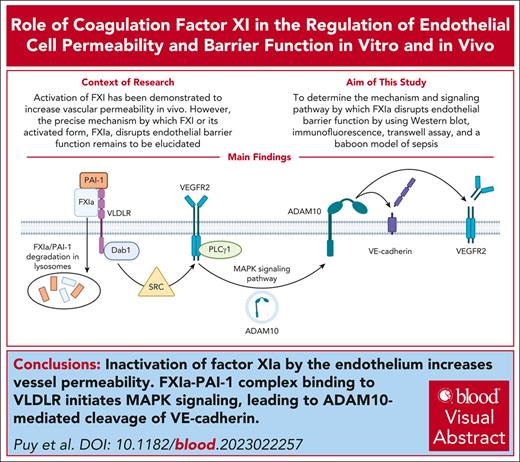
Key Points
Inactivation of factor XIa by the endothelium increases vessel permeability.
FXIa–PAI-1 complex binding to very low–density lipoprotein receptor induces MAPK signaling, causing ADAM10-mediated cleavage of VE-cadherin.
Visual Abstract
Loss of endothelial barrier function contributes to the pathophysiology of many inflammatory diseases. Coagulation factor XI (FXI) plays a regulatory role in inflammation. Although activation of FXI increases vascular permeability in vivo, the mechanism by which FXI or its activated form FXIa disrupts endothelial barrier function is unknown. We investigated the role of FXIa in human umbilical vein endothelial cell (HUVEC) or human aortic endothelial cell (HAEC) permeability. The expression patterns of vascular endothelial (VE)-cadherin and other proteins of interest were examined by western blot or immunofluorescence. Endothelial cell permeability was analyzed by Transwell assay. We demonstrate that FXIa increases endothelial cell permeability by inducing cleavage of the VE-cadherin extracellular domain, releasing a soluble fragment. The activation of a disintegrin and metalloproteinase 10 (ADAM10) mediates the FXIa-dependent cleavage of VE-cadherin, because adding an ADAM10 inhibitor prevented the cleavage of VE-cadherin induced by FXIa. The binding of FXIa with plasminogen activator inhibitor 1 and very low–density lipoprotein receptor on HUVEC or HAEC surfaces activates vascular endothelial growth receptor factor 2 (VEGFR2). The activation of VEGFR2 triggers the mitogen-activated protein kinase (MAPK) signaling pathway and promotes the expression of active ADAM10 on the cell surface. In a pilot experiment using an established baboon model of sepsis, the inhibition of FXI activation significantly decreased the levels of soluble VE-cadherin to preserve barrier function. This study reveals a novel pathway by which FXIa regulates vascular permeability. The effect of FXIa on barrier function may be another way by which FXIa contributes to the development of inflammatory diseases.
Introduction
The vascular endothelium functions as a barrier to regulate the extravasation of water, plasma proteins, and leukocytes to maintain homeostasis. During inflammation, select mediators act on endothelial cells (ECs) to increase the permeability of the endothelial layer to facilitate resolution. Yet, vascular hyperpermeability in response to chronic inflammation such as in atherosclerosis, cancer, or certain infections can lead to edema, tissue damage, and EC dysfunction and death.1
Intercellular transport between ECs is the predominant form of extravasation and is tightly regulated by apical intercellular junctions including tight junctions, and adherens junctions. Specific to ECs, vascular endothelial (VE)-cadherin serves as a central regulator of adherens junction integrity. The 5 cadherin domains that comprise the extracellular region of VE-cadherin maintain cohesion through Ca2+-dependent homophilic interactions. The intracellular region of VE-cadherin binds several proteins of the catenin family, acting to stabilize the junction at the plasma membrane by way of mediating interaction with the actin cytoskeleton.2 VE-cadherin also forms key complexes with transmembrane proteins including platelet EC adhesion molecule 1, vascular endothelial growth factor receptor 2 (VEGFR2), Tie2, and VE-protein tyrosine phosphatase.3-5 Changes in the expression of these transmembrane proteins can affect the stability and expression of VE-cadherin.
Adherens junctions and vascular permeability are regulated through several mechanisms: (1) clustering junctional complexes and formation of focal adherent junctions to generate small gaps between ECs; (2) endocytosis of junctional proteins to reduce or disrupt the expression of adherens junctions; or (3) shedding of the extracellular part of VE-cadherin (ectodomain shedding).2 The cleavage of VE-cadherin is known to be mediated by neutrophil elastase and metalloproteases, including a disintegrin and metalloproteinase 10 (ADAM10), resulting in the shedding of soluble VE-cadherin (sVE-cadherin), which can be detected in the circulation.6-8 How VE-cadherin is regulated with respect to the blood microenvironment and activity of serine proteases within the coagulation cascade is not well defined.
We previously found that EC VE-cadherin expression levels were sensitive to activation of the coagulation factor XI (activated FXI [FXIa]) in vitro, whereas pharmacological inhibition of FXI in a mouse model of atherosclerosis preserved VE-cadherin expression in vivo.9 Along these lines, we have demonstrated that pharmaceutical targeting of FXI activation or FXIa activity has improved outcomes in models of atherosclerosis,9,10 ischemic stroke,11 myocardial infarction,12 multiple sclerosis,13,14 and sepsis.15-17 Because compromised endothelial permeability and barrier function is a commonality among these pathologies, we considered whether inhibition of FXI mitigates these inflammatory diseases, in part, through maintenance of VE-cadherin expression and barrier function. We explored indirect mechanisms by which FXIa acts to downregulate VE-cadherin expression. FXIa, dissimilar to the coagulation factors thrombin and FXa that induce ECs permeability by cleaving protease activated receptor 1 or 2 (PAR1/2), respectively, is incapable of directly cleaving PARs.18,19
Herein we explored a pathway by which FXIa induces ADAM10 maturation and translocation to the EC surface to facilitate the cleavage of VE-cadherin to regulate vascular permeability.
Methods
All reagent and cell information, and statistical analysis can be found in supplemental Data, available on the Blood website.
Immunofluorescence
Human umbilical vein endothelial cells (HUVECs) were grown on glass coverslips and fixed in 4% paraformaldehyde before blocking with 1% bovine serum albumin (BSA) and 2% fetal bovine serum. Primary antibodies (VE-cadherin N-terminal ectodomain [NTD], 2 μg/mL in 1% BSA) were incubated overnight at 4°C. Slides were washed and secondary Alexa Fluor 488 anti-mouse immunoglobulin G (4 μg/mL) and tetramethylrhodamine-phalloidin were incubated for 2 hours at room temperature in 1% BSA in the dark. Hoechst 33342 was incubated for 30 minutes before mounting onto glass slides using Fluoromount G. HUVECs were imaged using a Zeiss 20× Plan-APOCHROMAT 0.8 NA objective on a Zeiss Axio Imager M2 microscope. Representative images were collected from at least 3 fields of view.
Permeability assay
Cells were grown to confluence in gelatin-coated upper chambers of Transwell devices. Cells permeability was assessed by adding Evans Blue dye in 4% BSA to the upper chamber. Absorbance was measured at 650 nm using a spectrophotometer.
Western blotting, Phos-tag electrophoresis, immunoprecipitation, and cell surface biotinylation
Cells were lysed with Laemmli sample buffer containing 200 mM dithiothreitol, subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis, transferred to polyvinylidene fluoride membranes, and immunoblotted with antibodies for VE-cadherin, phospho-extracellular signal-regulated kinase 1/2 (ERK1/2), phospho-P38 mitogen-activated protein kinase (MAPK), phospho-protein kinase B (Akt), phosphor–endothelial nitric oxide synthase (eNOS), phospho-phospholipase C gamma 1 (PLCγ1), phospho-proto-oncogene tyrosine-protein kinase (Src), phospho–focal adhesion kinases (FAK), phospho-DAB adaptor protein 1 (Dab), phospho-vascular endothelial growth factor receptor 2 (VEGFR2), or tubulin and an horseradish peroxidase–conjugated secondary antibody. Proteins were detected using electrogenerated chemiluminescence. All western blot images are representative of 3 or 4 experiments. Densitometry analysis of protein bands imaged were done using ImageJ software.
