Key Points
This study illustrates the survival benefits of abatacept combined with CNI/MTX in patients with hematologic malignancies undergoing HCT.
The addition of abatacept may provide an approach for alternative donor pool expansion when HLA-identical sibling donors are unavailable.
Visual Abstract
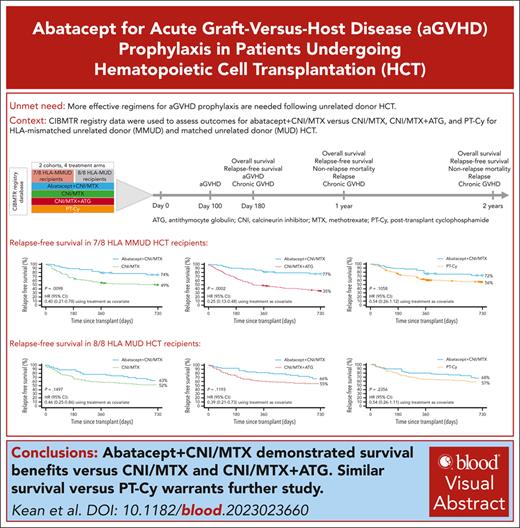
Abatacept plus calcineurin inhibitors/methotrexate (CNI/MTX) is the first US Food and Drug Administration (FDA)-approved regimen for acute graft-versus-host disease (aGVHD) prophylaxis during unrelated-donor hematopoietic cell transplantation (URD-HCT). Using Center for International Blood and Marrow Transplant Research data, we investigated its impact in patients receiving 7/8 HLA-mismatched unrelated donor (MMUD) or 8/8 HLA-matched unrelated donor (MUD) URD-HCT between 2011 and 2018. Primary outcomes included day-180, 1-year, and 2-year overall survival (OS) and relapse-free survival (RFS) for abatacept + CNI/MTX vs CNI/MTX, CNI/MTX + antithymocyte globulin (ATG), and posttransplant cyclophosphamide-based prophylaxis (PT-Cy). For 7/8 MMUDs, day-180 OS (primary end point supporting FDA approval) was significantly higher for abatacept + CNI/MTX vs CNI/MTX (98% vs 75%; P = .0028). Two-year RFS was significantly higher for abatacept + CNI/MTX vs CNI/MTX (74% vs 49%; P = .0098) and CNI/MTX + ATG (77% vs 35%; P = .0002), and similar vs PT-Cy (72% vs 56%; P = .1058). For 8/8 MUDs, 2-year RFS for abatacept + CNI/MTX was numerically higher vs CNI/MTX (63% vs 52%; P = .1497), with an improved hazard ratio (HR) of 0.46 (0.25-0.86), and vs CNI/MTX + ATG (66% vs 55%; P = .1193; HR, 0.39 [0.21-0.73]), and was similar vs PT-Cy (68% vs 57%; P = .2356; HR, 0.54 [0.26-1.11]). For 7/8 MMUD and 8/8 MUD recipients, abatacept + CNI/MTX prophylaxis improved survival outcomes vs CNI/MTX and CNI/MTX + ATG; outcomes were similar to PT-Cy–based regimens. Abatacept + CNI/MTX may facilitate unrelated donor pool expansion for HCT.
Introduction
Allogeneic hematopoietic cell transplantation (HCT) provides a potentially curative therapy for aggressive hematopoietic malignancies. Many HCT recipients do not have a suitable matched sibling donor and require an alternative donor, such as from a matched unrelated donor (MUD), a haploidentical donor, or cord blood. Access to fully HLA-MUDs varies considerably across racial and ethnic groups, often being limited or unavailable.1,2 HLA-mismatched related (haploidentical) or mismatched unrelated donor (MMUD) HCTs are associated with a significantly elevated risk of acute graft-versus-host disease (aGVHD).3,4 Largely dependent upon the degree of HLA disparity, severe aGVHD occurs in up to 50% of adult HCT recipients,5 and continues to be a primary cause of morbidity and mortality.6,7 Historically, one of the greatest unmet needs has been to identify more effective regimens for aGVHD prophylaxis, especially after HLA-MMUD HCT.
Since the 1980s, the standard regimen in the United States for aGVHD prophylaxis in HLA-MUD HCT recipients has been a combination of a calcineurin inhibitor (CNI) and methotrexate (MTX; CNI/MTX); however, this regimen is not as effective for HLA-MMUD HCT recipients.8-10 The absence of a standard regimen for HLA-MMUD HCT recipients and the introduction of several adjunctive therapies, including the addition of antithymocyte globulin (ATG) to CNI/MTX or the use of posttransplant cyclophosphamide (PT-Cy)-based regimens (often with tacrolimus and mycophenolate mofetil),11-14 adds greater variability to the standard of care for aGVHD prophylaxis.
Abatacept is a cytotoxic T-lymphocyte–associated 4 immunoglobulin G1 fusion protein that selectively modulates the costimulatory signal necessary for full T-cell activation with demonstrated efficacy in several autoimmune diseases.15-17 Recently, abatacept in combination with CNI/MTX was approved by the US Food and Drug Administration (FDA) for aGVHD prophylaxis in recipients of HLA-MUD or 1-allele HLA-MMUD HCT, aged ≥2 years.18,19 This represents the first drug regimen to receive FDA approval for prophylaxis of aGVHD. Approval was based on phase 2 trial data (ABA2; ClinicalTrials.gov identifier: NCT0174313120) of HLA-MUD recipients and data from the Center for International Blood and Marrow Transplant Research (CIBMTR) registry for HLA-MMUD recipients; the primary end point was day-180 overall survival (OS) comparing abatacept + CNI/MTX with CNI/MTX. To further validate abatacept for aGVHD prophylaxis, we report here an extension of the regulatory study with additional survival analyses to 2 years after HCT. Importantly, this study enabled new comparisons to be made that were not performed as part of the previously published ABA2 trial.
The key objectives of this analysis were to evaluate the effect of adding abatacept to CNI/MTX on OS and relapse-free survival (RFS) in 7/8 HLA-MMUD and 8/8 HLA-MUD HCT recipients, compared with CNI/MTX alone, CNI/MTX + ATG, or a PT-Cy–based regimen.
Methods
Study design and data source
This retrospective cohort study used data collected in the CIBMTR registry database. The CIBMTR database stores information on all allogeneic HCTs performed in the United States. Research conducted by the CIBMTR was performed in accordance with the Declaration of Helsinki. Data collection was performed according to the CIBMTR data sharing protocol (NCT01166009)21 in which all patients provided signed consent and all studies were approved and overseen by the National Marrow Donor Program central institutional review board. Deidentified data sets were provided to study investigators.
Patient population
Eligibility criteria included: first HCT in the United States between 2011 and 2018 from an 7/8 HLA-MMUD or 8/8 HLA-MUD; diagnosis of acute myeloid leukemia, acute lymphoblastic leukemia, chronic myelogenous leukemia (or chronic neutrophil leukemia), myelodysplastic syndrome, or lymphoma; bone marrow or peripheral blood graft source; age of ≥6 years; weight of ≥20 kg; Karnofsky/Lansky performance score of at least 80%; treatment with CNI/MTX (with or without ATG, and with or without abatacept) or PT-Cy–based GVHD prophylaxis without ATG (referred to as PT-Cy); and receipt of one of the following conditioning regimens: total body irradiation/Cy, busulfan/Cy, busulfan/fludarabine, or fludarabine/melphalan. Data regarding the brand and dose of ATG were not collected in the CIBMTR registry database, so could not be compared. Conditioning regimens specified in the FDA submission matched the ABA2 study eligibility criteria.
Exclusion criteria were lack of reporting for whether or not ATG was used, alemtuzumab use, lack of consent for data to be used for research purposes, treatment at a center not meeting CIBMTR data quality standards, having received both abatacept and ATG treatment, having received an HCT at a center that enrolled patients for an abatacept trial (for patients not treated with abatacept), and patients with missing information for propensity score variable analysis.
Controls were randomly selected from the eligible control populations. These were based on a ratio of 3 controls per case for the 7/8 HLA-MMUD cohort, and 5 controls per case for the 8/8 HLA-MUD cohort.
