Key Points
Eligibility criteria for lower-risk and higher-risk MDS often do not vary across trial phases and do not reflect known drug safety signals.
Inclusive eligibility criteria that are phase and drug specific will enhance access to MDS trials and applicability to the real world.
Visual Abstract
Excessively restrictive inclusion and exclusion criteria in clinical trials are one of many barriers to clinical trial enrollment for patients with myelodysplastic syndromes/neoplasms (MDSs). Many organizations are developing efforts to increase clinical trial eligibility; yet, several recent publications focused on patients with MDS suggest that many patients with this disease may be excluded from clinical trials unnecessarily. Clinical trial eligibility should reflect the phase of the study and risks of the agent being studied. Phase 3 trials should be less restrictive than early-phase trials to represent the real-world population as closely as possible. We hypothesize that many clinical trials, particularly phase 3 trials, have unnecessarily restrictive eligibility criteria. This study aims to evaluate the most common eligibility criteria according to phase of trial and to determine whether criteria correspond with drug safety signals. We identified MDS clinical trials registered on ClinicalTrials.gov from 1 January 2000 to 1 September 2023 and analyzed the eligibility criteria of 191 therapeutic MDS trials. We found that categorical inclusion and exclusion criteria are remarkably similar in representation across trial phases. Additionally, only 13% of trials are concordant with drug safety signals, suggesting that the eligibility criteria are often arbitrary. On behalf of the icMDS (International Consortium for Myelodysplastic Syndromes), an association of international MDS experts, we provide a position statement on restrictive eligibility criteria for MDS clinical trials that should be avoided with the aim of removing barriers to clinical trial enrollment.
Introduction
Myelodysplastic syndromes/neoplasms (MDSs) are hematologic cancers characterized by morphologic dysplasia of hematopoietic cell lineages and ineffective hematopoiesis. The median age at diagnosis is 71 years,1 and the yearly incidence rate is estimated to be 162 per 100 000 in patients aged ≥65 years (204/100 000 in those aged ≥80 years).2 There are limited therapeutic options for patients with MDS, and overall survival is poor, particularly once active treatment is required.3-5 The paucity of effective therapies for MDS underscores the importance of clinical trials for these diseases; however, many barriers to patient enrollment exist.6 These barriers have produced disparities in participation, and there are calls to address these inequities as a moral imperative in cancer research.7 One significant opportunity to improve clinical trial enrollment is thoughtful consideration of eligibility criteria.
Eligibility criteria are meant to assure the appropriate patients are enrolled on clinical trials. However, overly restrictive inclusion and exclusion criteria can significantly limit the number of patients eligible for a trial, and often misapproximate the patient population that the trial aims to serve. In screening log data from the National Cancer Institute Community Cancer Centers Program that included patients with solid and hematologic malignancies, the most common reason for nonenrolment in trials was ineligibility (51.6%).8 Within randomized clinical trials conducted in hematologic malignancies, 1 study demonstrated that eligibility criteria reflected neither known nor realized adverse events, whereas another study conducted using Southwest Oncology Group data showed that treated patients retroactively found to be ineligible for leukemia studies fared just as well as eligible patients.9,10 A recent study used artificial intelligence and a machine-learning algorithm to simulate 10 oncology clinical trials and concluded that the eligibility limits for laboratory values could be relaxed without affecting the hazard ratio of overall survival. In this simulation, changes in the eligibility criteria doubled the number of patients that would have been eligible for a trial, suggesting that less restrictive eligibility criteria can significantly increase the pool of eligible patients without affecting safety or efficacy.11
Many organizations have developed initiatives to expand enrollment in clinical trials through education, recommendations, and policy efforts. In 2016, the American Society of Clinical Oncology and Friends of Cancer Research assembled working groups for new recommendations on variables that often exclude participation in a trial: brain metastases, HIV/AIDS, organ dysfunction, prior or concurrent cancers, and minimum age for enrollment.12 The Cancer Therapy Evaluation Program of the National Cancer Institute implemented these recommendations into eligibility criteria requirements for their clinical trials.13 In 2020, the US Food and Drug Administration (FDA) released a guidance document with recommendations to limit restrictions on organ dysfunction, comorbid conditions, disabilities, extremes of weight, and conditions with low prevalence.14 In 2021, the American Society of Clinical Oncology–Friends targeted the broadening of eligibility criteria related to washout periods (eg, time required after previous therapies), concomitant medications, prior therapies, laboratory reference ranges and test intervals, and performance status.15 Most recently, in April 2024, the FDA issued 3 draft guidance documents on eligibility criteria for cancer clinical trials to address performance status, washout periods and concomitant medications, and laboratory values.16-18 It is paramount that trials for patients with MDS reflect this movement of broadening eligibility criteria.