For Phos-tag gel separation, 10% polyacrylamide gel electrophoresis gels were supplemented with 25 μM Phos-tag acrylamide and 50 μM MnCl2.20 For immunoprecipitation, cells were lysed with 10 mM Tris/HCl, pH 7.4, 150 mM NaCl, 2mM EDTA, 1% (v/v) Triton X-100, precleared with Protein A/G Sepharose and incubated with 2 μg of an anti-FXI antibody overnight at 4°C. Antibody-protein complexes were captured with Protein A/G PLUS agarose beads. For cell surface biotinylation, cells were incubated with 0.5 mg/mL sulfo-NHS-SS-biotin (30 minutes, 4°C) and washed 3 times with 50 mM glycine in phosphate-buffered saline/CaCl2/MgCl2, pH 7.4. Cells were lysed and precipitated using NeutrAvidin agarose beads (4 hours, 4°C).
Baboon model of Staphylococcus aureus sepsis
We analyzed stored plasma samples from a historical study15 that was approved by the interfaculty animal ethics committee of the University of the Free State, Bloemfontein, South Africa and the institutional care and use committee of Oklahoma Medical Research Foundation. Healthy Papio ursinus baboons were randomly distributed between the control and treatment groups. S aureus subspecies aureus Rosenbach was from American Type Culture Collection (Manassas, VA).
The study included 2 arms, as previously described,15: a control group (n = 3) and a treated group (n = 4) who received a bolus of the anti-FXI antibody 3G3 (1 mg/kg) 30 minutes before the bacterial infusion was started. Baboons in both groups were challenged with 3 × 1010 bacteria per kg by intravenous infusion over a 2-hour period. Plasma samples were collected before challenge (T0) and at 2, 4, 6, 8, and 24 hours after challenge.
Results
FXIa increases endothelial permeability by inducing cleavage of the VE-cadherin ectodomain
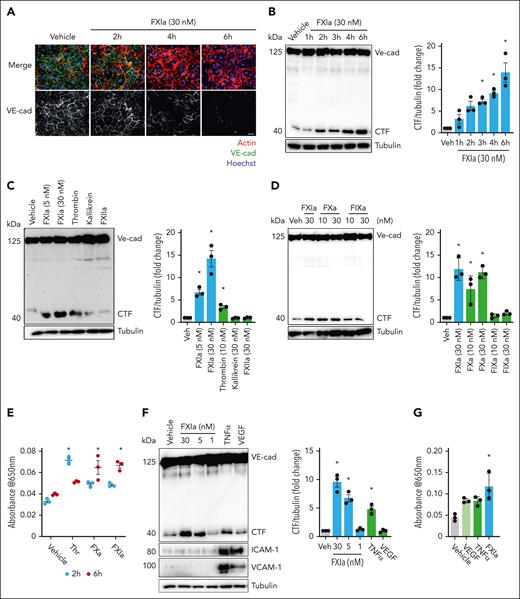
We previously found that inhibition of FXI prevents disruption of VE-cadherin expression in aortic sinus lesions.9 We, therefore, first confirmed that FXIa disrupts surface expression of VE-cadherin on ECs in vitro. We used a concentration of 30 nM FXIa for our study design based on physiologic plasma concentration of FXI. Incubation of HUVECs with FXIa decreased surface expression of VE-cadherin NTD (extracellular domain) in a time-dependent manner, leading to nearly undetectable levels of VE-cadherin on the EC surface after 6 hours of incubation (Figure 1A).
FXIa induces VE-cadherin shedding and EC permeability. (A) HUVECs were grown on gelatin-coated glass coverslips and incubated with FXIa (30 nM) for 2 to 6 hours. Cells were fixed and stained for VE-cadherin (green), actin (red), and nuclei (blue). Bar, 50 μm. (B) HUVECs were incubated with FXIa for 1 to 6 hours. Cells were lysed and analyzed by western blotting using an anti–C-terminal VE-cadherin antibody. Results are representative of 3 experiments. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± standard error of the mean (SEM; n = 3). (C) HUVECs were incubated with FXIa (5 or 30 nM), thrombin (10 nM), kallikrein (30 nM), or FXIIa (30 nM) for 6 hours. Cells were lysed and analyzed by western blotting using an anti–C-terminal anti–VE-cadherin antibody. Results are representative of 3 experiments. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3). (D) HUVECs were incubated with FXIa (30 nM), or FXa (10 or 30 nM), or FIXa (10 or 30 nM) for 6 hours. Cells were lysed and analyzed by western blotting using an anti–C-terminal VE-cadherin antibody. Results are representative of 3 experiments. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3). (E) HUVECs were grown to confluence on gelatin-coated Transwell filters and incubated with thrombin, FXa, or FXIa (5 nM), for 2 or 6 hours. Permeability of Evans Blue-BSA was measured after 60 minutes of incubation. Data are mean ± SEM (n = 3). Significance (∗P < .05) was determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. (F) HUVECs were incubated with FXIa (1, 5, or 30 nM), TNFα (10 ng/mL), or VEGF (100 ng/mL) for 6 hours. Cells were lysed and analyzed by western blotting using an anti–C-terminal VE-cadherin, anti–ICAM-1, or anti–VCAM-1 antibodies. Results are representative of 3 experiments. Data are mean ± SEM. Significance (∗P < .05) was determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. (G) HUVECs were grown to confluence on gelatin-coated Transwell filters and incubated with FXIa (30 nM), VEGF (100 ng/mL), or TNFα (10 ng/mL) for 6 hours. Permeability of Evans Blue-BSA was measured after 60 minutes of incubation. Data are mean ± SEM (n = 3). Significance (∗P < .05) was determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons.
FXIa induces VE-cadherin shedding and EC permeability. (A) HUVECs were grown on gelatin-coated glass coverslips and incubated with FXIa (30 nM) for 2 to 6 hours. Cells were fixed and stained for VE-cadherin (green), actin (red), and nuclei (blue). Bar, 50 μm. (B) HUVECs were incubated with FXIa for 1 to 6 hours. Cells were lysed and analyzed by western blotting using an anti–C-terminal VE-cadherin antibody. Results are representative of 3 experiments. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± standard error of the mean (SEM; n = 3). (C) HUVECs were incubated with FXIa (5 or 30 nM), thrombin (10 nM), kallikrein (30 nM), or FXIIa (30 nM) for 6 hours. Cells were lysed and analyzed by western blotting using an anti–C-terminal anti–VE-cadherin antibody. Results are representative of 3 experiments. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3). (D) HUVECs were incubated with FXIa (30 nM), or FXa (10 or 30 nM), or FIXa (10 or 30 nM) for 6 hours. Cells were lysed and analyzed by western blotting using an anti–C-terminal VE-cadherin antibody. Results are representative of 3 experiments. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3). (E) HUVECs were grown to confluence on gelatin-coated Transwell filters and incubated with thrombin, FXa, or FXIa (5 nM), for 2 or 6 hours. Permeability of Evans Blue-BSA was measured after 60 minutes of incubation. Data are mean ± SEM (n = 3). Significance (∗P < .05) was determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. (F) HUVECs were incubated with FXIa (1, 5, or 30 nM), TNFα (10 ng/mL), or VEGF (100 ng/mL) for 6 hours. Cells were lysed and analyzed by western blotting using an anti–C-terminal VE-cadherin, anti–ICAM-1, or anti–VCAM-1 antibodies. Results are representative of 3 experiments. Data are mean ± SEM. Significance (∗P < .05) was determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. (G) HUVECs were grown to confluence on gelatin-coated Transwell filters and incubated with FXIa (30 nM), VEGF (100 ng/mL), or TNFα (10 ng/mL) for 6 hours. Permeability of Evans Blue-BSA was measured after 60 minutes of incubation. Data are mean ± SEM (n = 3). Significance (∗P < .05) was determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons.
