Visual Abstract
Richter transformation (RT) is defined as an aggressive lymphoma emerging in patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL). Despite novel therapeutics developed in CLL, RT is associated with poor outcomes. In light of recent progress regarding the diagnostic procedures and therapeutic concepts of RT, an international group of experts, under the coordination of the European Research Initiative on CLL, has developed consensus recommendations for clinical procedures and future research on this disease. Patients with RT typically present with a rapid clinical decline, worsening B-symptoms, elevated lactate dehydrogenase, and/or rapidly enlarging lymphadenopathy. Workup should include a positron emission tomography–computed tomography scan for patients with suspected RT. An excisional biopsy should be taken from an accessible lesion, preferably with the highest fluorodeoxyglucose avidity, and analyzed for the presence of aggressive lymphoma. The molecular relationship to the original CLL clone(s) should be defined. Because no effective standard treatment for RT exists, patients should be treated in a clinical trial. Response of both RT and CLL should be assessed at an early time point, and survival end points should be prioritized in trial design. We hope that these recommendations can help to harmonize clinical and translational research and improve outcomes for patients with RT.
Introduction
Richter transformation (RT) is the development of aggressive lymphoma in the setting of chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL).1 CLL rarely transforms to Hodgkin lymphoma, plasmablastic lymphoma, or other histologies, whereas diffuse large B-cell lymphoma (DLBCL) remains the most common transformation event and is the focus of these recommendations. Literature reporting transformation events is sparse, especially for the rarer subtypes. DLBCL-RT, hereafter RT, is estimated to occur in 2% to 10% of patients with CLL, and is associated with a median overall survival (OS) of 6 to 12 months, unless an allogeneic stem cell transplant can be successfully performed.2-6 In recent years, there has been rapid development of clinical trials focused on RT; but there is no comprehensive recommendation regarding the essential end points in clinical research. Therefore, the European Research Initiative on CLL (ERIC) gathered an international group of experts to provide consensus recommendations on the diagnosis, management, and optimal clinical trial design for RT.
Methods
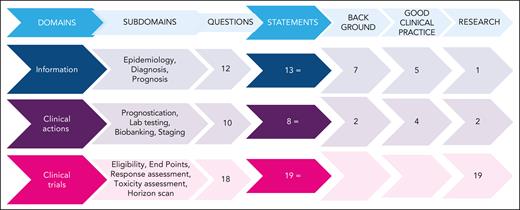
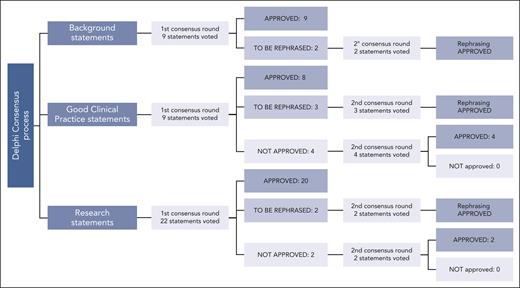
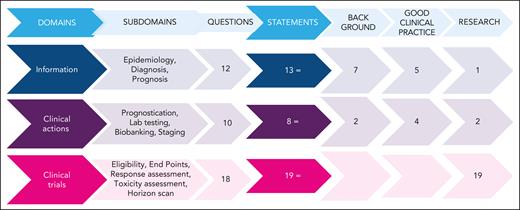
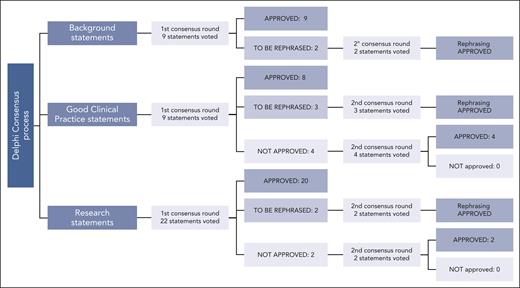
The ERIC assembled a core panel, which included 5 hematologists specifically devoted to CLL and RT (A.S.K., G.G., J.A.W., and P.G.) or guideline methodology (M.M.). The ERIC then convened an international expert panel of 24 additional hematologists from 10 countries and 3 continents. The core panel framed the problem into 3 domains and 12 subdomains (supplemental Table 1, available on the Blood website). Several questions were raised by the core panel for each subdomain; overall, 40 questions were listed (Figure 1). The core panel replied to each question by elaborating 40 draft statements. Statements underwent a careful classification according to GRADE (grades of recommendation, assessment, development, and evaluation)7: (1) background statements, (2) good clinical practice statements, and (3) research statements. We assigned to the first class, namely “background,” those statements not prompting specific clinical decisions or actions and those statements reporting consolidated practice. Rather, we assigned to the second class, namely “good clinical practice statements,” those recommendations that were expected to lead to a relevant health care practice improvement and were supported by direct or well-documented and explicit indirect evidence. Finally, “research statements” included a list of actionable and not-actionable statements that were not sufficiently equipped with evidence and for which net benefit estimation was not possible. The core panel also integrated the statements with “remarks” supporting their interpretation and to guide the user on the intervention options. A modified Delphi process was applied to achieve consensus on the statements (Figure 2).8,9 Google modules were developed by the methodologist (M.M.) and filled anonymously by the expert panel members. A 5-point scale was adopted to obtain panelist agreement on the statements. Consensus rates were calculated by summing up “absolutely-agree” and “agree” answers, and a threshold rate of >75% was adopted (supplemental Table 2). Comments were also recorded by a note field. Results were discussed during 3 virtual meetings. A second round of discussions was devoted to those items achieving a borderline consensus or requiring rewording; the threshold rate was refined (>60%) for rewordings.