This analysis used publicly available MDS clinical trial data to evaluate how inclusion and exclusion criteria vary according to different phases of study design. We assessed whether there was concordance between known drug toxicities and the eligibility criteria. We then used a modified Delphi process among members of the International Consortium on Myelodysplastic Syndromes (icMDS) to adjudicate final recommendations for removal of specific unnecessary eligibility criteria for MDS clinical trials, with an overall aim of streamlining eligibility and increasing MDS clinical trial enrollment.
Methods
The methods of this systematic review are registered on PROSPERO, an international database of prospectively registered systematic reviews (ID: CRD42024496724).
Data search
We searched ClinicalTrials.gov to identify all active clinical trials registered for adults with MDS. The database was queried on 1 September 2023. Trials span from 1 January 2000 to 1 September 2023. The following search terms were used: myelodysplastic syndromes, MDS, phase 1, phase 2, phase 3, adult, older adult, actively recruiting, active but not recruiting, and not yet recruiting trials. Clinical trials were first assessed by study title, summary, eligibility, and objective to screen for inclusion in this analysis. The trials selected for this analysis included patients with MDS and could also contain additional cohorts of patients with acute myeloid leukemia (AML), chronic myelomonocytic leukemia (CMML), blastic plasmacytoid dendritic cell neoplasm, clonal cytopenia of undetermined significance, or MDS/myeloproliferative neoplasm.
Trials excluded from this analysis were those without any MDS cohorts and trials that did not involve a drug. Trials focused on cellular therapy, transplant conditioning regimens, and maintenance after transplant were excluded as were studies incorporating solid malignancies or other hematologic malignancies (chronic myeloid leukemia, acute lymphoblastic leukemia, myeloproliferative neoplasm, lymphoma, and/or chronic lymphocytic leukemia). Studies allowing VEXAS (vacuoles, E1 enzyme, X-linked, autoinflammatory, somatic) syndrome, thalassemia, aplastic anemia, paroxysmal nocturnal hemoglobinuria, and/or other bone marrow failure disorders were excluded. Finally, trials that include pediatric patients (aged <18 years) were excluded.
Data extraction
We extracted the following variables from the included trials: ClinicalTrials.gov identifier, study title, phase, sponsor type, study drug or other therapeutic drugs involved, inclusion criteria, and exclusion criteria. In trials that included AML and/or CMML, inclusion and exclusion criteria specific to the AML or CMML cohort were not recorded to focus on the MDS eligibility criteria. Study protocols publicly available on ClinicalTrials.gov, included in publications, and available at The Ohio State University were also reviewed. When there was discrepancy in eligibility criteria between ClinicalTrials.gov and the protocol, the source that was most recently updated was used. Data extraction was performed independently and in parallel by 2 investigators followed by resolution of disagreements.
Validation
Any full manuscripts or abstracts for all clinical trials that met inclusion criteria were identified on Google Scholar, PubMed, and Google. Keywords from the clinical trial title, ClinicialTrials.gov identification number, and author were used to match the clinical trial with its publication. The patient eligibility portions of the published methods were reviewed to determine whether the inclusion and exclusion criteria in the publication were in line with the language found on ClinicalTrials.gov.
Analysis of criteria
Once all criteria were collected for all studies, the number of times each specific inclusion and exclusion criterion was present was tallied to determine the 10 most common inclusion and exclusion criteria in all of the represented studies. Criteria were reported using frequencies and percentages as early-phase trials (defined as phase 1 and 1/2) and late-phase trials (defined as phase 2, 2/3, and 3), and the results were compared by χ2 test or Fishers exact test when the counts were <5. Odds Ratios (ORs) were calculated using logistic regression, with phase and sponsor controlled and reported with 95% confidence intervals (95% CIs). It was also assessed whether the most common eligibility criteria were more or less restrictive in the included phase 3 studies compared with their earlier phases per eligibility criteria published on ClinicalTrials.gov and available protocols. All analyses were conducted using SAS, version 9.4 (SAS Institute Inc, Cary, NC, 2016), with a 1-sided type-1 error of <.05 indicating statistical significance. All statistical tests were not adjusted for multiple comparisons.