We then examined whether FXIa induced VE-cadherin NTD shedding. Confluent HUVEC monolayers were incubated with FXIa for up to 6 hours. We quantified the level of the C-terminal intracellular fragment (CTF; 40 kDa) of cleaved VE-cadherin by western blotting using an anti–VE-cadherin C-terminal domain (CTD) antibody. Incubation of HUVECs with FXIa led to the appearance of a lower molecular weight band at 40 kDa (Figure 1B), indicative of NTD VE-cadherin shedding. The total levels of full-length VE-cadherin remained constant because we were detecting both intracellular and membrane-bound VE-cadherin, because VE-cadherin is constitutively trafficked between intracellular compartments and the plasma membrane.21
Next, we determined whether other coagulation serine proteases could induce cleavage of the VE-cadherin NTD. Thrombin induced a slight cleavage of VE-cadherin as measured by appearance of the CTF (Figure 1C). Interestingly, FXa was also capable of significantly promoting the cleavage of VE-cadherin (Figure 1D). Neither FXIIa, kallikrein, nor FIXa induced cleavage of VE-cadherin (Figure 1C-D). We also compare the effect of 5 nM FXIa, thrombin or FXa on ECs permeability after 2 or 6 hours of incubation. We found that thrombin increased vascular permeability after 2 hours of incubation and that this effect diminished after 6 hours of incubation (Figure 1E). In contrast, FXa and FXIa promoted an increase in vascular permeability after 6 hours of incubation (Figure 1E). These experiments suggest that FXIa and FXa induce VE-cadherin NTD shedding by a different mechanism to that of thrombin.
We examined whether other inflammatory mediators were capable of inducing VE-cadherin cleavage or shedding. Although FXIa induced the cleavage of VE-cadherin in a dose-dependent manner, tumor necrosis factor α (TNFα) was only capable of inducing a slight cleavage of VE-cadherin (Figure 1E-F). VE-cadherin remained structurally intact even after 6 hours of incubation with VEGF (Figure 1E). We also measured the expression of vascular cell adhesion molecule 1 (VCAM-1) and intercellular adhesion molecule 1 (ICAM-1) after incubating HUVECs with FXIa, TNFα, or VEGF. Although FXIa did not induce the expression of either VCAM-1 or ICAM-1, TNFα and VEGF increased the expression of both adhesive proteins (Figure 1F). We evaluated the effect of FXIa, VEGF, and TNFα on ECs permeability. ECs were cultured on Transwell filter inserts in the presence of FXIa, VEGF, or TNFα. FXIa promoted an increase in leakage of Evans Blue dye compared with VEGF or TNFα (P < .05; Figure 1G). These experiments suggest that FXIa disrupts surface expression of VE-cadherin by inducing VE-cadherin NTD shedding by a different mechanism to that of VEGF or TNFα.
FXIa induces VE-cadherin shedding by increasing ADAM10 expression and activity
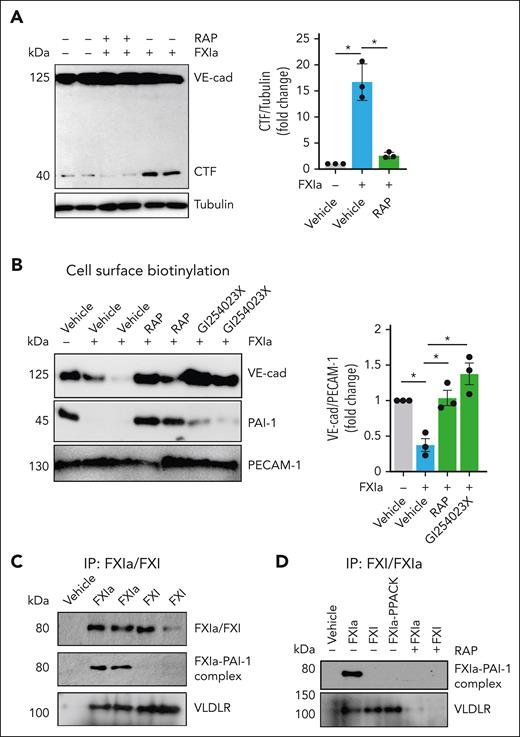
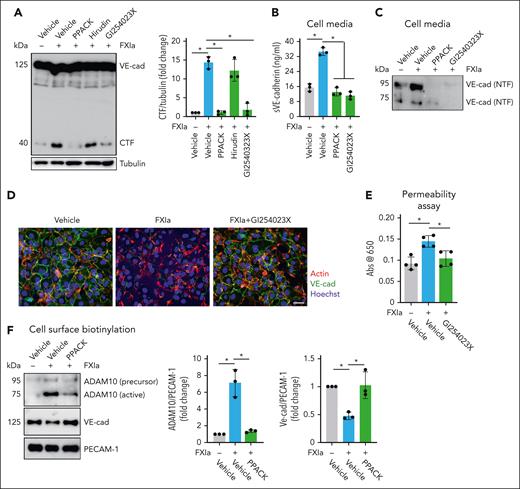
We studied the role of the FXIa catalytic domain on shedding of the NTD of VE-cadherin. The serine protease inhibitor, D-Phenylalanyl-L-prolyl-L-arginine chloromethyl ketone (PPACK), which binds irreversibly to the active site of FXIa, abolished the effect of FXIa on VE-cadherin proteolysis. The presence of hirudin (a specific thrombin inhibitor; Figure 2A), or rivaroxaban (a specific FXa inhibitor) did not affect the cleavage of VE-cadherin by FXIa (supplemental Figure 1A).
Effect of ADAM10 inhibitor on VE-cadherin cleavage induced by FXIa. HUVECs were incubated with FXIa for 6 hours in the presence or absence of the serine protease inhibitor, PPACK (100 μM); the thrombin inhibitor, hirudin (25 μg/mL); or the ADAM10 inhibitor, GI254023X (10 μM). (A) Cells were lysed and analyzed by western blotting using an anti–C-terminal VE-cadherin antibody. Results are representative of 3 experiments. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3). (B) Cell media were analyzed by using a sVE cadherin enzyme-linked immunosorbent assay. Data are mean ± SEM (n = 3). Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. (C) Cell media were also analyzed by western blot using an anti–N-terminal VE-cadherin antibody. Results representative of 3 experiments. (D) HUVECs were grown on gelatin-coated glass coverslips and incubated with FXIa (30 nM) for 6 hours in the absence or presence of GI254023X (10 μM). Cells were fixed and stained for VE-cadherin (green), actin (red), and nuclei (blue). Bar, 50 μm. (E) HUVECs were grown to confluence on gelatin-coated Transwell filters and incubated with FXIa (30 nM) in the absence or presence of GI254023X (10 μM). Permeability of Evans Blue-BSA was measured after 60 minutes of incubation. Data are mean ± SEM (n = 4). Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. (F) HUVECs were incubated with FXIa (30 nM) for 6 hours in the absence or presence of PPACK (100 μM). Cell surface was biotinylated and cell lysates were precipitated with NeutrAvidin agarose beads and probed with an anti-ADAM10, anti–N-terminal VE-cadherin, or anti-PECAM1 antibodies. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3).
Effect of ADAM10 inhibitor on VE-cadherin cleavage induced by FXIa. HUVECs were incubated with FXIa for 6 hours in the presence or absence of the serine protease inhibitor, PPACK (100 μM); the thrombin inhibitor, hirudin (25 μg/mL); or the ADAM10 inhibitor, GI254023X (10 μM). (A) Cells were lysed and analyzed by western blotting using an anti–C-terminal VE-cadherin antibody. Results are representative of 3 experiments. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3). (B) Cell media were analyzed by using a sVE cadherin enzyme-linked immunosorbent assay. Data are mean ± SEM (n = 3). Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. (C) Cell media were also analyzed by western blot using an anti–N-terminal VE-cadherin antibody. Results representative of 3 experiments. (D) HUVECs were grown on gelatin-coated glass coverslips and incubated with FXIa (30 nM) for 6 hours in the absence or presence of GI254023X (10 μM). Cells were fixed and stained for VE-cadherin (green), actin (red), and nuclei (blue). Bar, 50 μm. (E) HUVECs were grown to confluence on gelatin-coated Transwell filters and incubated with FXIa (30 nM) in the absence or presence of GI254023X (10 μM). Permeability of Evans Blue-BSA was measured after 60 minutes of incubation. Data are mean ± SEM (n = 4). Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. (F) HUVECs were incubated with FXIa (30 nM) for 6 hours in the absence or presence of PPACK (100 μM). Cell surface was biotinylated and cell lysates were precipitated with NeutrAvidin agarose beads and probed with an anti-ADAM10, anti–N-terminal VE-cadherin, or anti-PECAM1 antibodies. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3).