Questions and statements. The left side flowchart shows how many questions were generated within each domain. Each question generated at least 1 statement. Two questions were moved from “clinical actions” to “information” and “clinical trials” after discussion. Three classes of statements were elaborated: background, good clinical practice, and research statements. The right side of the figure shows how many statements were generated and their typology. The figure outlines the full process of the present project. Three domains were initially identified: the “information” domain addressed RT epidemiology, diagnosis, and prognosis; the “clinical actions” domain addressed RT testing, prognostication, and staging; and the “clinical trials” domain addressed clinical trials for patients with RT. Within the 3 domains, 12 subdomains were identified, which led to 40 clinical questions. The clinical questions were answered by 40 statements classified as “background” (9), “good clinical practice” (9), or “research” (22) statements, according to GRADE (grades of recommendation, assessment, development, and evaluation) classification. One or more Delphi consensus rounds allowed the group to reach agreement on the listed statements. Moreover, 16 corollary “remarks” completed the list of statements.
Questions and statements. The left side flowchart shows how many questions were generated within each domain. Each question generated at least 1 statement. Two questions were moved from “clinical actions” to “information” and “clinical trials” after discussion. Three classes of statements were elaborated: background, good clinical practice, and research statements. The right side of the figure shows how many statements were generated and their typology. The figure outlines the full process of the present project. Three domains were initially identified: the “information” domain addressed RT epidemiology, diagnosis, and prognosis; the “clinical actions” domain addressed RT testing, prognostication, and staging; and the “clinical trials” domain addressed clinical trials for patients with RT. Within the 3 domains, 12 subdomains were identified, which led to 40 clinical questions. The clinical questions were answered by 40 statements classified as “background” (9), “good clinical practice” (9), or “research” (22) statements, according to GRADE (grades of recommendation, assessment, development, and evaluation) classification. One or more Delphi consensus rounds allowed the group to reach agreement on the listed statements. Moreover, 16 corollary “remarks” completed the list of statements.
Delphi process. Approval process to the 3 classes of statements is reported: agreement by ≥75% of the panelists was requested. Rephrased, but previously approved, statements were accepted when at least 60% of the panelists agreed on the final phrasing. “Good clinical practice statements” involved actionable recommendations, which were expected to lead to a relevant health care practice improvement and were supported by direct or well-documented and explicit indirect evidence. Rather, “background statements” include nonactionable descriptive assertions or standardized practice actions. Finally, “research statements” include a list of actionable (or nonactionable) suggestions that are not sufficiently supported by evidence or cannot yet be estimated to lead to a large healthcare improvement.
Delphi process. Approval process to the 3 classes of statements is reported: agreement by ≥75% of the panelists was requested. Rephrased, but previously approved, statements were accepted when at least 60% of the panelists agreed on the final phrasing. “Good clinical practice statements” involved actionable recommendations, which were expected to lead to a relevant health care practice improvement and were supported by direct or well-documented and explicit indirect evidence. Rather, “background statements” include nonactionable descriptive assertions or standardized practice actions. Finally, “research statements” include a list of actionable (or nonactionable) suggestions that are not sufficiently supported by evidence or cannot yet be estimated to lead to a large healthcare improvement.