Study end points
The primary end points were day-180, 1-year, and 2-year OS and RFS. OS was defined as the time from HCT date to death from any cause, and RFS as the time from HCT date to the date of death or relapse, whichever occurred earlier. Secondary end points included 1-year and 2-year nonrelapse mortality (NRM), defined as the time from HCT date to death from any cause without prior relapse; 1-year and 2-year relapse, defined as the time from HCT date to date of disease relapse; grades 2 to 4 and 3 to 4 aGVHD at days 100 and 180 after transplant; and chronic GVHD (cGVHD) at day 180, 1 year, and 2 years after transplant. Data required for GVHD RFS were not collected by the CIBMTR for all patients during the years of transplant included in this study, and therefore GVHD RFS was not included as an end point.
All analyses were performed for 7/8 HLA-MMUD or 8/8 HLA-MUD HCT recipients treated with abatacept + CNI/MTX compared with combinations of CNI/MTX, CNI/MTX + ATG, and PT-Cy. An analysis was also done to evaluate whether the process for selection of the 7/8 HLA-MMUD recipient control arm in the CIBMTR was adequate.
Statistical analysis
OS was examined by a time-to-event analysis at day 180, 1 year, and 2 years after HCT. The weighted log-rank test was used to compare OS using inverse probability of treatment weighting based on calculation of propensity scores to control for treatment bias, in line with FDA guidance requesting bias control using inverse probability of treatment weighting based on propensity score (rather than matched-pair analysis) for the regulatory submission. Propensity scores were estimated using observed baseline covariates, such that patients whose propensity scores are equal will have similar baseline covariate values and will be comparable. Propensity scores were obtained from a logistic regression model including sex, disease, age (continuous), HCT graft source, conditioning intensity, Karnofsky/Lansky performance score, and CNI type (when both treatment arms included CNIs). The figures depicting propensity scores (Figures 1 and 2) show the corresponding number of weighted patients along the x-axis as a result of the propensity score–matching analytical approach. Demographics are provided for both the unweighted and weighted samples for all comparison cohorts. Analyses of the study end points for the weighted samples of patients are indicated in the relevant tables and figures. Marginal hazard ratios (HRs) and the corresponding 2-sided 95% confidence intervals (CIs) were estimated in a weighted Cox proportional hazards model with treatment only as a covariate, using a robust variance estimator that accounted for the sample weights. This comparison was evaluated at a 2-sided α = .05. The estimated OS probabilities are provided by weighted Kaplan-Meier curves up to 2 years after HCT. Based on 10 000 simulations with varying OS rates (nQuery Advisor 7.0 software), power estimates ranged from 76% to 99% with OS set at ≥95% for abatacept + CNI/MTX, and 75% to 80% for CNI/MTX.
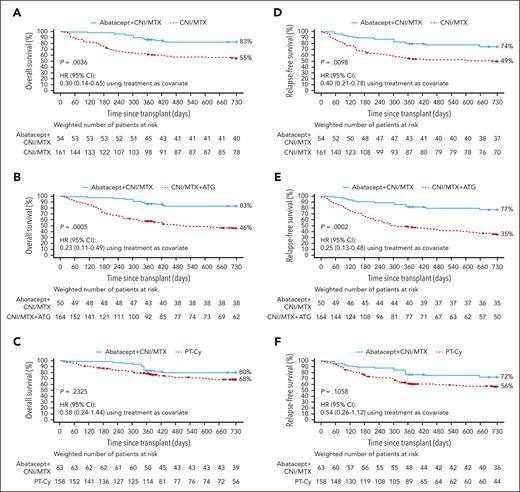
Two-year OS and RFS in 7/8 HLA-MMUD HCT weighted samples. OS with abatacept + CNI/MTX compared with CNI/MTX (A), CNI/MTX + ATG (B), and PT-Cy∗ (C) and RFS with abatacept + CNI/MTX compared with CNI/MTX (D), CNI/MTX + ATG (E), and PT-Cy∗ (F). Propensity scores were obtained from a logistic regression model including sex, disease, age, HCT graft source, conditioning intensity, Karnofsky/Lansky performance score, and CNI type as covariates. Based on weighted Kaplan-Meier method. Symbols represent censored observation. Patients were censored at 2 years after transplant or at time of last follow-up, whichever occurred earlier. Tables under each curve show the number of weighted patients at risk at each time point. ∗For PT-Cy, propensity scores obtained from a logistic regression model including sex, disease, age, HCT graft source, conditioning intensity, and performance score as covariates. Adapted from Kean et al.22-25
Two-year OS and RFS in 7/8 HLA-MMUD HCT weighted samples. OS with abatacept + CNI/MTX compared with CNI/MTX (A), CNI/MTX + ATG (B), and PT-Cy∗ (C) and RFS with abatacept + CNI/MTX compared with CNI/MTX (D), CNI/MTX + ATG (E), and PT-Cy∗ (F). Propensity scores were obtained from a logistic regression model including sex, disease, age, HCT graft source, conditioning intensity, Karnofsky/Lansky performance score, and CNI type as covariates. Based on weighted Kaplan-Meier method. Symbols represent censored observation. Patients were censored at 2 years after transplant or at time of last follow-up, whichever occurred earlier. Tables under each curve show the number of weighted patients at risk at each time point. ∗For PT-Cy, propensity scores obtained from a logistic regression model including sex, disease, age, HCT graft source, conditioning intensity, and performance score as covariates. Adapted from Kean et al.22-25
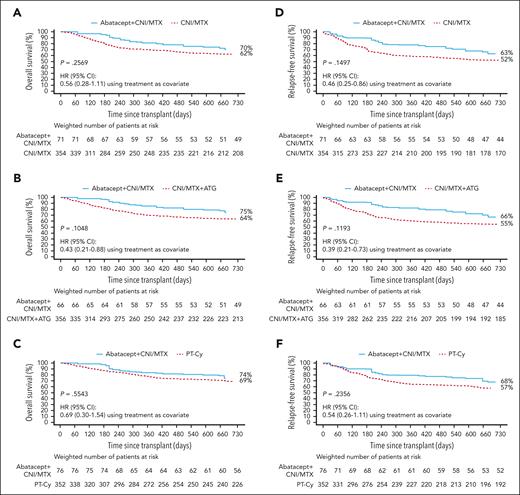
Two-year OS and RFS in 8/8 HLA-MUD HCT weighted samples. OS with abatacept + CNI/MTX compared with CNI/MTX (A), CNI/MTX + ATG (B), and PT-Cy∗ (C) and RFS with abatacept + CNI/MTX compared with CNI/MTX (D), CNI/MTX + ATG (E), and PT-Cy∗ (F). Propensity scores obtained from a logistic regression model including sex, disease, age, HCT graft source, conditioning intensity, Karnofsky/Lansky performance score, and CNI type as covariates. Based on weighted Kaplan-Meier method. Symbols represent censored observation. Patients were censored at 1 year after transplant or at time of last follow-up, whichever occurred earlier. Tables under each curve show the number of weighted patients at risk at each time point. ∗For PT-Cy, propensity scores obtained from a logistic regression model including age and conditioning intensity as covariates. Adapted from Kean et al.22-25
Two-year OS and RFS in 8/8 HLA-MUD HCT weighted samples. OS with abatacept + CNI/MTX compared with CNI/MTX (A), CNI/MTX + ATG (B), and PT-Cy∗ (C) and RFS with abatacept + CNI/MTX compared with CNI/MTX (D), CNI/MTX + ATG (E), and PT-Cy∗ (F). Propensity scores obtained from a logistic regression model including sex, disease, age, HCT graft source, conditioning intensity, Karnofsky/Lansky performance score, and CNI type as covariates. Based on weighted Kaplan-Meier method. Symbols represent censored observation. Patients were censored at 1 year after transplant or at time of last follow-up, whichever occurred earlier. Tables under each curve show the number of weighted patients at risk at each time point. ∗For PT-Cy, propensity scores obtained from a logistic regression model including age and conditioning intensity as covariates. Adapted from Kean et al.22-25
RFS was examined by a time-to-event analysis at day 180, 1 year, and 2 years after HCT. Marginal HRs and the corresponding 2-sided 95% CIs were estimated in weighted Cox proportional hazards models. Estimated RFS probabilities are provided by weighted Kaplan-Meier curves up to 2 years after HCT.