Assessment of concordance between drug safety profile and eligibility criteria
The therapeutic drug(s) involved in each trial were recorded. The safety profiles of each standard-of-care drug were investigated on the drug websites Lexicomp and MicroMedex.19,20 When available, safety signals for investigational agents were extracted from the corresponding study investigational brochures. The safety profiles of the drugs and concordance with eligibility criteria then underwent a second rigorous independent review by a specialty hematology pharmacist for validation. Concordance was defined as the presence of an eligibility criterion with a drug safety signal or the absence of a criterion without a signal,21 and focused specifically on the following variables: renal function, alanine aminotransferase (ALT)/aspartate aminotransferase (AST), total bilirubin, HIV, hepatitis B, hepatitis C, left ventricular ejection fraction (EF), and corrected QT interval (QTc). A safety signal was defined as a drug safety flag determined by literature and pharmacist review that could warrant a restriction on eligibility, and a limit was defined as an eligibility criterion that restricted certain patients based on a cutoff. Concordance was recorded as no safety signal/no limit, safety signal/limit, and discordance was recorded as no safety signal/limit, or safety signal/no limit. For chronic viral diseases, a limit was not imposed if the eligibility criteria aligned with the recommendations set forth by the FDA (eg, requiring a negative hepatitis C viral load was not considered a limit whereas restricting all patients with a history of hepatitis C was a limit). If some variables were concordant and others discordant, the overall concordance was ultimately classified as discordant. Discordance because of a limit in the trial that did not represent a safety signal was considered the most restrictive, and this was prioritized in classification as more discordant compared with a safety signal without a limit. We grouped analysis based on phase of study, recognizing that later phases of study should better reflect the drug profile. Logistic regression was applied to test the association between specific criteria and relevant drug safety signals. Phase was controlled in the logistic regression. It was assessed whether there was an association between sponsor type (pharmaceutical vs investigator-initiated trials [IITs] and concordance.
Modified Delphi process
We performed a mixed-method Delphi adjudication approach among members of the icMDS to identify consensus on eligibility criteria that should be avoided in MDS clinical trials. First, we polled a focus group of 4 experts to pilot the language of the survey. The survey was then distributed to the entire group and included yes/no agreement statements for each possible recommendation with the option for comments (round 1). The survey was updated with recurrent ideas and distributed in round 2 for final consensus, and ≥75% agreement resulted in a recommendation.
Results
Characteristics of clinical trials
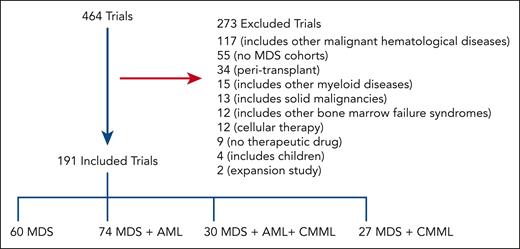
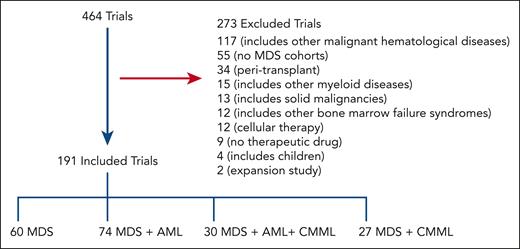
Our initial search yielded 464 trials of which 191 were included for analysis after screening as shown in Figure 1; 273 trials were excluded from additional analyses as outlined in Methods. Of 191 included trials, 34% are phase 1 (n = 64), 25% are phase 1/2 (n = 48), 30% are phase 2 (n = 57), 3% are phase 2/3 (n = 6), and 8% are phase 3 (n = 16). Overall, 112 were classified as early phase and 79 as late phase.
Schematic outlining the selection process of MDS trials. A total of 464 trials were selected for further screening after initial search results; 191 trials were included in the final analysis. Most trials incorporated MDS with AML.
Schematic outlining the selection process of MDS trials. A total of 464 trials were selected for further screening after initial search results; 191 trials were included in the final analysis. Most trials incorporated MDS with AML.
Trials most commonly included MDS and AML (n = 74); followed by MDS only (n = 60); MDS, AML, and CMML (n = 30); then MDS and CMML (n = 27; Figure 1). Table 1 shows general patient characteristics. Trials more commonly focused on higher-risk MDS (HR-MDS; 110 trials) as opposed to lower-risk MDS (LR-MDS; 38 trials) and on patients with at least 1 first-line prior therapy (86 trials) as opposed to treatment-naïve disease (12 trials).