Epidemiology, diagnosis, and prognosis of RT
Epidemiology and prevalence
RT can occur at any time point in the CLL disease history, regardless of CLL treatment status, with studies reporting a median time to RT from CLL diagnosis ranging between 2 to 4 years.4,6,10 Approximately 2% to 10% of patients with CLL will develop RT, at a rate of 0.5% per year.4-6 Contemporary first-line phase 3 trials comparing chemoimmunotherapy to targeted therapy have reported low rates of RT in both arms, suggesting development of RT is not affected by the choice of therapy. Longer follow-up is necessary to determine the true incidence of RT in the modern era of targeted agents.11-15 Furthermore, receiving CLL-directed treatment before an indication per International Workshop on CLL (iwCLL) guidelines (eg, early intervention clinical trials typically targeting high-risk patients) has not been associated with an increased or reduced risk of RT, compared with observation or placebo.16-18 Thus, no therapeutic strategy has been proven to effectively prevent or reduce the risk of RT (Table 1).
Diagnosis
Risk of RT
Multiple studies have reported risk factors observed in preexisting CLL cells for development of RT, including complex karyotype (CK), defined by the presence of ≥3 chromosomal abnormalities, with increasing complexity associated with higher risk.6,19,20 These data led to the development of a new RT risk model delineating patients as low, medium, and high-risk for RT; with high-risk characterized by patients with either high CK (defined by the presence of ≥5 chromosomal lesions), and/or CK2 (CK with major structural abnormalities defined as unbalanced translocations; chromosome additions; insertion; duplications; and ring, dicentric, and marker chromosomes).21 These individuals had a hazard ratio of 9.2 for risk of RT compared with those patients with low-risk, defined as mutated immunoglobulin heavy chain variable (IGHV) without CK or TP53 abnormalities. Patients with unmutated IGHV gene, del(11q), TP53 abnormalities, or Binet stage B to C were considered intermediate risk and had a hazard ratio of 3.4 compared with the low-risk group. In this study, 63% of patients classified as high risk by cytogenetics for risk of RT development carried a TP53 abnormality.21 A rare subset of patients at high risk of RT are patients with CLL with expression of stereotyped B-cell receptors belonging to subset no. 8, comprising cases with unmutated IGHV4-39/IGHD6-13/IGHJ5 gene rearrangements.22,23 Additionally, one-third of patients with RT carry NOTCH1 mutations, with 1 large series describing 40% of patients with NOTCH1-mutated CLL developing RT.24 Subset no. 8 and NOTCH1 mutations have not been validated in a modern cohort of patients with RT, and their presence should not influence the choice of CLL-directed therapy25; thus further research on the impact of these abnormalities and risk of transformation is needed. These risk factors inform clinicians of the chance of RT development and may warrant closer follow-up for particularly high-risk individuals. Because early intervention has not been shown to make a difference in RT development, and modern molecular testing requires further validation, early intervention for patients considered to be at high-risk of transformation to RT is not warranted.
When to suspect RT
Role of imaging (PET-CT)
Fluorodeoxyglucose (FDG) positron emission tomography–computed tomography (PET-CT) is a standard method to evaluate lymphoma at initial staging and at response assessments.28,29 A high metabolic activity in a lymph node, as evaluated by a high maximum standardized uptake (SUVmax), typically reflects aggressive rather than indolent lymphoma.30,31 Biopsies to document the development of RT should be performed on the most metabolically active, accessible lymph node (Table 1).32 In different studies, a SUVmax of ≥10 was associated with an adverse prognosis irrespective of histopathology and predictive of RT, including in a study after ibrutinib therapy.33-35 Because the ranges of SUVmax in histologically indolent vs aggressive disease can overlap, a biopsy is mandatory to establish a definitive diagnosis.36 With a threshold SUVmax of ≥5 to identify patients at risk of RT, a sensitivity of 88% for 96% for RT on biopsy is achieved, but the positive predictive value is only 38% to 51%.32,37 In the presence of a SUVmax of <5, the probability of RT is extremely low (3%-8%).32,37
Appropriate biopsy
Fine needle aspiration is inadequate for diagnosis. Multiple samples from a core needle biopsy may be sufficient, although excisional or incisional biopsy of the most clinically concerning/FDG-avid lesion is optimal,38 including when core needle biopsy does not reveal RT but there remains strong clinical concern. Obtaining sufficient diagnostic tissue is important for multiple reasons, including (1) having sufficient material to exclude pathologic mimics of RT, and (2) having tissue for genetic studies including fluorescence in situ hybridization, TP53 mutation, clonal relationship, and comprehensive next-generation sequencing (NGS).