Estimated NRM and relapse probabilities are provided by weighted cumulative incidence curves up to 2 years after HCT. The weighted incidence rates of grades 2 to 4 and 3 to 4 aGVHD are provided at days 100 and 180 after transplant, and cGVHD at day 180, 1 year, and 2 years after transplant.
Results
Demographics of unweighted and weighted samples
A patient disposition summary for 7/8 HLA-MMUD recipients is shown in supplemental Figure 1, available on the Blood website. A total of 216 and 215 7/8 HLA-MMUD recipients comprised the unweighted and weighted cohorts for the comparison of abatacept + CNI/MTX vs CNI/MTX, respectively (Table 1). The abatacept + CNI/MTX group included 42 patients from the ABA2 clinical trial (1 of 43 patients from the ABA2 clinical trial declined consent for their data to be used in the current study), in addition to 12 patients treated outside of the trial identified in the CIBMTR registry. For both groups (weighted samples; Table 1), acute myeloid leukemia was the most common disease, patients were predominantly male, had Karnofsky/Lanksy performance scores of 90% to 100%, had received myeloablative conditioning, were mostly peripheral blood stem cell graft recipients, and received tacrolimus as the CNI. For the groups of CNI/MTX + ATG (supplemental Table 1) and PT-Cy (supplemental Table 2), weighted demographics and disease characteristics were similar between treatment groups with the exception of stem cell source, with higher rates of peripheral blood in the PT-Cy group vs the abatacept + CNI/MTX group.
Demographics and disease characteristics at transplant for unweighted and weighted samples in the 7/8 HLA-MMUD HCT cohort for the abatacept + CNI/MTX vs CNI/MTX comparison
| Characteristic . | Unweighted samples . | Weighted samples . | ||
|---|---|---|---|---|
| Abatacept + CNI/MTX (n = 54)∗ . | CNI/MTX (n = 162) . | Abatacept + CNI/MTX (n = 54)∗ . | CNI/MTX (n = 161) . | |
| Age, y | ||||
| Mean (SD) | 35.9 (22.8) | 47.9 (16.2) | 47.1 (19.2) | 45.9 (17.0) |
| Median (range) | 36.5 (6-76) | 51.0 (10-74) | 51.0 (6-76) | 49.0 (10-74) |
| Age category (y), n (%) | ||||
| ≤21 | 23 (42.6) | 9 (5.6) | 8.6 (15.9) | 12.8 (7.9) |
| >21 | 31 (57.4) | 153 (94.4) | 45.3 (84.1) | 148.7 (92.1) |
| Sex, n (%) | ||||
| Male | 36 (66.7) | 87 (53.7) | 33.4 (62.1) | 95.5 (59.1) |
| Female | 18 (33.3) | 75 (46.3) | 20.4 (37.9) | 66.0 (40.9) |
| Race, n (%) | ||||
| White | 39 (72.2) | 135 (83.3) | 38.8 (72.1) | 133.4 (82.6) |
| Black or African American | 9 (16.7) | 6 (3.7) | 10.9 (20.3) | 6.7 (4.1) |
| Asian | 3 (5.6) | 6 (3.7) | 2.4 (4.5) | 5.4 (3.4) |
| Not reported | 3 (5.6) | 15 (9.3) | 1.7 (3.1) | 16.0 (9.9) |
| Karnofsky/Lansky performance score, n (%)† | ||||
| 70 or 80 | 15 (27.8) | 53 (32.7) | 16.3 (30.3) | 50.9 (31.5) |
| 90 to 100 | 39 (72.2) | 109 (67.3) | 37.5 (69.7) | 110.6 (68.5) |
| Disease, n (%) | ||||
| AML | 20 (37.0) | 81 (50.0) | 24.6 (45.6) | 74.4 (46.1) |
| ALL | 13 (24.1) | 31 (19.1) | 7.9 (14.6) | 29.3 (18.2) |
| MDS or MDS/MPN unclassifiable | 12 (22.2) | 32 (19.8) | 13.2 (24.4) | 34.2 (21.2) |
| CML or CNL | 6 (11.1) | 7 (4.3) | 4.3 (8.1) | 12.8 (8.0) |
| HL + NHL | 3 (5.6) | 11 (6.8) | 3.9 (7.3) | 10.7 (6.6) |
| Disease status, n (%)‡ | ||||
| Early | 33 (61.1) | 84 (51.9) | 38.7 (71.8) | 87.4 (54.1) |
| Intermediate | 13 (24.1) | 28 (17.3) | 8.2 (15.3) | 27.9 (17.3) |
| Advanced | 5 (9.3) | 39 (24.1) | 3.1 (5.7) | 35.6 (22.0) |
| Chemotherapy sensitive | 3 (5.6) | 6 (3.7) | 3.9 (7.3) | 6.5 (4.1) |
| Chemotherapy resistant | 0 | 5 (3.1) | 0 | 4.1 (2.5) |
| Conditioning intensity, n (%) | ||||
| Myeloablative | 41 (75.9) | 107 (66.0) | 38.2 (70.8) | 110.6 (68.5) |
| Reduced intensity or nonmyeloablative | 13 (24.1) | 55 (34.0) | 15.7 (29.2) | 50.9 (31.5) |
| Stem cell source, n (%) | ||||
| Bone marrow | 31 (57.4) | 44 (27.2) | 15.9 (29.5) | 50.2 (31.1) |
| Peripheral blood | 23 (42.6) | 118 (72.8) | 38.0 (70.5) | 111.3 (68.9) |
| CNI type, n (%) | ||||
| Tacrolimus | 33 (61.1) | 153 (94.4) | 46.7 (86.7) | 140.6 (87.1) |
| Cyclosporine | 21 (38.9) | 9 (5.6) | 7.2 (13.3) | 20.8 (12.9) |
| HCT-CI, n (%) | ||||
| 0 | 16 (29.6) | 50 (30.9) | 10.1 (18.8) | 49.4 (30.6) |
| 1 to 2 | 13 (24.1) | 32 (19.8) | 17.5 (32.5) | 33.9 (21.0) |
| ≥3 | 25 (46.3) | 80 (49.4) | 26.3 (48.7) | 78.2 (48.4) |
| Median (range) duration of follow-up, d§ | 1429 (359-2268) | 1470 (190-2839) | 1429 (359-2268) | 1471 (190-2839) |
| Characteristic . | Unweighted samples . | Weighted samples . | ||
|---|---|---|---|---|
| Abatacept + CNI/MTX (n = 54)∗ . | CNI/MTX (n = 162) . | Abatacept + CNI/MTX (n = 54)∗ . | CNI/MTX (n = 161) . | |
| Age, y | ||||
| Mean (SD) | 35.9 (22.8) | 47.9 (16.2) | 47.1 (19.2) | 45.9 (17.0) |
| Median (range) | 36.5 (6-76) | 51.0 (10-74) | 51.0 (6-76) | 49.0 (10-74) |
| Age category (y), n (%) | ||||
| ≤21 | 23 (42.6) | 9 (5.6) | 8.6 (15.9) | 12.8 (7.9) |
| >21 | 31 (57.4) | 153 (94.4) | 45.3 (84.1) | 148.7 (92.1) |
| Sex, n (%) | ||||
| Male | 36 (66.7) | 87 (53.7) | 33.4 (62.1) | 95.5 (59.1) |
| Female | 18 (33.3) | 75 (46.3) | 20.4 (37.9) | 66.0 (40.9) |
| Race, n (%) | ||||
| White | 39 (72.2) | 135 (83.3) | 38.8 (72.1) | 133.4 (82.6) |
| Black or African American | 9 (16.7) | 6 (3.7) | 10.9 (20.3) | 6.7 (4.1) |
| Asian | 3 (5.6) | 6 (3.7) | 2.4 (4.5) | 5.4 (3.4) |
| Not reported | 3 (5.6) | 15 (9.3) | 1.7 (3.1) | 16.0 (9.9) |
| Karnofsky/Lansky performance score, n (%)† | ||||
| 70 or 80 | 15 (27.8) | 53 (32.7) | 16.3 (30.3) | 50.9 (31.5) |
| 90 to 100 | 39 (72.2) | 109 (67.3) | 37.5 (69.7) | 110.6 (68.5) |
| Disease, n (%) | ||||
| AML | 20 (37.0) | 81 (50.0) | 24.6 (45.6) | 74.4 (46.1) |
