Abstract
The cellular and molecular mechanisms that regulate the most primitive hematopoietic stem cell are not well understood. We have undertaken a systematic dissection of the complex hematopoietic microenvironment to define some of these mechanisms. An extensive panel of immortalized stromal cell lines from murine fetal liver were established and characterized. Collectively, these cell lines display extensive heterogeneity in their in vitro hematopoietic supportive capacity. In the current studies, we describe a long-term in vitro culture system using a single stromal cell clone (AFT024) that qualitatively and quantitatively supports transplantable stem cell activity present in highly purified populations. We show multilineage reconstitution in mice that received the equivalent of as few as 100 purified bone marrow and fetal liver stem cells cultured for 4 to 7 weeks on AFT024. The cultured stem cells meet all functional criteria currently ascribed to the most primitive stem cell population. The levels of stem cell activity present after 5 weeks of coculture with AFT024 far exceed those present in short-term cytokine-supported cultures. In addition, maintenance of input levels of transplantable stem cell activity is accompanied by expansion of other classes of stem/progenitor cells. This suggests that the stem/progenitor cell population is actively proliferating in culture and that the AFT024 cell line provides a milieu that stimulates progenitor cell proliferation while maintaining in vivo repopulating activity.
MAMMALIAN BLOOD formation originates in a small population of hematopoietic stem cells. The hallmark features of these cells are (1) a hierarchic multilineage differentiation potential with the ability to clonally give rise to at least eight distinct cell lineages, (2) a self-renewal capacity that is reflected in the lifelong continuous activity of few, and in some cases single, stem cells, and (3) a dramatic proliferative potential that is ultimately responsible for the production of large numbers of mature blood cells.1-3 During the past decade, much progress has been made in providing a physical phenotype for this rare population of stem cells.4-6 However, currently, the only reliable functional assay system for the most primitive stem cell compartment is long-term in vivo transplantation. No in vitro system has been developed that adequately recapitulates stem cell behavior. Therefore, the cellular and molecular mechanisms that regulate the biology of stem cells have remained obscure.
A major challenge in stem cell research is the establishment of culture systems that facilitate in vitro maintenance of long-term transplantable stem cell activity. This is a necessary first step toward a cellular and molecular understanding of the regulatory mechanisms that mediate commitment versus self-renewal decisions. Moreover, establishment of such culture systems is a prerequisite for potential expansion of undifferentiated stem cell populations and for generation of stem/progenitor cells committed to selected lineages.
Efforts to develop culture systems for the maintenance of transplantable stem cells can be subdivided into two broad categories: (1) those using defined cytokine combinations as the only culture supplements and (2) those relying on a preestablished stromal monolayer as an additional supportive component (with or without exogenously added cytokines). Both of these strategies have met with limited success. In the first case, it has been repeatedly demonstrated that combinations of cytokines can exert potent stimulatory effects on stem/progenitor populations.7-9 In some studies, highly purified stem cells10,11 have been used, and the direct effects of cytokines have been demonstrated at the single-cell level.12,13 While informative, the vast majority of these studies are limited by their strictly in vitro nature. Thus, it is feasible to expand replatable in vitro progenitor populations10,14 and to stimulate colony formation by cells with both myeloid-erythroid and lymphoid potential12,13,15,16; however, the equivalence of these progenitor cells to the in vivo transplantable stem cell population remains speculative. Several studies have clearly demonstrated a dramatic loss of in vivo repopulating potential as a result of cytokine-driven in vitro proliferation.17-19 A small number of studies have shown that defined cytokine combinations promote the maintenance of transplantable activity.20 However, most of these are limited by the use of very short culture periods, the exact nature of the in vivo assay, and the use of nonenriched stem cell sources.21-23 This precludes interpretations suggesting a direct action of the given cytokine(s) in maintaining transplantable activity.