The diagnosis of RT requires the morphologic review of tissue by an expert pathologist (Table 1).1,39 The International Consensus Classification and World Health Organization require the presence of sheets of large cells for the correct identification of the transformed areas to differentiate RT from “CLL with enlarged proliferation centers.”39,40 RT typically expresses pan–B-cell antigens but may be negative for CD20 in the setting of prior anti-CD20 therapy. Non–germinal center B-cell phenotype is frequently identified by the Hans algorithm using immunohistochemistry; however, germinal B-cell phenotype can also be observed. CD5 and CD23, typically expressed in CLL, are variably expressed in RT.
Even with sufficient tissue, a confident diagnosis of RT may present a challenge, with false-positive diagnoses reported in up to 18% of centrally reviewed specimens.41 Therefore, great efforts should be made to involve an experienced hematopathologist with expertise in lymphoma to review RT biopsies and/or discussing the case within a pathology panel (Table 1). The most critical task for the pathologist is the differentiation from CLL with nonstandard morphology, sometimes termed accelerated CLL, aggressive CLL, or CLL with expanded proliferation centers.1,42 These aggressive CLL cases are distinct from RT and usually benefit from CLL-directed therapies, albeit often with a poorer outcome.43
Withdrawal of Bruton tyrosine kinase inhibitor (BTKi) therapy can mimic RT.44 However, BTKi reintroduction results in favorable responses and resolution of associated symptoms.44,45 International Consensus Classification criteria include the importance of recognizing reversible Richter-like proliferations in patients with CLL who have halted BTKi therapy and emphasizes distinguishing CLL from RT.39 Therefore, it is essential to convey the clinical history to the pathologist.
Appropriate determination of clonal relationship
RT must be differentiated from de novo large B-cell lymphoma arising as a second cancer by tracking the clonal relationship between the lymphoma and the background CLL (Table 2).46-52 To do this, one must show a clonal relationship between the CLL and the DLBCL by looking at immunoglobulin rearrangements in the 2 diseases.52 Ideally, biobanked CLL samples stored in compliance with country-specific regulations, or immunoglobulin gene rearrangement identification at any time point before RT will serve as a source of this information for the CLL, because this parameter is usually not changing through the disease course. Alternatively, peripheral blood or bone marrow samples collected at the time of RT can be used to track the clonotype of the CLL, as the small CLL cell infiltration usually persists in these compartments.52 The clonal relationship between CLL and the RT is currently evaluated by clonality assays based on fragment size analysis or by immunoglobulin sequencing according to the ERIC and EuroClonality guidelines.53,54 The finding of the same immunoglobulin rearrangement in both conditions denotes clonally related RT. Recently, surrogate approaches for clonal relationship analysis have been described, including analysis of programmed cell death protein 1 expression in RT tissue demonstrated by immunohistochemistry and a multiomics approach based on methylation and transcriptomic analysis,46,55 but these approaches have not been sufficiently validated for clinical use.
Prognosis
In the last decade, survival of patients with CLL has improved with the development of BTKis and B-cell lymphoma 2 inhibitors (BCL2is) but this progress has not translated to improved survival for RT. In large pooled cohorts, meta-analyses, and population-based studies, the median OS for patients with RT ranges from 8.7 to 12 months.3,4,56,57 Recent retrospective studies, evaluating RT cohorts in which some patients were treated with BTKis or BCL2is, reported median OS ranges from 3.3 to 19.9 months.10,26,58,59 These data highlight that patients with RT continue to have dismal outcomes in the era of targeted therapy. Hence, there is currently no effective treatment for RT. Chemoimmunotherapy regimens (ie, R-CHOP [rituximab, cyclophosphamide, doxorubicin, Oncovin (vincristine), and prednisone]) remain the de facto standard of care despite poor outcomes with its use.
Baseline laboratory testing, prognostication, and staging
Prognostication
Several variables have independent adverse prognostic significance for survival for patients with RT including advanced age, failure to achieve complete response with treatment, Eastern Cooperative Oncology Group performance status of >1, low hemoglobin and platelet count, lactate dehydrogenase of ≥1.5× the upper limit of normal, tumor size of >5 cm, and prior CLL therapy.3,6,27,51,60-63 High total metabolic tumor volume on staging FDG-PET, was also reported as a negative prognostic factor in a single, small series.61 One of the more established factors predicting a poor prognosis of RT is the receipt of CLL-directed therapy, both chemoimmunotherapy and targeted therapies.3,6,10,27,60,63 Additionally, TP53 aberrations, defined as presence of del(17p) or TP53 mutations, in either CLL or RT tissue are associated with significantly shorter survival.3,60,62,64,65 As clonal relationship status in RT tissue is missing from most published RT studies, it remains difficult to determine the independent prognostic value of the historically reported clinical variables without full genomic characterization of RT tissue.
