Abstract
The mechanism of megakaryocytic differentiation was investigated using human megakaryocytic leukemia cell line UT-7. Polyploidization of UT-7 cells was induced by the microtubule-depolymerizing agent, nocodazole, and 12-O-tetradecanoylphorbol-13-acetate (TPA), but the effect was much more striking with nocodazole. By contrast, induction of cytoplasmic maturation, as judged by β-thromboglobulin production and platelet factor 4 expression, was more prominent in TPA-treated cells than in nocodazole-treated cells. Nocodazole and TPA could act synergistically to increase ploidy and to enhance the expression of mature phenotypes. Human thrombopoietin induced functional maturation but not polyploidization in UT-7 cells and also acts synergistically with nocodazole. Cyclin-dependent kinase inhibitor p21 was upregulated at the early stage of megakaryocytic differentiation, and overexpression of p21 resulted in an increase in ploidy of UT-7 cells. This suggests that p21 is implicated in polyploidization via suppression of CDC2 activity at mitosis. UT-7 but not HL-60 cells could incorporate [3H]thymidine in the presence of TPA, indicating the presence of megakaryocyte-specific licensing factor to allow DNA replication during differentiation. Taking these data together, we propose that megakaryocytic differentiation consists of two distinct processes, polyploidization and functional maturation, and that these two processes are independently regulated.
MEGAKARYOCYTOPOIESIS is a complex process that involves proliferation of committed precursor cells and differentiation of their progeny leading to platelet formation. Terminal differentiation of megakaryocytes is characterized by nuclear polyploidization, cell size growth, and generation of specific cytoplasmic proteins such as platelet factor 4 (PF4) and β-thromboglobulin (β-TG).1,2 Polyploidization is a phenomenon unique to megakaryocytes among hematopoietic cells3 and is also observed in other cell types including liver, salivary glands, and urinary bladder epithelium.4 Although it has been known that endomitosis, a process of repeated nuclear replication without concomitant mitotic cell division, is a major cause of polyploidization in these cells, the underlying mechanism is largely unknown.4 Because polyploidization of megakaryocytes is believed to be essential for the efficient production and release of platelets, an attempt to understand the mechanism of this process has both clinical and scientific significance.
In the past, the study of megakaryocytic differentiation was hampered by the rarity of megakaryocytes in normal bone marrow (only 0.03% to 0.06% of the nucleated cells), the poorly defined cell populations, and inadequate assay methods.1,2 However, these methodological pitfalls have been circumvented in part by the establishment of continuous cell lines originating from normal or leukemic marrow that express a range of megakaryocytic phenotypic properties.1 UT-7 is one of these cell lines and was established from bone marrow cells of a patient with acute megakaryoblastic leukemia in 1991.5 This cell line possesses typical features of megakaryocytic progenitors and has been shown to differentiate into cells with more mature megakaryocytic phenotypes in response to phorbol ester.6 Since then, UT-7 has been used to investigate the mechanisms of hematopoietic cell growth and differentiation in many laboratories.7 Furthermore, this cell line was recently found to respond to thrombopoietin and, thus, will be a more useful tool for studying the unresolved problems in megakaryocytic development, including the mechanism of polyploidization and signal transduction of thrombopoietin ( Tpo ).8
p21 is a recently identified universal inhibitor of cyclin-dependent kinases (CDKs), including CDK2 and CDC2.9 p21 is known to be involved in p53-mediated G1 arrest by DNA damage via suppression of CDK2 activity.10 Recent investigations showed that p21 was expressed during hematopoietic and myogenic differentiation, thus potentially contributing to cell cycle exit associated with cell differentiation.11-14 However, the role of p21 in megakaryocyte development is at present unknown.
In this study, using UT-7 cell line as a model system, we investigated the mechanism of late stages of megakaryocytic differentiation including polyploidization and functional maturation. We present here evidence that cytoplasmic maturation and polyploidization are independently regulated during megakaryocytic differentiation and that p21 is involved in polyploidization.
MATERIALS AND METHODS
Reagents.Nocodazole was purchased from Janssen Biotech N.V. (Olen, Belgium), diluted at 50 μg/mL in dimethylsulfoxide (DMSO) and stored at −20°C until use. Butyrolactone I is a specific inhibitor of CDKs that was isolated from mycelia of Aspergillus terreus at Tsukuba Research Institute, Banyu Pharmaceutical Co Ltd (Ibaraki, Japan).15 16 All other chemicals, including 12-O-tetradecanoylphorbol-13-acetate (TPA), were purchased from Sigma Chemical Co (St Louis, MO). Recombinant human Tpo was provided by Kirin Brewery Co Ltd (Gunma, Japan). Recombinant human granulocyte-macrophage colony-stimulating factor (GM-CSF ) was a gift of Genetics Institute (Cambridge, MA).
Cells and cell culture.Human promyelocytic leukemia cell line HL-60 was obtained from American Type Culture Collections (Rockville, MD) and routinely maintained in RPMI 1640 medium with 10% heat-inactivated fetal calf serum (FCS; HyClone Laboratories, Logan, UT). UT-7 cell line was maintained in Iscove's modified Dulbecco's medium (IMDM; Life Technologies, Inc, Grand Island, NY) containing 10% FCS and 1 ng/mL of GM-CSF. For induction of megakaryocytic differentiation, UT-7 cells were seeded at the initial concentration of 3 to 5 × 105 cells/mL and cultured in the presence of various inducers. The percentage of hyperploid cells was determined microscopically by counting more than 200 cells on Wright-Giemsa staining cytospin slides. The cells containing more than two nuclei were defined as hyperploid cells.
Short-term liquid culture of human megakaryocytes was performed as previously described17 18 with minor modifications. CD34+ progenitor cells were isolated from normal bone marrow using a magnetic cell sorting system Mini-MACS (Miltenyl Biotec, Auburn, CA) according to the manufacturer's instruction. CD34+ enriched cells were plated at 1 × 106 cells/mL in megakaryocyte culture medium containing 20% serum derived from platelet-poor plasma of the patients with aplastic marrows in 24-well tissue culture plates (Falcon, Lincoln Park, NJ). They were cultured at 37°C with 5% CO2 for 10 days.
Flow cytometry.DNA histograms were determined by flow cytometry after staining DNA with propidium iodide as previously described.19 A CellFIT program (Becton Dickinson, San Jose, CA) was used for analyzing the results.
Cell proliferation assay.Cell proliferation was monitored by [3H]thymidine incorporation assay.20 The cells were pulse-labeled for a final 1 hour of the culture with 5 μCi/mL of [3H]thymidine (Amersham Life Science, Buckinghamshire, UK). [3H]thymidine uptake was well correlated with the proportion of S-phase cells in this condition.
Elutriation.Separation of the cells in each phase of the cell cycle and hyperploid cells was conducted by counterflow centrifugal elutriation using the SRR6Y elutriation system and a rotor equipped with a 4.5-mL chamber (Hitachi Koki Co Ltd, Tokyo, Japan), as previously described.21 At an initial flow rate of 15 mL/min at 4°C and a rotor speed of 2,000 rpm, UT-7 cells resuspended at 5 to 10 × 107 cells in 50 mL of phosphate-buffered saline (PBS) were injected. The flow rate was incrementally increased, and cell fractions were collected serially as follows: fraction 1 (fr. 1), 200 mL at 15 mL/min; fr. 2, 200 mL at 20 mL/min; fr. 3, 200 mL at 25 mL/min; fr. 4, 200 mL at 30 mL/min; fr. 5, 200 mL at 35 mL/min; fr. 6, 200 mL at 40 mL/min; fr. 7, 200 mL at 45 mL/min; fr. 8, 200 mL at 50 mL/min; and fr. 9, 200 mL at 55 mL/min.
β-Thromboglobulin assay.Harvested cells were washed twice with PBS and resuspended in 400 μL of PBS. Adherent cells were detached by rinse with PBS containing 0.02% EDTA. Cell lysates were obtained by two cycles of sonication (15 seconds at 40 W) and subjected to an enzyme-linked immunosorbent assay (ELISA) for the quantitative measurement of intracellular β-TG.