| ALL | 13 (24.1) | 31 (19.1) | 7.9 (14.6) | 29.3 (18.2) |
| MDS or MDS/MPN unclassifiable | 12 (22.2) | 32 (19.8) | 13.2 (24.4) | 34.2 (21.2) |
| CML or CNL | 6 (11.1) | 7 (4.3) | 4.3 (8.1) | 12.8 (8.0) |
| HL + NHL | 3 (5.6) | 11 (6.8) | 3.9 (7.3) | 10.7 (6.6) |
| Disease status, n (%)‡ | ||||
| Early | 33 (61.1) | 84 (51.9) | 38.7 (71.8) | 87.4 (54.1) |
| Intermediate | 13 (24.1) | 28 (17.3) | 8.2 (15.3) | 27.9 (17.3) |
| Advanced | 5 (9.3) | 39 (24.1) | 3.1 (5.7) | 35.6 (22.0) |
| Chemotherapy sensitive | 3 (5.6) | 6 (3.7) | 3.9 (7.3) | 6.5 (4.1) |
| Chemotherapy resistant | 0 | 5 (3.1) | 0 | 4.1 (2.5) |
| Conditioning intensity, n (%) | ||||
| Myeloablative | 41 (75.9) | 107 (66.0) | 38.2 (70.8) | 110.6 (68.5) |
| Reduced intensity or nonmyeloablative | 13 (24.1) | 55 (34.0) | 15.7 (29.2) | 50.9 (31.5) |
| Stem cell source, n (%) | ||||
| Bone marrow | 31 (57.4) | 44 (27.2) | 15.9 (29.5) | 50.2 (31.1) |
| Peripheral blood | 23 (42.6) | 118 (72.8) | 38.0 (70.5) | 111.3 (68.9) |
| CNI type, n (%) | ||||
| Tacrolimus | 33 (61.1) | 153 (94.4) | 46.7 (86.7) | 140.6 (87.1) |
| Cyclosporine | 21 (38.9) | 9 (5.6) | 7.2 (13.3) | 20.8 (12.9) |
| HCT-CI, n (%) | ||||
| 0 | 16 (29.6) | 50 (30.9) | 10.1 (18.8) | 49.4 (30.6) |
| 1 to 2 | 13 (24.1) | 32 (19.8) | 17.5 (32.5) | 33.9 (21.0) |
| ≥3 | 25 (46.3) | 80 (49.4) | 26.3 (48.7) | 78.2 (48.4) |
| Median (range) duration of follow-up, d§ | 1429 (359-2268) | 1470 (190-2839) | 1429 (359-2268) | 1471 (190-2839) |
Weighted numbers of patients were categorized using stabilized inverse probability of treatment weighing with propensity scores. Propensity scores were obtained from a logistic regression model including sex, disease, age, HCT graft source, conditioning intensity, Karnofsky/Lansky performance score, and CNI type as covariates. Primary end point cohort comprised patients receiving abatacept + CNI/MTX vs CNI/MTX alone.
Adapted from Kean et al.22-25
ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; CML, chronic myeloid leukemia; CNL, chronic neutrophilic leukemia; HCT-CI, HCT-specific comorbidity index; HL, Hodgkin lymphoma; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; NHL, non-Hodgkin lymphoma; SD, standard deviation.
The abatacept + CNI/MTX group included 42 patients from the ABA2 clinical trial, in addition to 12 patients treated outside of the trial.
Two patients treated with abatacept in the ABA2 trial had reported performance scores of 80%; however, the performance score reported in the CIBMTR database for these patients was 70%. Due to the small number of patients treated with abatacept, these patients were included with performance score based on that reported from the ABA2 trial.
chemotherapy-sensitive/chemotherapy-resistant distinction is specific to patients with lymphoma.
Duration of follow-up was defined as the date of transplant to the date of death or last follow-up. Median was estimated using the reverse Kaplan-Meier method; minimum and maximum values are summarized among patients who were alive at the end of follow-up.
A patient disposition summary for 8/8 HLA-MUD recipients is shown in supplemental Figure 2. A total of 426 and 425 patients comprised the primary 8/8 HLA-MUD unweighted and weighted cohorts for the comparison of abatacept + CNI/MTX vs CNI/MTX, respectively (Table 2). The 71 patients in the abatacept + CNI/MTX group included 70 patients from the ABA2 clinical trial (3 of 73 patients from the ABA2 clinical trial were not identified in the CIBMTR registry) plus 1 patient treated outside of the trial identified in the registry. Weighted demographic and disease characteristics were similar between treatment groups (Table 2); unweighted characteristics are also shown. For the CNI/MTX + ATG (supplemental Table 3) and PT-Cy (supplemental Table 4) comparison groups, weighted demographics and disease characteristics were similar between treatment groups, with the exception of stem cell source, as noted for the 7/8 HLA cohort.
Demographics and disease characteristics at transplant for unweighted and weighted samples in the 8/8 HLA-MUD HCT cohort for the abatacept + CNI/MTX vs CNI/MTX comparison
| Characteristic . | Unweighted samples . | Weighted samples . | ||
|---|---|---|---|---|
| Abatacept + CNI/MTX (n = 71)∗ . | CNI/MTX (n = 355) . | Abatacept + CNI/MTX (n = 71)∗ . | CNI/MTX (n = 354) . | |
| Age, y | ||||
| Mean (SD) | 41.4 (19.5) | 51.4 (15.4) | 50.4 (16.0) | 50 (16.2) |
| Median (range) | 46 (6.9-71.9) | 54.4 (6.8-77.8) | 56.7 (6.9-71.9) | 53.1 (6.8-77.8) |
| Age category (y), n (%) | ||||
| ≤21 | 16 (22.5) | 11 (3.1) | 5.4 (7.7) | 15.9 (4.5) |
| >21 | 55 (77.5) | 344 (96.9) | 65.1 (92.3) | 338.2 (95.5) |
| Sex, n (%) | ||||
| Male | 40 (56.3) | 207 (58.3) | 36.5 (51.7) | 203.5 (57.5) |
| Female | 31 (43.7) | 148 (41.7) | 34.1 (48.3) | 150.5 (42.5) |
| Race, n (%) | ||||
| White | 64 (90.1) | 327 (92.1) | 63.6 (90.2) | 325.3 (91.9) |
| Black or African American | 3 (4.2) | 6 (1.7) | 3.7 (5.2) | 6.4 (1.8) |
| Asian | 3 (4.2) | 9 (2.5) | 2.6 (3.7) | 8.9 (2.5) |
| American Indian or Alaska Native | 1 (1.4) | 1 (0.3) | 0.6 (0.8) | 0.9 (0) |
| Not reported | 0 (0.0) | 12 (3.4) | 0 (0) | 12.6 (3.5) |
| Karnofsky/Lansky performance score, n (%)† | ||||
| 70 or 80 | 22 (31.0) | 131 (36.9) | 25.8 (36.5) | 126.8 (35.8) |
| 90 to 100 | 49 (69.0) | 224 (63.1) | 44.8 (63.5) | 227.3 (64.2) |
| Disease, n (%) | ||||
| AML | 29 (40.8) | 180 (50.7) | 37.5 (53.1) | 173.9 (49.1) |
| ALL | 24 (33.8) | 62 (17.5) | 14.1 (20.0) | 70.3 (19.8) |
| MDS or MDS/MPN unclassifiable‡ | 16 (22.5) | 83 (23.4) | 15.2 (21.6) | 83.3 (23.5) |