Laboratory testing
Importance of NGS and molecular testing
RT typically retains the genetic alterations observed in the antecedent CLL cells. RT is characterized by genetic complexity, manifesting through aneuploidy, chromothripsis, and events of whole-genome doubling.47,66 Additional genetic abnormalities that disrupt the DNA damage response pathway, cell cycle, and metabolism can also be acquired in RT tissue (including TP53 abnormalities, CDKN2A/B deletions, MGA mutations, and MYC translocations).46-48,63,67 Specific molecular testing, beyond clonal relationship and TP53 mutation, might be considered for mutations in the BTK, PLCG2, and BCL2 genes in the CLL cells of patients with RT after BTKi and BCL2i treatment,68 although no evidence for a clinical effect exists (Table 2).
Role for additional molecular or genetic testing in RT
Although multiple other cytogenetic aberrations, along with CK, occur more frequently with RT, they have unclear prognostic significance once RT develops.47,51,64,69 Thus, there is no established role for the assessment of cytogenetic aberrations, except for 17p deletion and/or TP53 aberrations, at the development of RT in RT tissue (Table 2). Biobanking of samples from RT tissue and the associated CLL cells is highly recommended to help advance our understanding of the pathobiology and prognosis of this disease. Recently, data from detailed molecular studies in RT, including single-cell analysis, provided a deeper insight into the pathogenesis of the disease,46,47 including the detection of RT molecular events many years preceding RT diagnosis.47,66 It is recommended to collect samples of both RT tissue and associated CLL cells, peripheral blood mononuclear cells, and plasma for the potential detection of circulating tumor DNA (ctDNA).
Staging/pretreatment assessment
Clinical trial recommendations
Over the past 20 years, RT has been largely excluded from trials testing novel therapies for both CLL and DLBCL. As the prognosis of RT remains poor, it is essential to conduct prospective clinical trials specifically dedicated to this disease either by academic investigators, the pharmaceutical industry, or cooperative groups. We provide recommendations for trial development in RT in 4 specific areas: (1) eligibility criteria, (2) end points, (3) response criteria, and (4) toxicity assessment.
Eligibility criteria for clinical trials
Every effort should be made to treat patients with RT on clinical trials. New concepts and the concerted effort to accumulate additional evidence hold the potential to improve outcomes. Several unique obstacles exist for patients with RT to enroll in clinical trials (eg, delays in the diagnosis, relative lack of clinical trial sites, and quickly deteriorating physical status because of RT). Thus, flexibility in eligibility criteria is required to enable better trial participation (eg, using pragmatic trial approaches that limit the exclusion criteria, including organ dysfunction, which often complicates RT course, decreasing the burden of screening procedures, and allowing patients with impaired performance status to be enrolled). The expert panel recommends that concurrent CLL that meets indication to treat, or is under ongoing treatment, should not be considered an exclusion criterion for enrollment onto RT trials (Table 3). As in clinical trials for highly aggressive lymphoid malignancies, prephase therapies should be allowed during the screening period to improve patients’ clinical status. Similarly, patients who are benefiting from an ongoing CLL-directed therapy should be permitted to be enrolled and the wash-out period minimized (ie, patients could be screened for the trial while on CLL-directed therapy). These flexible alterations of protocols should be reported within trial findings.
End points of clinical trials
In all trials, to reliably assess the contribution of novel treatments in RT, survival end points such as progression-free survival, time to next treatment, or OS, should be included. In phase 3 trials, and in phase 2 trials when appropriate (eg, randomized phase 2 trials), progression-free survival or OS should be the primary end point used. In early phase and single-arm trials, response rate may be considered as the primary end point. Overall and complete response rates, along with duration of response, should also be collected and correlated with survival outcomes to explore the possibility of surrogate end points for future trials. Data on postprotocol care, including information on autologous and allogeneic stem cell transplantation, should be captured.
Biobanking and exploratory end points
Prospective sample collection, at baseline and at the time of progression, should be mandated in trials to allow exploratory analyses and/or centralized review for clonality assessment. Measurable residual disease and/or the collection of ctDNA should also be used to allow future exploration of these techniques.