Eligibility criteria
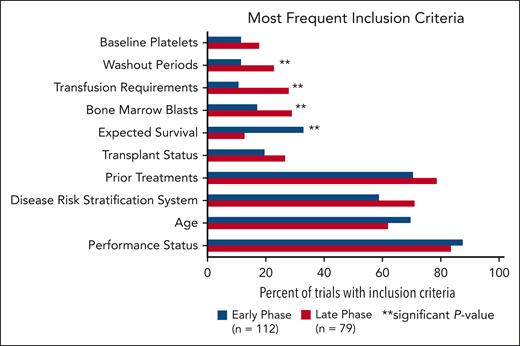
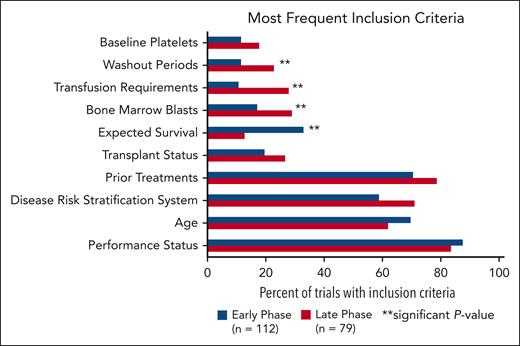
The most common inclusion criteria were performance status, age, disease risk stratification system, prior treatments, transplant status, expected survival, bone marrow blasts, transfusion requirements, washout periods, and baseline platelets (Figure 2). Performance status scores included Eastern Oncology Cooperative Group performance status, Karnofsky performance scale, and World Health Organization performance status. Risk stratification included scores based on the International Prognostic Scoring System (IPSS; n = 44 trials) and Revised IPSS (n = 84 trials). No trials required scoring per the Molecular IPSS (IPSS-M). Nine trials had an upper age limit in this analysis whereas 46 trials incorporated expected survival (range, 28 days to 12 months). Eligibility criteria for bone marrow blasts, transfusion requirements, and washout periods were more frequently present in late trials than in early phases (P = .0459; P = .0023; and P = .0391, respectively). Expected survival criteria was more often represented in early-phase trials compared with late-phase trials (P = .0013).
Most common inclusion criteria in early-phase vs late-phase trials. There is a significant difference between early- and late-phase trials for inclusion criteria categorized as expected survival, bone marrow blasts, transfusion requirements, and washout periods.
Most common inclusion criteria in early-phase vs late-phase trials. There is a significant difference between early- and late-phase trials for inclusion criteria categorized as expected survival, bone marrow blasts, transfusion requirements, and washout periods.
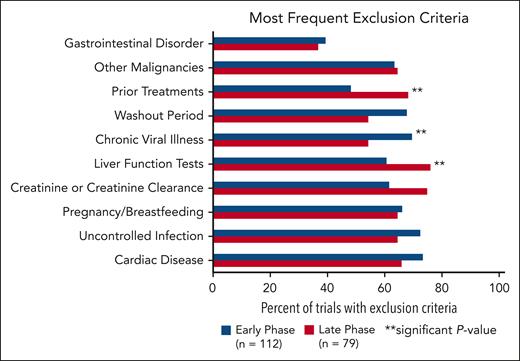
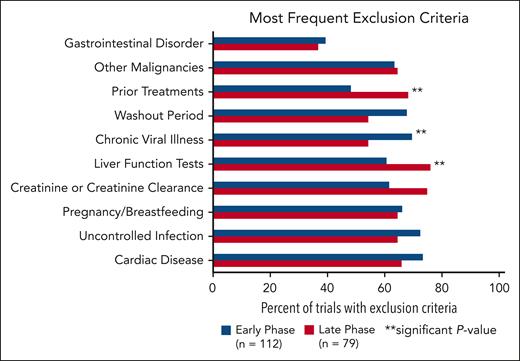
The following categories were the most common exclusion criteria (Figure 3): cardiac disease, uncontrolled infection, pregnancy/breastfeeding, creatinine or creatinine clearance, liver function tests, chronic viral illness, washout periods, prior treatments, other malignancies, and gastrointestinal disorders. Cardiac disease was the most common comorbidity used as an exclusion criterion and included specifications for uncontrolled hypertension, QTc, prior myocardial infarction, or EF. Exclusion criteria for chronic viral illness referred to statements regarding HIV, hepatitis B, and hepatitis C. Early-phase trials were more likely to have exclusion criteria for chronic viral illness (P = .0316). Late-phase trials were significantly more likely to have exclusion criteria for prior treatment (P = .0057) and liver function tests (P = .0274).