| CML | 1 (1.4) | 17 (4.8) | 2.7 (3.8) | 15.0 (4.2) |
| HL + NHL | 1 (1.4) | 13 (3.7) | 1.1 (1.5) | 11.6 (3.3) |
| Disease status, n (%)§ | ||||
| Early | 52 (73.2) | 235 (66.2) | 56.6 (80.3) | 234.0 (66.1) |
| Intermediate | 14 (19.7) | 54 (15.2) | 9.9 (14.0) | 57.4 (16.2) |
| Advanced | 4 (5.6) | 53 (14.9) | 3.0 (4.2) | 51.0 (14.4) |
| Chemo-sensitive | 1 (1.4) | 13 (3.7) | 1.1 (1.5) | 11.6 (3.3) |
| Chemo-resistant | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Conditioning intensity, n (%) | ||||
| Myeloablative | 59 (83.1) | 236 (66.5) | 51.6 (73.2) | 245.3 (69.3) |
| Reduced intensity or nonmyeloablative | 12 (16.9) | 119 (33.5) | 18.9 (26.8) | 108.8 (30.7) |
| Stem cell source, n (%) | ||||
| Bone marrow | 31 (43.7) | 72 (20.3) | 19.1 (27.0) | 84.8 (23.9) |
| Peripheral blood | 40 (56.3) | 283 (79.7) | 51.5 (73.0) | 269.3 (76.1) |
| CNI type, n (%) | ||||
| Tacrolimus | 59 (83.1) | 335 (94.4) | 65.7 (93.2) | 329.0 (92.9) |
| Cyclosporine | 12 (16.9) | 20 (5.6) | 4.8 (6.8) | 25.1 (7.1) |
| HCT-CI, n (%) | ||||
| 0 | 16 (22.5) | 69 (19.4) | 15.8 (22.5) | 74.3 (21.0) |
| 1 to 2 | 26 (36.6) | 104 (29.3) | 25.2 (35.8) | 100.2 (28.3) |
| ≥3 | 29 (40.8) | 182 (51.3) | 29.5 (41.8) | 179.5 (50.7) |
| Median (range) duration of follow-up, d|| | 1470 (575-2919) | 1181 (91-3001) | 1473 (575-2919) | 1361 (91-3019) |
| Characteristic . | Unweighted samples . | Weighted samples . | ||
|---|---|---|---|---|
| Abatacept + CNI/MTX (n = 71)∗ . | CNI/MTX (n = 355) . | Abatacept + CNI/MTX (n = 71)∗ . | CNI/MTX (n = 354) . | |
| Age, y | ||||
| Mean (SD) | 41.4 (19.5) | 51.4 (15.4) | 50.4 (16.0) | 50 (16.2) |
| Median (range) | 46 (6.9-71.9) | 54.4 (6.8-77.8) | 56.7 (6.9-71.9) | 53.1 (6.8-77.8) |
| Age category (y), n (%) | ||||
| ≤21 | 16 (22.5) | 11 (3.1) | 5.4 (7.7) | 15.9 (4.5) |
| >21 | 55 (77.5) | 344 (96.9) | 65.1 (92.3) | 338.2 (95.5) |
| Sex, n (%) | ||||
| Male | 40 (56.3) | 207 (58.3) | 36.5 (51.7) | 203.5 (57.5) |
| Female | 31 (43.7) | 148 (41.7) | 34.1 (48.3) | 150.5 (42.5) |
| Race, n (%) | ||||
| White | 64 (90.1) | 327 (92.1) | 63.6 (90.2) | 325.3 (91.9) |
| Black or African American | 3 (4.2) | 6 (1.7) | 3.7 (5.2) | 6.4 (1.8) |
| Asian | 3 (4.2) | 9 (2.5) | 2.6 (3.7) | 8.9 (2.5) |
| American Indian or Alaska Native | 1 (1.4) | 1 (0.3) | 0.6 (0.8) | 0.9 (0) |
| Not reported | 0 (0.0) | 12 (3.4) | 0 (0) | 12.6 (3.5) |
| Karnofsky/Lansky performance score, n (%)† | ||||
| 70 or 80 | 22 (31.0) | 131 (36.9) | 25.8 (36.5) | 126.8 (35.8) |
| 90 to 100 | 49 (69.0) | 224 (63.1) | 44.8 (63.5) | 227.3 (64.2) |
| Disease, n (%) | ||||
| AML | 29 (40.8) | 180 (50.7) | 37.5 (53.1) | 173.9 (49.1) |
| ALL | 24 (33.8) | 62 (17.5) | 14.1 (20.0) | 70.3 (19.8) |
| MDS or MDS/MPN unclassifiable‡ | 16 (22.5) | 83 (23.4) | 15.2 (21.6) | 83.3 (23.5) |
| CML | 1 (1.4) | 17 (4.8) | 2.7 (3.8) | 15.0 (4.2) |
| HL + NHL | 1 (1.4) | 13 (3.7) | 1.1 (1.5) | 11.6 (3.3) |
| Disease status, n (%)§ | ||||
| Early | 52 (73.2) | 235 (66.2) | 56.6 (80.3) | 234.0 (66.1) |
| Intermediate | 14 (19.7) | 54 (15.2) | 9.9 (14.0) | 57.4 (16.2) |
| Advanced | 4 (5.6) | 53 (14.9) | 3.0 (4.2) | 51.0 (14.4) |
| Chemo-sensitive | 1 (1.4) | 13 (3.7) | 1.1 (1.5) | 11.6 (3.3) |
| Chemo-resistant | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Conditioning intensity, n (%) | ||||
| Myeloablative | 59 (83.1) | 236 (66.5) | 51.6 (73.2) | 245.3 (69.3) |
| Reduced intensity or nonmyeloablative | 12 (16.9) | 119 (33.5) | 18.9 (26.8) | 108.8 (30.7) |
| Stem cell source, n (%) | ||||
| Bone marrow | 31 (43.7) | 72 (20.3) | 19.1 (27.0) | 84.8 (23.9) |
| Peripheral blood | 40 (56.3) | 283 (79.7) | 51.5 (73.0) | 269.3 (76.1) |
| CNI type, n (%) | ||||
| Tacrolimus | 59 (83.1) | 335 (94.4) | 65.7 (93.2) | 329.0 (92.9) |
| Cyclosporine | 12 (16.9) | 20 (5.6) | 4.8 (6.8) | 25.1 (7.1) |
| HCT-CI, n (%) | ||||
| 0 | 16 (22.5) | 69 (19.4) | 15.8 (22.5) | 74.3 (21.0) |
| 1 to 2 | 26 (36.6) | 104 (29.3) | 25.2 (35.8) | 100.2 (28.3) |
| ≥3 | 29 (40.8) | 182 (51.3) | 29.5 (41.8) | 179.5 (50.7) |
| Median (range) duration of follow-up, d|| | 1470 (575-2919) | 1181 (91-3001) | 1473 (575-2919) | 1361 (91-3019) |
Weighted samples were characterized using stabilized inverse probability of treatment weighing with propensity scores. Propensity scores were obtained from a logistic regression model including sex, disease, age, HCT graft source, conditioning intensity, Karnofsky/Lansky performance score, and CNI type as covariates.
ALL, acute lymphocytic leukemia; AML, acute myeloid leukemia; CML, chronic myeloid leukemia; HCT-CI, HCT-specific comorbidity index; HL, Hodgkin lymphoma; MDS, myelodysplastic syndrome; MPN, myeloproliferative neoplasm; NHL, non-Hodgkin lymphoma; SD, standard deviation.
The abatacept + CNI/MTX group included 70 patients from the ABA2 clinical trial, and 1 patient treated outside of the trial.
MDS/MPN unclassifiable patient: n = 1 in abatacept group, n = 0 in non-abatacept group.
Two patients treated with abatacept in the ABA2 trial had reported performance scores of 80%; however, the performance score reported in the CIBMTR database for these patients was 70%. Because of the small number of patients treated with abatacept, these patients were included with performance score based on that reported from the ABA2 trial.
Chemotherapy-sensitive/chemotherapy-resistant distinction is specific to patients with lymphoma.
Duration of follow-up was defined as the date of transplant to the date of death or last follow-up. Median was estimated using the reverse Kaplan-Meier method; minimum and maximum values are summarized among patients who were alive at the end of follow-up.