Semiquantitative reverse transcription-polymerase chain reaction (RT-PCR).Total cellular RNA extracted from a pellet containing 1 × 105 cells was reverse transcribed into cDNA using SuperScript reverse transcriptase and oligo(dT) primers in a 20 μL mixture (Life Technologies Inc). Subsequent PCR amplification was performed with 1 μL cDNA solution in a 50 μL reaction mixture containing 5 U of Taq polymerase, 10 mmol/L Tris-HCl (pH 8.5), 50 mmol/L KCl, 1.5 mmol/L MgCl2 , and 100 μmol/L dNTPs in the presence of specific primer pairs (200 nmol/L each). Each cycle of PCR consisted of 1 minute of denaturation at 94°C, 1 minute of annealing at 60°C, and 2 minutes of extension at 72°C. Control experiments were performed to determine the range of PCR cycles over which amplification efficiency remained constant and to show that the amount of amplified PCR product was directly proportional to the amount of input RNA (data not shown).22 The following oligonucleotides were used as primers (nucleotide positions in the respective sequences are shown in parentheses): p21waf1/cip1 (based on El-Deiry et al10 ): sense primer, 5′-AAGTCAGTTCCTTGTGGAGCCGGA-3′ (−71 ∼ −48), antisense primer, 5′-TTCCAGGACTGCAGGCTTCCTGTG-3′ (502-525); PF4 (based on Poncz et al23 ): sense primer, 5′-AGCATGAGCTCCGCAGCCGGGTTC-3′ (−3 ∼ 21), antisense primer, 5′-GGCAGCTAGTAGCTAACTCTCCAA-3′ (395-318); and glyceraldehyde-3-phosphate dehydrogenase (GAPDH; based on Tso et al24 ): sense primer, 5′-CCACCCATGGCAAATTCCATGGCA-3′ (146-169), antisense primer, 5′-TCTAGACGGCAGGTCAGGTCCACC-3′ (720-743).
Northern blotting.Total cellular RNA was isolated by cesium chloride ultracentrifugation using CS120FX ultracentrifuge and S100AT5 fixed-angle rotor (Hitachi Koki, Co Ltd) at 80,000 rpm for 5 hours at 15°C. Ten micrograms of RNA was electrophoresed in a 1% agarose gel containing 6% formaldehyde, 20 mmol/L 4-morpholinepropane-sulfonic acid, 5 mmol/L sodium acetate, and 1 mmol/L EDTA. To confirm the equal loading of RNA in each lane, ethidium bromide (EtBr) staining of the gels was performed. RNA was then blotted onto Hybond N+ nylon membranes (Amersham Life Science) in 20× SSC (1× SSC = 150 mmol/L NaCl, 15 mmol/L sodium citrate). The membranes were hybridized with each cDNA probe, which was labeled with [32P]dCTP by the oligonucleotide random priming method. p21waf1/cip1 and PF4 cDNA probes were generated by RT-PCR as described above. Densitometric scanning of autoradiograms was performed to quantitate the amounts of each mRNA transcript.
Expression vectors.p21 expression vector was generated by ligation of full-length p21waf1/cip1 cDNA into the EcoRI site of pcDNA3 mammalian expression vector (Invitrogen, San Diego, CA). Orientation of the insert was determined by restriction mapping and sequencing analysis, and sense clone was used in this study. To monitor the transfection efficiencies, pSV-β-galactosidase plasmid (Promega, Madison, WI), which constitutively expresses β-galactosidase under the control of the SV40 early promoter and enhancer, was simultaneously transfected (see below).25 All plasmids were linealized by appropriate restriction enzymes and purified by ethanol precipitation before transfection.
Transient transfection.Electroporation was used for transient transfection of plasmids into UT-7 cells as previously described.26 In brief, 20 μg of either pcDNA3 containing p21 cDNA or empty vector (mock) was introduced with 2 μg of pSV-β-galactosidase plasmid into 1 × 107 UT-7 cells resuspended in 0.25 mL of RPMI 1640 medium containing 10% FCS by electropulse at 250 V, 960 μF. Transfected cells were seeded at 3 to 5 × 105 cells/mL in IMDM medium containing 10% FCS and 1 ng/mL of GM-CSF. The culture was continued for 3 days and then harvested for the morphologic examination. β-Galactosidase activity was determined by in situ staining27 and by standard assay using cell lysates according to the manufacturer's instructions.
RESULTS
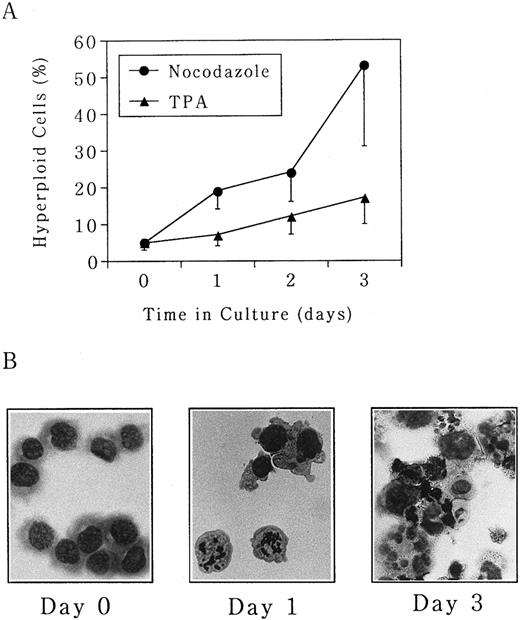
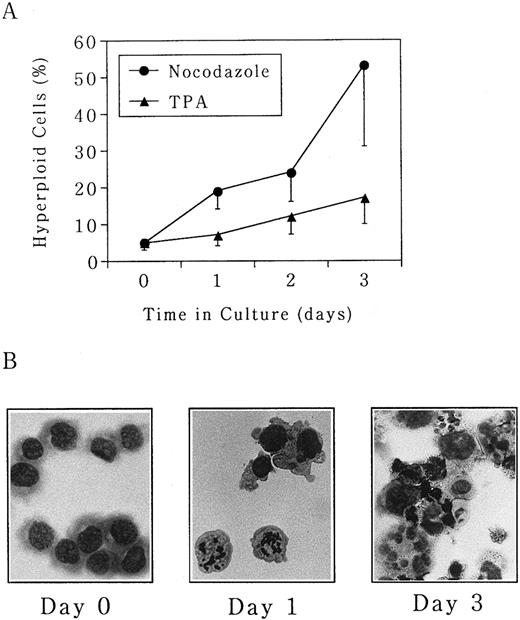
Polyploidization of UT-7 cells is predominantly induced by the microtubule depolymerizing agent nocodazole.In this study, three different types of pharmacologic modulators of the cell cycle (nocodazole, butyrolactone I, and TPA) were used to investigate the mechanism of megakaryocytic differentiation. Nocodazole arrests cells at M phase by inhibition of actin polymerization. Butyrolactone I arrests cells at both G1 and G2 phases by inhibition of CDK2 and CDC2 kinases.15,16 TPA is a well-known inducer of differentiation, thereby arresting cells in G1 phase.26 UT-7 cells were seeded at an initial concentration of 5 × 105 cells/mL in the absence or presence of either nocodazole (50 ng/mL), butyrolactone I (10 ng/mL), or TPA (10 ng/mL), and morphologic changes were observed over a 3-day period. As shown in Fig 1A, the percentage of polyploid cells increased from 5% ± 2% at day 0 to 53% ± 22% at day 3 in the presence of nocodazole. Morphologically, M-phase arrest was seen at day 1, and mature megakaryocyte-like polyploid cells were detected after day 3 (Fig 1B). No increase in the percentage of polyploid cells was observed in untreated UT-7 cells during this culture period (data not shown). TPA also had a similar effect on UT-7 cells but it was much weaker than that of nocodazole, ie, the percentage of polyploid cells was maximal at day 3 with 17% ± 2% (Fig 1A). Polyploidization-inducing effect of butyrolactone I was weaker than that of TPA (data not shown).
Polyploidization was induced by nocodazole and TPA in UT-7 cells. Human megakaryocytic leukemia cell line UT-7 was seeded at 5 × 105 cells/mL and cultured in the absence or presence of either (•) nocodazole (50 ng/mL) or (▴) TPA (10 ng/mL) for 3 days. (A) The cells were harvested at given time points, and the proportion of hyperploid cells (cells containing more than 2 nuclei) was determined by morphologic examination on the cytospin specimens. The mean ± SD (bar) of seven independent experiments is shown. (B) Wright-Giemsa staining of each specimen is shown. (Original magnification × 400).
Polyploidization was induced by nocodazole and TPA in UT-7 cells. Human megakaryocytic leukemia cell line UT-7 was seeded at 5 × 105 cells/mL and cultured in the absence or presence of either (•) nocodazole (50 ng/mL) or (▴) TPA (10 ng/mL) for 3 days. (A) The cells were harvested at given time points, and the proportion of hyperploid cells (cells containing more than 2 nuclei) was determined by morphologic examination on the cytospin specimens. The mean ± SD (bar) of seven independent experiments is shown. (B) Wright-Giemsa staining of each specimen is shown. (Original magnification × 400).