Response assessments
Given the short survival of patients with RT, we recommend designing clinical trials that include an early response assessment, typically within 1 to 3 cycles of treatment. We recommend PET-CT for the response assessment, and a PET-CT scan to confirm progression if clinical suspicion arises. Although we recognize a lack of evidence on appropriate interval or utility of PET-CT as surveillance, monitoring with PET-CT at prespecified intervals on trial can be considered. The nature of RT poses challenges when trying to assess response to therapy, in particular how the iwCLL70 and Lugano criteria29 should be used to allow an accurate definition of response, especially considering that these 2 diseases typically co-occur and may exhibit a different response to therapy. We recommend response of RT treated in routine practice and on clinical trials be assessed using the contrast-enhanced PET-CT–based response per Lugano criteria for FDG-avid lymphomas, with the addition of the LYRIC (lymphoma response to immunomodulatory therapy criteria) criteria if immunotherapy is tested.71 Response of the underlying CLL must follow iwCLL criteria,70 including performing a bone marrow biopsy after treatment to determine the extent of CLL disease status for those who had a pretreatment bone marrow involvement by CLL. If there is detectable CLL disease by flow cytometry in the peripheral blood at the end of treatment, a bone marrow biopsy to assess CLL response is not warranted. Further research is needed to determine whether CLL status at completion of RT treatment is predictive of survival and/or RT relapse risk. We recommend that the persistent CLL component should be explicitly reported, based on iwCLL criteria, but should not affect the response definition specific to the RT. Additionally, if it is unclear whether residual disease is CLL vs RT, a biopsy is warranted.
Toxicity assessment
We recommend using the common terminology criteria for adverse events to assess nonhematologic toxicity, and the iwCLL criteria70 for hematologic toxicity. When T-cell–redirecting therapies are used in RT, we recommend the American Society for Transplantation and Cellular Therapy guidelines to grade cytokine release syndrome and immune effector cell–associated neurotoxicity syndrome.72
Sample size planning
To ensure the appropriate number of confirmed patients with clonally related disease, we recommend adjusting the trial sample size according to a preplanned estimation of classification into de novo DLBCL after the analysis of clonal relationship (∼20%).
Discussion: future directions and exploratory end points
Additional understanding of the pathobiology of RT is expected to improve therapeutic options and outcomes. Future efforts aimed at combining multiomics data sets (genomic, epigenomic, and spatial) are thus essential to further stratify disease subtypes, identify subtype-specific molecular vulnerabilities, improve power to identify recurrent RT events, and link tumor-intrinsic properties with microenvironmental characteristics. As such, collection of viably cryopreserved primary RT samples, paired with antecedent CLL, will be fundamental to facilitate experimental applications (including single-cell technologies, proteomics, and drug screening) that are currently limited by scarce material availability or quality, particularly because most RT tissue samples are preserved as paraffin embedded. Recent advances in the manipulation of primary samples and mouse models with methods based on CRISPR-Cas9 have allowed a more faithful recapitulation of the complex disease features characterizing RT in vitro and in vivo,48,73,74 and we envision future studies using these technologies to dissect the mechanistic relevance of recurrent disease drivers in disease pathogenesis. We expect that a more complete characterization of the targetable vulnerabilities in RT will pave the way to tailored therapeutic options for patients.
Another essential biological aspect is the identification of initiating events of RT. Recent studies have shown that although RT is often a late event in CLL, subclones (so-called “RT seeds”) that lead to RT can be detected much earlier, potentially preceding diagnosis66 because RT cells may have a common ancestor with CLL cells that can be detected years before diagnosis.75 However, additional research on larger and longitudinal cohorts of CLL and RT samples is needed to fully understand which subclones expand, which genomic and epigenetic changes are driving such subclones, and which microenvironmental stimuli are required for RT development, to reveal therapeutic vulnerabilities. This approach could identify new biomarkers to predict RT development. If paired with further validation of known molecular features that predict RT, developing trials exploring opportunities for early intervention to stop the evolution toward RT would be reasonable.
Additional research is needed to further unify the workup and pathologic evaluation of RT. Although the determination of clonality is highly valuable, access to testing is variable across institutions. Harmonization of the acceptable methods to prove clonality is needed to ensure valid results. Newer methods such as NGS and approaches guided by artificial intelligence may play a future role in diagnosis of RT and assessment of clonality. Additionally, TP53 aberrations reported different predictive yields on risk of transformation to RT vs risk of death after RT transformation. Therefore, additional research on larger and longitudinal cohorts of CLL and RT samples is needed to fully understand the interplay of TP53 aberrations found in the CLL and RT compartments.