Study outcomes for weighted samples
7/8 HLA-MMUD HCT recipients
OS
Abatacept + CNI/MTX was associated with statistically significantly higher day-180, 1-year, and 2-year OS vs CNI/MTX (day 180: 98 [95% CI, 78-100] vs 75 [95% CI, 67-82], P = .0028; 1-year: 86 [95% CI, 68-95] vs 61 [95% CI, 53-69], P = .0046; 2-year: 83 [95% CI, 64-92] vs 55 [95% CI, 46-63], HR: 0.30 [95% CI, 0.14-0.65], P = .0036; Figure 1A; Table 3). Abatacept + CNI/MTX was associated with statistically significantly higher OS vs CNI/MTX + ATG through 2 years after HCT (83% [95% CI, 62-93] vs 46% [95% CI, 37-54], HR = 0.23 [0.11-0.49]; Figure 1B); higher OS was also evident at 1 year after HCT (87% [95% CI, 67-95] vs 58% [95% CI, 49-66]) and day 180 (98% [95% CI, 76-100] vs 74% [95% CI, 65-80]). OS with abatacept + CNI/MTX was similar to PT-Cy through 2 years (80% [95% CI, 59-91] vs 68% [95% CI, 59-76], HR 0.58 [0.24-1.44]; P = .2325; Figure 1C), at 1 year after HCT (86% [95% CI, 66-94] vs 79% [95% CI, 70-85]; P = .3376; Figure 1C), and at day 180 (98% [95% CI, 77-100] vs 88% [95% CI, 81-92]).
OS for the 7/8 HLA-MMUD HCT weighted samples in the for the abatacept + CNI/MTX vs CNI/MTX comparison cohort
| . | Abatacept + CNI/MTX (n = 54) . | CNI/MTX (n = 161) . | P value∗ . |
|---|---|---|---|
| Day 180 (primary end point) | |||
| OS (95% CI) | 98 (78-100) | 75 (67-82) | .0028 |
| HR (95% CI)† | 0.06 (0.01-0.27) | ||
| 1 y | |||
| OS (95% CI) | 86 (68-95) | 61 (53-69) | .0046 |
| HR (95% CI)† | 0.28 (0.12-0.68) | ||
| 2 y | |||
| OS (95% CI) | 83 (64-92) | 55 (46-63) | .0036 |
| HR (95% CI)† | 0.30 (0.14-0.65) |
| . | Abatacept + CNI/MTX (n = 54) . | CNI/MTX (n = 161) . | P value∗ . |
|---|---|---|---|
| Day 180 (primary end point) | |||
| OS (95% CI) | 98 (78-100) | 75 (67-82) | .0028 |
| HR (95% CI)† | 0.06 (0.01-0.27) | ||
| 1 y | |||
| OS (95% CI) | 86 (68-95) | 61 (53-69) | .0046 |
| HR (95% CI)† | 0.28 (0.12-0.68) | ||
| 2 y | |||
| OS (95% CI) | 83 (64-92) | 55 (46-63) | .0036 |
| HR (95% CI)† | 0.30 (0.14-0.65) |
OS is defined as the time between the date of transplant to the physician documented date of death. Patients were censored at 181 days and 1 year after transplant or at time of last follow-up, whichever is earlier. Propensity scores obtained from a logistic regression model including sex, disease, age, HCT graft source, conditioning intensity, Karnofsky/Lansky performance score, and CNI type, as covariates.
Based on weighted Kaplan-Meier method.
Marginal HR based on weighted Cox proportional hazards model with treatment as the only covariate using a robust variance estimator that accounts for the sample weights. Ties are handled using the Breslow method.
RFS
In 7/8 HLA-MMUD recipients, abatacept + CNI/MTX was associated with statistically significantly higher RFS vs CNI/MTX through 2 years after HCT (74% [95% CI, 55-87] vs 49% [95% CI, 41-57], HR: 0.40 [0.21-0.78], P = .0098; Figure 1D); RFS was also higher at 1 year after HCT (79% [95% CI, 60-90] vs 54% [95% CI, 46-62]) and at day 180 (89% [95% CI, 72-96] vs 67% [95% CI, 58-74]). RFS with abatacept + CNI/MTX was statistically significantly higher vs CNI/MTX + ATG through 2 years (77% [95% CI, 55-89] vs 35% [95% CI, 27-43], HR: 0.25 [0.13-0.48]; P = .0002; Figure 1E); RFS was also higher at 1 year after HCT (81% [95% CI, 60-92] vs 48% [95% CI, 39-56]) and at day 180 (91% [95% CI, 71-97] vs 66% [95% CI, 57-74]). RFS was similar with abatacept + CNI/MTX and PT-Cy through 2 years after HCT (72% [95% CI, 51-86] vs 56% [95% CI, 48-64]; HR: 0.54 [0.26-1.12], P = .1058; Figure 1F), at 1 year after HCT (75% [95% CI, 56-87] vs 62% [95% CI, 52-70]), and at day 180 (85% [95% CI, 67-94] vs 77% [95% CI, 68-83]).
NRM
Weighted cumulative incidence rates for NRM at 1 and 2 years are shown in supplemental Table 5. The addition of abatacept to CNI/MTX resulted in lower NRM at 2 years after HCT vs CNI/MTX alone (11% [95% CI, 4-29] vs 26% [95% CI, 20-34]; P = .0225). Abatacept + CNI/MTX was also associated with lower NRM at 2 years compared with CNI/MTX + ATG (11% [95% CI, 5-25] vs 30% [95% CI, 22-39]; P = .0096) and similar NRM vs PT-Cy (13% [95% CI, 5-38] vs 12% [95% CI, 8-18]; P = .8578).
Relapse
Weighted cumulative incidence rates for relapse at 1 and 2 years are shown in supplemental Table 5. The addition of abatacept to CNI/MTX resulted in numerically lower relapse occurrence 2 years after HCT (14% [95% CI, 7-28] vs 24% [95% CI, 17-33]; P = .1319). Abatacept + CNI/MTX was associated with lower relapse incidence 2 years after HCT compared with CNI/MTX + ATG (12% [95% CI, 6-23] vs 34% [95% CI, 27-43]; P = .0060) and PT-Cy (13% [95% CI, 7-27] vs 29% [95% CI, 23-37]; P = .0250).
aGVHD and cGVHD
Weighted incidence rates for aGVHD and cGVHD are shown in supplemental Table 5. At both time points (days 100 and 180) and for both severities (grade 2 to 4 and 3 to 4) the incidence rate for aGVHD was lower with abatacept + CNI/MTX vs CNI/MTX, CNI/MTX + ATG, and PT-Cy. At day 180, rates of cGVHD were lower with abatacept + CNI/MTX compared with CNI/MTX, CNI/MTX + ATG, and PT-Cy. Rates of cGVHD at 1 and 2 years were similar for patients treated with abatacept + CNI/MTX vs CNI/MTX, and higher for abatacept + CNI/MTX vs CNI/MTX + ATG, and vs PT-Cy–based prophylaxis.
8/8 HLA-MUD HCT recipients
OS
In 8/8 HLA-MUD recipients, OS was similar with abatacept + CNI/MTX and CNI/MTX through 2 years after HCT (70% [95% CI, 55-80] vs 62% [95% CI, 56-67], HR: 0.56 [0.28-1.11]; P = .2569; Figure 2A), at 1 year after HCT (81% [95% CI, 68-89] vs 70% [95% CI, 65-74]), and at day 180 (95% [95% CI, 84-99] vs 80% [95% CI, 75-84]). Abatacept + CNI/MTX displayed similar OS vs CNI/MTX + ATG through 2 years after HCT (75% [95% CI, 60-84] vs 64% [95% CI, 58-68], HR: 0.43 [0.21-0.88]; P = .1048; Figure 2B), at 1 year after HCT (86% [95% CI, 72-93] vs 70% [95% CI, 65-75]), and at day 180 (97% [95% CI, 86-99] vs 82% [95% CI, 78-86]). OS was similar with abatacept + CNI/MTX and PT-Cy through 2 years after HCT (74% [95% CI, 55-86] vs 69% [95% CI, 64-74], HR: 0.69 [0.30-1.54]; P = .5543; Figure 2C), at 1 year after HCT (84% [95% CI, 66-93] vs 78% [95% CI, 73-82]), and at day 180 (97% [95% CI, 81-100] vs 87% [95% CI, 83-90]).