Most common exclusion criteria in early-phase vs late-phase trials. There is a significant difference between early- and late-phase trials for exclusion criteria categorized as liver function tests, chronic viral illness, and prior treatments.
Most common exclusion criteria in early-phase vs late-phase trials. There is a significant difference between early- and late-phase trials for exclusion criteria categorized as liver function tests, chronic viral illness, and prior treatments.
There was no significant difference between the number of eligibility criteria in phase 3 trials compared with earlier phase 1, 1/2, and 2 trials (P = .2251). There was a wide variation in the number of reported eligibility criteria across all trial phases, ranging from 3 to 30 distinct inclusion criteria and from 2 to 57 distinct exclusion criteria.
When comparing early and late-phase trials, 13 of 20 of the criteria categories did not significantly differ in representation. Specific eligibility criteria outlined in the trials are listed in the supplemental Material, available on the Blood website. Some trials had multiple stipulations for each category, and each specification is counted individually in the supplement tables. On analysis of the top 10 most common inclusion and exclusion criteria that differed between individual phase 3 trials and their respective earlier phase trials, approximately half of these criteria were more restrictive and half were less restrictive. Expected survival was part of inclusion criteria in much more industry trials than in IITs (P = .009). IITs tended to have cardiac disease (P = .0017), pregnancy/breastfeeding (P < .0001, creatinine (P < .0001), and liver function (P < .0001) in their exclusion criteria more than industry trials.
Drug safety data
A total of 107 trials had agents that were not FDA approved. Of these, 35 (33%) had investigational brochures available for review. Overall, 23 trials had safety data incorporated from online publications.
Concordance
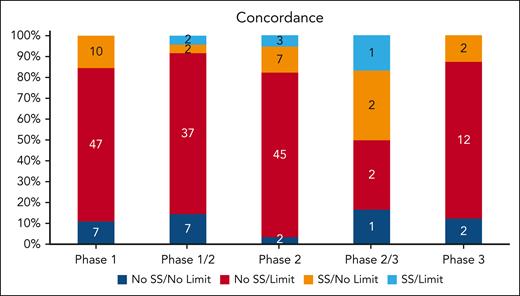
We evaluated the concordance (blue) and discordance (red and orange) of criteria with known drug safety data available for ALT/AST, total bilirubin, HIV, hepatitis B, hepatitis C, EF, and QTc (Figure 4). Overall, 25 trials (13%) had complete concordance between drug safety data and eligibility criteria. Phase 1 studies had a 10.9% concordance between drug safety data and eligibility limits. In phase 1/2 studies, 18.8% were concordant. Only 8.8% were concordant in phase 2, 33.3% in phase 2/3, and 12.5% in phase 3. Overall, there was no significant improvement in concordance between drug safety and trial eligibility moving from earlier- to later-phase studies (P = .125). Most trials (76%) included an eligibility criterion without a corresponding drug safety rationale, as determined by literature and pharmacist review. Only 12% of trials evaluated a drug with known safety concerns without a corresponding eligibility criterion to account for potential toxicity. For these trials, the safety signals were for renal (14 trials), bilirubin (13 trials), ALT/AST (11 trials), hepatitis C (11 trials), hepatitis B (10 trials), HIV (7 trials), QTc (5 trials), and EF (4 trials). After controlling for phase and sponsor type, only EF and QTc drug safety data were positively related to their eligibility limits in trials (OR, 4.81; 95% CI, 1.44-16.04; P = .0106; OR, 4.16; 95% CI, 1.45-11.87; P = .0078; respectively). However, after controlling for phase and sponsor type, renal function drug safety data were negatively related to eligibility limits in trials (OR, 0.35; 95% CI, 0.15-0.81; P = .0146; Table 2). Industry trials had much higher concordance rate than IITs (20/94 [21.3%] vs 5/97 [5.2%]; χ2P = .0010).
Overall concordance and discordance of eligibility criteria with drug safety signals. A safety signal (SS) is defined as the presence of a potential drug safety issue as identified by literature and pharmacist review. A limit is defined as eligibility criteria that is restrictive. The presence or absence of SS and limits for the following variables in each trial were assessed: EF, QTc limits, HIV, hepatitis B, hepatitis C, creatinine, ALT, AST, and total bilirubin. Stacked bars show concordance (shades of blue) and discordance (red and orange) between eligibility criteria and relevant drug safety signals. Numbers within the bars indicate the number of trials.
