-B2 occurs in the plasma compartment. Consistent with this premise, our PK/PD modeling and mouse infusion studies indicate FXIII-A2 stabilizes FXIII-B2 in circulation. This observation establishes a previously unrecognized reciprocal relationship between the FXIII-A2 and -B2 subunits. This relationship underlies an impressive example of coevolution, in which both the potent affinity of the FXIII-A2/B2 interaction and the synthesis rates of each subunit work in concert to ensure sufficient functional FXIII-A2B2 is present in circulation to support essential biological functions, including hemostasis and wound healing. Taken together, these findings uncover a novel mechanism mediating the concentrations of these subunits in plasma and provide insight into the effects of clinical rFXIII-A2 administration.
The reciprocal dependence of the FXIII-A2 and -B2 subunits for circulation in plasma underscores the importance of the FXIII-A2/FXIII-B2 interface for maintaining hemostatic levels of FXIII-A2B2 in plasma. FXIII-A2 binds FXIII-B2 with high affinity (KD ∼10-10 M)2; however, the specific regions mediating the interaction between these dimers are unclear. FXIII-B consists of a string of 10 consensus repeat (“sushi”) domains based on their disulfide bonding pattern; the FXIII-B subunits dimerize via interactions between sushi domains 4 and 9.38 Experiments using truncated FXIII-B variants implicated the N-terminal sushi domain 1 in FXIII-A2 binding.38 However, an anti–FXIII-B antibody that disrupts the FXIII-A2/FXIII-B2 interaction recognizes a peptide (residues, 96-103) in sushi domain 2, suggesting this domain may also contribute to this interaction directly or through steric interactions with sushi domain 1.2 Accordingly, atomic force microscopy visualized 2 FXIII-B sushi domains interacting with FXIII-A.39 Congenital FXIII-B deficiency is rare, and disentangling the effects of F13B mutations on FXIII-B synthesis and secretion versus heterotetramer formation with FXIII-A2 and FXIII-A2 stabilization in vivo, has been difficult. However, in vitro expression studies identified FXIII-B variants that have normal antigen production and stability but reduced binding to FXIII-A2. These include FXIII-BIle81Asn in sushi domain 2 and FXIII-BVal401Glu in sushi domain 7, consistent with a broad interface and/or cooperative assembly mechanisms for the FXIII-A2B2 heterotetramer.40 Several heterozygous mutations in F13B have been identified that appear to exert a dominant negative effect by destabilizing the formation of either the FXIII-B2 dimer and/or FXIII-A2B2 heterotetramer and lead to mild FXIII deficiency.41 FXIII-A consists of 5 domains: an N-terminal activation peptide, a β-sandwich domain, a catalytic core, and 2 β-barrel domains. Residues mediating FXIII-A2 dimerization are spread across a large interface that includes the activation peptide and residues within the catalytic domain.42-47 Little is known about natural F13A1 mutations that disrupt heterotetramer formation. A FXIII-ATyr283Cys mutation causes folding and secretion defects and is unable to dimerize, and purified FXIII-ATyr283Cys protein binds FXIII-B subunits and forms heterotetramers at only 10% of that seen for wild-type FXIII-A.46 An integrative/hybrid approach using crosslinking mass spectrometry and molecular modeling of the heterotetramer suggested the FXIII-A2/FXIII-B2 interface is asymmetric, with intersubunit contacts between multiple domains in the FXIII-A and FXIII-B subunits.48 Such extensive interactions between the 2 subunits may obscure the motifs on each that promote clearance from circulation when exposed. Ultimately, high-resolution FXIII-A2B2 structural studies are required to fully understand FXIII-A2/FXIII-B2 interactions and illuminate structure-function mechanisms mediating the reciprocal stabilization of these subunits.