After diagnosis, additional research is needed to ensure uniform and accurate strategies for monitoring. Follow-up of patients with RT can be a challenge because these patients typically have 2 active diseases (CLL and RT). Clinical trials must monitor responses for both DLBCL and CLL, with both iwCLL and Lugano criteria as described, to understand the efficacy of specific therapies and allow the trial data to be generalized to the real-world setting. Novel strategies of disease monitoring, including ctDNA and novel imaging strategies incorporating total metabolic tumor volume could prove helpful with this regard.
Ultimately, progress in the therapy of RT will need clinical trials. Some recent clinical trials, and retrospective studies, reported promising results with the combined use of targeted agents, cellular therapies, and immunotherapies.76-81 Additional work will confirm the relevance of these novel strategies. Certainly, there will not be a 1-size-fits-all approach to this disease. Therefore, research will need to identify strategies for different biological subgroups. When designing future clinical trials, attempts should be made to enroll representative patient populations and reduce screening failures and early withdrawals. Importantly, studies on RT should avoid long screening periods and have broad inclusion criteria, because RT often proceeds fast and requires rapid action. Because of the rarity of RT, it is difficult for single centers to perform designated RT studies in the timeframe necessary to allow progress. Therefore, cooperative clinical trials are the preferred mode of operation. Additionally, separate cohorts of patients with RT could be subspecified and included in studies of other aggressive lymphomas to investigate novel therapies.
Conclusion
Considering recent advances in understanding the biology of RT, meaningful progress by plausible pathology-mechanistic–based therapies now seems possible. Further laboratory investigations using primary longitudinal samples, molecular analyses, and novel in vivo models are needed to develop rational therapeutic strategies. Current clinical research should incorporate CLL therapies for RT, and clinical trials should monitor the response of both the aggressive lymphoma and the CLL. Through collaborative basic, translational, and clinical research, improvement in outcomes for patients with RT may be achieved.
Acknowledgments
A.S.K. is a recipient of the Conquer Cancer, the American Society of Clinical Oncology Foundation, career development award. O.A.-S. receives support from the Deutsche Forschungsgemeinschaft (DFG [German Research Foundation]), project no. 524342988. A.V.D., M.S.D., and J.A.W. are, or have been, Leukemia and Lymphoma Society scholars in clinical research. A.V.D. is supported by National Institutes of Health (NIH), National Cancer Institute (NCI) grant 1R01CA283171. M.S.D. is supported by NIH, NCI grants 1R01CA266298-03 and P01CA206978 (principal investigator: Catherine Wu). B.E. receives support from the DFG (project no. 455784452). M.H. receives support from the DFG (SFB 1530 projects Z01 and B01). A.P.K. is supported by an ERC consolidator grant 864815/BOOTCAMP. E.P. is supported by NIH, NCI grant K08 CA270085. G.G. and P.G. receive support from Associazione Italiana per la Ricerca sul Cancro Foundation Medicine, Italy, under the “5 per Mille 2018” program (identity 21198; principal investigators: Foà Roberto, G. L. Gaidano Gianluca, and Ghia Paolo). G.G. acknowledges support from Piano Nazionale di Ripresa e Resilienza (PNRR)-MAD-2022-12375673 (Next Generation EU, M6/C2_CALL 2022), Italian Ministry of Health, Rome, Italy; and receives support from the PNRR project PNRR-MAD-2022-12376441 sponsored by the Italian Ministry of Health.
Authorship
Contribution: All authors wrote, edited, and revised the manuscript, provided critical intellectual content, participated in the consensus surveys, and approved the final manuscript.
Conflict-of-interest disclosure: A.S.K. has received research funding from AstraZeneca and BeiGene; has performed speaking engagements for AstraZeneca, BeiGene, and Eli Lilly; and has participated in advisory boards for AbbVie, AstraZeneca, BeiGene, Bristol Myers Squibb (BMS), and Galapagos. M.M. has consulted for Gilead, Novartis, Roche, Sanofi, and MSK. O.A.-S. received honoraria and personal fees from AbbVie, Adaptive, Ascentage, AstraZeneca, BeiGene, Eli Lilly, Genmab, Gilead, Janssen, and Roche and has received research funding from AbbVie, BeiGene, Janssen, and Roche. O.B. has consulted for AbbVie, Janssen, AstraZeneca, and Lilly and has participated on advisory boards for AbbVie, Janssen, and Lilly. A.V.D. has received consulting fees from AbbVie, ADC Therapeutics, AstraZeneca, BeiGene, BMS, Genentech, Genmab, Incyte, Janssen, Lilly Oncology, MEI Pharma, Merck, Nurix, Prelude, and Regeneron and has ongoing research funding from AbbVie, AstraZeneca, Bayer Oncology, BMS, Cyclacel, Genmab, Lilly Oncology, MEI Pharma, TG Therapeutics, and Nurix. M.S.D. has received institutional research funding from Ascentage Pharma, MEI Pharma, and Novartis and reports personal fees from AbbVie, Adaptive Biosciences, Ascentage Pharma, AstraZeneca, BeiGene, BMS, Eli Lilly, Genentech, Genmab, Janssen, Merck, MingSight Pharmaceuticals, Nuvalent, Ono Pharmaceutical, Secura Bio, TG Therapeutics, and Takeda. B.E. has consulted for AbbVie, AstraZeneca, BeiGene, Galapagos, Janssen, Loxo/Lilly, and Merck Sharp & Dohme (MSD) and receives research funding from AbbVie, AstraZeneca, BeiGene, Janssen, and Roche. T.A.E. has received honorarium and/or performed advisory work for Roche, Gilead, Kite, Janssen, AbbVie, AstraZeneca, Loxo Oncology, BeiGene, Incyte, Autolus, Galapagos, BMS, Medscape, PeerView, Clinical Care Options, and Limbic; has received research funding from AstraZeneca, BeiGene, and Gilead; and has received travel support from Roche, AstraZeneca, and AbbVie. A.M.F. has received honoraria from Janssen, BeiGene, AbbVie, and AstraZeneca. M.H. has received institutional research funding from AbbVie, AstraZeneca, BeiGene, Eli Lilly, Gilead, Janssen, and Roche. R.J.H. is a shareholder in Telix Pharmaceuticals but has no conflicts with respect to the current manuscript. A.P.K. has received research funding from AbbVie, AstraZeneca, BMS, Janssen, and Roche/Genentech; has served on advisory committees for AstraZeneca, BMS, Roche/Genentech, Janssen, AbbVie, and LAVA; and received speakers fees from AbbVie, AstraZeneca, and Janssen. C.O. has received honoraria from AbbVie, AstraZeneca, Janssen, BeiGene, Roche, Lilly, and Incyte. T.S. receives research funding from BMS; has participated in advisory boards for AstraZeneca, AbbVie, BeiGene, BMS, Genmab, and Gilead/Kite; and has participated in speakers bureau for AstraZeneca. S.S. has received honoraria and travel and research support from AbbVie, Amgen, AstraZeneca, BeiGene, BMS, Galapagos, Gilead, GlaxoSmithKline, Hoffmann-La Roche, Janssen, Lilly, Novartis, and Sunesis. P.A.T. has consulted for and/or received honoraria from AbbVie, Adaptive Biotechnologies, Ascentage, AstraZeneca, BeiGene, Genentech, Genmab, Janssen, Lilly, and Merck; received speaker fees from AbbVie, Adaptive Biotechnologies, AstraZeneca, Janssen, and Merck; and received travel support from Roche, Genmab, and Merck. G.G. has participated in advisory boards for AbbVie, AstraZeneca, BeiGene, Incyte, Johnson and Johnson, and Eli Lilly and has performed speaking engagements for AbbVie, AstraZeneca, BeiGene, Hikma, Incyte, and Johnson and Johnson. J.A.W. has consulted for AbbVie, AstraZeneca, BeiGene, Genentech, Janssen, Loxo/Lilly, Merck, Newave, and Pharmacyclics and receives research funding from AbbVie, BeiGene, Janssen, Schrodinger, and Pharmacyclics. P.G. has consulted for AbbVie, AstraZeneca, BeiGene, BMS, Galapagos, Johnson and Johnson, Lilly/Loxo Oncology, MSD, and Roche and receives research funding from AbbVie, AstraZeneca, BeiGene, BMS, Johnson and Johnson, and MSD. The remaining authors declare no competing financial interests.
Correspondence: Adam S. Kittai, Hematology/Oncology, Icahn School of Medicine at Mount Sinai, 1425 Madison Ave, Lobby Level, Room L1-01H, New York, NY 10029; email: adam.kittai@mssm.edu.
References
Author notes
A.S.K. and M.M. contributed equally to this study.
G.G., J.A.W., and P.G. contributed equally to this study.
Data supporting the findings of this study are available on reasonable request from the corresponding author, Adam S. Kittai (adam.kittai@mssm.edu).
The online version of this article contains a data supplement.
There is a Blood Commentary on this article in this issue.