Serine proteases such as neutrophils elastase are known to cleave the ectodomain of VE-cadherin in regions near the C-terminus, which contains small hydrophobic residues, including alanine, valine, serine, glycine, leucine, or isoleucine. In contrast, FXIa selectively cleaves substrates at distinct peptide bonds between arginine or lysine and alanine, valine, or serine residues.22 Upon analysis of the sequence of the VE-cadherin ectodomain, we could not find a peptide sequences that would be susceptible to cleavage by FXIa, suggesting that the FXIa catalytic domain does not directly cleave the ectodomain of VE-cadherin.
On ECs, shedding of VE-cadherin is mainly regulated by ADAM10,6-8 which is localized predominantly at adherens junctions.23 ADAM10 cleaves VE-cadherin near the cell membrane, releasing a 90-kDa fragment. We performed experiments in the presence of the ADAM10 inhibitor GI254023X and showed formation of the VE-cadherin CTF and release of a 90-kDa NTD fragment (sVE-cadherin) into the cell media induced by FXIa was prevented (Figure 2A-C). GI254023X also prevented the reduction in cell surface expression of VE-cadherin caused by FXIa (Figure 2D), and the leakage of Evans Blue dye induced by FXIa (Figure 2E). The addition of the inhibitors PPACK, hirudin, rivaroxaban, or GI254023X alone were unable to induce VE-cadherin cleavage (supplemental Figure 1A-B). The ADAM10 inhibitor GI254023X also inhibited the formation of the VE-cadherin CTF induced by FXa (supplemental Figure 2B).
Furthermore, we found that FXIa induced the expression of active ADAM10 on the cell surface (Figure 2F) while concomitantly decreasing the expression of the VE-cadherin NTD, indicative of a decrease in plasma membrane full-length VE-cadherin. The addition of PPACK eliminated these effects. These data suggest that FXIa induces VE-cadherin shedding by promoting the expression of active ADAM10 on the surface of ECs.
FXIa induces ADAM10 activation and VE-cadherin cleavage by activation of Src kinase and the PLCγ1-ERK signaling pathway
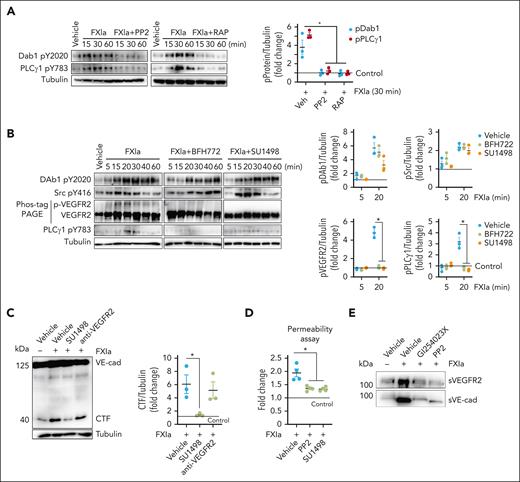
Prior studies have outlined a mechanism for ADAM10 activation and cellular transport, with roles for mitogen-activated protein kinases, ERK, and Src kinase in the brain microenvironment.24,25 We examined whether FXIa incited a similar signaling pathway to induce ADAM10 activation in ECs. FXIa induced ERK1/2 T202/Y204 and Src Y416 phosphorylation. In contrast, FXIa did not induce P38 MAPK T180/Y182 phosphorylation (Figure 3A). Incubation of ECs with FXIa induced phosphorylation of Akt S473 and eNOS S1177 (Figure 3A), 2 substrates downstream of phosphatidylinositol 3-kinase (PI3K) and in line with prior studies linking the PI3K pathway to ADAM10 activation.26
Role of the PI3K-Akt-eNOS and PLCγ1-ERK signaling pathways on VE-cadherin cleavage induced by FXIa. (A) HUVECs were incubated with FXIa (30 nM). Cells were lysed and immunoblotted with antibodies for phosphorylated ERK1/2 T202/Y204, p38 MAPK T180/Y182. Akt S473, eNOS S1177, PLCγ1 Y783, Src Y416, FAK Y397, or tubulin (n = 3). Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3). (B) HUVECs were incubated with FXIa, FXIa-PPACK, or FXI (30 nM) for 15 or 30 minutes. Cells were lysed and immunoblotted with antibodies for phosphorylated ERK1/2 T202/Y204 or Akt S473. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Results are representative of 3 experiments. (C) HUVECs were incubated with vehicle (dimethyl sulfoxide [DMSO]) or FXIa for 6 hours in the absence or presence of the Src inhibitor, PP2 (10 μM); the protein kinase A (PKA) inhibitor, H-89 (10 μM); the eNOS inhibitor, L-NAME (5 μM); or the ERK1/2 inhibitor, LY3214996 (1 μM). Cells were lysed and analyzed by western blotting using an anti–C-terminal VE-cadherin antibody. Results are representative of 3 experiments. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3).
Role of the PI3K-Akt-eNOS and PLCγ1-ERK signaling pathways on VE-cadherin cleavage induced by FXIa. (A) HUVECs were incubated with FXIa (30 nM). Cells were lysed and immunoblotted with antibodies for phosphorylated ERK1/2 T202/Y204, p38 MAPK T180/Y182. Akt S473, eNOS S1177, PLCγ1 Y783, Src Y416, FAK Y397, or tubulin (n = 3). Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3). (B) HUVECs were incubated with FXIa, FXIa-PPACK, or FXI (30 nM) for 15 or 30 minutes. Cells were lysed and immunoblotted with antibodies for phosphorylated ERK1/2 T202/Y204 or Akt S473. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Results are representative of 3 experiments. (C) HUVECs were incubated with vehicle (dimethyl sulfoxide [DMSO]) or FXIa for 6 hours in the absence or presence of the Src inhibitor, PP2 (10 μM); the protein kinase A (PKA) inhibitor, H-89 (10 μM); the eNOS inhibitor, L-NAME (5 μM); or the ERK1/2 inhibitor, LY3214996 (1 μM). Cells were lysed and analyzed by western blotting using an anti–C-terminal VE-cadherin antibody. Results are representative of 3 experiments. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3).
Activation of the MAPK signaling pathway is mediated by activation of either phospholipase C gamma1 (PLCγ1) or FAK in ECs. In keeping with this, we observed that PLCγ1 Y783 but not FAK Y397 was phosphorylated after incubating ECs with FXIa (Figure 3A). The addition of PPACK eliminated FXIa-induced phosphorylation of Akt S473 and ERK1/2 T202/Y204, whereas zymogen FXI failed to induce phosphorylation of either substrate (Figure 3B).
We determined whether activation of the PI3K-Akt-eNOS or PLCγ1-ERK signaling pathways by FXIa induced VE-cadherin shedding. Experiments were performed by addition of the eNOS inhibitor L-NAME or the ERK1/2 inhibitor LY3214996 before incubation of ECs with FXIa. Pretreatment with LY3214996 but not L-NAME inhibited FXIa-induced cleavage of VE-cadherin (Figure 3C). Pretreatment with the Src kinase inhibitor PP2 inhibited the formation of the VE-cadherin CTF. In contrast, the inhibition of protein kinase A did not block VE-cadherin shedding (Figure 3C). These data suggest that FXIa incites cleavage of VE-cadherin by promoting the activation of Src kinase and the PLCγ1-ERK signaling pathway. The addition of the inhibitors L-NAME, LY3214996, PP2, or H-89 alone were insufficient to induce VE-cadherin cleavage (Figure 3C).