Polyploidization is followed by functional maturation during megakaryocytic differentiation.The expression of differentiation-related phenotypes was then examined in UT-7 cells treated with nocodazole, TPA, and butyrolactone I as shown above. We used β-TG and PF4 as specific markers for megakaryocytic differentiation. Intracellular β-TG was quantitatively measured by ELISA. It was under the detection limits in untreated UT-7 cells by this method. As shown in Fig 2A, β-TG production was significantly induced by nocodazole and TPA after 3 days of the culture. There was no increase in intracellular β-TG in butyrolactone I-treated UT-7 cells (data not shown). In contrast to the weak ability to induce polyploidization, TPA could produce more β-TG than nocodazole did. Expression of PF4 mRNA, another specific marker of mature megakaryocytes, was simultaneously examined by Northern blot analysis in UT-7 cells treated with TPA and nocodazole. PF4 mRNA was undetectable in untreated UT-7 cells (Fig 2B). As shown in Fig 2B, TPA could induce PF4 mRNA expression after 5 days of the treatment. Nocodazole showed a similar but much weaker effect (data not shown). Given that these reagents induced polyploidization after 2 days of the treatment (as shown in Fig 1), functional maturation is likely to occur after polyploidization. Moreover, there was a discrepancy between the inducibility of these two processes depending on the reagents, ie, polyploidization was preferentially induced by nocodazole and cytoplasmic maturation was mainly induced by TPA. These results suggest that polyploidization and functional maturation are separately regulated during megakaryocytic differentiation and that the former is associated with M-phase modulation and the latter is a G1-related event. Failure of butyrolactone I to induce both polyploidization and cytoplasmic maturation indicates that these processes are interrelated. To confirm this hypothesis, we performed sequential treatment of UT-7 cells with nocodazole and TPA.
Functional maturation was induced by nocodazole and TPA in UT-7 cells. (A) UT-7 cells were cultured in the absence or presence of either (•) nocodazole (50 ng/mL) or (▴) TPA (10 ng/mL) for 5 days. Cell lysates were isolated at the given time points and subjected to ELISA for quantitative measurement of intracellular concentration of β-TG. The mean ± SD (bar) of three independent experiments is shown. (B) (Left panel) UT-7 cells were cultured in the presence of TPA for 7 days as described above. Total cellular RNA was isolated at the indicated time points and subjected to Northern blot analysis for PF4 mRNA expression. (Right panel) PF4 mRNA expression was examined in UT-7 cells treated with nocodazole only [3], TPA only [2], and the combination of both [4] (see Fig 3 legend for details of the culture condition). EtBr-staining of the gels is shown as a loading control.
Functional maturation was induced by nocodazole and TPA in UT-7 cells. (A) UT-7 cells were cultured in the absence or presence of either (•) nocodazole (50 ng/mL) or (▴) TPA (10 ng/mL) for 5 days. Cell lysates were isolated at the given time points and subjected to ELISA for quantitative measurement of intracellular concentration of β-TG. The mean ± SD (bar) of three independent experiments is shown. (B) (Left panel) UT-7 cells were cultured in the presence of TPA for 7 days as described above. Total cellular RNA was isolated at the indicated time points and subjected to Northern blot analysis for PF4 mRNA expression. (Right panel) PF4 mRNA expression was examined in UT-7 cells treated with nocodazole only [3], TPA only [2], and the combination of both [4] (see Fig 3 legend for details of the culture condition). EtBr-staining of the gels is shown as a loading control.
Nocodazole and phorbol ester act synergistically on differentiation of megakaryocytes.UT-7 cells were cultured in four different conditions, as shown in Fig 3A. The combination of the reagents was based on the assumption that megakaryocytic differentiation consists of two orderly regulated processes; the first step is polyploidization and the second step is functional maturation. Therefore, UT-7 cells were first treated with either 0.1% DMSO (carrier) or 50 ng/mL of nocodazole (inducer of polyploidization) for 2 days. The culture was then continued for an additional 5 days after replacing the medium with the fresh one containing either 0.1% DMSO (carrier) or 10 ng/mL of TPA (inducer of maturation). At day 7, the cells were harvested for morphologic examination (Fig 3B), β-TG assay (Fig 3C), and Northern blot analysis for PF4 mRNA expression (Fig 2B). As anticipated, the most mature phenotype was obtained when UT-7 cells were first treated with nocodazole and then treated with TPA (the condition [4]).
Nocodazole and phorbol ester act synergistically on maturation of megakaryocytes. (A) Schematic presentation of the culture conditions. The details are as follows. [1] UT-7 cells were cultured for 7 days in the presence of 0.1% DMSO, which was used as a solvent for nocodazole and TPA. [2] The cells were cultured for 2 days with DMSO. TPA was then added at the concentration of 10 ng/mL, and the culture was subsequently continued for 5 days. [3] The cells were treated with 50 ng/mL of nocodazole for 2 days, and the medium was replaced with those containing 0.1% DMSO. The culture was continued for additional 5 days. [4] The cells were treated with 50 ng/mL of nocodazole for 2 days, and the medium was replaced with those containing 10 ng/mL TPA. The culture was continued for an additional 5 days. (B) Wright-Giemsa staining of UT-7 cells was performed after the culture with four different conditions as shown above. No morphologic change was noted in the condition [1] (not shown). (Original magnification × 400). (C) The intracellular concentration of β-TG was determined by ELISA after the culture in each condition. The mean ± SD (bar) of three independent experiments is shown.
Nocodazole and phorbol ester act synergistically on maturation of megakaryocytes. (A) Schematic presentation of the culture conditions. The details are as follows. [1] UT-7 cells were cultured for 7 days in the presence of 0.1% DMSO, which was used as a solvent for nocodazole and TPA. [2] The cells were cultured for 2 days with DMSO. TPA was then added at the concentration of 10 ng/mL, and the culture was subsequently continued for 5 days. [3] The cells were treated with 50 ng/mL of nocodazole for 2 days, and the medium was replaced with those containing 0.1% DMSO. The culture was continued for additional 5 days. [4] The cells were treated with 50 ng/mL of nocodazole for 2 days, and the medium was replaced with those containing 10 ng/mL TPA. The culture was continued for an additional 5 days. (B) Wright-Giemsa staining of UT-7 cells was performed after the culture with four different conditions as shown above. No morphologic change was noted in the condition [1] (not shown). (Original magnification × 400). (C) The intracellular concentration of β-TG was determined by ELISA after the culture in each condition. The mean ± SD (bar) of three independent experiments is shown.
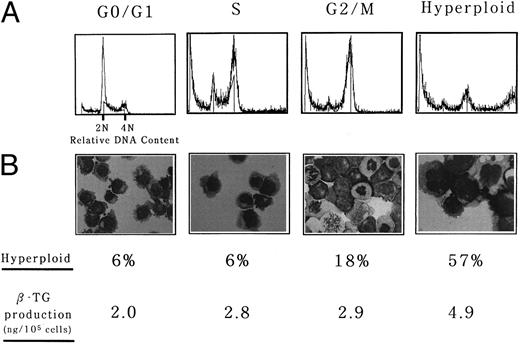
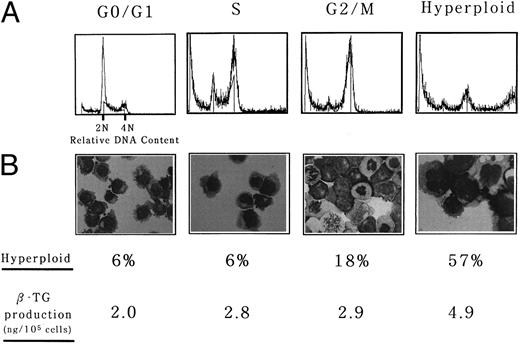
To further confirm the synergistic effect of nocodazole and TPA on megakaryocytic differentiation, hyperploid cells were separated from nonhyperploid cells after nocodazole treatment and the effects of TPA on each fraction were compared. UT-7 cells were treated with nocodazole for 2 days and subjected to counterflow centrifugal elutriation for separation of the fractions enriched for cells in each phase of the cell cycle and hyperploid cells. Figure 4A shows a representative result that indicates effective enrichment of the cells at various phases of the cell cycle. Efficiencies of the enrichment were as follows: more than 90% for G0/G1-phased cells, more than 80% for S-phased cells, approximately 90% for G2/M-phased cells, and more than 60% for polyploid cells. These cells were resuspended in the medium containing TPA and incubated for an additional 5 days. Obviously, the percentage of polyploid cells was highest in hyperploid cell-rich fraction after 5 days of the culture (Fig 4B). Intracellular β-TG content was also highest in the hyperploid cell-enriched fraction (4.9 ± 0.9 ng/105 cells v 2.0 ± 0.5 ng/105 cells for the G0/G1-phased cell-enriched fraction, 2.8 ± 0.8 ng/105 cells for the S-phased cell-enriched fraction, and 2.9 ± 0.6 ng/105 cells for the G2/M-phased cell-enriched fraction). This clearly indicates that hyperploid cells are already on the way of differentiation and can express more mature phenotypes in response to TPA than other nonhyperploid cells.