Overall concordance and discordance of eligibility criteria with drug safety signals. A safety signal (SS) is defined as the presence of a potential drug safety issue as identified by literature and pharmacist review. A limit is defined as eligibility criteria that is restrictive. The presence or absence of SS and limits for the following variables in each trial were assessed: EF, QTc limits, HIV, hepatitis B, hepatitis C, creatinine, ALT, AST, and total bilirubin. Stacked bars show concordance (shades of blue) and discordance (red and orange) between eligibility criteria and relevant drug safety signals. Numbers within the bars indicate the number of trials.
Survey results
The modified Delphi process resulted in 14 final recommendations. The final recommendations are listed with supporting background information are listed in Table 3. We have provided loose recommendations on eligibility criteria categories for specific categories (LR-MDS, HR-MDS, frontline, and relapsed/refractory) in Table 4 agreed upon by authors. Final survey results are provided in the supplemental.
Discussion
Overly restrictive eligibility criteria are 1 of many factors that prevent patients from accessing potential benefit from participation in trials with novel therapies. A key consideration of eligibility criteria is to limit enrollment of patients at higher risk of toxicity that may be erroneously attributed to investigational therapy. We contend that, although earlier phase trials should have more restrictive eligibility criteria, this should be relaxed in later-phase trials, such that drug risk in a broader patient population may be better appreciated. One key to optimizing patient eligibility for clinical trials is to select inclusion and exclusion criteria concordant with drug safety profiles. In particular, eligibility criteria within phase 3 trials should be the most concordant with drug safety signals identified in earlier phase studies, and criteria that are not supported by known pharmacologic risk should be avoided. However, our study revealed that most trials were not concordant with drug safety signals, including in the phase 2 and phase 3 settings.
Restrictive eligibility criteria may be particularly limiting in the field of MDS given the high frequency of older patients with comorbid conditions including often a history of another nonmyeloid neoplasm resulting in therapy-related MDS.28 One study evaluating clinical trial enrollment between 1991 to 2017 reviewed enrollment data for 1919 patients with MDS, and found only 449 patients (23%) participated in a clinical trial.22 Another study evaluated 47 MDS trials conducted in a center between 1987 and 2016 and found that only 18% of 1809 patients in the MDS registry were eligible. Karyotype, comorbidities, and prior therapies were the main reasons for exclusion.29 These eligibility criteria that are excluding patients from clinical trials may be unnecessarily restrictive. One study tested the feasibility of a clinical trial for 30 patients with AML or MDS who are typically excluded from trials. Patients were enrolled if they met 1 of the following inclusion criteria: serum creatinine of ≥2 mg/dL, total bilirubin of ≥2 mg/dL, Eastern Oncology Cooperative Group performance status of 3 to 4, or ineligibility to participate on a protocol of higher priority because of comorbidities or other malignancies with a remission period of <2 years. In this study, a majority of patients were able to complete at least 1 cycle of therapy without major toxicity and obtain clinical benefit, supporting the concept that these patients can be included in clinical trials.30
Notably, laboratory values assessed including creatinine, ALT, AST, and total bilirubin were not concordant with drug safety data. These laboratory criteria require careful scrutiny when designing future clinical trials. This is particularly important because restrictive criteria for creatinine and liver function tests tend to eliminate ethnic and racial minorities that have reported higher baseline values than White individuals.26,31 The diversity of the enrolled patient population in clinical trials is historically already limited. From 2008 to 2018, only 3% of cancer treatment trial participants leading to FDA oncology drug approvals were Black and 6% were Hispanic.32 Another opportunity to expand clinical trial eligibility is use of the modification of diet in renal disease equation or the chronic kidney disease epidemiology collaboration formula instead of the Cockcroft-Gault equation. One study demonstrated that the Cockcroft-Gault equation gives lower estimates of renal function when compared with the modification of diet in renal disease equation and the chronic kidney disease epidemiology collaboration formula in adults aged >70 years.33 This is highly relevant in MDS, a disease mostly of older adults. In accordance with ongoing efforts by the FDA to increase inclusion of minority patients in clinical trials,34 addressing eligibility criteria specifically related to laboratory values will lead to increased diversity and closer representation of the real-world population.