Endothelial VLDLR receptor mediates FXIa–PAI-1 complex binding to induce cell signaling and VE-cadherin cleavage
We determined the EC surface receptor that mediates FXIa binding and signaling. We have shown that FXIa forms a complex with plasminogen activator inhibitor-1 (PAI-1) on the surface of ECs, inducing FXIa internalization and depletion of PAI-1 from the cell surface. The addition of PPACK prevented FXIa–PAI-1 complex formation and the depletion of PAI-1.27 Prior studies found that internalization of uPA–PAI-1 complexes can initiate intracellular signaling to regulate VE-cadherin function in a low-density lipoprotein receptor-related protein (LRP)-dependent manner.28 First, we examined the role of very low–density lipoprotein receptor (VLDLR) and PAI-1 in FXIa-induced VE-cadherin shedding. Pretreatment of ECs with a pan-LDLR antagonist, receptor-associated protein (RAP), prevented generation of VE-cadherin CTF by FXIa (Figure 4A). We observed that RAP prevented loss of cell surface expression of VE-cadherin and PAI-1 caused by FXIa (Figure 4B). These findings suggest that a member of the LDLR family binds FXIa–PAI-1 complexes to the EC surface. We confirmed that FXIa but not thrombin or FXa was able to decrease the expression of PAI-1 (supplemental Figure 2A-B). Also, the presence of RAP did not inhibit VE-cadherin cleavage by FXa (supplemental Figure 2A-B), suggesting that FXa promotes VE-cadherin NTD shedding by a different mechanism to that of FXIa, probably through the activation of PAR2.18,29
FXIa–PAI-1 complex interaction with VLDLR induces VE-cadherin shedding. (A) HUVECs were incubated with FXIa (30 nM) for 6 hours in the absence or presence of the low-density lipoprotein receptor antagonist, RAP (50 ng/mL). Cells were lysed and analyzed by western blotting using an anti–C-terminal VE-cadherin antibody. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3). (B) HUVECs were incubated with FXIa (30 nM) in the absence or presence of PPACK (100 μM), GI254023X (5 μM), or RAP (50 ng/mL) for 6 hours. HUVECs cell surfaces were biotinylated, and cell lysates were precipitated with NeutrAvidin agarose beads. The precipitates were probed with an anti–N-terminal VE-cadherin or anti–PAI-1 antibodies. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3). (C) HUVECs were incubated with FXIa (30 nM) at 4°C for 2 hours. Cells were washed and incubated with PPACK (100 μM) for 30 minutes; lysed in the presence of PPACK; followed by immunoprecipitation with an anti-FXI LC and western blotting with an anti–PAI-1, anti-VLDLR, or anti–platelet EC adhesion molecule 1 (PECAM-1) antibodies. Results are representative of 3 experiments. (D) HUVECs were incubated with FXIa, FXI, or FXIa-PPACK (30 nM) at 4°C for 2 hours in the absence or presence of RAP (50 ng/mL). Cells were washed and incubated with PPACK (100 μM) for 30 minutes, lysed in the presence of PPACK followed by with an anti-FXI light chain immunoprecipitation and western blotting with an anti–PAI-1 antibody or anti-VLDLR antibodies. Results are representative of 3 experiments.
FXIa–PAI-1 complex interaction with VLDLR induces VE-cadherin shedding. (A) HUVECs were incubated with FXIa (30 nM) for 6 hours in the absence or presence of the low-density lipoprotein receptor antagonist, RAP (50 ng/mL). Cells were lysed and analyzed by western blotting using an anti–C-terminal VE-cadherin antibody. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3). (B) HUVECs were incubated with FXIa (30 nM) in the absence or presence of PPACK (100 μM), GI254023X (5 μM), or RAP (50 ng/mL) for 6 hours. HUVECs cell surfaces were biotinylated, and cell lysates were precipitated with NeutrAvidin agarose beads. The precipitates were probed with an anti–N-terminal VE-cadherin or anti–PAI-1 antibodies. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3). (C) HUVECs were incubated with FXIa (30 nM) at 4°C for 2 hours. Cells were washed and incubated with PPACK (100 μM) for 30 minutes; lysed in the presence of PPACK; followed by immunoprecipitation with an anti-FXI LC and western blotting with an anti–PAI-1, anti-VLDLR, or anti–platelet EC adhesion molecule 1 (PECAM-1) antibodies. Results are representative of 3 experiments. (D) HUVECs were incubated with FXIa, FXI, or FXIa-PPACK (30 nM) at 4°C for 2 hours in the absence or presence of RAP (50 ng/mL). Cells were washed and incubated with PPACK (100 μM) for 30 minutes, lysed in the presence of PPACK followed by with an anti-FXI light chain immunoprecipitation and western blotting with an anti–PAI-1 antibody or anti-VLDLR antibodies. Results are representative of 3 experiments.
Endothelial PAI-1 is known to interact with 2 members of the LDLR family, VLDLR and LRP-1.30 We found that the FXIa–PAI-1 complex coimmunoprecipitated only with VLDLR (Figure 4C). Moreover, the zymogen form of FXI, or FXIa that had been inactivated with PPACK, and are unable to complex with PAI-1, were also pulled down with VLDLR (Figure 4D), suggestive of a binding site for VLDLR on FXI. We confirmed this by showing that RAP was capable of blocking FXIa, FXI, or FXIa-PPACK binding to VLDLR. In addition, we showed that PPACK and RAP prevented FXIa–PAI-1 complex formation (Figure 4D). These data suggest that the interaction of FXIa with VLDLR is required for the FXIa–PAI-1 complex formation and the depletion of PAI-1 from the surface of ECs.
Prior studies determined that pharmacological targeting of PAI-1 is sufficient to induce shedding of VE-cadherin.31 We confirmed that targeting EC PAI-1 with a blocking anti–PAI-1 antibody induced a slight cleavage of VE-cadherin inferred by the appearance of the VE-cadherin CTF and diminished surface expression of VE-cadherin (supplemental Figure 3A-B). The addition of GI254023X prevented the cleavage of VE-cadherin induced by the anti–PAI-1 antibody (supplemental Figure 3A-B).
FXIa signals through Dab1 and VEGFR2 to induce cleavage of VE-cadherin
Next, we defined the signaling cascade linking FXIa-PAI1complex ligation of VLDLR and downstream activation of the Src kinase and PLCγ1-ERK signaling pathways. The best-characterized VLDLR-signaling pathway is the reelin pathway. Binding of reelin to VLDLR induces phosphorylation of the adapter protein Dab1Y220 in a Src kinase-dependent manner.32 We tested the hypothesis that FXIa binding to VLDLR induces Dab1 Y220 phosphorylation. FXIa induced Dab1 Y220 phosphorylation after 15 minutes of incubation (Figure 5A). Preincubation with RAP or PP2 prevented Dab1 Y220 and PLCγ1 Y783 phosphorylation (Figure 5A). These data suggest that binding of FXIa to VLDLR induces activation of Dab1 and PLCγ1 in a process that requires Src kinase activation. The total levels of Dab1 or ERK1 were insensitive to FXIa (supplemental Figure 4B).
Role of FXIa on Dab1 and VEGFR2 activation. (A) HUVECs were incubated with FXIa (30 nM) in the absence or presence of (A) the Src inhibitor, PP2 (10 μM), or the low-density lipoprotein receptor antagonist, RAP (50 ng/mL). Cells were lysed and immunoblotted with antibodies for Dab1 Y2020, PLCγ1 Y783, or tubulin. Results are representative of 3 experiments. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. (B) HUVECs were incubated with FXIa (30 nM) in the absence or presence of the VEGFR2 inhibitors, BFH772 (1 μM) or SU 1498 (5 μM). Cells were lysed and immunoblotted with antibodies for Dab1 Y2020, Src Y416, PLCγ1 Y783, or tubulin, or lysates were separated by Phos-tag sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted for VEGFR2 phosphorylation (n = 3). Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3). (C) HUVECs were incubated with vehicle or FXIa for 6 hours in the absence or presence of the VEGFR2 kinase activity inhibitor, SU1498 (5 μM), or the blocking anti-VEGF antibody, ramucirumab (10 μg/mL). Cells were lysed and analyzed by western blotting using an anti–C-terminal VE-cadherin antibody. Results are representative of 3 experiments. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3). (D) HUVECs were grown to confluence on gelatin-coated Transwell filters and incubated with FXIa (30 nM), for 6 hours in the absence or presence of PP2 (10 μM) or SU1498 (5 μM). Permeability of Evans Blue-BSA was measured after 60 minutes of incubation. Data are mean ± SEM (n = 4). Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. (E) HUVECs were incubated with FXIa (30 nM) for 6 hours in the absence or presence of the ADAM10 inhibitor, GI254023X (5 μM), or the Src inhibitor, PP2. Cell media were collected and immunoblotted with antibodies for N-terminal VE-cadherin or N-terminal VEGFR2. Results are representative of 3 experiments.
Role of FXIa on Dab1 and VEGFR2 activation. (A) HUVECs were incubated with FXIa (30 nM) in the absence or presence of (A) the Src inhibitor, PP2 (10 μM), or the low-density lipoprotein receptor antagonist, RAP (50 ng/mL). Cells were lysed and immunoblotted with antibodies for Dab1 Y2020, PLCγ1 Y783, or tubulin. Results are representative of 3 experiments. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. (B) HUVECs were incubated with FXIa (30 nM) in the absence or presence of the VEGFR2 inhibitors, BFH772 (1 μM) or SU 1498 (5 μM). Cells were lysed and immunoblotted with antibodies for Dab1 Y2020, Src Y416, PLCγ1 Y783, or tubulin, or lysates were separated by Phos-tag sodium dodecyl sulfate–polyacrylamide gel electrophoresis and immunoblotted for VEGFR2 phosphorylation (n = 3). Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3). (C) HUVECs were incubated with vehicle or FXIa for 6 hours in the absence or presence of the VEGFR2 kinase activity inhibitor, SU1498 (5 μM), or the blocking anti-VEGF antibody, ramucirumab (10 μg/mL). Cells were lysed and analyzed by western blotting using an anti–C-terminal VE-cadherin antibody. Results are representative of 3 experiments. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3). (D) HUVECs were grown to confluence on gelatin-coated Transwell filters and incubated with FXIa (30 nM), for 6 hours in the absence or presence of PP2 (10 μM) or SU1498 (5 μM). Permeability of Evans Blue-BSA was measured after 60 minutes of incubation. Data are mean ± SEM (n = 4). Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. (E) HUVECs were incubated with FXIa (30 nM) for 6 hours in the absence or presence of the ADAM10 inhibitor, GI254023X (5 μM), or the Src inhibitor, PP2. Cell media were collected and immunoblotted with antibodies for N-terminal VE-cadherin or N-terminal VEGFR2. Results are representative of 3 experiments.
Prior work has shown that activation of endothelial PLCγ1 is dependent on activation of tyrosine kinase receptors, such as VEGFR2 or VEGFR1.33 These receptors can be activated by interactions with ligands such as by VEGF, or via intracellular signaling pathways that trigger Src kinase activation.34-37 As FXIa induces PLCγ1 Y783 phosphorylation in a Src-kinase dependent manner; we investigated whether FXIa induced VEGFR2 or VEGFR1 activation by way of Src kinase activation. We observed that FXIa promoted VEGFR2 but not VEGFR1 phosphorylation (Figure 5B and data not shown). Pretreatment of ECs with 2 distinct VEGFR2 tyrosine kinase activity inhibitors, BFH772 or SU1498, attenuated the phosphorylation of VEGFR2 and PLCγ1 Y783 by FXIa, but did not inhibit Dab1 Y2020 or Src Y416 phosphorylation (Figure 5B). The addition of BFH772 or SU1498 alone were insufficient to induce any changes in phosphorylation (data not shown). These data suggest that the activation of Dab1 and Src kinases by FXIa occurs upstream of VEGFR2 activation, suggesting FXIa-induces Dab1 and Src kinase activation, which in turn actives VEGFR2 and PLCγ1.
We evaluated whether activation of VEGFR2 is required for FXIa-induced cleavage of endothelial VE-cadherin. When VEGFR2 kinase activation was blocked with the inhibitor SU1498 FXIa was incapable of inducing VE-cadherin cleavage as measured by appearance of the CTF (Figure 5C). In contrast, cleavage of VE-cadherin by FXIa was insensitive to pretreatment of ECs with the anti-VEGF antibody ramucirumab (Figure 5C), ruling out the possibility that the observed effect was due to FXIa inducing EC release of endogenous VEGF. Ramucirumab inhibited VEGFR2, PLCγ1, and the MAPK pathway activation by VEGF (supplemental Figure 4A). In addition, pretreatment of ECs with the PP2 or SU1498 prevented FXIa-induced leakage of Evans Blue dye (Figure 5D). These data suggest that the cleavage of VE-cadherin induced by FXIa requires VEGFR2 activation.
We explored whether the addition of an anti–PAI-1 antibody alone activated Dab1, because we had observed a slight signal for VE-cadherin cleavage in the presence of the anti–PAI-1 antibody. Although we observed a slight phosphorylation signal for ERK1/2 T202/Y204 induced by ligation of PAI-1 with an antibody, we were unable to detect phosphorylation of either Dab1 Y220 or Src Y416 (supplemental Figure 4C).
Because VEGFR2 activation by VEGF is known to stimulate ADAM10-dependent shedding of VEGFR2,7 we evaluated whether FXIa induced shedding of the NTD of VEGFR2. Western blot analysis using an anti-VEGFR2 NTD antibody revealed that incubation with FXIa led to the appearance of a 100-kDa fragment of VEGFR2 (Figure 5E) in media. The release of the NTD fragment (sVEGFR2) induced by FXIa was impeded by the presence of an ADAM10 or Src kinase inhibitor (Figure 5E).
FXIa induces human aortic EC permeability
Building on our previous work showing that FXIa forms a complex with PAI-1 and decreases the expression of PAI-1 over time in aortic ECs,27 we determined whether FXIa increases human aortic endothelial cells (HAECs) permeability in a PAI-1– and VLDLR-dependent manner.
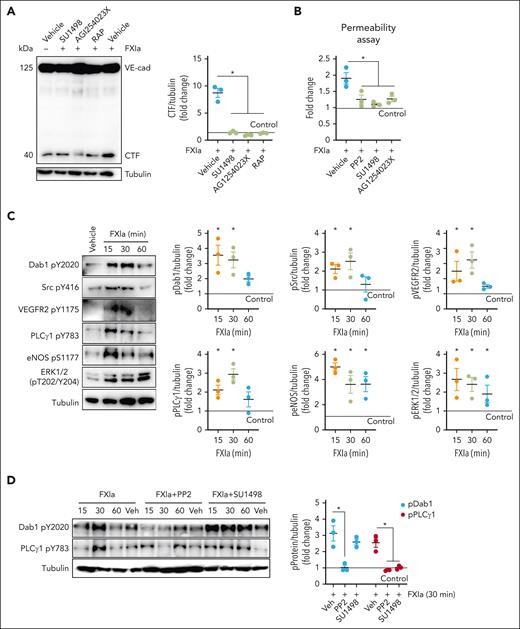
Western blot analysis revealed that incubation of HAECs with FXIa led to the appearance of the VE-cadherin CTF (40 kDa; Figure 6A). The addition of GI254023X, SU1498, or RAP prevented FXIa-induced cleavage of VE-cadherin (Figure 6A). These data suggest that FXIa induces VE-cadherin cleavage in HAECs in an ADAM10, VEGFR2, and VLDLR-dependent manner. FXIa promoted a twofold increase in permeability in HAECs (Figure 6B). Pretreatment of ECs with PP2, SU1498, or GI254023X prevented FXI-induced leakage of Evans Blue dye (Figure 6B). We confirmed that FXIa induces Dab1 Y220 and VEGFR2 Y1175 phosphorylation as evidenced by activating the MAPK pathway in HAECs (Figure 6C). The addition of PP2 prevented Dab1 Y220 and PLCγ1 Y783 phosphorylation induced by FXIa. In contrast, the presence of the VEGFR2 inhibitor, SU1498, only prevented PLCγ1 Y783 phosphorylation (Figure 6D), indicating that, as observed in HUVECs, the activation of Dab1 and Src kinases by FXIa occurred upstream of VEGFR2 activation.
Effect of FXIa on EC permeability on HAECs. (A) HAECs were incubated with vehicle or FXIa for 6 hours in the absence or presence of the VEGFR2 kinase activity inhibitor, SU1498 (5 μM), the ADAM10 inhibitor, GI254023X (10 μM), or RAP (50 ng/mL). Cells were lysed and analyzed by western blotting using an anti–C-terminal VE-cadherin antibody. Results are representative of 3 experiments. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. (B) HAECs were grown to confluence on gelatin-coated Transwell filters and incubated with FXIa (30 nM) for 6 hours in the absence or presence of PP2 (10 μM), SU1498 (5 μM), or GI254023X (10 μM). Permeability of Evans Blue-BSA was measured after 60 minutes of incubation. Data are mean ± SEM (n = 4). Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. (C) HAECs were incubated with FXIa (30 nM) for 15 to 60 minutes. Cells were lysed and immunoblotted with antibodies for phosphorylated Dab1 Y2020, Src Y416, VEGFR2 Y1175, ERK1/2 T202/Y204, eNOS S1177, PLCγ1 Y783, or tubulin. Results are representative of 3 experiments. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3). (D) HAECs were incubated with FXIa (30 nM) for 15 to 60 minutes in the presence or absence of PP2 or SU1498. Cells were lysed and immunoblotted with antibodies for phosphorylated Dab1 Y2020, PLCγ1 Y783, or tubulin. Results are representative of 3 experiments. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3).
Effect of FXIa on EC permeability on HAECs. (A) HAECs were incubated with vehicle or FXIa for 6 hours in the absence or presence of the VEGFR2 kinase activity inhibitor, SU1498 (5 μM), the ADAM10 inhibitor, GI254023X (10 μM), or RAP (50 ng/mL). Cells were lysed and analyzed by western blotting using an anti–C-terminal VE-cadherin antibody. Results are representative of 3 experiments. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. (B) HAECs were grown to confluence on gelatin-coated Transwell filters and incubated with FXIa (30 nM) for 6 hours in the absence or presence of PP2 (10 μM), SU1498 (5 μM), or GI254023X (10 μM). Permeability of Evans Blue-BSA was measured after 60 minutes of incubation. Data are mean ± SEM (n = 4). Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. (C) HAECs were incubated with FXIa (30 nM) for 15 to 60 minutes. Cells were lysed and immunoblotted with antibodies for phosphorylated Dab1 Y2020, Src Y416, VEGFR2 Y1175, ERK1/2 T202/Y204, eNOS S1177, PLCγ1 Y783, or tubulin. Results are representative of 3 experiments. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3). (D) HAECs were incubated with FXIa (30 nM) for 15 to 60 minutes in the presence or absence of PP2 or SU1498. Cells were lysed and immunoblotted with antibodies for phosphorylated Dab1 Y2020, PLCγ1 Y783, or tubulin. Results are representative of 3 experiments. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3).
Pharmacological inhibition of FXI activation prevents cleavage of VE-cadherin in a nonhuman primate model of S aureus–induced sepsis
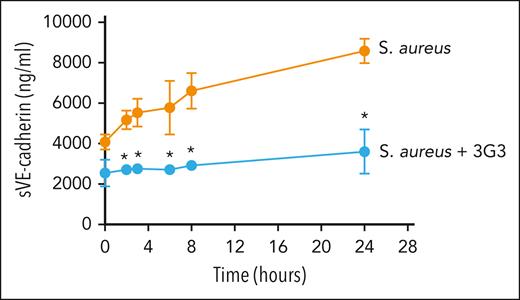
Elevated levels of circulating sVE-cadherin are used as a biomarker of endothelial dysfunction in patients. For instance, elevated levels of sVE-cadherin are associated with atherosclerosis and severe acute kidney injury as well as severe organ dysfunction in patients with sepsis.8,38,39 Thus, as an in vivo proof-of-concept experiment, we determined whether sVE-cadherin levels were elevated in response to a bacterial challenge in a nonhuman primate model of sepsis known to induce EC permeability and loss of barrier function. A rapid and robust 2.5-fold increase in sVE-cadherin levels were observed in animals after a challenge with S aureus (Figure 7).
Effect of the blocking anti-FXI antibody, 3G3, on sVE-cadherin levels after infusion of heat-inactivated S aureus into baboons. Time course change of sVE-cadherin levels. Data are mean ± standard deviation (SD) (n = 3). Same time points are compared between baboons challenged with 3 × 1010 colony forming units of S aureus per kg, and S aureus plus the blocking anti-FXI antibody, 3G3 (SA + 3G3) using 2-tailed student t test; ∗P < .05.
Effect of the blocking anti-FXI antibody, 3G3, on sVE-cadherin levels after infusion of heat-inactivated S aureus into baboons. Time course change of sVE-cadherin levels. Data are mean ± standard deviation (SD) (n = 3). Same time points are compared between baboons challenged with 3 × 1010 colony forming units of S aureus per kg, and S aureus plus the blocking anti-FXI antibody, 3G3 (SA + 3G3) using 2-tailed student t test; ∗P < .05.
Next, we examined whether inhibiting FXI activation with the blocking anti-FXI antibody 3G3 could prevent this increase in sVE-cadherin levels a nonhuman primate model of sepsis. Using this model we had previously reported a rapid increase in FXIa generation as measured by increased levels of FXIa-antithrombin complexes as well as an increase in FXIa–PAI-1 complexes after the bacterial challenge.27 In this model, inhibition of FXI activation with 3G3 abrogated both of these biomarkers as well as provided a survival advantage. Herein, we analyzed whether 3G3 administration correlated with a reduction in sVE-cadherin levels; indeed, we found that sVE cadherin levels remained near baseline in animals receiving the anti-FXI monoclonal antibody (Figure 7).
Discussion
Whether coagulation FXI interacts with, or regulates the function of, the endothelium has been a controversial and unsettled matter of discussion over the years,40-42 in part because of a lack of a mechanistic understanding. In this study, we identify the cellular targets and pathway by which FXIa signals to regulate vascular permeability. FXIa induces VE-cadherin proteolysis via an interaction with PAI-1 and VLDLR, thus promoting the VEGFR2-MAPK signaling pathway inducing the expression of active ADAM10 on the cell surface.
Previously, we observed that FXI inhibition reduced vascular permeability in aortic sinus lesions in a mouse model of atherosclerosis.9 Observational data from this study showed that incubation of ECs with FXIa decreased VE-cadherin expression, suggesting a connection between FXIa and EC-cell adhesion and barrier function.9 Here, we provide a mechanistic understanding of the receptors and pathways by which FXIa induces shedding of VE-cadherin to regulate EC barrier function.
Several epidemiologic studies reported that levels of sVE-cadherin increase in patients with coronary atherosclerosis,38 sepsis,8,39,43 rheumatoid arthritis,44 and malignancy,45 suggesting that endothelial dysfunction may contribute to, or at least is associated with, the pathogenesis of these inflammatory diseases. Here, we observed that FXI inhibition reduced the levels of sVE-cadherin in a baboon model of sepsis. These pilot data provide a rationale for targeting FXI to protect the vascular endothelium from damage during systemic inflammation. Several enzymes trigger the ectodomain shedding of VE-cadherin. Neutrophil elastase, cathepsin G, and matrix metalloproteinases released from cancer cells have all been shown to incite the cleavage of VE-cadherin.46,47 It was not until 2008 that Schulz et al used specific inhibitors and RNA interference experiments to show that ADAM10 is the enzyme responsible for cleavage of VE-cadherin in ECs.6 Based on the data shown here, FXIa can be added to the list of inflammatory mediators, alongside lipopolysaccharide, TNF-α, and VEGF, that upregulate ADAM10 activity in ECs.5,48
The physiological role of ADAM10 has been most extensively studied in the brain. ADAM10 plays crucial roles during neurogenesis; mediates Notch signaling’ and contributes to brain disorders, in part, because 1 of the substrates of ADAM10 being the β-amyloid precursor protein.49 The pathways regulating ADAM10 activity in the brain have been described at the transcriptional, translational, and posttranslational levels. Inflammatory mediators trigger ADAM10 maturation and translocation by initiating activation of the PI3K/Akt or MAPK signaling pathways that increases the concentration of cytoplasmic Ca2+.49 We found that FXIa can trigger the PI3K/Akt and MAPK signaling pathways in ECs in a Src kinase–dependent manner. This is congruent with previous studies in pituitary adenomas linking Src kinase activity with ADAM10 activation.25
FXIa induced ADAM10 activation and VE-cadherin cleavage through the MAPK signaling pathway in a process dependent on VEGFR2 activation. One of the primary activators of VEGFR2 is its ligand VEGF. Ligand binding to VEGFR2 induces an outside-in signaling cascade leading to ADAM10 activation on ECs. Alternatively, VEGFR2 is activated solely intracellularly in a Src kinase–dependent and ligand-independent manner. The intracellular signaling pathway leading to VEGFR2 activation is triggered in ECs by fluid shear stress3,35; high-density lipoprotein36; bone morphogenetic protein 437; and now, FXIa.
VE-cadherin and VEGFR2 collaborate to regulate EC barrier function. The proteins form a complex in quiescent cells, in which VE-cadherin inhibits VEGFR2 tyrosine phosphorylation and MAPK pathway activation as well as its degradation and internalization.5 Importantly, PAI-1 appears to inhibit endocytosis and activation of VEGFR2 in a process dependent upon binding to VLDLR. This is based on the observation that depleting PAI-1 from the endothelium promotes formation of complexes between VEGFR2 and β3 integrins, inducing VEGFR2 activation.50 On pulmonary microvascular ECs, PAI-1 forms a complex with uPA that disrupts VE-cadherin expression through interaction with LRP1,28 or acts alone to regulate VE-cadherin trafficking, resulting in a loss of surface expression and increased vascular permeability.31 Here, we now add a third mechanism by which PAI-1 regulates VE-cadherin expression. Having previously shown that PAI-1 plays an anticoagulant and anti-inflammatory role by binding and inactivating FXIa,27 we now show that the functional consequences of formation and internalization of FXIa–PAI-1 complex. Binding of this complex to VLDLR triggers activation of Dab1 and downstream activation of Src kinase, inducing VEGFR2 activation to incite cleavage of VE-cadherin. As PAI-1 inhibition or depletion enhances VEGFR2 signaling through integrin interactions, perhaps the pathway by which FXIa regulates VEGFR2 activation reflects a conserved combination between Src kinase activation and interaction with β3 integrins.
VLDLR is a member of the LDL receptor family, which is associated with cholesterol homeostasis.51 VLDLR is present in the endothelium and other tissues but is primarily recognized for its role in neural development through the extracellular matrix protein reelin.52 The interaction of reelin with VLDLR induces binding of the adapter protein Dab1 on the cytoplasmic tail of VLDLR, leading to Src activation.52 Although our understanding of the role of VLDLR in regulating EC function and signaling is limited, prior work indicates that fibrin binding to VLDLR decreases VE-cadherin cell surface expression to promote leukocyte transmigration.53 Akin to this observation, we found that interaction of FXIa–PAI-1 complexes with endothelial VLDLR induces ERK activation downstream of Dab1 phosphorylation to increase EC permeability. This pathway seems to parallel observations made in MCF-7 breast cancer epithelial cells, in which the interaction of the uPA–PAI-1 complex with VLDLR promoted a rapid complex internalization to initiate cell signaling leading to ERK activation and cell growth.54 Whether binding of fibrin or the uPA–PAI-1 complex to VLDLR, like FXIa, also induces activation of Dab1 remains to be explored.
In summary, FXIa now joins the list of regulators of EC activation, permeability, and barrier function. Whether the effects of FXIa extend to angiogenesis, proliferation, or migration, remains to be determined.
Acknowledgments
This work is supported, in part, by the National Institutes of Health (NIH), National Heart, Lung, and Blood Institute (R01HL144133, R01HL101972, R01HL151367, and R35HL140025) and the NIH, National Institute of Allergy and Infectious Diseases (R01AI157037).
Authorship
Contribution: C.P., D.G., and O.J.T.M. conceptualized the study; C.P., J.P., H.H.V., A.R.M., R.S.K., and F.L. developed the methodology; C.P. and O.J.T.M. drafted the manuscript; C.P., S.A.M, C.U.L, E.I.T, J.J.S, F.L., D.G., and O.J.T.M. edited and revised manuscript; and all authors approved final version of manuscript.
Conflict-of-interest disclosure: C.U.L. and E.I.T. are employees of Aronora Inc, a company that may have a commercial interest in the results of this research. J.J.S. serves as a medical consultant for Aronora, Inc; this potential conflict of interest has been reviewed and managed by the Oregon Health and Science University conflict of interest in research committee. The remaining authors declare no competing financial interests.
Correspondence: Cristina Puy, Department of Biomedical Engineering, Oregon Health and Science University, 3303 South Bond Ave, Portland, OR 97239; email: puygarci@ohsu.edu.
References
Author notes
Presented, in part, at the American Society of Hematology annual meeting, 10 - 13 December 2022, New Orleans, LA; at the Arteriosclerosis, Thrombosis, and Vascular Biology, Peripheral Vascular Disease, 12 May 2023, Boston, MA; and at the International Society of Thrombosis and Hemostasis meeting, 24 - 28 June 2023, Montreal, Canada.
For original data, please contact the corresponding author, Cristina Puy (puygarci@ohsu.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

![Role of the PI3K-Akt-eNOS and PLCγ1-ERK signaling pathways on VE-cadherin cleavage induced by FXIa. (A) HUVECs were incubated with FXIa (30 nM). Cells were lysed and immunoblotted with antibodies for phosphorylated ERK1/2 T202/Y204, p38 MAPK T180/Y182. Akt S473, eNOS S1177, PLCγ1 Y783, Src Y416, FAK Y397, or tubulin (n = 3). Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3). (B) HUVECs were incubated with FXIa, FXIa-PPACK, or FXI (30 nM) for 15 or 30 minutes. Cells were lysed and immunoblotted with antibodies for phosphorylated ERK1/2 T202/Y204 or Akt S473. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Results are representative of 3 experiments. (C) HUVECs were incubated with vehicle (dimethyl sulfoxide [DMSO]) or FXIa for 6 hours in the absence or presence of the Src inhibitor, PP2 (10 μM); the protein kinase A (PKA) inhibitor, H-89 (10 μM); the eNOS inhibitor, L-NAME (5 μM); or the ERK1/2 inhibitor, LY3214996 (1 μM). Cells were lysed and analyzed by western blotting using an anti–C-terminal VE-cadherin antibody. Results are representative of 3 experiments. Significance (∗P < .05) determined by Kruskal-Wallis testing with Dunns correction for multiple comparisons. Data are mean ± SEM (n = 3).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/144/17/10.1182_blood.2023022257/1/m_blood_bld-2023-022257-gr3.jpeg?Expires=1769279424&Signature=WFhmWU4gQoWJeeo0~UAzj~af-oWFj8svJN2cvprLCWQgKER2Z4AP6-UGkl-o4C-IsVh2EVEOISZEpLyFhPYQgdiciozDQbfm82-vv1AvzUhpYkn250I937KrgO9c-OmzkidAxMTh5WeuIJt5gcSPWnl~ADKF5xXWKZR2PEqMkfEjdcbKQ3qwWdd6rZgD5vEEa-xuawLFTx1KzA9LgqhlDet~P1ESzdnpZe9f017OCNnRk3X~pOOc9UV0BszVEhm3aA9nURnNvkdEp5FFp5Sa5Kdf4UiDrmeY6MyJ3ZIDaPjp-Loti0iaxpUzQIkU5qFUNsZGvDvzmuxALJ2sdYaQRA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)