Hyperploid UT-7 cells could produce more β-TG in response to phorbol ester than nonhyperploid cells. Counterflow centrifugal elutriation was performed as described in the Materials and Methods. (A) Representative DNA histogram of each fraction used in the subsequent experiments. (B) Wright-Giemsa staining of each fraction was performed after the culture with TPA for 5 days. (Original magnification × 400). The proportion of hyperploid cells was determined by counting more than 200 cells on cytospin specimens. Intracellular concentration of β-TG was determined by ELISA. The mean of three independent experiments is shown.
Hyperploid UT-7 cells could produce more β-TG in response to phorbol ester than nonhyperploid cells. Counterflow centrifugal elutriation was performed as described in the Materials and Methods. (A) Representative DNA histogram of each fraction used in the subsequent experiments. (B) Wright-Giemsa staining of each fraction was performed after the culture with TPA for 5 days. (Original magnification × 400). The proportion of hyperploid cells was determined by counting more than 200 cells on cytospin specimens. Intracellular concentration of β-TG was determined by ELISA. The mean of three independent experiments is shown.
Tpo is a factor that acts like TPA to induce functional maturation of megakaryocytes.The results given above indicate that megakaryocytic differentiation consists of polyploidization and functional maturation and that the former is induced by nocodazole and the latter is regulated by TPA in vitro. However, these inducers are artificial and do not exist in vivo. Thus, we sought to determine the factors implicated in these processes in vivo. First, we investigated the role of Tpo, a recently cloned major regulator of thrombopoiesis,28 in each process of megakaryocytic differentiation. UT-7 cells were cultured in three different conditions shown in Fig 5A. [1] The cells were cultured for 2 days with 0.1% DMSO (carrier). Tpo was then added at the concentration of 10 ng/mL, and the culture was subsequently continued for 5 days. [2] The cells were cultured for 2 days with 10 ng/mL of Tpo, and the medium was replaced with those containing only DMSO. The culture was continued for additional 5 days. [3] The cells were treated with 50 ng/mL of nocodazole for 2 days, and the medium was replaced with those containing 10 ng/mL Tpo. The culture was continued for an additional 5 days. Cell ploidy and β-TG production were compared with each condition. As shown in Fig 5B, cell ploidy was not increased by Tpo alone ([1] and [2]), ie, the percentage of polyploid cells was 4% ± 1% in condition [1] and 5% ± 2% in condition [2]. However, in combination with nocodazole, Tpo could greatly increase ploidy of UT-7 cells and reached 29% ± 5%. Moreover, as shown in Fig 5B [3], specific features of mature megakaryocytes, such as emperiporesis (indicated by large arrow) and demarcation membrane-like structure (small arrows), were observed in some cells after the combined treatment. Intracellular content of β-TG was also significantly high in these cells (3.3 ± 0.1 ng/105 cells; Fig 5C [3]). However, Tpo alone could induce β-TG production in a time-dependent manner, ie, intracellular β-TG was 1.1 ± 0.2 ng/105 cells by 2 days of treatment (Fig 5C [2]) and 2.8 ± 0.3 ng/105 cells by 5 days of treatment (Fig 5C [1]). These results clearly indicate that Tpo, like TPA, acts mainly on the late process of megakaryocytic differentiation, namely functional maturation.
Effect of Tpo on megakaryocytic differentiation. (A) UT-7 cells were cultured with either Tpo alone or the combination of nocodazole and Tpo as follows. [1] The cells were cultured for 2 days in the presence of 0.1% DMSO, which was used as a solvent for nocodazole. The medium was then replaced with those containing Tpo at the concentration of 10 ng/mL, and the culture was continued for an additional 5 days. [2] The cells were treated with Tpo for 2 days, and the medium was replaced with those containing 0.1% DMSO. The culture was continued for additional 5 days. [3] The cells were treated with 50 ng/mL of nocodazole for 2 days, and the medium was replaced with those containing Tpo. The culture was continued for an additional 5 days. (B) Wright-Giemsa staining of UT-7 cells was performed after the culture with three different conditions as shown above. (Original magnification × 400). (C) The intracellular concentration of β-TG was determined by ELISA after the culture in each condition. The mean ± SD (bar) of three independent experiments is shown.
Effect of Tpo on megakaryocytic differentiation. (A) UT-7 cells were cultured with either Tpo alone or the combination of nocodazole and Tpo as follows. [1] The cells were cultured for 2 days in the presence of 0.1% DMSO, which was used as a solvent for nocodazole. The medium was then replaced with those containing Tpo at the concentration of 10 ng/mL, and the culture was continued for an additional 5 days. [2] The cells were treated with Tpo for 2 days, and the medium was replaced with those containing 0.1% DMSO. The culture was continued for additional 5 days. [3] The cells were treated with 50 ng/mL of nocodazole for 2 days, and the medium was replaced with those containing Tpo. The culture was continued for an additional 5 days. (B) Wright-Giemsa staining of UT-7 cells was performed after the culture with three different conditions as shown above. (Original magnification × 400). (C) The intracellular concentration of β-TG was determined by ELISA after the culture in each condition. The mean ± SD (bar) of three independent experiments is shown.
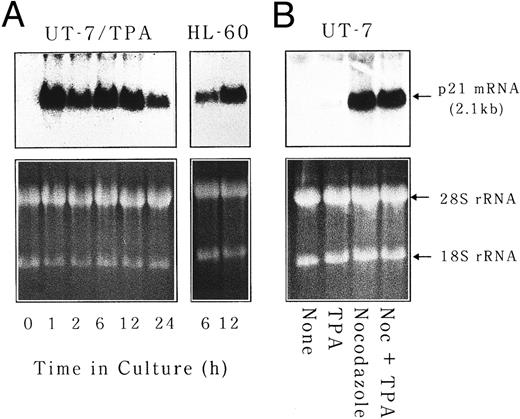
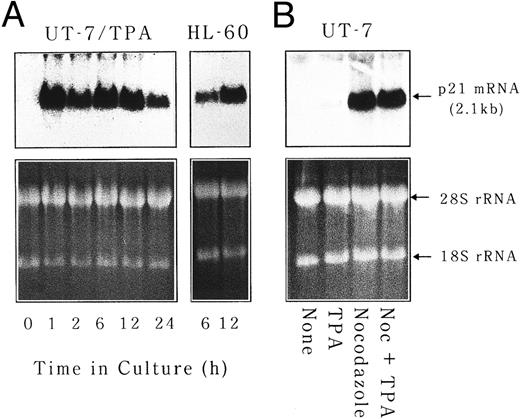
p21waf1/cip1 mRNA is induced at very early stage of megakaryocytic differentiation and is implicated in polyploidization.Because Tpo was not considered to be a major regulator of polyploidization, at least in our experimental condition, we next tried to identify the factor(s) that controls polyploidization in vivo. Taking into account the fact that M-phase skip is required for polyploidization, we tested whether p21 was implicated in this process. p21 is known to inhibit the activity of CDC2 kinase that is essential for G2/M transition as a major component of M-phase promoting factor.29 Total cellular RNA was isolated from UT-7 cells cultured in the absence or presence of either TPA (10 ng/mL), nocodazole (50 ng/mL), or Tpo (10 ng/mL) for 24 hours and subjected to Northern blot analysis for p21waf1/cip1 mRNA expression. As shown in Fig 6A, p21waf1/cip1 mRNA transcript was readily detected after 1 hour of the culture with TPA. The induction of p21waf1/cip1 mRNA was as rapid as that observed in HL-60 cells differentiated into monocytic lineage by phorbol ester (Fig 6A). It was also transient, ie, the amounts of p21waf1/cip1 mRNA decreased after 24 hours and became almost undetectable after 48 hours. Nocodazole could also induce p21waf1/cip1 mRNA expression and the level of induction was higher than that of TPA (Fig 6B). There was no synergistic effect on p21waf1/cip1 mRNA induction between TPA and nocodazole. On the other hand, p21waf1/cip1 mRNA induction by Tpo was negligible (data not shown). This pattern of p21 induction (nocodazole > TPA > Tpo) is well correlated with the ability to induce polyploidization of these reagents, suggesting that p21 may play some roles in polyploidization during megakaryocytic differentiation.
p21waf1/cip1 mRNA is induced at very early stage of megakaryocytic differentiation. (A) UT-7 and HL-60 cells were cultured with 10 ng/mL of TPA for 24 hours. RNA was isolated at the given time points and subjected to Northern blot analysis for p21waf1/cip1 mRNA expression. EtBr-stained 28S and 18S rRNAs are shown as a loading control. (B) UT-7 cells were cultured in the absence (none) or presence of TPA, nocodazole, or a combination of both. p21 mRNA expression was examined after 24 hours of culture.
p21waf1/cip1 mRNA is induced at very early stage of megakaryocytic differentiation. (A) UT-7 and HL-60 cells were cultured with 10 ng/mL of TPA for 24 hours. RNA was isolated at the given time points and subjected to Northern blot analysis for p21waf1/cip1 mRNA expression. EtBr-stained 28S and 18S rRNAs are shown as a loading control. (B) UT-7 cells were cultured in the absence (none) or presence of TPA, nocodazole, or a combination of both. p21 mRNA expression was examined after 24 hours of culture.
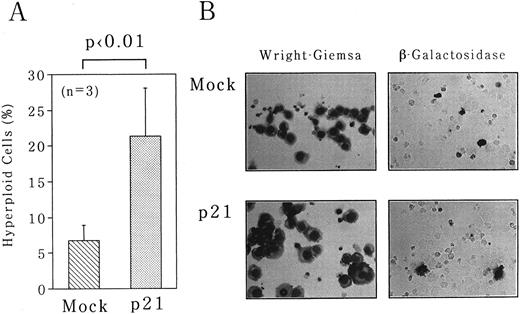
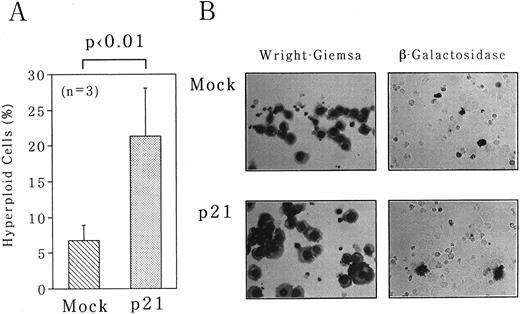
To obtain the convincing evidence that p21 is implicated in polyploidization, we overexpressed p21 in UT-7 cells under the control of cytomegarovirus promoter. Either p21 expression vector or empty vector was introduced into UT-7 cells with pSV-β-galactosidase control vector by electroporation. After 24 hours of transfection, the cells were harvested for morphologic assessment of hyperploid cells and for β-galactosidase assays. There was no significant difference in transfection efficiencies between p21 and mock transfections as monitored by β-galactosidase expression in transfectants (20% ± 8% for p21 and 24% ± 6% for mock). As shown in Fig 7, overexpression of p21 resulted in the significant increase in hyperploid cells (21% ± 7%), whereas no increase was observed in mock-transfected cells (6.7% ± 2%). This increase is truly attributable to p21 overexpression, because in situ detection showed that most hyperploid cells express β-galactosidase activity (Fig 7B).
Overexpression of p21-induced polyploidization of UT-7 cells. pcDNA3 expression plasmid containing full-length p21 cDNA was introduced into UT-7 cells by electroporation. pSV-β-gal vector was simultaneously transfected to monitor the transfection efficiencies. As a control, empty pcDNA3 vector was transfected in the same manner (Mock). The cells were harvested after 3 days of the culture. (A) The percentage of polyploid cells was determined as described in the legend to Fig 1. The mean ± SD (bar) of three independent experiments is shown. (B) Wright-Giemsa staining of Mock- and p21-transfected cells is shown in the left panel. The result of in situ detection of β-galactosidase activity is shown in the right panel. (Original magnification × 400 and × 250, respectively).
Overexpression of p21-induced polyploidization of UT-7 cells. pcDNA3 expression plasmid containing full-length p21 cDNA was introduced into UT-7 cells by electroporation. pSV-β-gal vector was simultaneously transfected to monitor the transfection efficiencies. As a control, empty pcDNA3 vector was transfected in the same manner (Mock). The cells were harvested after 3 days of the culture. (A) The percentage of polyploid cells was determined as described in the legend to Fig 1. The mean ± SD (bar) of three independent experiments is shown. (B) Wright-Giemsa staining of Mock- and p21-transfected cells is shown in the left panel. The result of in situ detection of β-galactosidase activity is shown in the right panel. (Original magnification × 400 and × 250, respectively).
UT-7 cells but not HL-60 cells can undergo DNA replication during differentiation.The results given above suggest that suppression of CDC2 kinase activity by p21 may inhibit normal G2/M transition, thereby allowing cells to skip M-phase and become hyperploid. However, as shown in Fig 6 and in the previous studies,11-14 induction of p21 during differentiation is not specific for megakaryocytic lineage cells. Therefore, it can be speculated that megakaryocytes possess a specific mechanism that permits DNA replication during differentiation. To corroborate this hypothesis, we performed [3H]thymidine incorporation assay in the presence of TPA using UT-7 and HL-60 cells. As shown in Fig 8A, UT-7 could synthesize DNA until day 3 of the culture even in the presence of TPA as in untreated control. It is of note that these periods correspond to the time of polyploidization, as shown in Fig 1. In contrast, HL-60 cells could not in corporate thymidine in the presence of TPA (Fig 8B), which is consistent with the fact that nonmegakaryocytic cells such as HL-60 do not become hyperploid during differentiation. Intriguingly, DNA replication ceased after day 2, with a sharp increase in [3H]thymidine uptake at day 1 in butyrolactone I-treated UT-7 cells (Fig 8A). This might be due to simultaneous inhibition of S-phase promoting CDKs (CDK2 and CDK4) and M-phase promoting factor (CDC2) by butyrolactone I. These results indicate that megakaryocytes have licensing factor to allow DNA replication in the absence of concomitant mitosis (endomitosis) during differentiation. Given that butyrolactone I shortened the period of endomitosis, it is strongly suggested that this licensing factor is closely related to CDKs that regulate G1/S transition such as CDK2 and CDK4.
UT-7 cells but not HL-60 cells could synthesize DNA during TPA-induced differentiation. UT-7 (A) and HL-60 (B) cells were seeded at an initial concentration of 1 × 104 cells/mL and cultured for 4 days in the presence of 0.1% DMSO (as a control [•]), TPA (10 ng/mL [▪]), or butyrolactone I (10 ng/mL [▴]). DNA synthesis was serially determined by [3H]thymidine incorporation assay. The cells were pulse-labeled for the final 1 hour of the culture with 5 μCi/mL of [3H]thymidine.
UT-7 cells but not HL-60 cells could synthesize DNA during TPA-induced differentiation. UT-7 (A) and HL-60 (B) cells were seeded at an initial concentration of 1 × 104 cells/mL and cultured for 4 days in the presence of 0.1% DMSO (as a control [•]), TPA (10 ng/mL [▪]), or butyrolactone I (10 ng/mL [▴]). DNA synthesis was serially determined by [3H]thymidine incorporation assay. The cells were pulse-labeled for the final 1 hour of the culture with 5 μCi/mL of [3H]thymidine.
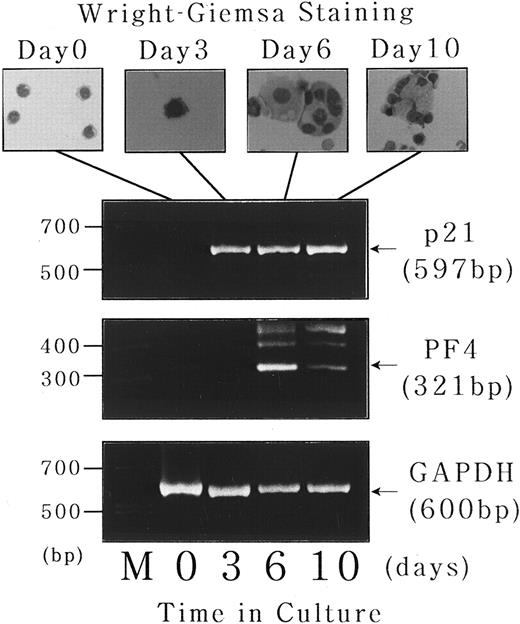
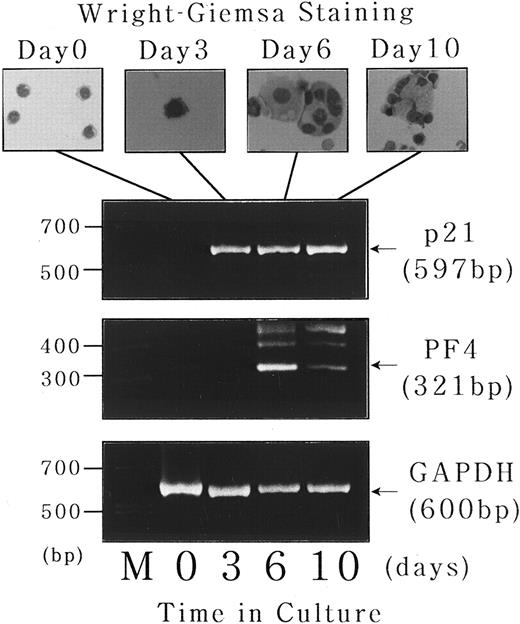
Expression of p21waf1/cip1 and PF4 mRNA during short-term liquid culture of normal human megakaryocytes.Finally, to investigate the physiologic relevance of the findings obtained with UT-7 cell line, we examined the expression of p21waf1/cip1 and PF4 mRNA transcripts during the course of normal human megakaryocyte development in a short-term liquid culture. CD34+ cells were isolated from normal bone marrow using a magnetic cell sorting system and cultured for 10 days in megakaryocyte culture medium as previously described.17 18 As shown in the upper panel of Fig 9, the cells with morphologic features of immature megakaryocytes (basophilic cytoplasm and budding) begun to appear after 3 days of the culture. Mature megakaryocytes with polyploid nuclei were observed after 6 days, followed by cytoplasmic maturation after 10 days. Total cellular RNA was isolated at each time point, and expression of p21waf1/cip1 and PF4 mRNA was examined by semiquantitative RT-PCR analysis. As expected, CD34+ bone marrow cells expressed neither p21 nor PF4 mRNA transcript just after the isolation, whereas GAPDH mRNA, which was used as an internal control, was readily detectable (Fig 9, lower panel, day 0). p21waf1/cip1 mRNA expression was observed after 3 days of the culture, which apparently preceded the induction of polyploidization. On the other hand, PF4 mRNA transcript was not present at day 3 and induced after 6 days of the culture in parallel with the appearance of mature megakaryocytes. This chronologic order of p21 and PF4 expression during normal megakaryocytes development is consistent with the findings obtained with UT-7 differentiation system.
Expression of p21waf1/cip1 and PF4 mRNA during short-term liquid culture of normal human megakaryocytes. Short-term liquid culture of normal human megakaryocytes was performed as described.17 18 The cells were harvested at the indicated time points and subjected to morphologic examination on Wright-Giemsa–staining cytospin slides (upper panel) and semiquantitative RT-PCR analysis for p21, PF4, and GAPDH mRNA expression (lower panel). The numbers of PCR cycles were set in each gene to show that the amount of amplified PCR product was directly proportional to the amount of input RNA (35 cycles for each gene). Amplified products were analyzed on 2% agarose gels followed by ethidium bromide staining. M, molecular size marker (BioMarker Low; Bio Ventures, Inc, Murfreesboro, TN).
Expression of p21waf1/cip1 and PF4 mRNA during short-term liquid culture of normal human megakaryocytes. Short-term liquid culture of normal human megakaryocytes was performed as described.17 18 The cells were harvested at the indicated time points and subjected to morphologic examination on Wright-Giemsa–staining cytospin slides (upper panel) and semiquantitative RT-PCR analysis for p21, PF4, and GAPDH mRNA expression (lower panel). The numbers of PCR cycles were set in each gene to show that the amount of amplified PCR product was directly proportional to the amount of input RNA (35 cycles for each gene). Amplified products were analyzed on 2% agarose gels followed by ethidium bromide staining. M, molecular size marker (BioMarker Low; Bio Ventures, Inc, Murfreesboro, TN).
DISCUSSION
In this study, we have investigated the mechanism of megakaryocytic differentiation using UT-7 cell line that has been proven to be a nice model to study hematopoietic cell growth and differentiation.5-8 UT-7 differentiation is considered to correspond to the late stages of megakaryocytic differentiation, because this cell line already express glycoprotein IIb/IIIa and has some properties of erythroid progenitors.5 Late stages of megakaryocytic differentiation are characterized by polyploidization and cytoplasmic maturation, including the appearance of demarcation membranes and production of specific proteins such as β-TG and PF4.1-3 In this report, we show that treatment with the microtubule depolymerizing agent, nocodazole, preferentially induces polyploidization of UT-7 cells with a relatively small increase in intracellular content of β-TG. This finding is in line with the previous observations in normal megakaryocytes30 and in another megakaryocytic cell line ELF-15331 showing that inhibition of actin polymerization by cytochalasin B or leptomycin B resulted in polyploidization. In contrast, TPA provoked a dramatic increase in intracellular β-TG and PF4 mRNA expression. Induction of polyploidization was also observed in TPA-treated UT-7 cells, but it was less striking than in nocodazole-treated cells. In UT-7 cells, Tpo mainly affected functional maturation and had little ability to promote polyploidization. The significance of this observation is currently unknown, although similar findings have been observed in other human megakaryocytic cell lines such as MEG-01 and CMK.32 These results raise the idea that two distinct processes of the late stages of megakaryocytic differentiation, polyploidization and cytoplasmic maturation, may occur independently, although they must be interrelated. Chronologically, polyploidization (peaking at day 3) is followed by functional maturation (occurring after day 3). This idea is further supported by our finding that nocodazole and TPA acted synergistically on UT-7 cells to enhance both polyploidization and cytoplasmic maturation. The dissociation of these two processes was also observed during ontogenesis33 and in vitro culture of normal megakaryocytes.34 Furthermore, this is consistent with the recent report by Shivdasani et al35 in which NF-E2–deficient mice lack platelet production, but polyploidization of bone marrow megakaryocytes is intact in these mice. Thus, it is likely that some NF-E2–regulated target genes have critical functions to cytoplasmic maturation and platelet formation independently of polyploidization, although functionally relevant NF-E2 binding sites have not been identified in the promoter of any megakaryocyte-specific genes.
Polyploidization is resulted from the disturbances of mitotic division and subsequent entry into DNA reduplication cycles (endomitosis).4 Although endomitosis has long been recognized as a unique feature of megakaryocytes,3 the exact mechanism is still unclear. As for the mechanism of mitotic disturbances, many hypotheses have been proposed based mainly on the morphologic observations. Those hypotheses include fusion of anaphase and telophase chromosomes, refusion of daughter cells during cytokinesis, and blocking in the middle phases of mitosis.4 This last hypothesis was substantiated by the absence of patterns of the late stages of mitosis in normal megakaryocytes.36 However, to date, few data are available regarding the molecules that regulate endomitosis despite extensive investigations. Recently, Grafi and Larkins37 reported that endoreduplication in maize endosperm, although it is not identical to endomitosis, is associated with inhibition of M-phase promoting factor (CDC2/cyclin B complex) and induction of E2F-related S-phase kinase activity. In some systems, loss of CDC2 kinase activity due to a lack of cyclin B protein was shown to be sufficient to drive endomitosis. For example, most postmitotic cells in Drosophila eventually enter endoreduplication cycles in which they progress through several rounds of S phase without intervening mitoses. The absence of cyclin B expression has been described in these cells.38 Moreover, Zhang et al39 also reported that endomitosis was closely associated with reduced levels of cyclin B protein and loss of CDC2 activity in murine megakaryocytic cell line MegT derived from SV40 large T-antigen–transgenic mice. These findings seem to establish that inhibition of CDC2/cyclin B activity plays a causative role in endomitotic cell cycle. This is compatible with the previous observation that induction of polyploidization by protein kinase inhibitor K-252a was closely related to inhibition of CDC2 kinase activation during G2/M transition.40 In this regard, our present data on p21 are very interesting.
p21 was originally identified in a quaternary complex that contains CDK, cyclin, and the proliferating cell nuclear antigen.41 Subsequently, molecular cloning of cDNA encoding p21 was achieved by various approaches in different laboratories.10,42,43 Reconstitution of the active complex in vitro and its enforced expression in mammalian cells showed that p21 could act as a universal inhibitor of CDKs that is capable of inducing cell cycle arrest.44 It is well known that p21 is inducible by DNA damage in a p53-dependent manner and arrests the cells in G1 through inactivation of CDK2/cyclin E complex during DNA repair.10 On the other hand, p21 has an affinity to CDC2/cyclin B complex (M-phase promoting factor), but the biologic significance of this complex formation remains to be elucidated.44,45 Recent investigations showed that p21 is expressed during embryogenesis primarily in a subset of cells that are postmitotic, thus potentially contributing to cell cycle exit during differentiation.46 Its induction has also been observed in cells undergoing differentiation11-14 or cellular senescence43 in vitro. In our study, p21 mRNA was induced immediately (1 hour) after the differentiation induction of UT-7 cells, suggesting a role in the early part of megakaryocytic differentiation, namely polyploidization. We have also observed that p21 mRNA expression was induced before polyploidization during the course of normal human megakaryocytes development in a short-term liquid culture. Furthermore, overexpression of p21 could promote polyploidization of UT-7 cells, suggesting that p21 is indeed implicated in this process. Although we did not show any direct evidence, the underlying mechanism of p21-induced polyploidization might involve suppression of CDC2 activity. Recently, Cross et al47 reported that p53 participated in a mitotic checkpoint to ensure the maintenance of cell diploidy. Fibroblasts from p53-deficient mice underwent a multiple round of DNA synthesis without completing chromosome segregation in the presence of spindle inhibitors, thus forming polyploid cells.47 A similar finding was reported in colon cancer cells with p53 abnormalities.48 Given that parts of p53 functions are mediated through p21,10 it is possible that p21 has some roles in mitotic checkpoint, especially via its interaction with CDC2 kinase.
Because our conclusion is mainly based on in vitro experiments using an immortalized cell line, its physiologic relevance to normal hematopoiesis is unknown. However, the involvement of p21 in polyploidization in vivo was recently demonstrated by Wu et al49 using a targeted expression technique. They observed the appearance of large polyploid nuclei in hepatocytes of the transgenic mice that abundantly express p21 specifically in the liver. Because the hepatocyte is, like the megakaryocyte, one of the cell types that commonly become hyperploid, it is highly possible that p21 is also implicated in polyploidization of megakaryocytes in vivo. To confirm this hypothesis, we are currently generating the transgenic mice that selectively overexpress p21 in megakaryocytes under the control of PF4 promoter.
Finally, we have presented the evidence suggesting the presence of licensing factor that allows DNA synthesis during differentiation in UT-7 cells, ie, UT-7 cells could synthesize DNA in the presence of TPA. Other nonmegakaryocytic cell lines did not possess such an activity. The presence of this factor is required for effective endomitotic cycles. Although we did not know much about the licensing factor in megakaryocytes, it must be related to G1-cyclins, because butyrolactone I, a potent inhibitor of G1-cyclin–dependent kinases, failed to induce polyploidization in UT-7 cells. Indeed, E2F-related S-phase kinase has been proposed as licensing factor in maize endosperm.37 Similarly, periodic activation of cyclin E-dependent CDK was reported in endoreduplication cycles of Drosophila embryogenesis.50 Therefore, cyclin E- and/or cyclin D-dependent CDKs may be candidates for licensing factors to promote polyploidization in human megakaryocytes. In this context, it is intriguing that cyclin D1 mRNA was upregulated during TPA-induced differentiation of Dami megakaryocytic cell line.51 However, overexpression of cyclin D1 failed to increase ploidy in this cell line.51 Characterization of licensing factor in megakaryocytes is currently underway in our laboratory.
Supported in part by a grant-in-Aid from the Ministry of Education, Science and Culture of Japan and by a grant from the Ichiro Kanehara Foundation (Y.F.).
Address reprint requests to Yusuke Furukawa, MD, Division of Hemopoiesis, Institute of Hematology, Jichi Medical School, 3311-1 Yakushiji, Minamikawachi-machi, Kawachi-gun, Tochigi 329-04, Japan.

![Fig. 2. Functional maturation was induced by nocodazole and TPA in UT-7 cells. (A) UT-7 cells were cultured in the absence or presence of either (•) nocodazole (50 ng/mL) or (▴) TPA (10 ng/mL) for 5 days. Cell lysates were isolated at the given time points and subjected to ELISA for quantitative measurement of intracellular concentration of β-TG. The mean ± SD (bar) of three independent experiments is shown. (B) (Left panel) UT-7 cells were cultured in the presence of TPA for 7 days as described above. Total cellular RNA was isolated at the indicated time points and subjected to Northern blot analysis for PF4 mRNA expression. (Right panel) PF4 mRNA expression was examined in UT-7 cells treated with nocodazole only [3], TPA only [2], and the combination of both [4] (see Fig 3 legend for details of the culture condition). EtBr-staining of the gels is shown as a loading control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.3980/4/m_bl_0005f2.jpeg?Expires=1768048683&Signature=j5TLiZ009NmK9tLlfxmuhQZk5--AwubFil4ZfAnwwV5cs9u~s-6OjLnFa1wlXd5kTLFVAHqAZqRKgm0b7XR7bCG0q~JzFnmcF-neFnOSnsBjaroixixfSnSngCqzRrnLhR6sC155xcMVbEvibOyW3js1MbRh93Nqriyq3qnQ-ciDD-dqldte7hGLpOwqUWFYzTUZ29oc7OqNUzx2jl6ImSf2gdhrnXakxs8LBSgxYJZl2iLQbBOvVTTfkvxXlg26t1gnYNvGoEQoRTRcIbDtK0b7E6MNoXGa1WQ4PwRS6T63brf8VcvODxxCckNuJqzrAfz~wBOVbEPwRIWytdzGfw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Nocodazole and phorbol ester act synergistically on maturation of megakaryocytes. (A) Schematic presentation of the culture conditions. The details are as follows. [1] UT-7 cells were cultured for 7 days in the presence of 0.1% DMSO, which was used as a solvent for nocodazole and TPA. [2] The cells were cultured for 2 days with DMSO. TPA was then added at the concentration of 10 ng/mL, and the culture was subsequently continued for 5 days. [3] The cells were treated with 50 ng/mL of nocodazole for 2 days, and the medium was replaced with those containing 0.1% DMSO. The culture was continued for additional 5 days. [4] The cells were treated with 50 ng/mL of nocodazole for 2 days, and the medium was replaced with those containing 10 ng/mL TPA. The culture was continued for an additional 5 days. (B) Wright-Giemsa staining of UT-7 cells was performed after the culture with four different conditions as shown above. No morphologic change was noted in the condition [1] (not shown). (Original magnification × 400). (C) The intracellular concentration of β-TG was determined by ELISA after the culture in each condition. The mean ± SD (bar) of three independent experiments is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.3980/4/m_bl_0005f3.jpeg?Expires=1768048683&Signature=dsyQbalu9qeFAjJoZTn~GLmCKNkbEpDUiPPpPVJaQIXFD3MtLRbW4nfZvGabrIw4Rf6XJnOfRWIrKNk5NLUcdyLvMYejGq2hgWo-p-SvlVAbzh4SApIw9KJk1AQbvTuJ1UTYE8-TxJJzO7fk~57Qf8TXz4~mt9mKtgWbZyIviQcI1W4omxB2dkv7gWQfQg1GmX0mO~Q8ASTJdPjxVwV59sUSUw1L5KMpYxDqmq5cGKS~AeXckqocrUXoUTUId0brCI2XLhz8uqN8QAY7p7GFwZ7ldvmV47ajG93Efx8Ydab0qFrxUsiTdbnET2qv7hj~oiO7z-DOOtFbsFKlJPY2gA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Effect of Tpo on megakaryocytic differentiation. (A) UT-7 cells were cultured with either Tpo alone or the combination of nocodazole and Tpo as follows. [1] The cells were cultured for 2 days in the presence of 0.1% DMSO, which was used as a solvent for nocodazole. The medium was then replaced with those containing Tpo at the concentration of 10 ng/mL, and the culture was continued for an additional 5 days. [2] The cells were treated with Tpo for 2 days, and the medium was replaced with those containing 0.1% DMSO. The culture was continued for additional 5 days. [3] The cells were treated with 50 ng/mL of nocodazole for 2 days, and the medium was replaced with those containing Tpo. The culture was continued for an additional 5 days. (B) Wright-Giemsa staining of UT-7 cells was performed after the culture with three different conditions as shown above. (Original magnification × 400). (C) The intracellular concentration of β-TG was determined by ELISA after the culture in each condition. The mean ± SD (bar) of three independent experiments is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.3980/4/m_bl_0005f5.jpeg?Expires=1768048683&Signature=kDu1YS2VNG8yb9MT0GAshYrDDpxY5rGM8GGDwMNkNEkOB9wvp7o1a1HKIvR4EV7rakObbpaoVyihYDDDIR273KibrOoa4zdf2KQLTwSJ5BsQtqRYaiqHXUC8rkUhlzO9qeK7xT0tXXvF7Bj79SCVntPQjA~Rx8fqrtUC6cQFMKR4EAwBUMoakCOXP3SZ4jjjts5KwwPmCIsgEMW8tfBl7GSKQ4RjjZ7mOHhu4RV4XGRYHcWzN-~O2bzEZv-Oz--12VJ4NpfpQwGsDeeKRp8s4R56TyN7YQJMx6WL~e-Axu30Prvhl42eWe2CliMaSu2uYGsev9rEAkV2trEV5DsB7g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. UT-7 cells but not HL-60 cells could synthesize DNA during TPA-induced differentiation. UT-7 (A) and HL-60 (B) cells were seeded at an initial concentration of 1 × 104 cells/mL and cultured for 4 days in the presence of 0.1% DMSO (as a control [•]), TPA (10 ng/mL [▪]), or butyrolactone I (10 ng/mL [▴]). DNA synthesis was serially determined by [3H]thymidine incorporation assay. The cells were pulse-labeled for the final 1 hour of the culture with 5 μCi/mL of [3H]thymidine.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.3980/4/m_bl_0005f8.jpeg?Expires=1768048683&Signature=mBnZiQ48zyZZgmMJ5LpWoA4CTIy~FN4hae7e6EPyk4NpSOkX7cYBfvcSMYcysZQth5F1FJLc9xWYAUMwGOoEz6NQJ-ea69DBC6A6dROdmBTwXBpJ5q5BpyncMMOfSOH1TWHmsR7JVY-BpAyR90p49-9Yr9j3o2rk3WYVIdkpYAoD4LO6d59Ol~8O6u3jtK613vvb5jCcHiyQ226RbcIiSGSH145~omyJX6RRsdK9oC8VWh9iKu-HrQnlbaBh9O0SAz2YakJib8ZDSLsMfGws8CpgQZZgw0yFH9HpnfDwQ0QbxnYDSKogxIROZgj4RxvAlt2ylFX5RnHKxkQlBgB49w__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 2. Functional maturation was induced by nocodazole and TPA in UT-7 cells. (A) UT-7 cells were cultured in the absence or presence of either (•) nocodazole (50 ng/mL) or (▴) TPA (10 ng/mL) for 5 days. Cell lysates were isolated at the given time points and subjected to ELISA for quantitative measurement of intracellular concentration of β-TG. The mean ± SD (bar) of three independent experiments is shown. (B) (Left panel) UT-7 cells were cultured in the presence of TPA for 7 days as described above. Total cellular RNA was isolated at the indicated time points and subjected to Northern blot analysis for PF4 mRNA expression. (Right panel) PF4 mRNA expression was examined in UT-7 cells treated with nocodazole only [3], TPA only [2], and the combination of both [4] (see Fig 3 legend for details of the culture condition). EtBr-staining of the gels is shown as a loading control.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.3980/4/m_bl_0005f2.jpeg?Expires=1768355986&Signature=0I56AArARPVTFIDKfJPNnE7iHJUl6OC8lJFbdlL3UfnAH1b3J2nu4ETqakBatMAZDA~msHuzYxMK-6i17ObcTrpezTPc3hgUbNf4PrIV8-nBHnK96ORjqSuTfhTQKCtHWGquDXU4JRh9UAH-L7FG~p-zXYYttPpMCqasc7wSaGn~ZWCgFjkfaa6~thGHhDk50n1JrYyBka-CeSshBHswVdnnbjRQPm35oSadrFivL6gke5T1HAVPIwFZKeFqcsDF9ddI0L~--V7RYA77SOvthGjYE52sIsjnsE8ZXNWf66ec7LR8BRx1tfZNHVM30xo~4QrPGQ1EwsLBmp4~k5OYpg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 3. Nocodazole and phorbol ester act synergistically on maturation of megakaryocytes. (A) Schematic presentation of the culture conditions. The details are as follows. [1] UT-7 cells were cultured for 7 days in the presence of 0.1% DMSO, which was used as a solvent for nocodazole and TPA. [2] The cells were cultured for 2 days with DMSO. TPA was then added at the concentration of 10 ng/mL, and the culture was subsequently continued for 5 days. [3] The cells were treated with 50 ng/mL of nocodazole for 2 days, and the medium was replaced with those containing 0.1% DMSO. The culture was continued for additional 5 days. [4] The cells were treated with 50 ng/mL of nocodazole for 2 days, and the medium was replaced with those containing 10 ng/mL TPA. The culture was continued for an additional 5 days. (B) Wright-Giemsa staining of UT-7 cells was performed after the culture with four different conditions as shown above. No morphologic change was noted in the condition [1] (not shown). (Original magnification × 400). (C) The intracellular concentration of β-TG was determined by ELISA after the culture in each condition. The mean ± SD (bar) of three independent experiments is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.3980/4/m_bl_0005f3.jpeg?Expires=1768355986&Signature=WV1xb04s5fJBy6cTFb~Sxt9reK62UIn1rDknSUoDCBh26ePrbw5YmiB9lC2-BRJBMWPCVMtAJLzmb0l8SIFi2MIpBFyTqMJNLyLPml9sKMDG3f1v-BCwNbq8re2UO62ZVhFxbracEsfWrzqPlfkKC2obmCU~jSKJT1sYIrRyT1fv8ITN2fInWtmhCp9cbeFgAnE8Q5MCqUD9Ps3Wo7VlmrvYxfogYqGMKwTpxdExHv8CWVszpf5SHtsYWVRE05b6x4LglxXsNTW~wM5RwjfdLvSQgDMZXUzo2iHhkTSP0WA~ZqL5NwGCfqIYkIA-YSTEyjylEZH3UHlU8K4x8llGwg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 5. Effect of Tpo on megakaryocytic differentiation. (A) UT-7 cells were cultured with either Tpo alone or the combination of nocodazole and Tpo as follows. [1] The cells were cultured for 2 days in the presence of 0.1% DMSO, which was used as a solvent for nocodazole. The medium was then replaced with those containing Tpo at the concentration of 10 ng/mL, and the culture was continued for an additional 5 days. [2] The cells were treated with Tpo for 2 days, and the medium was replaced with those containing 0.1% DMSO. The culture was continued for additional 5 days. [3] The cells were treated with 50 ng/mL of nocodazole for 2 days, and the medium was replaced with those containing Tpo. The culture was continued for an additional 5 days. (B) Wright-Giemsa staining of UT-7 cells was performed after the culture with three different conditions as shown above. (Original magnification × 400). (C) The intracellular concentration of β-TG was determined by ELISA after the culture in each condition. The mean ± SD (bar) of three independent experiments is shown.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.3980/4/m_bl_0005f5.jpeg?Expires=1768355986&Signature=L3YPajIAW0fTMLND6CitJXogsJAhzgW-Tjet5GYjBDZq4Tatqjff4QGjfv0MVH8lqM3-P96ZkHZ3NsEy36X2Il-yxlEl9hK-y04XSSi4Sz7Vpj~ns8iN60gzCRTOKb-TRxiaNuUrVSarMVMRlv2CFjoIM-HyMQnvSt7rqg2lsalhGLluHr-6xVQCZh4zymyxbvlv41VmZsaL8QyoFwN8GoyzMcHznU6vNzqplsqcuoOlr9Y4uhep83Y5nQKAELSOEbTkGkT2m3iaDg-gnW4eFod6QRiE4JCnYFFz~178YigsNLJIfsdD0puDASCOYYUshwN8fF7ytpR9Ak1PA2qf0g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 8. UT-7 cells but not HL-60 cells could synthesize DNA during TPA-induced differentiation. UT-7 (A) and HL-60 (B) cells were seeded at an initial concentration of 1 × 104 cells/mL and cultured for 4 days in the presence of 0.1% DMSO (as a control [•]), TPA (10 ng/mL [▪]), or butyrolactone I (10 ng/mL [▴]). DNA synthesis was serially determined by [3H]thymidine incorporation assay. The cells were pulse-labeled for the final 1 hour of the culture with 5 μCi/mL of [3H]thymidine.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/11/10.1182_blood.v89.11.3980/4/m_bl_0005f8.jpeg?Expires=1768355986&Signature=zoqnn5l7jHSde0ds-wUB~RaIFTu~Lgez8pRY0jW7ywnozKhHy5~p-2GXfGaOIQmZCsY~mhWD44de0I7WeZgK89-xKQekvAEo~q2EGRTMIaaPubv5y57OveGaNIKWl2fyn4sZuf7kHRxHCRBPr8FJC7SoHlIeDkR5uU0PcgsBMrla-GrjoOFjqenw1~xR4pJ8QjoVbbKWx7F9TYNB7fRHzXwBYd9CFsAgjA6rPSHjSZeyNe3JThk43gP2c4HWXxWzlqu1-4ZOTeyZtEXkADgvDmKLEeNVV3lwL~PLtzD4RuniwGHJIJnm6R1zGLerAED0OYdmKet5f90CmIBJGxwZrA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)