Our analysis of MDS trials on ClinicalTrials.gov elicits several other key points. Eligibility criteria regarding HIV and hepatitis were deemed not concordant with drug safety data, and special consideration to FDA recommendations in designing future clinical trials is indicated. Additionally, no trials in this analysis yet required IPSS-M assessment for eligibility. Clinical trials should start including baseline molecular testing, and as the field develops more targeted therapies, baseline IPSS-M may further refine eligibility. A significant proportion of trials mandated expected survival of several months. This is subjective, and we encourage use of more objective data to determine eligibility. It is also noteworthy that 50% of patients with MDS with del(5q) relapse after 2 to 3 years of treatment with lenalidomide35; therefore, these patients with del(5q) MDS with relapsed/refractory disease after lenalidomide should be eligible and included in clinical trials.
Our analysis shows that industry-sponsored trials and IITs mostly had similar representation of criteria in 15 of 20 eligibility criteria categories. We acknowledge that many sponsors and government agencies have protocol templates for specific drugs that come with significantly prewritten eligibility sections even when the trial is technically an IIT. One concern of including patients with comorbidities is falsely attributing adverse events as drug related. This can be circumvented by (1) involvement of experienced pharmacist and FDA guidance on when it is reasonable to relax safety parameters in a phase 2 setting and (2) allowing for a cohort of patient with specific comorbidities (renal or liver function abnormalities) to provide more real-world data that can be used to inform phase 3 studies. It is difficult to adjust safety criteria for phase 3 trials if patients with poor organ function are not included in phase 2 trials, and problematic to extrapolate to a real-world setting if a drug has no data in patient with compromised laboratory test results.
Based on the analysis above and with the intent of maximizing MDS clinical trial enrollment, the icMDS recommends avoiding restrictive eligibility criteria delineated in Table 3. We recognize that for some trials, more restrictive eligibility criteria will be necessary. Our preference is to use language such as “minimize” to avoid being overly didactic in our recommendations. For each trial, eligibility criteria should align with the expected toxic effects of the drug that may necessitate more restrictive recommendations than proposed in this manuscript. We advise careful consideration of these recommendations to soften eligibility criteria as appropriate recognizing that disease characteristics and pharmacology of the specific trial drug must also inform final eligibility criteria. We recognize that these recommendations may not be applicable in certain trial scenarios. For example, when a trial attempts to improve the results of azacitidine by adding a new compound, the health authorities generally require that the inclusion criteria be identical to those of the azacitidine approval. We recommend aligning with regulators diplomatically to loosen historical clinical trial eligibility criteria that may no longer be applicable to future trials for that agent. We also recommend reporting in publications how many patients were screened for clinical trials and the reasons for ineligibility.
Although eligibility criteria are 1 piece of how to improve clinical trial accrual, this is unfortunately unlikely to revolutionize new drug approvals in MDS. Development of effective drugs particularly in HR-MDS has been slow with several recent phase 3 trial failures for MDS. The PANTHER trial, which evaluated pevonedistat plus azacitidine as a first-line treatment for patients with MDS, CMML, and low-blast AML, did not reach its primary end point.36 In the ENHANCE trial evaluating the first-line combination of magrolimab and azacitidine for higher-risk MDS, the primary analysis demonstrated futility with an increased risk of death in the magrolimab-treated arm.37 Most recently, the phase 3 STIMULUS-MDS2 trial testing sabatolimab in combination with azacitidine did not meet its primary end point and its development in MDS has been discontinued.38 There are many reasons for these phase 3 failures including potential overestimation of response with previous International Working Group 2006 response criteria.39 Earlier phase studies with overly restrictive criteria may erroneously suggest an active drug that is disproven in phase 3 settings with expanded eligibility. The progress is ultimately halted by a lack of efficacious drugs. However, there have been new drug approvals in LR-MDS despite more restrictive exclusion criteria than recommended in this manuscript. This is potentially because of different end points in these trials compared with HR-MDS trials and more effective drugs that combat anemia. Although unlikely to significantly alter clinical trial outcomes, avoiding overly restrictive eligibility criteria in clinical trials from the outset may increase patient access and more closely represent the real world.