A further complication with defined cytokine studies is the inability to ascribe in vivo physiologic relevance to the observed effects. It has long been accepted that in the intact animal, stem cells are found in close association with discrete cellular microenvironments.24-27 These observations suggest both the existence of stem cell niches and the notion that in vivo stem cell regulatory mechanisms are likely to require cell-cell contact or short-range interactions.28 Efforts to understand the features of the hematopoietic microenvironment began with the establishment of the Dexter long-term culture (LTC) system.29 In this culture system, hematopoiesis is maintained for weeks or months by a heterogeneous adherent cell monolayer derived from bone marrow (BM). Some degree of transplantable stem cell maintenance and self-renewal30 has been demonstrated, but a general feature of the Dexter LTC is a dramatic net decrease of stem cell activity over time.31,32 Although much progress has been made, especially in studies of human stem/progenitor cells,33-35 a further drawback of this system is the heterogeneity of the stromal cell types present in the supportive monolayer. This hampers identification of regulatory mechanisms. Numerous studies have been reported in which the heterogeneous stromal monolayer is replaced with cloned stromal cell lines.36-39 Many of these cell lines can support in vitro myelopoiesis,40-43 B-lymphopoiesis,43,44 or in some cases both.39,45-47 However, few studies have focused on in vitro maintenance of the most primitive transplantable stem cell compartment. Moreover, with one exception,46 studies that have focused on in vitro maintenance of this stem cell population begin with heterogeneous unpurified sources of hematopoietic activity.39,42 45 Such populations contain numerous nonhematopoietic stromal cell types. Therefore, it has not yet been possible to assign a direct stem cell supporting phenotype to a given stromal cell line.
We have hypothesized that the rare frequency of primitive stem cells may suggest an equally rare frequency of stem cell–supporting microenvironmental niches. Accordingly, we established and characterized a large panel of conditionally immortalized, cloned stromal cell lines from midgestation fetal liver. This organ was chosen because during development it is where the stem cell compartment undergoes self-renewal expansion in addition to differentiation.48 The cell lines were generated as previously described45 by immortalization with a temperature-sensitive simian virus 40 T-antigen (SV40TAg).49 The clonal nature of the AFT024, 2018, and 2012 cell lines was verified by Southern blot analysis, which detected a single, unique proviral integration locus in the genomic DNA. As a first screen to identify potentially interesting cell lines, we used a “cobblestone area” (CA) assay50 initiated with BM taken from mice injected 2 days previously with 5-fluorouracil. It has been suggested that CA colonies that appear after a prolonged culture period are derived from more primitive stem cells, possibly identical to some in vivo transplantable entities.51 Therefore, we were most interested in identifying cell lines that support such late-arising CAs. Of 225 lines, 77 (34%) were capable of supporting limited in vitro hematopoiesis, whereas, consistent with our initial hypothesis, only 2% were able to maintain long-term (>6 weeks) hematopoietic CA activity. Subsequent studies with a selected subset of these lines showed that the ability to effectively support reconstituting BM stem cells in vivo is infrequently observed.45 Two of 16 cell lines maintained significant levels of long-term reconstituting stem cell activity for an in vitro culture period of 3 weeks. Several other cell lines supported low levels of such activity or transiently repopulating stem cells. The cell inoculum in these studies was whole BM unenriched for stem cell activity. Therefore, it is not possible to suggest that the effective stromal cell lines were directly supporting stem cell activity. In the studies described, we demonstrate that a single clonal cell line, designated AFT024, can maintain quantitative levels of transplantable stem cell activity present in highly purified stem cell populations. We have used a competitive repopulation assay system that uses uncompromised competitor BM cells. The in vitro–maintained stem cells satisfy all criteria that currently define the most primitive stem cell population, including the ability to reconstitute secondary recipients. We also show that in vitro maintenance of primitive transplantable stem cells is compatible with concurrent generation of large numbers of committed progenitors.
MATERIALS AND METHODS
Mice.Timed-pregnant mice and 5- to 7-week-old female mice (C57Bl/6J, Ly5.2) were purchased from the Jackson Laboratory (Bar Harbor, ME). Congenic C57Bl/6, Ly5.1 female mice were purchased from the National Cancer Institute (Frederick, MD). All mice were housed at the Princeton University Barrier Animal Facility in autoclaved microisolator cages on ventilated cage racks. The animals received sterile irradiated food and acidified autoclaved water ad libitum.
Stromal cell lines and culture conditions.Fetal liver stromal cell lines used in this study were derived as previously described.45 Stromal cell lines were routinely cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 5 × 10−5 mol/L β-mercaptoethanol (2-ME) at 32°C, 5% CO2 , and 100% humidity. Sera were obtained from Hyclone (Logan, UT). Other biochemical reagents were obtained from Sigma (St Louis, MO). Two of the lines used in this study (2012 and 2018) were previously characterized for the ability to support long-term repopulating activity present in whole unfractionated BM.45 The AFT024 cell line was identified as an additional long-term (>4 weeks) CA supporter. Subclones of 2012 and AFT024 were isolated and used in these studies. The AFT024 cell line has remained stable and demonstrated consistent stem cell supporting ability for over 4 years.
Hematopoietic stem cell purification.Stem cells were purified from day 14 fetal livers essentially as previously described,52 with inclusion of c-kit expression as an additional parameter. Briefly, AA4.1-positive (AA4.1+) cells were isolated by immunopanning on petri dishes coated with AA4.1 antibody (10 μg/mL). The AA4.1+ fraction has been shown to contain all repopulating stem cell activity present in day 14 fetal liver.53 AA4.1+ cells were collected and stained with saturating concentrations of fluorescein isothiocyanate (FITC)-labeled rat monoclonal antibodies to lineage markers (CD3, CD4, CD5, CD8, B220, Gr-1, Mac-1, and TER-119). The cells were simultaneously stained with phycoerythrin (PE)-labeled Ly6A/E (Sca-1) antibody and biotinylated antibody to c-kit. The latter was developed with streptavidin allophycocyanin (APC). The AA4.1 hybridoma was a kind gift from Dr J. McKearn of Monsanto (St Louis, MO). AA4.1 antibody was purified by ImClone Systems Inc (New York, NY). The TER-119 antibody was initially obtained from Dr T. Kina of Kyoto University (Kyoto, Japan) and subsequently purchased from PharMingen (San Diego, CA). All other antibodies were purchased from PharMingen. Streptavidin APC was purchased from Molecular Probes Inc (Eugene, OR).
Three-color fluorescence-activated cell sorting for lineage-negative to low (lin−/lo), Sca-1+, c-kit+ cells was initially performed on a dual-laser Epics 753 cell sorter (Coulter Electronics, Hialeah, FL) interfaced with Cicero software (Cytomation Inc, Fort Collins, CO), and subsequently on a multilaser FACS Vantage with Cell Quest software (Becton Dickinson Immunocytometry Systems, San Jose, CA).
Enriched fractions of BM stem cells were obtained from C57Bl/6 Ly5.1 mice as previously described.54 Briefly, BM mononuclear cells were isolated by density centrifugation over Ficoll Hypaque (<1.077; Pharmacia, Piscataway, NJ). Lineage-negative or -low staining cells (lin−/lo) were obtained by magnetic bead depletion (anti–rat Ig coated beads; Dynal, Oslo, Norway) of mononuclear cells using the same lineage cocktail described already. The cells were further stained with antibodies to Sca-1 and c-kit as described. Sorting for lin−/lo, Sca-1+, and c-kit+ cells was accomplished with the Epics 753 as already described.
Stem cell/stromal cell cocultivation and cytokine-supplemented suspension culture.Stromal cell lines were seeded on tissue culture dishes that had been coated with 1% gelatin (Specialty Media, Lavallette, NJ) and were grown at 32°C, 5% CO2 , and 100% humidity. Confluent monolayers were irradiated (20 Gy, Cesium 137 source, Gammacell 40; Nordion International Inc, Ontario, Canada) and cultured in modified Dexter55 media (DMEM, 10% FBS, 10% horse serum, 5 × 10−5 mol/L 2-ME, and 1 × 10−7 mol/L hydrocortisone). For Dexter LTC, enriched hematopoietic stem cells were added and the cultures were maintained at 37°C, 5% CO2 , and 100% humidity with weekly media changes. The specific number of purified stem cells added to stromal cell cocultures is given in the appropriate figure or table legend. In some experiments, week 4 AFT024/stem cell cultures were harvested and replated in limiting dilution onto fresh, irradiated (20 Gy) AFT024 monolayers in 96-well trays (Dexter LTC conditions). CAs were scored weekly (as described earlier) for an additional 5 weeks. Irradiated (20 Gy) 2018 monolayers in 96-well trays were used in limiting-dilution Whitlock-Witte44 assays (LD-WW) to assess stromal-dependent B-lymphopoiesis content of both freshly purified and AFT024 cultured fetal liver stem cells. These cultures were established in RPMI media with 5% FBS, 2 mmol/L glutamine, 1 mmol/L Na pyruvate, and 5 × 10−5 mol/L 2-ME at 37°C, 5% CO2 , and 100% humidity. Cell line 2018 has been previously identified as a potent B-lymphopoiesis–supporting line in WW conditions.47 Short-term cytokine-supported suspension cultures and short-term AFT024/stem cell cocultures were established in Iscove's modified Dulbecco's Medium, 10% FBS, 1% BSA, and 5 × 10−5 mol/L 2-ME at 37°C, 5% CO2 , and 100% humidity. Cytokine concentrations were as follows: rmflk2/flt3-ligand (FL) 30 ng/mL, rmSteel factor (SL) 20 ng/mL, and recombinant human interleukin-6 (IL-6) 10 ng/mL. FL was obtained from ImClone Systems Inc (New York, NY). SL was purchased from Genzyme Corp (Cambridge, MA). IL-6 was purchased from Upstate Biotechnology Inc (Lake Placid, NY).