RFS
Abatacept + CNI/MTX in 8/8 HLA-MUD recipients was associated with numerically higher RFS vs CNI/MTX through 2 years after HCT (63% [95% CI, 48-74] vs 52% [95% CI, 47-57]; P = .1497; Figure 2D) with an improved HR (0.46 [95% CI, 0.25-0.86]), at 1 year after HCT (78% [95% CI, 64-87] vs 59% [95% CI, 53-64]), and at day 180 (90% [95% CI, 77-95] vs 70% [95% CI, 64-74]). Abatacept + CNI/MTX also demonstrated numerically higher RFS vs CNI/MTX + ATG through 2 years after HCT (66% [95% CI, 51-78] vs 55% [95% CI, 49-60]; P = .1193; Figure 2E) with an improved HR (0.39 [0.21-0.73]), at 1 year after HCT (82% [95% CI, 69-90] vs 61% [95% CI, 55-66]), and at day 180 (92% [95% CI, 80-97] vs 73% [95% CI, 68-77]). RFS was similar with abatacept + CNI/MTX and PT-Cy through 2 years after HCT (68% [95% CI, 49-81] vs 57% [95% CI, 52-63]; P = .2356; Figure 2F), HR = 0.54 (0.26-1.11), at 1 year after HCT (79% [95% CI, 61-90] vs 65% [95% CI, 60-70]), and at day 180 (90% [95% CI, 73-96] vs 77% [95% CI, 72-81]).
NRM
Weighted cumulative incidences for NRM at 1 and 2 years are shown in supplemental Table 6. The addition of abatacept to CNI/MTX resulted in similar NRM at 2 years after HCT (13% [95% CI, 7-22] vs 17% [95% CI, 14-21]; P = .3694). Abatacept + CNI/MTX was also associated with similar NRM at 2 years compared with CNI/MTX + ATG (12% [95% CI, 5-22] vs 16% [95% CI, 12-19]; P = .3775) and PT-Cy (9% [95% CI, 4-16] vs 11% [95% CI, 8-15]; P = .4253).
Relapse
Weighted cumulative incidences for relapse at 1 and 2 years are shown in supplemental Table 6. The addition of abatacept to CNI/MTX resulted in similar relapse occurrence 2 years after HCT (24% [95% CI, 13-36] vs 31% [95% CI, 26-36]; P = .2805). Abatacept + CNI/MTX was also associated with similar relapse incidence 2 years after HCT compared with CNI/MTX + ATG (22% [95% CI, 11-35] vs 29% [95% CI, 24-34]; P = .2623) and PT-Cy (23% [95% CI, 12-37] vs 31% [95% CI, 26-36]; P = .2779).
aGVHD and cGVHD
Weighted incidence rates for aGVHD and cGVHD are shown in supplemental Table 6. At both time points (days 100 and 180) for grade 2 to 4 aGVHD, rates were similar with abatacept + CNI/MTX, CNI/MTX, and CNI/MTX + ATG, and higher for abatacept + CNI/MTX vs PT-Cy. At both time points (days 100 and 180) for grade 3 to 4, rates for aGVHD were lower for abatacept + CNI/MTX vs CNI/MTX; rates were lower at day 100 and similar at day 180 for abatacept + CNI/MTX vs CNI/MTX + ATG. At day 100, the rates of grade 3 to 4 aGVHD were similar with abatacept + CNI/MTX vs PT-Cy, whereas at day 180 they were higher with abatacept. At day 180, rates of cGVHD were lower with abatacept + CNI/MTX than with CNI/MTX and CNI/MTX + ATG; rates were higher than with PT-Cy. Rates of cGVHD at 1 and 2 years were higher for patients treated with abatacept + CNI/MTX vs CNI/MTX, CNI/MTX + ATG, and PT-Cy-based prophylaxis.
Discussion
Several lines of evidence from the published literature have demonstrated the aGVHD protective effect of abatacept.20,26-33 On that basis, abatacept received breakthrough therapy designation and, subsequently, FDA approval for prophylaxis of aGVHD in unrelated donor HCT recipients aged ≥2 years. CIBMTR registry data provided key evidence in support of the regulatory approval by validating the observed outcomes from the clinical trial (ClinicalTrials.gov identifier: NCT01743131), in which benefits of the addition of abatacept to CNI/MTX on OS and RFS were initially observed. The current further analyses of CIBMTR registry data underscore the potential for this regimen to provide an alternative additional donor source option to patients for whom a related or 8/8 HLA-MUD donor, or a haploidentical donor, is not available. Moreover, this is the first analysis of abatacept + CNI/MTX vs CNI/MTX + ATG in 8/8 HLA-MUD recipients, and the first analysis of a comparison with PT-Cy–based regimens.
The findings from this retrospective cohort study with recipients of 7/8 HLA-MMUD HCT who received CNI/MTX or CNI/MTX + ATG support previous observations from the ABA2 trial, which used a matched-pair design.20 In line with FDA guidance requesting bias control for the regulatory submission, this prospectively designed study used inverse probability of treatment weighting-based propensity score matching, rather than matched-pair analysis. The findings obtained via this different statistical approach demonstrate that addition of abatacept to CNI/MTX for aGVHD prophylaxis was associated with improved OS and RFS vs both CNI/MTX and CNI/MTX + ATG comparator regimens at 180 days, 1 year, and 2 years after HCT in recipients of grafts from 7/8 HLA-MMUDs. Additional analyses showed that treatment with abatacept + CNI/MTX resulted in numerically lower rates of aGVHD grades 2 to 4 and 3 to 4, NRM, and relapse in 7/8 HLA-MMUD recipients. Furthermore, we showed that 8/8 HLA-MUD HCT recipients treated with abatacept + CNI/MTX also had an improved HR for RFS compared with those treated with CNI/MTX, and an improved HR for both OS and RFS compared with CNI/MTX + ATG at 2 years. As demonstrated previously,20 the benefits of the 4-dose regimen of abatacept were primarily observed for the outcomes of aGVHD, NRM, and relapse, without a benefit for cGVHD. We are currently performing a randomized trial of 4 abatacept doses (with abatacept treatment through day +28 after transplant) vs 8 doses of abatacept (with abatacept treatment through day +150 after transplant) added to CNI/MTX (ClinicalTrials.gov identifier: NCT04380740) to determine whether an extended dosing regimen of abatacept can also improve rates of cGVHD in this patient cohort.
In light of recent phase 3 study results,34 PT-Cy–based regimens have increasingly become part of the standard of care for GVHD prophylaxis. However, new data suggest that immune reconstitution defects may be associated with PT-Cy prophylaxis,35 and recent long-term follow-up of MMUD transplant recipients treated with PT-Cy demonstrate higher-than-expected relapse rates36 after myeloablative pretransplant conditioning. To our knowledge, this is the first comparison between abatacept-based and PT-Cy–based aGVHD prophylaxis regimens and showed similar survival outcomes for both treatment strategies with a consistent trend toward numerically higher OS and RFS with abatacept for both 7/8 HLA-MMUD and 8/8 HLA-MUD HCT recipients, and lower relapse rates in 7/8 HLA-MMUD recipients treated with abatacept + CNI/MTX. These results suggest that a sufficiently powered comparative trial between abatacept-based and PT-Cy–based prophylaxis is warranted, especially given new data suggesting that PT-Cy may be superior to CNI/MTX for reduced-intensity HLA-MUD HCT using peripheral blood stem cells.34 Moreover, the comparable survival outcomes observed between patients receiving abatacept + CNI/MTX and PT-Cy–based prophylaxis also suggest that >1 regimen may now be available for use in further limiting morbidity and mortality after HLA-MMUD HCT.