The results of this review should be interpreted within the context of the following limitations. The data collection was done manually, which was addressed by parallel review by investigators. Our main source for identification of studies was ClinicalTrials.gov, which relies on investigator updates and sometimes has abbreviated or summarized enrollment criteria. Lack of access to full enrollment criteria may have limited the ability to assess consistence with known drug safety signals and led to false "concordant/discordant" assessments. This was mitigated by reviewing protocols and recent publications for the latest criteria as available. Additionally, this study is limited through the use of a single clinical trials registry based in the United States, which may not reflect trends in enrollment criteria outside of the United States.
Authorship
Contribution: K.P., A.M.B., and A.H.W. performed research; U.B., K.P., and R.L.W. performed analyses; A.M.Z., U.B., K.P., A.M.B., J.P.B., and M.S. were responsible for conception and design of the study; U.B. and K.P. drafted the initial version of the manuscript; A.M.Z. and A.M.B. supervised the study; and all authors contributed to the subsequent drafts of the manuscript, and have reviewed and approved the submitted version of the manuscript.
Conflict-of-interest disclosure: A.M.Z. participated in advisory boards, and/or had a consultancy with, and received honoraria from, AbbVie, Pfizer, Celgene/Bristol Myers Squibb (BMS), Jazz, Incyte, Agios, Servier, Boehringer-Ingelheim, Novartis, Astellas, Daiichi Sankyo, Geron, Taiho, Seattle Genetics, Otsuka, BeyondSpring, Takeda, Ionis, Amgen, Janssen, Genentech, Epizyme, Syndax, Gilead, Kura, Chiesi, ALX Oncology, BioCryst, Notable, Orum, Mendus, Zentalis, Schrodinger, Regeneron, Syros, and Tyme and served on clinical trial committees for Novartis, AbbVie, Gilead, Syros, BioCryst, ALX Oncology, Kura, Geron, and Celgene/BMS. M.S. served on the advisory board for Novartis, Kymera, Sierra Oncology, GlaxoSmithKline, Rigel, BMS, Sobi, and Syndax; consulted for Boston Consulting, Gerson Lehrman Group, and Dedham group; and participated in continuing medical education activity for Novartis, Curis Oncology, Haymarket Media, and Clinical Care Options. A.E.D. participated in advisory boards, and/or had a consultancy with, and received honoraria from, Celgene/BMS, Agios, Novartis, Astellas, Gilead and served on clinical trial committees or data and safety monitoring boards for Novartis, AbbVie, Kura, Geron, Servier, Keros, and Celgene/BMS. U.P. reports research support and honoraria from BMS, Geron, Curis, AbbVie, and Janssen. D.A.S. reports research funding from Aprea Therapeutics and reports consulting for, and/or advisory board participation with, AbbVie, Agios, Akesobio, Avencell Europe GmbH, Bluebird Bio, BMS, Geron, Gilead Sciences, Janssen Global Services, Kite Pharma, Molecular Partners AG, Novartis, Servier Pharmaceuticals, Shattuck Labs, Syndax Pharmaceuticals, Syros Pharmaceuticals, and Takeda Pharmaceutical. F.E. reports consultancy or advisory role with AbbVie, Incyte, Novartis, and Jazz Pharmaceuticals and reports research support (institution) from Daiichi Sankyo, all outside the submitted work. E.A.G. declares honoraria for continuing medical education activity from Aplastic Anemia and MDS International Foundation, MedscapeLIVE, MediCom Worldwide, MJH Health, American Society of Hematology, MDS International Foundation, and Physicians Educational Resource; reports consulting fees from AbbVie, Alexion Pharmaceuticals, Apellis Pharmaceuticals, Takeda Oncology, Astex Pharmaceuticasls/Taiho Oncology, Alexion/AstraZeneca Rare Disease, Celgene/BMS, CTI BioPharma, Novartis, Partner Therapeutics, Picnic Health, and Servier; and reports research funding (to Roswell Park Comprehensive Cancer Center) from Alexion Pharmaceuticals, Apellis, Astex/Otsuka Pharmaceuticals/Taiho Oncology, Blueprint Medicines, Celldex Pharmaceuticals, Genentech, and NextCure Inc. M.L.X. served as consultant to Treeline Biosciences and Pure Marrow. A.W. served on the advisory board of Syndax Medical in October 2024, and served on the advisory board of Jazz Pharmaceuticals, Inc. The remaining authors declare no competing financial interests.
Correspondence: Uma Borate, The James Cancer Hospital and Solove Research Institute, 460 W 10th Ave, Columbus, OH 43210; email: uma.borate@osumc.edu.
References
Author notes
Original data are available on request from the corresponding author, Uma Borate (uma.borate@osumc.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.