The finding that FXIII-B2 has a short half-life is consistent with PK/PD modeling studies of rFXIII-A2 infusion in monkeys in which the half-life of uncomplexed FXIII-B2 was estimated to be ∼4 hours.37 This short half-life is surprising because FXIII-B2 has been shown to bind several plasma proteins that have relatively stable half-lives. Both FXIII-B2 and FXIII-A2B2 circulate bound to fibrinogen,21 which has a half-life ∼4 days.49 Accordingly, fibrinogen binding was thought to underlie the observation that conjugation of coagulation FIX (half-life, ∼18 h) to FXIII-B increases the FIX half-life 3.9-fold in mice.50 However, our finding that FXIII-B antigen is not reduced in fibrinogen-deficient mice (Figure 6B) suggests that an alternate mechanism may extend this half-life. Immunoprecipitation studies suggest FXIII-B2 can also bind α2-macroglobululin, which has a half-life of approximately 2 to 3 days.51 Isolated FXIII-B protein is prone to spontaneous precipitation,21,36 such that structural instability and/or reduced solubility of FXIII-B2 may promote its clearance from circulation. Alternately, FXIII-B2 may possess a clearance-promoting epitope that is obscured in the FXIII-A2B2 heterotetramer and perhaps also in the FIX:FXIII-B conjugate. Removal of glycosylation moieties from FXIII-B2 shortens its half-life in F13b-deficient mice, which could reflect disruption of charge-charge interactions that mediate FXIII-B2 renal clearance.52,53 The molecular weight of FXIII-B2 (∼163 kDa) is above the glomerular membrane permeability threshold (∼36 kDa)54 and is similar to complement factor H, which contains 20 sushi domains and has a half-life of ∼6 days.55 Thus, the mechanism mediating the clearance of isolated (free) FXIII-B2 from circulation is currently unknown and warrants further study.
An intriguing observation in both humans and mice is the rate at which circulating FXIII-B2 increases in plasma after rFXIII-A2 infusion. Given the short half-life of FXIII-B2 and our data showing FXIII-A2 does not induce de novo FXIII-B synthesis, the rapid increase in FXIII-B2 suggests hepatocytes constitutively produce FXIII-B protein at an impressive rate. A previous study in monkeys estimated the FXIII-B production rate to be 12.1 μg/kg per hour,37 ∼20-fold higher than the estimated rate of FXIII-A production (0.622 μg/kg per hour).37 Such disparate rates of subunit production are surprising but may stem from differences in mRNA expression, secretory signals inherent to each subunit, and/or the cell of origin. The basal level of F13B expression in hepatocytes is approximately fourfold higher than F13A1 expression in macrophages.56 Moreover, FXIII-B bears a signal peptide and is produced in the liver, which also secretes most circulating coagulation factors at relatively high levels. Conversely, FXIII-A arises from cells of bone marrow origin, lacks a signal peptide, and has a poorly characterized release mechanism.7,57 Thus, despite the short half-life of both subunits, differences in production may explain the observation that FXIII-B2 circulates at an approximately twofold molar excess relative to FXIII-A2 in healthy individuals and can circulate even in the absence of FXIII-A2, albeit at lower levels.2,9 Because the FXIII-A2 and FXIII-B2 dimers must bind in circulation to avoid clearance, excess FXIII-B2 may be needed to ensure newly released FXIII-A2 can quickly encounter FXIII-B2 in circulation and form stable FXIII-A2B2 complexes. This biology may have implications for the therapeutic administration of plasma-derived FXIII-A2B2 (Corifact [North America], Fibrogammin P [international]), approved as a replacement therapy for patients with congenital FXIII-A or -B deficiency vs rFXIII-A2 (Tretten [North America], NovoThirteen [International]), which is approved specifically for patients with A-subunit mutations. In situations in which rapid restoration of functional FXIII is desired (eg, active bleeding from trauma and/or consumptive loss of FXIII), administration of FXIII-A2B2 may provide faster hemostasis because it does not depend on endogenous FXIII-B2 production and complex formation. Currently FXIII-A2B2 is only available as a plasma-derived product. Development of rFXIII-B2, including strategies to stabilize this material that is prone to precipitation,21,36 would be necessary to produce a fully recombinant product.
In addition to advancing the understanding of coagulation mechanisms mediated by FXIII-A2B2, our data identify a previously unrecognized mechanism involving intertissue regulation of 2 subunits of a plasma protein. Examples of proteins exhibiting intracellular, reciprocal regulation abound, from the balance of kinases and phosphatases that regulate the cell cycle23 to ubiquitin ligases and deubiquitinases controlling protein degradation.58 However, these instances of reciprocal regulation occur within the confines of an individual cell and typically involve the 2 regulatory elements modifying a target substrate. The FXIII-A2/FXIII-B2 relationship is unique, not only in that each subunit directly regulates the level of the other, but also that this mechanism occurs at a systemic scale. Certain cell populations, such as immune cell subsets, are known to exhibit systemic regulatory relationships.25 However, we are unaware of another example of 2 proteins synthesized in discrete tissues that directly regulate each other in the large spatial context of the plasma compartment. Within the coagulation system, von Willebrand factor (VWF) stabilizes coagulation FVIII and increases its level in plasma.59 However, VWF and FVIII undergo constitutive synthesis by the same cell type (endothelial cells, albeit in different ratios in cells located in larger or smaller vessels),60,61 and FVIII is not known to enhance VWF stability. The FXIII-A and FXIII-B subunits are estimated to have coevolved ∼500 million years ago in primitive fish, coinciding with evolution of the circulatory system.62 The selective pressures that led to the development of complementary structural epitopes in these subunits are unknown, but may relate to an enhanced need to localize FXIII-A2 on fibrinogen via the FXIII-B2 subunits21 for effective fibrin crosslinking and hemostasis. Collectively, our findings expose an example of coevolution in which both the high affinity of the FXIII-A2/FXIII-B2 interaction and the differential synthesis rates of each subunit have been finely tuned to ensure these subunits find each other in plasma and quickly reconstitute a functional complex.
Our study had potential limitations. First, we used human, not mouse, rFXIII-A2 in our mouse infusion experiments. However, human rFXIII-A2 is active in mice,20,29 and we and others30 showed mouse FXIII-B enhances circulation of human rFXIII-A2, demonstrating a functional interaction between human rFXIII-A2 and mouse FXIII-B2. Moreover, our findings suggesting costabilization are consistent with data from both humans and mice, suggesting a preservation of mechanisms regulating FXIII-A2B2 in both settings. Second, the rFXIII-A2 doses tested in mice (4 and 8 mg/kg) were ∼10-20–fold higher than a human therapeutic dose. However, these doses are well below doses that are toxic in monkeys (22.5 mg/kg)22 and are well tolerated in mice.30 Third, the sparsity of early data points from humans infused with rFXIII-A2 prevented us from accurately capturing the initial, rapid loss of uncomplexed FXIII-A2, and therefore, precluded fitting to a 2-compartment model or testing combined models. However, additional support for indirect response model 2 (FXIII-A2 stabilizes FXIII-B2) is derived from parallel, complementary pharmacologic analysis and functional testing in mice. Fourth, because F13a1–/– mice have ∼10% of normal FXIII-B levels, we were not able to perform the FXIII-B subunit clearance studies in a completely FXIII-B–free system. However, we observed a significant difference in FXIII-B levels 6 hours after infusion, and these levels were all above baseline FXIII-B levels observed in F13a1–/– mice.
In summary, our findings show FXIII-A2 potently increases plasma FXIII-B2 levels. The effect of FXIII-A2 on FXIII-B2 is independent of transcription or translation, but instead relies on reciprocal stabilization, representing a unique example of a coevolved, intercellular mechanism regulating a circulating hemostatic protein. Knowledge of this mechanism, which underlies both endogenous and pharmacologic effect of FXIII-A2 on FXIII-B2 levels, is essential for understanding congenital and acquired FXIII deficiency and improving FXIII-targeting treatment strategies in diverse clinical settings, including bleeding, venous thrombosis, cerebral amyloid angiopathy, myocardial infarction, arthritis, and cancer.63-67
Acknowledgments
The authors acknowledge Arijit Biswas, Sneha Singh, Dafna Groeneveld, and J. Charles Jennette for helpful conversations. The authors also thank the authors of the prior publication reporting the results of the mentor2 trial and Yaqiu Sang and Stéphanie Reitsma for helpful comments on the manuscript. Select figures were created using BioRender.com.