Although some patients have a suitable matched sibling donor, many do not and require an alternative donor, of which options include a fully HLA-matched or HLA-MMUD, a haploidentical donor, or cord blood. Given the substantially greater risk for aGVHD after HLA-MMUD HCT,3 our results, along with those of others,20,37 indicate that abatacept + CNI/MTX may allow for substantial expansion of the unrelated alternative donor pool available to patients with hematologic malignancies, thus filling an important and urgent unmet medical need.1,2 Of considerable importance in this regard, a greater number of patients who are members of underserved racial and ethnic minorities could now have access to HCT from a 7/8 HLA-MMUD, previously an important barrier to care, despite recent increases in the size of HCT registries1 and the expansion of haploidentical donor transplantation. Thus, abatacept + CNI/MTX provides an alternative therapeutic option that can expand the donor pool, thereby granting further access for patients in need.
This study has notable strengths, such as the incorporation of key outcomes from the CIBMTR registry, which stores detailed information regarding all allogeneic HCT recipients in the United States, using established data collection procedures routinely monitored for quality control. Moreover, given the clinical reach of the CIBMTR registry, the study population provides results that inform key survival outcomes over 2 years for both 7/8 HLA-MMUD and 8/8 HLA-MUD HCT recipients. To fully understand the longitudinal impact of abatacept-containing regimens, longer-term effectiveness of this treatment approach warrants further study. This study also has some limitations that should be acknowledged, such as the lack of data on adherence to abatacept treatment regimens in the registry. Also, because abatacept use was reported in a free-text field in the CIBMTR registry database, there is potential for underreporting. As such, patients who received abatacept may not have been identified, or could have been assigned to the control group if abatacept use was not reported. It should also be noted that some information about one of the comparison cohorts, the ATG cohort, is incomplete, because data regarding the brand and dose of ATG were not collected by the CIBMTR and were therefore unavailable for this analysis. The potential lack of uniformity in ATG dosing may have contributed to the poor performance of the ATG cohort observed in this analysis, which was worse than that reported previously for a standardized dose of anti-Jurkat ATG-Fresenius used in a European clinical trial.38 There is also the potential for enrollment or selection bias in this study, because most of the patients treated with abatacept in the CIBMTR registry analysis were also enrolled in the aforementioned ABA2 trial. Notably, an analysis of outcomes among control patients enrolled in the ABA2 trial (who received CNI/MTX + placebo) vs patients receiving CNI/MTX who were not enrolled in ABA2 yielded no differences in OS between the 2 groups (supplemental Figure 3). Additionally, the use of abatacept off-study is currently increasing, with early data supporting survival outcomes similar to those observed in the ABA2 trial39; and with preliminary data also supporting continued evaluation of abatacept as an aGVHD prophylactic after HCT in nonmalignant diseases.40
This database study illustrates the survival benefits of abatacept when combined with CNI/MTX in patients with hematologic malignancies undergoing HCT. Abatacept + CNI/MTX was associated with improved survival indicators compared with CNI/MTX in both recipients of 7/8 HLA-MMUD and 8/8 HLA-MUD HCTs. Exploratory analyses documented the survival benefit of abatacept + CNI/MTX vs CNI/MTX + ATG and demonstrated similar outcomes, with trends toward improved survival, vs PT-Cy. Importantly, the addition of abatacept may provide an additional approach capable of expanding the unrelated alternative donor pool when MUDs are unavailable.
Acknowledgments
The authors acknowledge the patients who participated in this study and their families. The authors thank the Center for International Blood and Marrow Transplant Research (CIBMTR). member centers in the United States and throughout the world for reporting patients to the CIBMTR41 and contributing to this study. The authors thank the clinical research staff and caregivers at all participating sites. The authors acknowledge Anne Torbeyns for her contributions to the study.
This study was supported by Bristol Myers Squibb (Princeton, NJ). Professional medical writing and editorial assistance was provided by Ryan Miller of Caudex, a division of IPG Health Medical Communications, and was funded by Bristol Myers Squibb.
Authorship
Contribution: L.S.K., T.D.K., M.P., and B.G. conceptualized and designed the study; L.J.B., A.L., J.T.H., X.-Y.T., B.W., M.Q., B.B., and K.B. acquired data; L.J.B., T.D.K., X.-Y.T., M.-J.Z., M.C.P., and R.K. analyzed the data; L.S.K., L.J.B., T.D.K., X.-Y.T., M.-J.Z., K.L., S.E.C., M.P., B.G., A.G.-C., M.C.P., R.K., A.L., J.T.H., B.W., M.Q., B.B., and K.B. interpreted the data; L.J.B., T.D.K., R.K., and M.P. verified the data; and all authors contributed to writing the manuscript, agree on the decisions for it, have seen and approved the final manuscript.
Conflict-of-interest disclosure: L.S.K. is on the scientific advisory board for Mammoth Biosciences and HiFiBio; received research funding from Magenta Therapeutics, Tessera Therapeutics, Novartis, EMD Serono, Gilead Pharmaceuticals, and Regeneron Pharmaceuticals; received consulting fees from Vertex; received grants/personal fees from Bristol Myers Squibb; and reports royalties/partial funding for this study from Bristol Myers Squibb. L.S.K.’s conflict-of-interest with Bristol Myers Squibb is managed under an agreement with Harvard Medical School. L.J.B. received research collaborations with Astellas, bluebird bio, Gamida Cell, Kyowa Kirin, Mesoblast, Sanofi, and Vertex; and received a research contract through her institution for this study with Bristol Myers Squibb. S.E.C. and M.P. are employees and shareholders of Bristol Myers Squibb. R.K. is a current employee of Cytel; was on a work-share agreement with Bristol Myers Squibb during the time of analysis; and is a shareholder of Bristol Myers Squibb. A.L. received a medical writing support and a research contract for this study with Bristol Myers Squibb. M.Q. received honoraria from Novartis and Vertex; and received medical writing support from Bristol Myers Squibb. X.-Y.T. received support and payment to their institution from Bristol Myers Squibb; and is an immediate family member of an employee and shareholder of Kite Pharma. B.G. received consulting fees from WebMD, LLC; and is a former employee of (within 36 months) and shareholder of Bristol Myers Squibb. M.C.P. received research support from Bristol Myers Squibb; received grants/contracts from Bristol Myers Squibb, Kite Pharma, Novartis, and Janssen; and reports payments/honoraria for lectures from Kite Pharma. K.L., T.D.K., and A.G.-C. are former Bristol Myers Squibb employees (within 36 months) and shareholders of Bristol Myers Squibb. The remaining authors declare no competing financial interests.
Correspondence: Leslie S. Kean, Division of Hematology/Oncology, Boston Children’s Hospital and Department of Pediatric Oncology, Dana-Farber Cancer Institute, 300 Longwood Ave, Boston, MA 02115; email: leslie.kean@childrens.harvard.edu.
References
Author notes
L.S.K. and L.J.B. are joint first authors.
Presented in poster form at the 63rd annual meeting of the American Society of Hematology, Atlanta, GA, 11 to 14 December 2021; and the Tandem Meetings Transplantation and Cellular Therapy meetings of the American Society for Transplantation and Cellular Therapy and CIBMTR, Salt Lake City, UT, 23 to 26 April 2022; as an oral presentation at the 64th annual meeting of the American Society of Hematology, New Orleans, LA 10 to 13 December 2022; and the 49th annual meeting of the European Society for Blood and Marrow Transplantation, Paris, France, 23 to 26 April 2023.
Bristol Myers Squibb policy on data sharing may be found at https://www.bms.com/researchers-and-partners/clinical-trials-and-research/disclosure-commitment.html. Requests for clinical trial data from qualified researchers with a clearly defined scientific objective will be considered after publication of the primary results. Sharing is subject to protection of patient privacy and respect for the patient’s informed consent. Data considered for sharing may include nonidentifiable patient-level and study-level clinical trial data, full clinical study reports, and protocols. In-scope proposals are sent to an independent review committee to review and provide the final decision on the requests. The independent review committee ensures that qualifying requests for patient-level data have a complete, consistent, and fair assessment. Before data being released, the researcher(s) will be expected to sign the Vivli data use agreement. Upon execution of an agreement, the deidentified and/or anonymized data sets will be available within the Vivli research environment. Data requests can be submitted at https://vivli.org/ourmember/bristol-myers-squibb/.
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal