Key Points
We describe the phenotypes of mouse models of XLSA, the SLC25A38 anemia, and XLPP.
XLSA and SLC25A38-CSA are conditionally synthetically lethal with pyridoxine deficiency.
Visual Abstract
X-linked sideroblastic anemia (XLSA) and X-linked protoporphyria (XLPP) are uncommon diseases caused by loss-of-function and gain-of-function mutations, respectively, in the erythroid form of 5-aminolevulinic acid synthetase (ALAS), ALAS2, which encodes the first enzyme in heme biosynthesis. A related congenital sideroblastic anemia (CSA) is due to mutations in SLC25A38 (solute carrier family 25 member A38), which supplies mitochondrial glycine for ALAS2 (SLC25A38–CSA). The lack of viable animal models has limited the studies on pathophysiology and development of therapies for these conditions. Here, using CRISPR-CAS9 gene editing technology, we have generated knockin mouse models that recapitulate the main features of XLSA and XLPP; and using conventional conditional gene targeting in embryonic stem cells, we also developed a faithful model of the SLC25A38-CSA. In addition to examining the phenotypes and natural history of each disease, we determine the effect of restriction or supplementation of dietary pyridoxine (vitamin B6), the essential cofactor of ALAS2, on the anemia and porphyria. In addition to the well-documented response of XLSA mutations to pyridoxine supplementation, we also demonstrate the relative insensitivity of the XLPP/EPP protoporphyrias, severe sensitivity of the XLSA models, and an extreme hypersensitivity of the SLC25A38-CSA model to pyridoxine deficiency, a phenotype that is not shared with another mouse hereditary anemia model, Hbbth3/+ β-thalassemia intermedia. Thus, in addition to generating animal models useful for examining the pathophysiology and treatment of these diseases, we have uncovered an unsuspected conditional synthetic lethality between the heme synthesis–related CSAs and pyridoxine deficiency. These findings have the potential to inform novel therapeutic paradigms for the treatment of these diseases.
Introduction
5-aminolevulinic acid synthase (ALAS) is the first enzyme in heme biosynthesis. ALAS condenses glycine and succinyl-coenzyme A in mitochondria to form 5-aminolevulinic acid (5ALA), the sole precursor of porphyrins and heme. ALAS uses pyridoxal 5-phosphate, the active form of pyridoxine (vitamin B6), as a cofactor (Figure 1A). In mammals, there are 2 isoforms of ALAS. ALAS2 is expressed in erythroid cells, whereas all other cells express ALAS1. Extraordinarily high-level heme synthesis is required to sustain hemoglobin (Hb) production in erythroblasts, representing ∼75% of mammalian heme.1
Establishment and characterization of the male erythroid phenotypes of XLSA and XLPP mouse models. (A) Schematic of key erythroid heme biosynthetic substrates and intermediates (left); mouse models used in this study (right). (B) Targeted mutations in the human ALAS2 genomic locus. XLSA mutations are indicated in blue, and XLPP mutations are in red. (C) Chromatograms of targeted mutations, including the protospacer-associated motif (PAM) site silent mutation (in black) that were created in addition to the coding sequence variants introduced (in blue or red). (D) Selected RBC indices for male mice of XLSA, XLPP, and EPP models maintained on conventional rodent chow at ages 5, 8, 24, and 54 weeks. (E) Spleen-to-body mass ratio at ages 8 and 54 weeks. (F) Serum erythropoietin (EPO) at ages 8 and 54 weeks. The dotted line in each panel indicates the average value in control animals. n = 3 to 6 mutants per group; n = 13 to 23 controls. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001, compared with control animals at the same age. B6, pyridoxine; B-thal, beta-thalassemia; CTL, control; CHr, hemoglobin of the reticulocyte; HBA/HBB, hemoglobin α/β; MCV, mean corpuscular volume.
Establishment and characterization of the male erythroid phenotypes of XLSA and XLPP mouse models. (A) Schematic of key erythroid heme biosynthetic substrates and intermediates (left); mouse models used in this study (right). (B) Targeted mutations in the human ALAS2 genomic locus. XLSA mutations are indicated in blue, and XLPP mutations are in red. (C) Chromatograms of targeted mutations, including the protospacer-associated motif (PAM) site silent mutation (in black) that were created in addition to the coding sequence variants introduced (in blue or red). (D) Selected RBC indices for male mice of XLSA, XLPP, and EPP models maintained on conventional rodent chow at ages 5, 8, 24, and 54 weeks. (E) Spleen-to-body mass ratio at ages 8 and 54 weeks. (F) Serum erythropoietin (EPO) at ages 8 and 54 weeks. The dotted line in each panel indicates the average value in control animals. n = 3 to 6 mutants per group; n = 13 to 23 controls. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001, compared with control animals at the same age. B6, pyridoxine; B-thal, beta-thalassemia; CTL, control; CHr, hemoglobin of the reticulocyte; HBA/HBB, hemoglobin α/β; MCV, mean corpuscular volume.
Two uncommon diseases are caused by mutations in ALAS2: X-linked sideroblastic anemia (XLSA) and X-linked protoporphyria (XLPP; Figure 1B). Loss-of-function mutations in ALAS2 cause XLSA, the most common form of congenital sideroblastic anemia (CSA).2,3 Abnormal iron deposits in the mitochondria of erythroblasts (ring sideroblasts [RSs]) and red blood cells ([RBC], siderocytes) are the defining morphologic features of CSA. Male patients with XLSA have a hypochromic, microcytic anemia and can present with iron overload due to ineffective erythropoiesis.4 In approximately two-thirds of patients with XLSA, the anemia is partially or completely corrected by supplementation with oral pyridoxine.4
In contrast, gain-of-function mutations in ALAS2 cause XLPP, a subset (∼5%) of erythropoietic protoporphyria (EPP).5 EPP largely results from recessive mutations in ferrochelatase (FECH) that decrease activity by at least ∼65%.6 FECH is the last enzyme in heme biosynthesis and incorporates ferrous iron into metal-free protoporphyrin IX (mf-PPIX) to form heme. mf-PPIX is phototoxic, and tissue accumulation causes acute photosensitivity and, occasionally, cholestatic liver failure.7 XLPP mutations disrupt the C-terminal autoinhibitory domain of ALAS2.5,8-10 In XLPP, excess ALAS2 activity and 5ALA production outstrips the enzymatic capacity of FECH such that it becomes limiting, leading to mf-PPIX accumulation.5,8 In XLPP, heme synthesis is also partially limited by iron abundance, resulting in a distinctive buildup of zinc protoporphyrin IX (Zn-PPIX) in RBCs.7,11
XLPP is among a very few X-linked diseases in which female carriers are typically as severely affected as hemizygous males.5,10,12,13 By contrast, female carriers of XLSA can have diverse phenotypes, ranging from normal Hb levels to profound anemia, depending on the severity of the mutation and the extent of skewing of X-chromosome inactivation toward the mutant allele in bone marrow (BM).14,15 Whereas RBCs in males with XLSA are characteristically microcytic, in affected females with XLSA, the anemia is typically normocytic or macrocytic.
Due to the extreme glycine requirement for 5ALA synthesis, autosomal recessive mutations in SLC25A38 (solute carrier family 25 member A38), the high-affinity erythroid mitochondrial glycine importer, also cause a heme deficiency–related CSA that is more severe than XLSA16-18 (Figure 1A). Most patients with SLC25A38-CSA carry null alleles and do not respond to pyridoxine.17,18
Other than pyridoxine for XLSA, few disease-modifying treatments are available for each of these diseases and only hematopoietic stem cell (HSC) transplantation can be curative. Afamelanotide, an US Food Drug and Administration–approved drug, and dersimelagon, have shown efficacy in increasing pain-free light tolerance in patients with protoporphyria.19-21 Bitopertin, an inhibitor of the erythroid cell surface glycine importer, has been advanced to phase 2 clinical trials to reduce mf-PPIX production and light photosensitivity in EPP.22
Attempts to model XLSA by creating null and erythroid-specific enhancer ALAS2 mutations have been made but are embryonic lethal.23-25 Using CRISPR-CAS9 gene editing,26 we created an allelic series of viable ALAS2 knockin mice with either XLSA or XLPP mutations. We also derived a conditional Slc25a38 null allele. With the aim of understanding the pathophysiology of the disorders and metabolic dependencies that might be used for therapeutic benefit, we characterized the hematological phenotypes of these models and uncovered an unsuspected conditional synthetic lethality between pyridoxine deficiency and the heme-related ALAS2- and SLC25A38-CSAs.
Methods
Animals
All animal studies were performed under protocols approved by the institutional animal care and use committee at Boston Children’s Hospital. CRISPR-CAS9–mediated ALAS2 targeting of mouse blastocysts was performed in the Boston Children’s Hospital mouse Embryonic Stem Cell and Gene Targeting Core using established protocols.27 The Slc25a38 floxed allele (Slc25a38fl) was rescued from C57BL/6NTac Slc25a38tm1a(EUCOMM)Wtsi/IcsOrl embryonic stem cells (Mouse Genome Informatics ID: 5781840). See supplemental Methods, available on the Blood website, for details.
Diets
Unless otherwise specified, animals were bred, weaned, and raised on Prolab IsoPro RMH 3000 (22% protein diet, 8.3-ppm pyridoxine, 1.2-ppm folate, and 360-ppm iron). Pyridoxine-defined purified diets (Teklad/Envigo; series TD.200252-TD.200255) were based on casein as a protein source and contained 3-ppm folate, 50-ppm iron, and pyridoxine levels of 0, 2, 10, 100, or 300 ppm. The synthetic diet (Teklad/Envigo; TD.170300) fed to Slc25a38BMKO animals for longitudinal studies provided amino acids as a protein source and contained 1% glycine, 0.37% serine, 20-ppm pyridoxine, 8-ppm folate, and 50-ppm iron.
Phenotyping
Complete blood counts (CBCs), photomicroscopy, electron microscopy, tissue and serum iron studies, erythropoietin and hepcidin immunoassays, and flow cytometry experiments (see supplemental Methods) were performed as previously described.28-35 Qualitative mf-PPIX levels were determined by flow cytometry using the violet excitation laser and recording fluorescence at 610/20 nm on a BD Celesta cytometer and expressed as mean fluorescence intensity of cells within the RBC forward scatter/side scatter (FSC/SSC) gate.
Porphyrin and ALAS activity analyses
Zn-PPIX was detected on a Helena Laboratories ProtoFluorZ instrument. Tissue/RBC porphyrin and ALAS activity36 protocols are described in supplemental Methods.
Statistics were performed with Prism 10
The Brown-Forsythe and Welch analysis of variance tests, assuming a Gaussian distribution and unequal standard deviations, were used when comparing ≥3 groups. The unpaired Mann-Whitney test was used when comparing 2 groups.
Results
Establishment of XLSA and XLPP mouse models
Approximately 40% of XLSA mutations in males occur at 3 amino acids: p.R170, p.R411, and p.R452 (Figure 1B).14,37 Male patients with these mutations have variably severe anemia and sensitivity to pyridoxine supplementation.37 Using gene editing techniques in mouse blastocysts, we generated mice with p.R170H, p.R411H, and p.R452H variants (Figure 1C), as well as a line with the p.Q548X variant (Figure 1B-C), a recurrent XLPP mutation with very high activity in vitro.10 We also obtained several insertion/deletion (indel) mutations resulting from nonhomologous end-joining at the CAS9 cleavage site that resulted in XLSA null alleles and other XLPP variants (supplemental Table 1).
XLSA and XLPP phenotypes in male mice
We performed CBCs at ages 5, 8, 24, and 54 weeks in the R170H, R411H, R452H, and Q548X male mutants as well as a viable C-terminal indel allele with an XLSA phenotype, c.1646_1647ins14 (p.V550Efs∗23, referred to as “ins14”; supplemental Figure 1). We compared them with control animals and a preexisting mouse model of EPP, Fechm1PAS/m1PAS.38
XLSA animals had a range of RBC abnormalities of varying severity on conventional rodent chow containing 8.3-ppm pyridoxine (Figure 1D; supplemental Figure 2). Apart from a mildly reduced cellular hemoglobin of the reticulocyte (CHr) at 5 and 8 weeks and a decreased mean corpuscular volume (MCV) volume at 5 weeks, R170H males were not different from controls. R411H, R452H, and ins14 males were variably microcytic and hypochromic with an increased RBC distribution width (RDW). Only the R411H and the ins14 alleles were anemic, and in both cases, the anemia became more severe with age. Reticulocytes were significantly increased in R411H and ins14 animals. White blood cell and platelet counts were unaffected in all mutants (supplemental Figure 2). Commensurate with the anemia, R411H and ins14 animals had variable splenomegaly and/or increased erythropoietin levels at age 8 or 54 weeks (Figure 1E-F). Other than a significantly increased transferrin saturation in the R452H line, there was no secondary iron overload in any of the XLSA mutants (supplemental Figure 3A-D). At 8 weeks, splenic iron was slightly increased in the R411H mutant, suggesting hemolysis. Overall, R411H and ins14 males had the most severe blood phenotypes, R452H animals were less severe, and the R170H animals had nearly no phenotype at all.
By contrast, Q548X XLPP RBCs were macrocytic and better hemoglobinized than control littermates, and aged Q548X animals were polycythemic compared with controls (Figure 1D; supplemental Figure 2). This phenotype is distinct from EPP animals, which had reduced CHr, increased RDW, and splenomegaly (Figure 1E). Although there was no change in serum hepcidin levels at age 54 weeks, XLPP animals had a decrease in liver and splenic iron, likely secondary to the increased erythroid mass (supplemental Figure 3A-C,E-F).
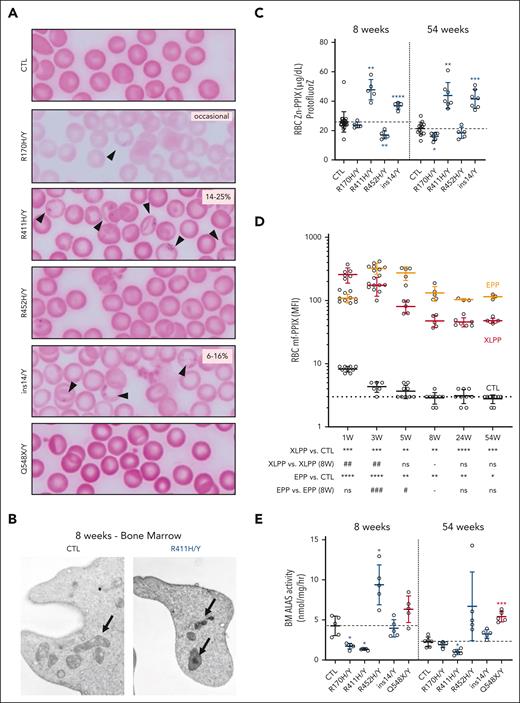
The hallmarks of XLSA and XLPP are RS/siderocytes and excess RBC mf-PPIX production, respectively. XLSA mutants had peripheral blood siderocytes that were present roughly proportional to the increase in RDW (Figure 2A-B; supplemental Figure 3E). Similar to other mouse models of CSA,35,39-42 RSs were not present in erythroid precursors by Prussian blue staining or electron microscopy in adult marrow or spleen. The Zn-PPIX/heme ratio was increased in the R411H and ins14 lines, suggesting, despite the mitochondrial iron deposits, a defect in mitochondrial iron available for incorporation into mf-PPIX (Figure 2C).
Siderocytes and mf-PP in XLSA and XLPP mouse models. (A) Representative photomicrographs of Perls iron-stained peripheral blood smears of control, XLSA, and XLPP males at age 8 weeks. Arrows indicate siderocytes. The range of siderocyte frequency is indicated in the upper right of each image. Original magnification, 1000×. (B) Representative transmission electron micrographs of reticulocytes from wild-type and p.R411H mice at age 8 weeks. Note the electron-dense deposits in the p.R411H mitochondria. Original magnification, 20 000×. (C) RBC Zn-PPIX determined by hematofluorometry. (D) RBC mf-PPIX mean fluorescence intensity (MFI) determined by flow cytometry. (E) ALAS activity in control, XLSA, and XLPP mutant BM determined in vitro. The dotted line in each panel indicates the average value in control animals. For all panels, n = 3 to 10 animals per group. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001, compared with control animals at the same age. #P < .05; ##P < .01; ###P < .001; ###P < .0001, compared with the same mutant animals at 8 weeks. CTL, control.
Siderocytes and mf-PP in XLSA and XLPP mouse models. (A) Representative photomicrographs of Perls iron-stained peripheral blood smears of control, XLSA, and XLPP males at age 8 weeks. Arrows indicate siderocytes. The range of siderocyte frequency is indicated in the upper right of each image. Original magnification, 1000×. (B) Representative transmission electron micrographs of reticulocytes from wild-type and p.R411H mice at age 8 weeks. Note the electron-dense deposits in the p.R411H mitochondria. Original magnification, 20 000×. (C) RBC Zn-PPIX determined by hematofluorometry. (D) RBC mf-PPIX mean fluorescence intensity (MFI) determined by flow cytometry. (E) ALAS activity in control, XLSA, and XLPP mutant BM determined in vitro. The dotted line in each panel indicates the average value in control animals. For all panels, n = 3 to 10 animals per group. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001, compared with control animals at the same age. #P < .05; ##P < .01; ###P < .001; ###P < .0001, compared with the same mutant animals at 8 weeks. CTL, control.
In the XLPP model, the mf-PPIX fluorescence in RBCs and the percentage of fluorescent RBCs (fluorocytes) was initially very high but decreased and stabilized over the first 8 weeks of life (Figure 2D; supplemental Figure 4A). mf-PPIX was detected in XLPP fetal liver erythroblasts as early as E14.5. Differences in mf-PPIX by flow cytometry were corroborated by quantitative mf-PPIX measurements at ages 8 and 54 weeks (supplemental Figure 4B). Except for the ins14 allele, other C-terminal Alas2 indel alleles had modest increases in RBC mf-PPIX fluorescence (supplemental Figure 1). Age-related changes in RBC mf-PPIX were also observed in the Fechm1PAS mutant. Adult EPP animals retained more mf-PPIX and had more fluorocytes than XLPP animals. RBC Zn-PPIX was quantified by hematofluorometry and splenic Zn-PPIX determined by ultra-performance liquid chromatography (UPLC; supplemental Figure 4D-E). RBC Zn-PPIX was increased in both XLPP and EPP, suggesting that the ProtoFluorZ device is not specific for Zn-PPIX in the presence of high mf-PPIX. Nevertheless, Zn-PPIX was more elevated in XLPP RBCs and detected only in XLPP spleens, indicating that the animals are indeed accumulating Zn-PPIX. Unlike EPP animals, XLPP mutants did not accumulate substantial hepatic mf-PPIX nor did they develop liver disease (supplemental Figure 4C). However, occasional XLPP and EPP animals (<5%) developed jaundice associated with extremely high mf-PPIX levels. These outliers, which likely developed cholestatic liver disease similar to a subset of human patients, were excluded from the analyses.
XLSA and XLPP are attributed to hypomorphic and hypermorphic ALAS2 alleles, respectively. ALAS (ALAS1 + ALAS2) activity normalized to total protein in the BM was significantly decreased in XLSA R170H and R411H males (Figure 2E). In the R452H mutant, ALAS activity was approximately twice that of controls. An erythroid hyperplasia cannot account for this increase in ALAS2 activity; in human studies, mutations at R452 consistently have normal or increased activity in vitro.14,43 Furthermore, consistent with in vitro experiments, Q548X BM ALAS activity was increased approximately twofold (Figure 2E).
XLSA and XLPP phenotypes in female mice
Unlike many X-linked disorders, the XLSA and XLPP phenotypes are commonly expressed in female carriers of pathogenic variants.10,44,45 Consequently, we also investigated the effect of XLSA and XLPP alleles in heterozygous female mice.
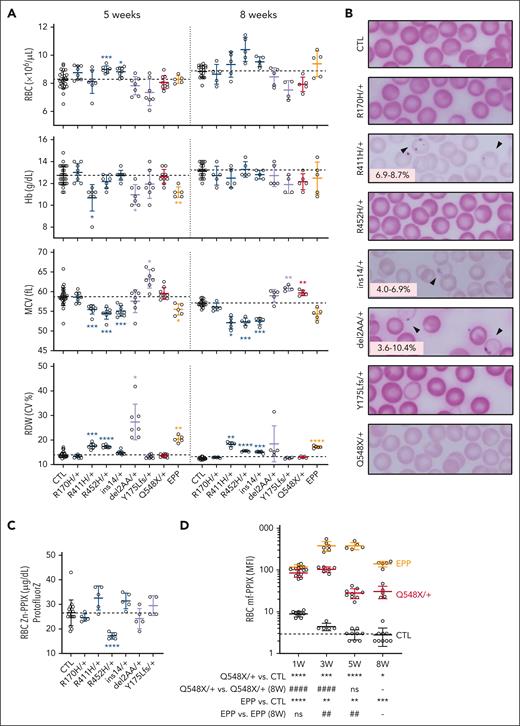
Similar to hemizygous males, heterozygous R411H, R452H, and ins14 females were microcytic, had an increased RDW, and decreased CHr (Figure 3A; supplemental Figure 5A). Only the R411H females were anemic at age 5 weeks. The only hematological phenotypes observed in R170H females were a decreased CHr and increased RDW. Variable numbers of siderocytes were present in R411H and ins14 heterozygous female mutants (Figure 3B).
Characterization of the female erythroid phenotypes of XLSA and XLPP mouse models. (A) Selected hematological indices for female control, XLSA, XLPP, and EPP mice maintained on conventional rodent chow at ages 5 and 8 weeks. (B) Representative photomicrographs of Perls iron-stained peripheral blood smears from heterozygous female carriers of XLSA or XLPP alleles at age 8 weeks. Arrows indicate siderocytes. The range of siderocyte frequency is indicated in the lower left of each image. Original magnification, 1000×. (C) RBC Zn-PPIX determined with by hematofluorometry. (D) RBC mf-PPIX MFI determined by flow cytometry. n = 5 to 9 mutant animals per group; n = 18 to 28 control animals. The dotted line in each panel indicates the average value in control animals. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001, compared with control animals at the same age. #P < .05; ##P < .01; ###P < .001; ####P < .0001, compared with the same mutant animals at 8 weeks. CTL, control; MCV, mean corpuscular volume.
Characterization of the female erythroid phenotypes of XLSA and XLPP mouse models. (A) Selected hematological indices for female control, XLSA, XLPP, and EPP mice maintained on conventional rodent chow at ages 5 and 8 weeks. (B) Representative photomicrographs of Perls iron-stained peripheral blood smears from heterozygous female carriers of XLSA or XLPP alleles at age 8 weeks. Arrows indicate siderocytes. The range of siderocyte frequency is indicated in the lower left of each image. Original magnification, 1000×. (C) RBC Zn-PPIX determined with by hematofluorometry. (D) RBC mf-PPIX MFI determined by flow cytometry. n = 5 to 9 mutant animals per group; n = 18 to 28 control animals. The dotted line in each panel indicates the average value in control animals. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001, compared with control animals at the same age. #P < .05; ##P < .01; ###P < .001; ####P < .0001, compared with the same mutant animals at 8 weeks. CTL, control; MCV, mean corpuscular volume.
Two other XLSA alleles, both resulting from nonhomologous end-joining, were also obtained: c.522_528del (Tyr175_Leufs∗26) and c.523_528del (Tyr175_Pro176del), referred to as Y175Lfs and del2AA, respectively. No live-born males were observed for either of these alleles, which is consistent with the predicted severe loss-of-function or null effects of these variants. Females heterozygous for these mutations had an increased RDW (Figure 3A). del2AA/+ females also had a transitory anemia, whereas Y175Lfs/+ animals had a higher CHr and macrocytosis, the latter finding being typical of human female carriers of the most severe (eg, null) ALAS2 alleles.46-48 del2AA female carriers had occasional circulating siderocytes (Figure 3B). Zn-PPIX levels were decreased in R452H/+ females similar to R452H/Y males (Figure 3C).
The expression of wild-type and mutant alleles was determined in the peripheral blood (reticulocytes) and BM (erythroblasts and reticulocytes) at 8 weeks (supplemental Figure 5D-E). We found no skewing in the expression of XLSA missense alleles. Y175Lfs messenger RNA (mRNA) was not detected, likely due to mRNA nonsense-mediated decay. The del2AA mRNA was more abundant in the marrow than the peripheral blood, suggesting a selection for the wild-type allele during erythroid differentiation. This likely induces erythropoietic stress, which can account for the relative macrocytosis seen in females heterozygous for an XLSA null allele.
Other than a slight increase in mean MCV and CHr, RBC indices were not different between heterozygous Q548X females and controls (Figure 3A). The age-related changes in RBC mf-PPIX fluorescence paralleled those observed in hemizygous XLPP males but were quantitatively less (Figure 3D). RBC mf-PPIX fluorescence in homozygous females was not different from that in hemizygous males (data not shown). Total ALAS activity in the marrow was not increased (supplemental Figure 5B). By hematofluorometry, Zn-PPIX was comparable in XLPP and EPP female RBCs, but mf-PPIX accumulation was lower in XLPP females, suggesting Zn-PPIX accumulation in the XLPP model (supplemental Figure 5B). Overall, heterozygous females with XLSA and XLPP alleles also mimic the hematological phenotypes of their human counterparts.
SLC25A38 deficiency in mice
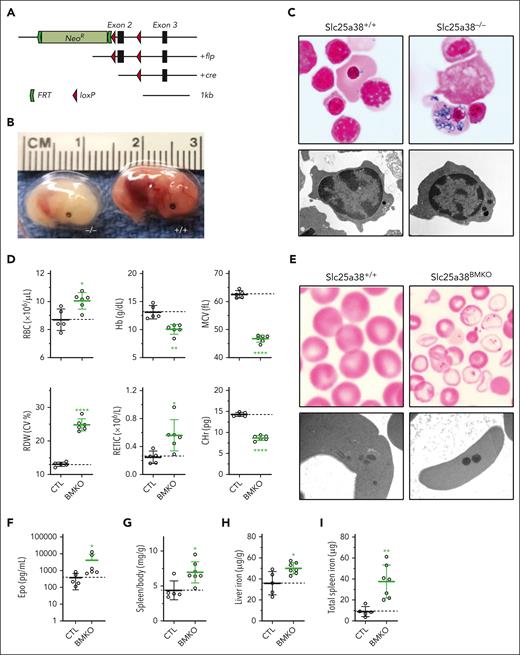
We also developed an Slc25a38 conditional null mutant allele (Slc25a38fl; Figures 1A and 4A). Unlike humans with null variants, germ line null mice (Slc25a38−/−) die in utero from severe anemia after E14.5 (Figure 4B), and many of the nucleated circulating embryonic erythrocytes contain abundant iron-laden mitochondria, reproducing the morphology of sideroblasts (Figure 4C). Conditional deletion in erythroid precursors with the erythropoietin receptor Cre similarly resulted in prenatal lethality. To circumvent this, we generated animals with pan-hematopoietic deletion of Slc25a38 using Vav1-Cre (B6.Cg-Tg(VAV1-cre)1Graf/MdfJ) that expresses a Cre recombinase under the control of the vav1 oncogene (VAV1) regulatory elements, expressed in HSCs (Slc25a38fl/−; Vav1-Cre+, referred to as Slc25a38BMKO). In our experience, Vav1-Cre deletes at a later stage in embryogenesis, often leading to viable erythroid phenotypes when the erythropoietin receptor Cre does not.35 Slc25a38BMKO animals were fully viable and had a moderately severe hypochromic, microcytic anemia (Figure 4D) with numerous siderocytes in the peripheral blood (Figure 4E), associated with splenomegaly, increased erythropoietin (Figure 4F-G), and iron overload (Figure 4H-I). The degree of anemia in the Slc25a38BMKO was similar to that of XLSA R411H animals (supplemental Figure 6). The Slc25a38BMKO anemia, microcytosis, and RDW become substantially worse with age on a conventional diet. However, switching the animals to a synthetic diet at 6 weeks of age stabilized the anemia and caused a rapid increase in CHr, a resolution of splenomegaly, and a decrease in erythropoietin (supplemental Figure 7A-H).
Murine Slc25a38 deficiency phenotype. (A) Slc25a38 gene targeting strategy. (B) Germ line Slc25a38-deficient E14.5 embryos show extreme pallor compared with the control. (C) Perls iron-stained peripheral blood smears (top; original magnification, 1000×) and transmission electron micrographs (TEMs) of peripheral blood (bottom; original magnification, 17 700×) from E14.5 embryos demonstrating iron-positive granules in mutant nucleated fetal erythrocytes confirmed to be electron-dense material within mitochondria by TEM. (D) RBC indices of control Slc25a38fl/− and Slc25a38fl/−; Vav1-cre+ (Slc25a38BMKO) animals. (E) Perls iron-stained peripheral blood smears (top; original magnification, 1000×) and TEMs of peripheral blood (bottom; original magnification, 22 000×) from 8-week-old animals demonstrating iron-positive granules in mutant erythrocytes confirmed to be electron-dense material within mitochondria on TEM. (F-I) Spleen-to-body mass ratio (F), EPO (G), liver iron (H), and splenic iron (I) in 8-week-old animals. For all panels, n = 5 or 6 mixed males and females in each group, aged 8 weeks, fed conventional mouse chow. The dotted line in each panel indicates the average value in control animals. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001, compared with control animals.
Murine Slc25a38 deficiency phenotype. (A) Slc25a38 gene targeting strategy. (B) Germ line Slc25a38-deficient E14.5 embryos show extreme pallor compared with the control. (C) Perls iron-stained peripheral blood smears (top; original magnification, 1000×) and transmission electron micrographs (TEMs) of peripheral blood (bottom; original magnification, 17 700×) from E14.5 embryos demonstrating iron-positive granules in mutant nucleated fetal erythrocytes confirmed to be electron-dense material within mitochondria by TEM. (D) RBC indices of control Slc25a38fl/− and Slc25a38fl/−; Vav1-cre+ (Slc25a38BMKO) animals. (E) Perls iron-stained peripheral blood smears (top; original magnification, 1000×) and TEMs of peripheral blood (bottom; original magnification, 22 000×) from 8-week-old animals demonstrating iron-positive granules in mutant erythrocytes confirmed to be electron-dense material within mitochondria on TEM. (F-I) Spleen-to-body mass ratio (F), EPO (G), liver iron (H), and splenic iron (I) in 8-week-old animals. For all panels, n = 5 or 6 mixed males and females in each group, aged 8 weeks, fed conventional mouse chow. The dotted line in each panel indicates the average value in control animals. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001, compared with control animals.
Response of XLSA and XLPP to dietary pyridoxine supplementation and deprivation
Because of the hematological response of certain XLSA alleles to supplemental pyridoxine, we explored the effect of pyridoxine-replete (10 ppm), pyridoxine-deficient (0 or 2 ppm), and pyridoxine-supplemented (100 or 300 ppm) synthetic diets on the CBCs of male XLSA mutants. XLPP and EPP animals were also subjected to dietary pyridoxine restriction to determine whether this would decrease RBC mf-PPIX accumulation. At age 3 weeks, mutant and control animals were weaned onto pyridoxine-defined diets and maintained for 8 weeks (supplemental Figure 8A). Although 2-ppm pyridoxine was sufficient to sustain normal growth in all cases, absolute pyridoxine restriction (0 ppm) affected growth in all genotypes (Figure 5A-B). Growth restriction was most severe in the R411H and ins14 mutants.
On the 10-ppm pyridoxine diet, XLSA phenotypes were largely similar to those on conventional rodent chow; the R411H, R452H, and ins14 alleles were mildly to moderately anemic, and all, including the R170H variant, were microcytic (Figure 5B-supplemental Figure 8C). Very high levels of dietary pyridoxine (300 ppm) nearly completely or completely normalized the anemia in R411H and R452H animals, respectively. However, the animals remained microcytic. Moderate pyridoxine restriction (2 ppm) resulted in mild and moderate anemia in the ins14 and R411H alleles, respectively. Although control animals developed a mild-moderate anemia on a completely pyridoxine-deficient diet (0 ppm), all XLSA alleles developed a lethal, severe microcytic, reticulocytopenic anemia with extreme anisopoikilocytosis after 8 weeks (Figure 5B; supplemental Figure 8E). The anemia and microcytosis were comparable in all pyridoxine-deficient mutants but differed in the extent of compensatory splenomegaly. R411H animals were the most extreme; their spleens grew to ∼12% of body weight after 8 weeks on a 0-ppm pyridoxine diet (Figure 5C-D; supplemental Figure 8D).
Pyridoxine deficiency also induced microcytosis, anemia, and siderocytes in XLPP and EPP animals. Only XLPP animals had a slightly decrease in RBC mf-PPIX (Figure 5B,E; supplemental Figure 8B-D,F). Moderate pyridoxine restriction (2 ppm) had little or no effect on RBC parameters or mf-PPIX levels.
Taken together, these data quantitatively demonstrate the pyridoxine responsiveness of R411H and R452H alleles and demonstrate a conditional synthetic lethality between XLSA and pyridoxine deprivation.
Conditional synthetic lethality between pyridoxine deficiency and SLC25A38-CSA
To examine the pyridoxine requirement of other hereditary anemias dependent on heme, we performed similar experiments on the β-thalassemia intermedia (Hbbth3/+)49 and the Slc25a38BMKO mouse models. During the first weeks of pyridoxine depletion, the Hbbth3/+ anemia improved slightly but ultimately returned to baseline. Nonetheless, there was a significant decrease in splenomegaly, suggesting decreased ineffective erythropoiesis characteristic of the disease (supplemental Figure 9A-B). By contrast, after only 2 weeks on a pyridoxine-deficient diet, Slc25a38BMKO animals had a marked decrease in reticulocytes and a rapid progression of their anemia, requiring euthanasia by week 3 (Figure 6A-B; supplemental Figure 9C,E). The extreme effect on erythropoiesis was further illustrated by a marked reduction in the spleen-to-body weight ratio. Pyridoxine deficiency also depressed white blood cell and platelet counts, indicating a pan-hematopoietic sensitivity to severe pyridoxine deficiency in the Slc25a38BMKO (supplemental Figure 9D). Slc25a38BMKO animals fed the low pyridoxine diet (2 ppm) had a phenotype similar to XLSA animals fed the pyridoxine-deficient diet (Figure 6A-B; supplemental Figure 9C-F).
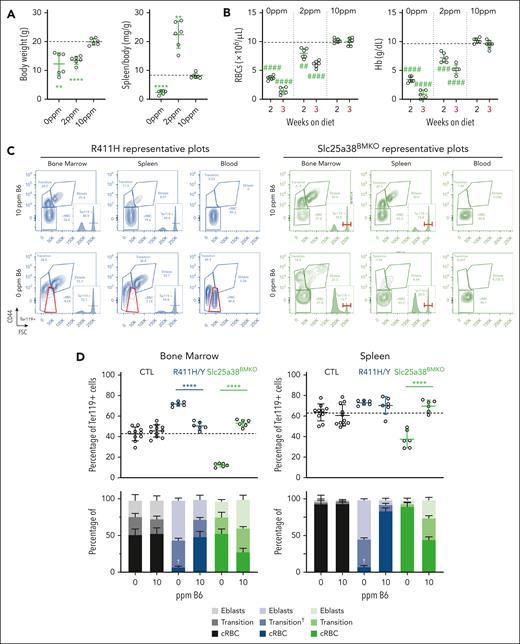
The erythropoietic response to pyridoxine deprivation in Slc25a38BMKO and p.R411H XLSA male mice. Male mice with the designated genotypes were weaned at age 3 weeks onto a synthetic diet containing defined amounts of pyridoxine. The experiment was discontinued after 3 weeks for Slc25a38BMKO due to the severity of the phenotype. (A-B) Body weight and spleen-to-body weight ratios (A) and selected RBC indices (B) of Slc25a38BMKO mice fed defined pyridoxine diets for 3 weeks. (C) Representative flow cytometry plots of erythroid differentiation determined as CD44 expression as a function of FSC in Ter119+ cells in peripheral blood, BM, and spleen in p.R411H and Slc25a38BMKO animals fed diets containing 10- or 0-ppm pyridoxine. (D) Enumeration of erythroid cell (ter119+) populations in control, p.R411H, and Slc25a38BMKO animals fed diets containing 10- or 0-ppm pyridoxine. †Indicates the unique characteristics of the “transitional population” seen in R411H/Y animals. n = 5 to 7 mutants and n = 9 to 12 control animals per diet. All animals are males, except SLC25A38BMKO mice fed 2 ppm, which are a mix of males and females. The dotted line in each panel indicates the average value in control animals. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001, compared with control animals at the same age. #P < .05; ##P < .01; ###P < .001; ####P < .0001, compared with animals of the same genotype on the 10-ppm pyridoxine control diet. B6, pyridoxine; cRBC, circulating red blood cells; CTL, control; Eblasts, erythroblasts.
The erythropoietic response to pyridoxine deprivation in Slc25a38BMKO and p.R411H XLSA male mice. Male mice with the designated genotypes were weaned at age 3 weeks onto a synthetic diet containing defined amounts of pyridoxine. The experiment was discontinued after 3 weeks for Slc25a38BMKO due to the severity of the phenotype. (A-B) Body weight and spleen-to-body weight ratios (A) and selected RBC indices (B) of Slc25a38BMKO mice fed defined pyridoxine diets for 3 weeks. (C) Representative flow cytometry plots of erythroid differentiation determined as CD44 expression as a function of FSC in Ter119+ cells in peripheral blood, BM, and spleen in p.R411H and Slc25a38BMKO animals fed diets containing 10- or 0-ppm pyridoxine. (D) Enumeration of erythroid cell (ter119+) populations in control, p.R411H, and Slc25a38BMKO animals fed diets containing 10- or 0-ppm pyridoxine. †Indicates the unique characteristics of the “transitional population” seen in R411H/Y animals. n = 5 to 7 mutants and n = 9 to 12 control animals per diet. All animals are males, except SLC25A38BMKO mice fed 2 ppm, which are a mix of males and females. The dotted line in each panel indicates the average value in control animals. ∗P < .05; ∗∗P < .01; ∗∗∗P < .001; ∗∗∗∗P < .0001, compared with control animals at the same age. #P < .05; ##P < .01; ###P < .001; ####P < .0001, compared with animals of the same genotype on the 10-ppm pyridoxine control diet. B6, pyridoxine; cRBC, circulating red blood cells; CTL, control; Eblasts, erythroblasts.
In sum, control, protoporphyric, and thalassemic animals were relatively resistant to pyridoxine deficiency compared with XLSA and Slc25a38BMKO animals. The rapidity of the decline in Hb and RBC counts in the Slc25a38BMKO animals fed a completely pyridoxine-deficient diet suggests that pyridoxine may have a role in promoting RBC survival as well as maturation. To this end, we directly compared the effect of pyridoxine restriction on erythropoiesis in the R411H XLSA and the Slc25a38BMKO models using a ter119+/CD44/FSC flow cytometry strategy.28,29 A subset of ter119+ RBCs with CD44int/FSClo parameters intermediate between erythroblasts (Hoechst positive) and RBCs (Hoechst negative) were variably positive for Hoechst staining. We termed this group the “transitional population,” because it appears to contain the most immature reticulocytes admixed with very mature, Hoechst-positive erythroblasts and/or pyrenocytes. A FSClo RBC population emerges from the transitional population (referred to as transition†) in the peripheral blood of pyridoxine-depleted XLSA and 2-ppm diet–treated Slc25a38BMKO animals but not in Slc25a38BMKO animals on the 0-ppm diet, (Figure 6C-D; supplemental Figures 9F and 10A). This population likely represents the extremely small anisopoikylocytes seen on peripheral smears (supplemental Figures 8E and 9F). In the R411H XLSA line fed the 0-ppm diet and Slc25a38BMKO animals fed the 2-ppm diet, there was a massive accumulation of erythroblasts and transitional cells in the marrow and the spleen, which, in combination with the splenomegaly and reduced reticulocyte count, is evidence of very severe ineffective erythropoiesis (Figure 6C-D; supplemental Figure 10B). The oxidative stress of RBCs of control and R411H animals was determined by quantifying reactive oxygen species, the pool of reduced thiols, and lipid peroxidation (supplemental Figure 12). When taking into account the XLSA microcytosis, the XLSA RBCs had more oxidative stress. Decreased pyridoxine availability worsened the oxidative stress, which could explain the increased ineffective erythropoiesis. The effect on BM erythropoiesis was similar in the R170H, R452H, and ins14 lines and roughly equivalent to the degree of splenomegaly (Figure 5C; supplemental Figure 11). Pyridoxine depletion in Slc25a38BMKO animals caused a profound decrease in Ter119+ cells both in the BM and spleen, nearly eliminating all erythroblasts (Figure 6C-D). This difference can account for the rapidly fatal anemia in pyridoxine-deficient Slc25a38BMKO animals. The proportion of HSCs in the marrow was unchanged in the absence of pyridoxine, and GR1+ neutrophils, which have a short half-life, were enriched, suggesting that some of the hematopoietic lineages were less affected (supplemental Figure 13A-G).
Discussion
We have established mouse models of XLSA, XLPP, and SLC25A38-CSA that recapitulate key features of each of these diseases. In males, the novel mouse allelic series of XLSA mutations has variably severe microcytic, hypochromic anemia and demonstrate differential sensitivity to pyridoxine supplementation. In female mice, null ALAS2 mutations result in anemia that can be macrocytic, similar to their human counterparts. Slc25a38BMKO animals have moderately severe anemia. We also determine that XLSA and SLC25A38BMKO models are profoundly sensitive to pyridoxine restriction. Finally, XLPP ALAS2 Q548X animals have protoporphyria in both males and females.
There are significant differences between the mouse and human diseases. We did not observe RS in adult XLSA or Slc25a38BMKO mice, as is true of other sideroblastic anemia mouse models.39-42 This could be explained by the observation that, in adult mice, much of the cellular heme synthesis occurs in reticulocytes, whereas in humans, heme synthesis peaks in erythroblasts.50 RSs have been observed during ex vivo differentiation of human XLSA cells only after the addition of exogenous iron.51-54 Therefore, the lack of secondary iron overload in our models could explain the absence of RS. Similar to heat shock cognate B (HSCB)-null animals,35 iron-laden mitochondria were present in nucleated fetal erythrocytes from SLC25A38-null animals, mimicking RSs. Further studies need to be conducted to explain the differences between fetal and adult animals and the ontogeny of RS in CSA. In addition, despite having created the clinically most severe XLPP mutation, p.Q548X, which is typically as severe as EPP due to most FECH mutations in humans, the animal model resulted in milder protoporphyria than the EPP mouse model. This may be attributed to the fact that the Fechm1PAS allele has only 2% to 5% of residual activity, which is substantially more severe than that of most patients with EPP, which typically retain >10% and <35% of residual FECH activity.6,38
We observed a change in the rodent diseases over time when maintained on conventional mouse chow: XLSA and Slc25a38-CSA models got more anemic and microcytic, while the XLPP and EPP RBC mf-PPIX stabilized at lower levels over time. This could be explained by ongoing developmental changes in ALAS2 expression/activity or other factors. Interestingly, placing Slc25a38BMKO animals on a control synthetic diet resulted in a prompt improvement in anemia and microcytosis. This indicates that there are dietary factors modulating the Slc25a38BMKO phenotype. Whether this is dietary glycine, folate, pyridoxine content, or other factors is uncertain. However, if identified, they may lead to dietary interventions for SLC25A38-CSA.
It is well established that pyridoxine supplementation at least partially corrects the anemia of two-thirds of patients with XLSA. It generally improves the Hb levels, whereas other parameters remain largely unchanged.4 The XLSA mouse models recapitulate these observations. It has been suggested that pyridoxine supplementation increases the stability or activity of the mutant ALAS2 enzyme; however, only a limited number of pyridoxine-responsive XLSA mutations affect residues critical to pyridoxal phosphate binding at the active site.55 Interference with pyridoxine metabolism, including repurposing drugs such as isoniazid, which inhibits pyridoxine activation to pyridoxal phosphate, has been a strategy to attempt to limit mf-PPIX accumulation in EPP.56,57 We, however, evaluated the consequences of pyridoxine depletion on both XLPP and EPP mice and found that a pyridoxine-free diet decreased RBC mf-PPIX content only partially in XLPP and not at all in EPP. These results suggest that the effect of isoniazid in EPP mice56 may be independent of its effect on pyridoxine metabolism and may explain why targeting pyridoxine metabolism may not be a productive therapeutic approach in EPP/XLPP.56,58
A novel concept that emerges from this study is the conditional synthetic lethality between heme-related CSAs and pyridoxine depletion. Pyridoxine is abundant in a wide variety of foods, which are often fortified. The prevalence of low serum pyridoxal phosphate (<20 nmol/L) in the United States ranges from 2.1% in infants to 16% in people aged ≥60 years.59 Nevertheless, severe pyridoxine deficiency is rare. It is likely that a subset of individuals with pyridoxine-responsive XLSA and the reported variability of certain mutations to respond to pyridoxine supplementation are highly dependent on pyridoxine consumption and/or metabolism at baseline. Our study also calls into question whether the SLC25A38-CSA should be regarded as “pyridoxine nonresponsive,” because our mouse model is highly dependent on pyridoxine. Because SLC25A38-CSA typically presents in infancy, when pyridoxine deficiency is unusual, pyridoxine may not be limiting in patients when they are diagnosed. Furthermore, the SLC25A38-CSA is severe, nearly uniformly necessitating transfusion; it is unlikely that a patient would be observed long enough to evaluate the effect of supplemental pyridoxine, which might take months to reach maximal effect.
In addition to heme synthesis, pyridoxine is also essential for multiple other mitochondrial and cytosolic pathways that are uniquely important for erythroid proliferation and survival (Figure 7). These pathways include folate-dependent 1-carbon metabolism necessary for DNA synthesis, the glycine cleavage system that generates mitochondrial reduced folate equivalents, and glutathione synthesis, which is one of the principal defenses against oxidative stress in the enucleated mature RBC. Severe ineffective erythropoiesis occurs in XLSA and SLC25A38BMKO animals fed the 0-ppm and 2-ppm pyridoxine diets, respectively. Complete pyridoxine restriction results in erythroid aplasia in SLC25A38BMKO animals. Pyridoxine limitation or depletion may affect different subsets of enzymes in both models. Ineffective erythropoiesis could be caused by an inability to manage excess oxidative stress in these CSAs or prevent ferroptosis, which has been reported in human models,54 whereas erythroid aplasia could be linked to a lack of intramitochondrial glycine. Indeed, SLC25A38 is not the only source of mitochondrial glycine, because serine hydroxymethyltransferase 2 can convert serine to glycine in the mitochondrion; serine hydroxymethyltransferase 2 is a also pyridoxine-dependent enzyme. Thus, it is likely that pyridoxine restriction further diminishes the mitochondrial supply of glycine for 5ALA synthesis, and this is particularly critical in the absence of SLC25A38. The rapidity of the decline in Hb and the other cytopenias observed in pyridoxine-deficient Slc25a38BMKO mice suggest that the effect is not entirely attributable to heme deficiency, and other pathophysiologies, such as hemolysis due to a defective response to oxidative stress, may contribute to the phenotype. In addition, an imbalance of folate-dependent 2-carbon metabolism and the glycine cleavage system in the absence of SLC25A38 may underlie why the addition of folate promotes the effect of glycine supplementation in zebrafish models of SLC25A38 deficiency.59,60 Based on our work, it is certainly possible that abnormalities in pyridoxine metabolism induced by SLC25A38 deficiency are the ultimate cause of the lack of a clinical response to dietary glycine in this disorder. Future studies will focus on the metabolomics of the erythroid cell in XLSA and SLC25A38 with the aim of designing rational treatments.
Erythroid heme metabolism and its dependence on glycine and pyridoxine. Glycine is imported into erythroid precursors by the surface transporter SLC6A9 and then into mitochondria by SLC25A38. Serine can readily be converted to glycine in the cytosol and mitochondrion by serine hydroxymethylase 1 (SHMT1) and SHMT2, respectively, which are pyridoxal phosphate-dependent enzymes that provide 1 carbon fragment to convert tetrahydrofolate (THF) to form methylene-THF (CH2-THF), promoting multiple pathways important to erythrocytes, including DNA and glutathione synthesis that require other PLP enzymes. Serine can be transported into mitochondria by members of the sideroflexin (SFXN) family, which may support the synthesis of the heme precursor ALA by ALAS2 by indirectly providing a source of glycine in the absence of SLC25A38. CoA, coenzyme A; GCS, glycine cleavage system; PLP, pyridoxal 5 phosphate or vitamin B6; SAH, S-adenosylhomocysteine hydrolase; SAM, S-adenosyl methionine.
Erythroid heme metabolism and its dependence on glycine and pyridoxine. Glycine is imported into erythroid precursors by the surface transporter SLC6A9 and then into mitochondria by SLC25A38. Serine can readily be converted to glycine in the cytosol and mitochondrion by serine hydroxymethylase 1 (SHMT1) and SHMT2, respectively, which are pyridoxal phosphate-dependent enzymes that provide 1 carbon fragment to convert tetrahydrofolate (THF) to form methylene-THF (CH2-THF), promoting multiple pathways important to erythrocytes, including DNA and glutathione synthesis that require other PLP enzymes. Serine can be transported into mitochondria by members of the sideroflexin (SFXN) family, which may support the synthesis of the heme precursor ALA by ALAS2 by indirectly providing a source of glycine in the absence of SLC25A38. CoA, coenzyme A; GCS, glycine cleavage system; PLP, pyridoxal 5 phosphate or vitamin B6; SAH, S-adenosylhomocysteine hydrolase; SAM, S-adenosyl methionine.
Acknowledgments
Azra Atabay is acknowledged for preliminary technical work. The authors thank Amy Dickey, Matthew Heeney, and other Fleming laboratory members for ongoing discussions; members of the Boston Children’s Hospital (BCH) Mouse Embryonic Stem Cell and Gene Targeting Core at BCH, including Minh Nguyen and Davide Seruggia, for technical advice and expertise in the construction of the CRISPR-CAS9 mutants. The authors acknowledge Hector Bergonia and John Phillips for the UPLC analyses of ALAS activity, PPIX, and Zn-PPIX at The University of Utah Iron and Heme Core Facility, which is part of the National Institute of Health (NIH), National Institute of Diabetes and Digestive and Kidney Disease (NIDDK)–sponsored Center for Iron and Heme Disorders (U54DK110858). The authors thank Ronald Mathieu (BCH) and Michelle Lifton (Beth Israel Deaconess Medical Center) for running the CBC. Maria Ericsson and Anja Nordstrom in the Harvard Medical School Electron Microscopy Core Facility performed transmission electron microscopy. The Dana-Farber Cancer Institute Molecular Biology Core Facilities performed the next-generation sequencing. The authors thank the employees of the Animal Resource at BCH for the extraordinary animal care they provided during the COVID-19 pandemic.
The work was supported by an NIH/NIDDK Cooperative Centers for Excellence in Hematology (CCEH) External Pilot and Feasibility grant type 1 (S.D.), an American Society of Hematology (ASH) Restart Award (S.D.), a CCEH-NIH/NIDDK U24 Type A award (S.D.), an ASH Bridge Grant (M.D.F.), and the Children’s Hospital Pathology Foundation, Inc (M.D.F.). Part of this work was funded by Forma Therapeutics, Inc, Watertown, MA, which was subsequently acquired by Novo Nordisk, Watertown, MA, in October 2022.
Authorship
Contribution: S.D. and M.D.F. contributed equally to the overall conception, design, interpretation, and presentation of the work; S.D. designed and performed all the experiments with 5-aminolevulinic acid synthetase 2 mutants and all pyridoxine diet studies; A.K.S. designed and performed all basic characterization of the SLC25A38BMKO line, which was supervised by M.D.F.; D.R.C. supported S.D. and A.K.S. with animal husbandry and phenotyping and was supervised by S.D., P.J.S., and M.D.F.; D.W.L.C. provided advice regarding the oxidative stress studies; Y.F. performed gene editing in mouse blastocysts; P.J.S. performed phenotyping assays; S.D. drafted the manuscript and figures; S.D. and M.D.F. wrote and finalized the manuscript; and all authors have read and agreed to the final submitted version.
Conflict-of-interest disclosure: D.W.L.C. is employed by Novo Nordisk. P.J.S. receives sponsored research funding from Disc Medicine. M.D.F. is on the scientific advisory board of and has an equity interest in Disc Medicine; serves as a consultant for Vertex Pharmaceuticals, Affyimmune Therapeutics, EvolveImmune, Minerva Biotechnologies, and Anjarium Biosciences; and received sponsored research funding from Novo Nordisk. The remaining authors declare no competing financial interests.
The current affiliation for A.K.S. is School of Medicine and Public Health, University of Wisconsin–Madison, Madison, WI.
Correspondence: Sarah Ducamp, Department of Pathology, Boston Children's Hospital, 300 Longwood Ave, Boston, MA 02115; email: sarah.ducamp@childrens.harvard.edu; and Mark D. Fleming, Department of Pathology, Boston Children's Hospital, 300 Longwood Ave, Boston, MA 02115; email: mark.fleming@childrens.harvard.edu.
References
Author notes
S.D. and M.D.F. contributed equally to this study.
Requests for novel animal models may be made by email to the corresponding authors, Sarah Ducamp (sarah.ducamp@childrens.harvard.edu) and Mark D. Fleming (mark.fleming@childrens.harvard.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.