This study was supported by funding from the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (R01HL126974 [A.S.W.] and NIH, National Institute of Diabetes and Digestive and Kidney Diseases (R01DK122813 [J.P.L.]), Novo Nordisk (A.S.W.), and a Dissertation Completion Fellowship from The Graduate School at the University of North Carolina at Chapel Hill (J.R.B.). This study was supported, in part, by funding from Novo Nordisk; Novo Nordisk produces recombinant FXIII-A2 for therapeutic administration. F13a1-deficient mice on a mixed 129Ola/CBACa background were a gift from CSL-Behring; CSL-Behring produces plasma-derived FXIII-A2B2 for therapeutic administration.
The funders and suppliers had no role in study design, data collection, analysis, conclusions, decision to publish, or preparation or submission of the manuscript.
Authorship
Contribution: J.R.B., T.L., S.S., R.A.C., D.A.D., L.A.H., M.L., K.K., and J.W.H. performed experiments and/or analyzed data; J.R.B., M.M.A., J.P.L., B.A.K., J.B.D., and A.S.W. provided input on the study design, analysis, and presentation; J.R.B. and A.S.W. wrote the manuscript; and all authors reviewed and edited the manuscript and approved the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Alisa S. Wolberg, Department of Pathology and Laboratory Medicine, The University of North Carolina at Chapel Hill, 8018A Mary Ellen Jones Building, CB #7035, Chapel Hill, NC 27599-7035; email: alisa_wolberg@med.unc.edu.
References
Author notes
The authors will make data sets and protocols available to other investigators without unreasonable restrictions.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.



![FXIII-A2 stabilizes FXIII-B2 in circulation. (A) rFXIII-B2 was incubated in normal, pooled human plasma for 24 hours at 37°C in the absence or presence of rFXIII-A2 and visualized by immunoblotting with anti-FXIII-B antibody. Representative blot of N = 2 experiments. (B) Fibrinogen, FXIII-A, and FXIII-B antigen were visualized in plasma from wild-type, Fibγ390-396A, or afibrinogenemic (Fga–/–) mice by immunoblotting using anti-fibrinogen, anti–FXIII-A, and anti–FXIII-B antibodies. (C) Schematic of in vitro FXIII-A2B2 activation with thrombin (IIa) and CaCl2 to separate FXIII-A and -B subunits before infusion. Reactions were performed in the presence of the transglutaminase inhibitor T101 to inhibit activated FXIII-A2∗ (FXIII-A2∗-I [inhibited dimeric or monomeric FXIIIa]), and thrombin was quenched with hirudin before infusion into F13a1–/– mice via the tail vein. (D) Representative immunoblot of FXIII-A and -B antigen in mice infused with complexed (FXIII-A2B2) or uncomplexed FXIII-B2 at the indicated times after infusion. Subunits were visualized with anti–FXIII-A and -B antibodies. (E) FXIII-B antigen over time, after infusion, was visualized for each condition (solid line, complexed; dashed line, uncomplexed) using immunoblot with anti–FXIII-B antibody and quantified by densitometry. Data were quantified relative to FXIII-B antigen at the 0 hour timepoint immediately after infusion. Dots show mean ± standard deviation, N = 1-11/time point. (F) Relative FXIII-B antigen for each condition at the 6 hour timepoint. Dots represent individual mice, bars show mean ± standard deviation. Groups were compared using a 2-tailed, unpaired t test, ∗∗ P < .01. A.U., arbitrary units.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/143/5/10.1182_blood.2023022042/1/m_blood_bld-2023-022042-gr6.jpeg?Expires=1769646349&Signature=4LlNIzCEZo8tP2PvC-ejuABy1UZdE5Of9~qW56TH7gWACtjZiGqOFgB1U8U~I324VnB-eWwiZnp9nZwx7t~Qdjg72Ti0qPSlZpUhJ~OsKFIk~bPkG~Gcz-u2xvP~S0TG3F~hB8GMJKa6p4pJXlCoRgN3GI-R-bvxDTaTcJGOw9FhAl7PtuctGnTQ9wytiZ6HY10KG1YVzSphReF~xuiOzelM46GbQNRIqkPPRFW75Ln8OkxkCPSr-ssD7n5XG3fRFcxQAap4-cOlp6dj~2nx1QyMiAa6v8fZm2JAH55Y4lsGcoSp8eRDZpKl65kYFNJySaVjyIGHMws1Jc92fty9~A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal