Abstract
B-cell chronic lymphocytic leukemia (B-CLL) cells accumulate in vivo in the G0/G1 phase of the cell cycle, suggesting that their malignant expansion is due, at least in part, to a delay in cell death. However, the cellular or molecular factors responsible for a delay in B-CLL cell death are unknown. B-CLL cells do express receptors for interferon-α (IFN-α) and IFN-γ, and activation of both has been shown to promote B-CLL survival in vitro by preventing apoptosis. The interleukin-10 (IL-10) receptor is another member of the IFN receptor family, but its ligand, IL-10, has been reported to induce apoptosis in B-CLL cells. In the current study, we undertook a biochemical analysis of IL-10 receptor expression on freshly isolated B-CLL cells and characterized the functional responsiveness of IL-10 binding to its constitutively expressed receptor. We show that B-CLL cells bind IL-10 with significant specificity and express between 47 and 127 IL-10 receptor sites per cell, with a dissociation constant in the range of 168 to 426 × 10−12 mol/L. Ligand binding and activation of the IL-10 receptor expressed on B-CLL cells results in the phosphorylation of signal transducer and activator of transcription 1 (STAT1) and STAT3 proteins. This pattern of STAT protein phosphorylation is identical to IL-10 receptor activation on normal cells and similar to IFN-α (STAT1 and STAT3) and IFN-γ (STAT1) receptor activation in CLL. Further, in consecutive samples of fresh blood obtained from patients with B-CLL cells, the addition of IL-10 inhibited B-CLL proliferation, enhanced B-CLL differentiation, but did not induce apoptosis. Indeed, IL-10, like IFN-γ, was able to significantly reduce the amount of B-CLL cell death caused by hydrocortisone-induced apoptosis. We conclude that cytokines, which signal through the interferon family of receptors, have comparable functional effects on B-CLL cells.
NORMAL TISSUE homeostasis requires constant regulation of cell growth and cell death.1,2 In B-cell chronic lymphocytic leukemia (B-CLL), the malignant cells are arrested in the G0 /G1 phase of the cell cycle. Accumulation of immunologically incompetent B-CLL cells in vivo is therefore thought to result more from a delay in cell death rather than an increase in cell growth.3,4 The cause of this delay in cell death is not known, but may involve an imbalance in the expression of gene products that control programmed cell death.5,6 However, specific serologic factors or consistent genetic defects responsible for such imbalances in gene expression in vivo have not been identified in B-CLL. In vitro culture of B-CLL cells in the presence of human serum leads to a gradual decrease in bcl-2 expression and induction of programmed cell death.7,8 This process can be delayed in the presence of certain cytokines such as interleukin-4 (IL-4), IL-13, interferon-α (IFN-α), and IFN-γ.9-12
IL-10 is a Th2-type cytokine, initially identified by its ability to suppress macrophage cytokine production.13,14 IL-10 prevents programmed cell death of both normal human germinal center B cells and Epstein-Barr virus (EBV)-infected B cells.15,16 However, IL-10 has also been reported to induce apoptosis in B-CLL cells.17 Data on the production of IL-10 during progression of B-CLL have been equivocal.18 19 To further analyze this apparent difference in the function of IL-10 on B-CLL cells compared with other B cells, we undertook an extensive characterization of IL-10 receptor expression on freshly isolated B-CLL cells and studied the functional consequences of IL-10 binding to its receptor on this malignant B-cell population.
MATERIALS AND METHODS
Patients.Fresh blood samples were obtained from patients previously diagnosed with CD19/CD5 positive B-CLL,20 who had been off all treatment, including corticosteroids, for at least 30 days. The percent of CD19/CD5 positive cells in the mononuclear fraction was always ≥85%. All patients signed informed consent to participate in this study that was approved by the Institutional Review Board of Roswell Park Cancer Institute.
Binding of radiolabeled IL-10 to B-CLL cells.Mononuclear cells (MNCs) were isolated from fresh blood by Ficoll separation (Histopaque; Sigma, St Louis, MO), washed, and resuspended in RPMI 1640 containing 10% fetal calf serum (FCS) (Sigma), and shipped overnight on wet ice to Schering-Plough Research Institute (Kenilworth, NJ). Patient samples used for binding studies had a median CD19/CD5 expression of 97% by flow cytometric analysis. The viability following overnight shipment was greater than 85% by trypan blue exclusion. The cells (5 × 106) were tested for specific binding using 125I-labeled recombinant human IL-10 (125I-IL–10; DuPont-NEN, Boston, MA) as previously described.21 In saturation binding experiments, twofold serial dilutions (from 0.1 to 2.5 nmol/L) of 125I-IL–10 were used. Nonspecific binding was determined in a parallel series of experiments using identical dilutions of 125I-IL–10 in the presence of 1,000-fold molar excess of unlabeled IL-10 (Schering-Plough Research Institute). The dissociation constant (kd) and maximal concentration of ligand bound to cells (Bmax ) was calculated by Scatchard analysis, using linear regression analysis with the EBDA program (Elsevier-Biosoft, Cambridge, UK). The results of Scatchard analysis were replotted using the Cricket Graph program (Computer Associates International, San Diego, CA).
Electrophoretic mobility shift assay (EMSA) and supershift assay.Frozen patient MNC samples were thawed, ficolled, and washed to eliminate nonviable cells. Following overnight adherence, the viability was greater than 95% in all experiments, except one where it was 75%. Between 91% and 99% of cells had coexpression of CD19 and CD5 on flow cytometric analysis. Ten million cells were stimulated with IL-10 (100 ng/mL) for 15 minutes. EMSA with whole cell extracts was performed as previously described using end-labeled oligonucleotide substrates containing the sequence of the sis-inducible element (SIE) (upper strand, GATCCATTTCCCGTA-AATCA).22 In one experiment, the EMSA was performed in parallel on freshly isolated and frozen cells from the same patient with identical results. For the supershift studies, specific antibodies reactive against STAT1 (Transduction Laboratories, Lexington, KY) or STAT3 (Santa Cruz Biotechnology Inc, Santa Cruz, CA) were included in the reaction mixture as previously described.23
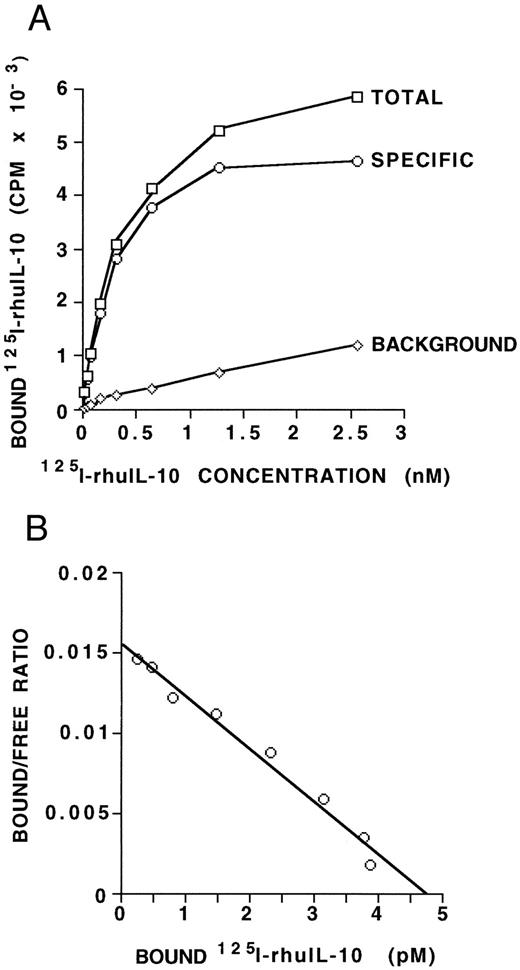
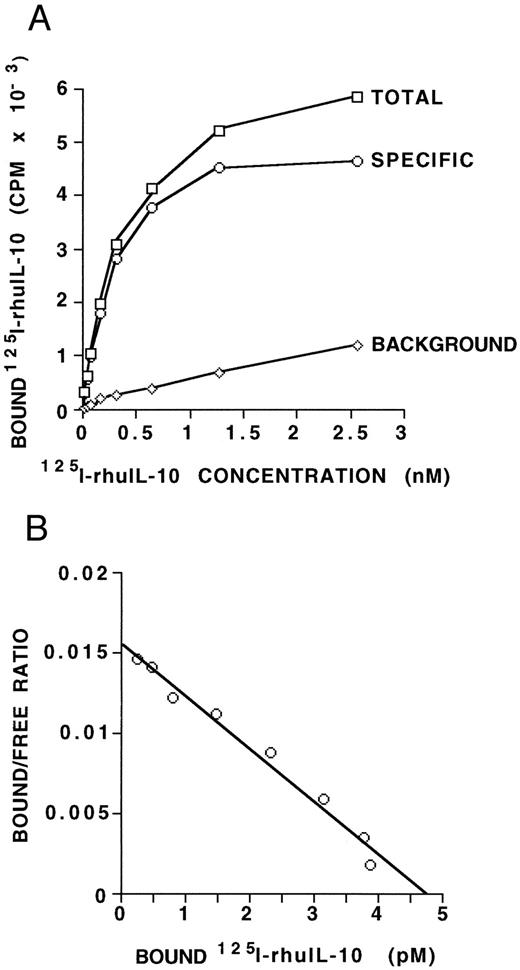
IL-10 receptor expression on B-CLL cells. (A) MNCs (5 × 106) from a B-CLL patient, containing 97% CD19/CD5 positive B-CLL cells, were incubated with increasing amounts of radiolabeled IL-10 with or without a 1,000-fold excess of unlabeled IL-10. Points shown are averages of duplicates, with a standard deviation of less than 15%. The specific binding was determined as the difference between the total CPM and the CPM in the presence of 1,000-fold excess unlabeled IL-10. Maximal binding occurs at 1.5 to 2.5 nmol/L. (B) Scatchard analysis of equilibrium binding data from the same patient sample as shown in (A). The kd derived is 306 pmol/L and the Bmax is 4.8 pmol/L, giving an estimate of 116 IL-10 receptors per cell. These data are representative of three such experiments from three different B-CLL patients.
IL-10 receptor expression on B-CLL cells. (A) MNCs (5 × 106) from a B-CLL patient, containing 97% CD19/CD5 positive B-CLL cells, were incubated with increasing amounts of radiolabeled IL-10 with or without a 1,000-fold excess of unlabeled IL-10. Points shown are averages of duplicates, with a standard deviation of less than 15%. The specific binding was determined as the difference between the total CPM and the CPM in the presence of 1,000-fold excess unlabeled IL-10. Maximal binding occurs at 1.5 to 2.5 nmol/L. (B) Scatchard analysis of equilibrium binding data from the same patient sample as shown in (A). The kd derived is 306 pmol/L and the Bmax is 4.8 pmol/L, giving an estimate of 116 IL-10 receptors per cell. These data are representative of three such experiments from three different B-CLL patients.
Determination of proliferation and differentiation.The median coexpression of CD5 and CD19 on MNCs used in these studies was 96.5%. For proliferation assays, cells were plated in triplicate in 96-well U-bottom plates at a concentration of 2 × 106 cells/mL in a total volume of 200 μL RPMI 1640 with 10% FCS and antibiotics. In addition, cells were cultured in the presence or absence of IL-10 at concentrations between 5 to 100 ng/mL, and the presence or absence of 100 ng/mL 12-O-tetradecanoyl 13-acetate (TPA) (Sigma). IL-10 and TPA were added together at the indicated amounts only at the initiation of cultures. Wells were pulsed with 1 μCi methyl-[3H]thymidine (ICN, Costa Mesa, CA) for the last 16 hours of the 7-day culture, incorporated thymidine was measured on a scintillation counter. For differentiation assays, fresh MNCs were plated in 24-well tissue culture plates (Sarstedt, Newton, NC) at 2 × 106/mL in 2 mL RPMI 1640 plus 10% FCS and antibiotics in the presence or absence of 25 ng/mL IL-10, with or without 100 ng/mL of TPA. Following culture for 7 days, IgM concentrations were determined in duplicate by enzyme-linked immunosorbent assay (ELISA) as previously described.24
Culture conditions for study of survival.MNCs were isolated from fresh blood by Ficoll separation (Histopaque), washed, resuspended in RPMI 1640 containing 10% FCS (Sigma), and brought to a final concentration of ≈2 × 106 cells/mL. Each well contained 2 mL. At initiation, cultures were supplemented with 25 ng/mL IL-10, 5 × 10−4 mol/L hydrocortisone (HC) (Sigma), IFN-γ (100 U/mL; Peprotech, Rocky Hill, NJ), combinations of HC + IL-10, HC + IFN-γ or neither, and then cultured at 37°C. The wells were harvested at 24 hours or 3 days and assayed for viability by analyzing 5 × 104 ungated events for exclusion of propidium iodide (PI) on a FacSort flow cytometer (Becton Dickinson, San Jose, CA). Two experiments were performed in replicates of eight wells to determine the interwell variation for each culture condition (estimated standard deviation ranged from 0.58% to 2.92%). Thereafter, a single well was analyzed for each culture condition in an additional five consecutive cases of B-CLL. Median coexpression of CD19 and CD5 on MNCs used in these studies was 91%.
Measurement of apoptosis and bcl-2 protein expression.For quantitation of programmed cell death, B-CLL nuclei were stained with PI and analyzed by flow cytometry as described.25 For analysis of bcl-2 protein expression, 1 × 106 cells were stained with phycoerythrin (PE)-conjugated anti-CD19 (Becton Dickinson, Mountain View, CA) or a directly-conjugated nonreactive isotype control monoclonal antibody (MoAb) and then permeabilized for 30 minutes on ice in Ortho Permea Fix (Ortho Diagnostic Systems, Raritan, NJ). Permeabilization, as examined by vital dye, was 100% in cells under all culture conditions. After washing, the cells were stained with fluorescein isothiocyanate (FITC) antihuman bcl-2 antibody (DAKO, Glostrup, Denmark), or a directly-conjugated nonreactive isotype control MoAb, washed twice and analyzed for simultaneous fluorescence of FITC and PE. Ten thousand events were collected and the median fluorescence intensity (MFI) was measured by flow cytometry. Background fluorescence with nonreactive control MoAbs was always within the first log of fluorescence intensity.
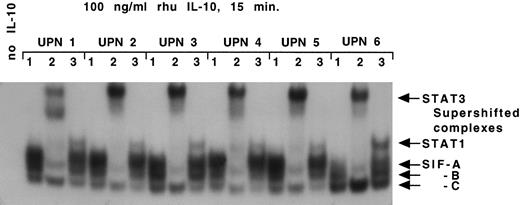
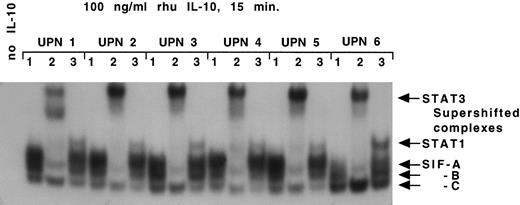
IL-10 receptor signal transduction in B-CLL cells. MNCs (1 × 107) containing a median of 93% CD19/CD5 positive B-CLL cells from 6 patients with B-CLL were stimulated with IL-10 (100 ng/mL) for 15 minutes. Whole cell extracts were prepared and subjected to EMSA using SIE as a probe as described in Materials and Methods. The lane to the far left identifies an EMSA with B-CLL cells (UPN 2) and the SIE probe in the absence of IL-10 (negative control). For each UPN, lane 1 identifies an EMSA with B-CLL cells and the SIE probe in the presence of IL-10, giving rise to SIF-A, -B, and -C complex as indicated on the right. For each UPN, lanes 2 and 3 identify an electrophoretic mobility supershift assay with B-CLL cells, the SIE probe, IL-10, and an antibody specific for STAT3 (lane 2) or STAT1 (lane 3). The arrows identify STAT1 and STAT3 MoAb-mediated supershift in all six patient samples.
IL-10 receptor signal transduction in B-CLL cells. MNCs (1 × 107) containing a median of 93% CD19/CD5 positive B-CLL cells from 6 patients with B-CLL were stimulated with IL-10 (100 ng/mL) for 15 minutes. Whole cell extracts were prepared and subjected to EMSA using SIE as a probe as described in Materials and Methods. The lane to the far left identifies an EMSA with B-CLL cells (UPN 2) and the SIE probe in the absence of IL-10 (negative control). For each UPN, lane 1 identifies an EMSA with B-CLL cells and the SIE probe in the presence of IL-10, giving rise to SIF-A, -B, and -C complex as indicated on the right. For each UPN, lanes 2 and 3 identify an electrophoretic mobility supershift assay with B-CLL cells, the SIE probe, IL-10, and an antibody specific for STAT3 (lane 2) or STAT1 (lane 3). The arrows identify STAT1 and STAT3 MoAb-mediated supershift in all six patient samples.
Statistical analysis.Both the matched-pairs t-test and the Wilcoxon signed rank sum test were used to compare assay results from patient samples with different conditions. The standard deviation of the within-patient variation of the percent viability determined by flow cytometric analysis of exclusion of PI for various conditions was estimated from the counts obtained using replicates of eight wells for each of two patients. The average of these two estimated values was used as the estimated standard deviation for the corresponding condition. The standard deviation of the difference in percent viability based on counts from a single well was conservatively estimated as the square root of the sum of the squares of the estimated standard deviations for the two conditions being compared.
RESULTS
IL-10 receptor expression on human B-CLL cells.To determine if IL-10 could bind specifically to B-CLL cells, fresh MNCs from nine patients were assayed for 125I-IL–10 binding. Results of this assay are shown in Table 1 and indicate that B-CLL cells bind IL-10 with a significant degree of specificity (P < .0005). To further assess for IL-10 receptor protein expression on B-CLL cells, we determined the binding affinity and estimated the number of binding sites per cell. Saturation binding was performed on freshly isolated B-CLL cells from three patients. Figure 1A and Fig 1B show the binding curve and the derived Scatchard plot, respectively, from one patient. The derived kd is 306 pmol/L and the Bmax is 4.8 pmol/L. The other two patient samples showed kd values of 168 and 426 pm and Bmax values of 1.9 and 5.3 pmol/L, respectively. Taken together, the results indicate that IL-10 binds its receptor on B-CLL cells with an affinity in the range of 1.9 to 5.3 pmol/L. Maximal binding of IL-10 occurs at approximately 1.5 to 2.5 nmol/L. If one assumes that each IL-10 receptor unit binds one dimer of human IL-10 ligand,21 then these data provide an estimate of approximately 47 to 120 IL-10 receptors per cell for B-CLL cells.
IL-10 receptor signal transduction in B-CLL cells.We next assessed whether the IL-10 receptor expressed on B-CLL cells was capable of intracellular signal transduction. The activation of signal transducer and activator of transcription (STAT) proteins following IL-10 stimulation of normal T cells and monocytes involves phosphorylation of STAT1 and STAT3 in both instances.26 Nonadherent MNCs containing a median of 93% B-CLL cells were isolated from six B-CLL patients and stimulated with IL-10 for 15 minutes. DNA-protein complexes were observed using the SIE probe in all six samples (Fig 2). The complexes were resolved into three bands generically termed SIF-A, -B, and -C.22 To define the identity of these IL-10–induced STAT proteins, the whole cell lysates were incubated in the presence of anti-STAT1 and anti-STAT3 antibodies in a supershift EMSA. A supershift identified SIF-A, -B, -C as STAT3 homodimer, STAT1-STAT3 heterodimer and STAT1 homodimer, respectively (Fig 2). Interestingly, STAT phosphorylation was not seen following stimulation with IL-2, despite functional evidence of IL-2 receptors on B-CLL cells (data not shown).27
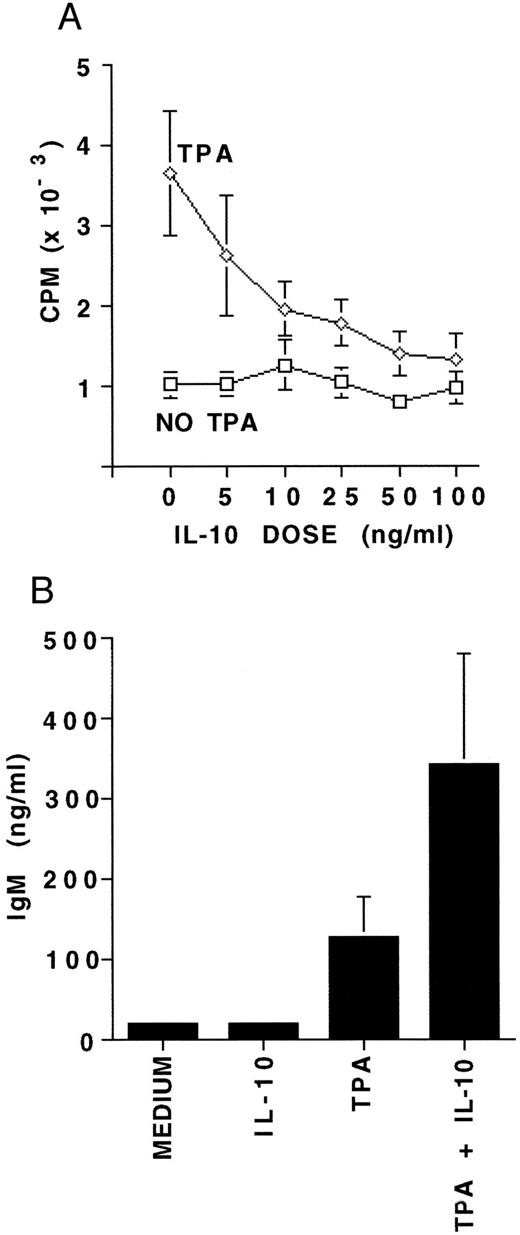
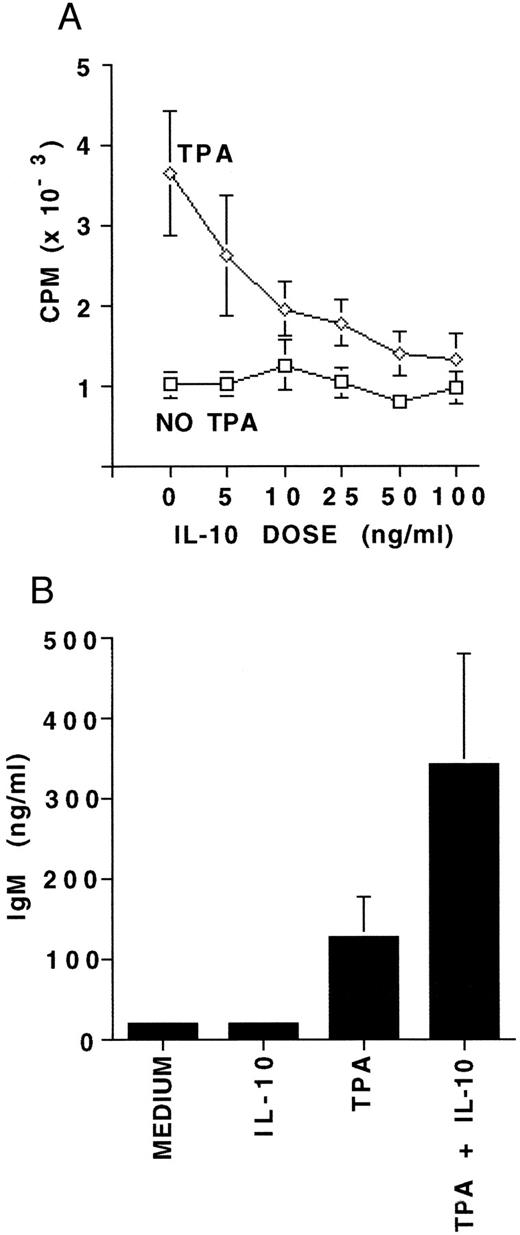
Proliferation and differentiation of B-CLL cells in response to IL-10.To further assess functional responsiveness of IL-10 receptor expression on B-CLL cells, we evaluated both proliferation and differentiation. MNCs from five patients containing a median of 96.5% B-CLL cells were incubated for 7 days in the presence or absence of 5 to 100 ng/mL of IL-10, with or without TPA. A 7-day culture was performed to compare our results with those reported for CD40-activated B-CLL cells and IL-10.28 Proliferation was assayed by 3H-thymidine incorporation. IL-10 alone did not induce any significant DNA synthesis in resting B-CLL cells at any concentration. However, IL-10 consistently and significantly inhibited the TPA-induced proliferation at IL-10 concentrations above 10 ng/mL, and did so in a dose-dependent fashion (P < .01, Fig 3A). Based on this, 25 ng/mL of IL-10 was chosen as the standard dose in further experiments. MNCs from 10 patients also containing a median of 96.5% B-CLL cells were cultured for 7 days in the presence or absence of IL-10 with or without simultaneous activation by TPA. IL-10 was noted to significantly enhance TPA-induced IgM production in all 10 patients samples (P < .05, Fig 3B).
The effects of IL-10 on proliferation and differentiation of B-CLL cells. (A) 3H-thymidine incorporation measured in 4 × 105 MNCs following culture for 7 days with either medium alone, 5 to 100 ng/mL of IL-10, 100 ng/mL TPA, or TPA plus 5 to 100 ng/mL of IL-10. Experiments were performed in triplicate and represent the mean ± SEM for five B-CLL patients. (B) IgM concentration in supernatants from 4 × 106 MNCs cultured under the same culture conditions as (A), but with 25 ng/mL of IL-10. Experiments in (B) were performed in triplicate and represent the mean ± SEM for 10 B-CLL patients. The MNCs studied for proliferation and differentiation contained a median of 96.5% CD19/CD5 positive B-CLL cells.
The effects of IL-10 on proliferation and differentiation of B-CLL cells. (A) 3H-thymidine incorporation measured in 4 × 105 MNCs following culture for 7 days with either medium alone, 5 to 100 ng/mL of IL-10, 100 ng/mL TPA, or TPA plus 5 to 100 ng/mL of IL-10. Experiments were performed in triplicate and represent the mean ± SEM for five B-CLL patients. (B) IgM concentration in supernatants from 4 × 106 MNCs cultured under the same culture conditions as (A), but with 25 ng/mL of IL-10. Experiments in (B) were performed in triplicate and represent the mean ± SEM for 10 B-CLL patients. The MNCs studied for proliferation and differentiation contained a median of 96.5% CD19/CD5 positive B-CLL cells.
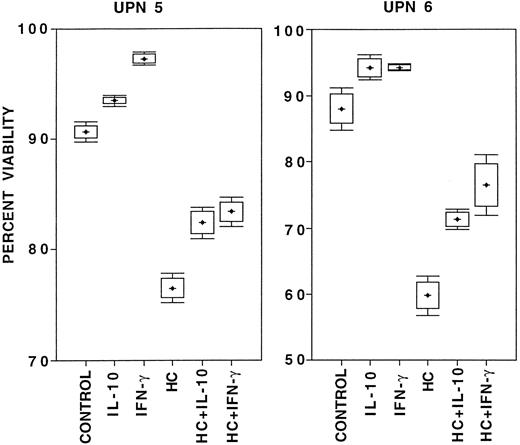
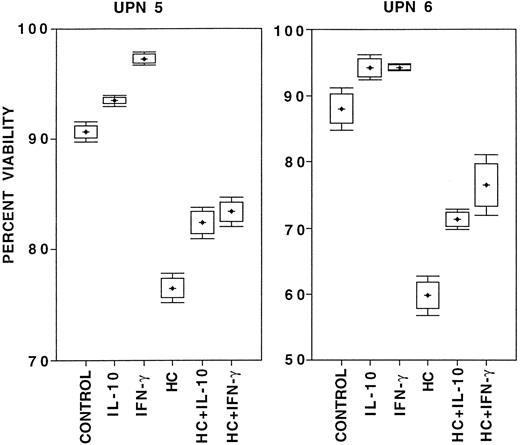
The effect of IL-10 on B-CLL cell survival.Survival was studied in the absence of exogenous factors, in the presence of IL-10, in the presence of HC as a positive control for the induction of apoptosis,9 in the presence of IFN-γ as a positive control for prolongation of survival,11 or in combinations of HC with either IL-10 or IFN-γ. The results are summarized in Table 2. IFN-γ always improved viability and HC always decreased viability, compared with B-CLL cells cultured for 24 hours in medium alone. In seven consecutive cases of B-CLL cultured for 24 hours, IL-10 did not decrease viability or induce apoptosis when compared with medium alone. Three of these cases were continued in culture for 72 hours with similar results (data not shown). While there was a trend toward improved survival of B-CLL cells cultured with IL-10 compared with cells cultured in medium alone in six of the seven cases studied, this did not reach statistical significance. There were individual cases of B-CLL in which survival was clearly higher in the presence of IL-10 , compared with cells cultured in medium alone. For example, unique patient numbers (UPNs) 5 and 6 were studied in eight parallel cultures, and IL-10 stimulation influenced viability in a manner that was significantly different from the controls, qualitatively comparable to that of IFN-γ, and in distinct contrast to that of HC (Fig 4). IL-10, like IFN-γ, always reduced HC-induced cell death when added at the onset of culture (P < .02, Table 2). Identical results were obtained when these studies were done in whole blood cultures from an additional 12 consecutive patients, only correcting for culture cell density with patients' autologous serum (data not shown).
Box and whiskers diagram of survival of 2 × 106/mL B-CLL cells from two patients (UPN 5 and 6 in Table 2) cultured in eight parallel cultures per condition for 24 hours in medium with 10% FCS under the listed conditions. Viability was assessed by PI-exclusion with acquisition of 5 × 104 ungated events on a flow cytometer. From these data the mean percent viability (crosses), the 95% confidence interval for estimation of the mean (boxes), and one standard deviation above and below the mean (bars) were calculated.
Box and whiskers diagram of survival of 2 × 106/mL B-CLL cells from two patients (UPN 5 and 6 in Table 2) cultured in eight parallel cultures per condition for 24 hours in medium with 10% FCS under the listed conditions. Viability was assessed by PI-exclusion with acquisition of 5 × 104 ungated events on a flow cytometer. From these data the mean percent viability (crosses), the 95% confidence interval for estimation of the mean (boxes), and one standard deviation above and below the mean (bars) were calculated.
The influence of IL-10 on the expression of bcl-2 protein.B-CLL cells constitutively express bcl-2, and a reduction of bcl-2 protein expression coincides with apoptosis of B-CLL cells in vitro.29 MNCs from six patients containing a median of 94.5% B-CLL cells were cultured under the conditions described above for viability studies. The expression of bcl-2 protein was analyzed on permeabilized CD19+ cells by flow cytometry. In three separate experiments, 100% of cells inside the lymphocyte gate maintained vital dye exclusion under all culture conditions. At 24 and 72 hours, there was no significant difference in the MFI of bcl-2 protein expression, regardless of culture conditions (data not shown).
DISCUSSION
In the current study, we assess the expression of IL-10 receptors on B-CLL cells and the functional consequences of IL-10 binding to these receptors. We provide the first documentation of specific IL-10 binding to freshly isolated B-CLL cells in all nine patient samples studied. Saturation binding experiments performed with 125I-IL–10 showed a range of 47 to 120 IL-10 receptor binding sites per cell, with a kd in the range of 168 pmol/L to 426 pmol/L, comparable to other IL-10–responsive cell types.21 30 Between 91% and 99% of the cells used in these binding experiments were B-CLL cells. Thus, our estimate of receptor number and affinity is likely to be representative of the malignant cell population.
The functional responsiveness of the IL-10 receptor to its ligand on B-CLL cells was first assessed by the phosphorylation of STAT proteins. The identity of STAT1 and STAT3 homodimers in all six patient samples studied, as well as one example of a STAT1/STAT3 heterodimer following IL-10 stimulation, was identical to that recently reported for normal human T-cell and monocyte preparations.26 Our failure to detect constitutive phosphorylation of either STAT1 or STAT3 in the absence of IL-10 suggests that B-CLL cells, like normal B cells, are likely to be dependent on IL-10 for IL-10 receptor signaling. Whether the IL-10 receptor on B-CLL cells is constitutively occupied and activated in vivo remains to be investigated.
It was also of interest to see that, at least in the six B-CLL samples tested, exogenous IL-2 was unable to induce an electrophoretic mobility shift with the TB-2 probe that binds STAT5.31 IL-2 receptor expression has been documented in B-CLL, as has functional responsiveness in the presence of IL-2.27,28,32 Whether the absence of STAT5 phosphorylation following IL-2 receptor activation will be a consistent finding in B-CLL is unknown, but these data suggest that alternate intracellular signal transduction may account for the effects of IL-2 on B-CLL cells.33 As IL-2 induces STAT5 in resting T cells,34 this finding makes it unlikely that contaminating T cells significantly contributed to STAT1 and STAT3 activation in our studies.
A number of reports have documented the role of IL-10 as a survival factor for human B-cells. Levy and Brouet15 showed germinal center B cells can be maintained in serum free medium supplemented with IL-10, which also enhanced bcl-2 expression. Baiocchi et al16 have shown that IL-10 maintains survival of EBV+ human B cells in serum free medium via a bcl-2–independent mechanism. However, Fluckiger et al17 reported that IL-10 could induce apoptosis in B-CLL cells. In that study, blood was processed in vitro with E rosetting, MoAbs, and immunomagnetic bead depletions before culture in medium containing 10% FCS. It was not stated if survival studies were performed with patient material that was fresh or frozen, but in our experience, frozen B-CLL cells display a substantial reduction in IL-10 binding specificity compared with fresh cells (J. Tan, unpublished observations, March 1995). Our survival studies were performed on both fresh blood obtained from 12 consecutive B-CLL patients without further separation of cellular components, as well as seven fresh, consecutive patients' mononuclear cells cultured in medium with 10% FCS. The differences in cell selection and cryopreservation alone could account for the discrepancy in our results and those of Fluckiger et al.
The IL-10 receptor belongs to the same family as the IFN-α and IFN-γ receptors.33 All three members of this cytokine receptor family can phosphorylate STAT1, and receptors for IL-10 and IFN-α can also phosphorylate STAT3, in normal and B-CLL cells.33,35 IFN-α and IFN-γ have previously been shown to enhance B-CLL differentiation.36 IFN-α has been shown to inhibit lipopolysaccharide (LPS)- and phytohemagglutinin (PHA)-induced proliferation in IFN-responsive B-CLL cells.37 These results are compatible with our assessment of the functional consequences of IL-10 binding to its receptor on B-CLL cells and also with the previously reported IL-10–induced differentiation of CD40-activated B-CLL cells.28 Furthermore, IFN-α and, as confirmed in this report, IFN-γ inhibit apoptosis in B-CLL cells.11,12 Although IL-10 was reported to induce apoptosis in four of 12 B-CLL samples,17 another report by the same group demonstrated that IL-10 enhanced viable B-cell recovery when added to anti-CD40 activated B-CLL cells without and with IL-2.28 We could not demonstrate that IL-10 induced apoptosis of B-CLL cells. Rather, IL-10 influenced apoptosis in a manner opposite to that of HC and similar to that of IFN-γ. We, therefore, conclude that activation of the IFN family of receptors on B-CLL cells elicit comparable functional responses. The mechanism by which each factor was able to significantly inhibit HC-induced apoptosis as observed in the current study is unknown, but does not appear to involve an isolated alteration in the expression of bcl-2. Whether IL-10 receptor activation leads to alterations in other members of the bcl-2 family of polypeptides that promote or inhibit apoptosis remains to be determined. If phosphorylation of STAT proteins proves to be critical to this process, as has recently been reported for pro-B cells,38 then targeting these molecules might offer novel therapeutic targets for chemoresistant B-CLL.
Supported by the Danish Medical Research Council (Copenhagen, Denmark), the Danish Cancer Society (Copenhagen, Denmark), The Elizabeth and Carl-Ejnar Nis-Hansen Foundation (Copenhagen, Denmark), The John and Birthe Meyer Foundation (Copenhagen, Denmark), The Spies Foundation (Copenhagen, Denmark), The Coleman Leukemia Research Foundation (Minneapolis, MN), and National Institutes of Health Grants No. CA26122, DK33886, CA65670, and CA68458.
Address reprint requests to Michael A. Caligiuri, MD, Division of Medicine, Roswell Park Cancer Institute, Buffalo, NY 14263.