Transplantation assays for hematopoietic stem cell activity. Competitive repopulation was used to measure stem cell activity present in both freshly isolated and cultured stem cell populations.56 This assay was performed using the congenic Ly5.1/5.2 mouse system.57 Enriched stem cells were seeded onto irradiated stromal monolayers and maintained in Dexter LTC conditions. At the end of 4 to 7 weeks, the cultures were harvested by vigorous trituration. Single-cell suspensions were prepared by passage through 22-gauge needles, mixed with fresh congenic BM, and transplanted into lethally irradiated congenic mice (10 Gy, split dose 3 hours apart, 1 Gy/min, Gammacell 40). All purified fetal liver stem cells were from Ly5.2 mice. The competitor BM and recipients were Ly5.1. Purified BM cells were from Ly5.1 mice; in this experiment, Ly5.2 BM was used as competitor and Ly5.2 mice as recipients. To assess reconstitution, mice were periodically bled by capillary puncture of the orbital venous plexus. Blood (0.1 mL) was collected into heparin-containing (10 U/mL) DMEM, and red blood cells were lysed with NH4Cl.58 For the experiments described in Fig 2 and Table 1, the nucleated cells were divided into two fractions, stained with the appropriate biotinylated Ly5 antibody, developed with streptavidin-peridinin chlorophyll protein (PerCP) and (1) CD4-FITC, CD8-PE and (2), B220-FITC, Mac-1-PE, Gr-1-PE. Cells from each fraction were analyzed on an Epics Profile II (Coulter Electronics). For the experiments described in Fig 3 and Tables 2 and 3, nucleated cells were stained with directly conjugated lineage antibodies (CD4-PE, CD8-PE, Mac-1-FITC, Gr-1-FITC, and B220-APC) and biotinylated Ly5.2 antibody developed with streptavidin Texas Red (TR). Four-color analysis of stained cells was performed on either the Coulter Epics 753 or Becton Dickinson FACS Vantage with the appropriate software interfaces. Anti-Ly5.1 was a kind gift from Dr H. Nakauchi, University of Tsukuba (Tsukuba, Japan). Purified and biotinylated Ly5.2 antibody was originally obtained from a hybridoma (AL14A2) kindly provided by Dr G. Spangrude, University of Utah Medical Center (Salt Lake City, UT). In later experiments, Ly5.2 antibody was purchased from PharMingen; CD4-PE, CD8-PE, and B220-APC were also purchased from PharMingen. Streptavidin-PerCP was purchased from Becton Dickinson Immunocytometry Systems. Streptavidin TR was purchased from Molecular Probes. Competitive repopulating units (CRU) per 105 were calculated according to the following formula59:
Reconstitution values of less than 2% of the test Ly5 donor allele were not considered sufficiently above background for calculation of CRU.
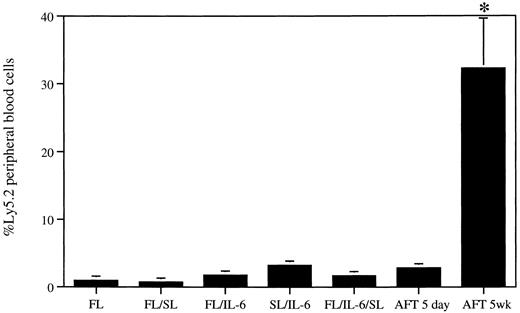
LTC on AFT024 maintains greater levels of repopulating stem cell activity than short-term cytokine- or short-term AFT024-supported cultures. Purified fetal liver cells were cultured for 5 days in suspension with cytokines or on an AFT024 monolayer (3,000/well, 12-well tray). Additional cells from the same purification were seeded onto AFT024 monolayers (3,000/6-cm dish) and maintained in Dexter LTC for 5 weeks. At completion of both culture periods, the cells were harvested, mixed with Ly5.1 BM, and used to transplant mice. Each mouse received 20% of each culture (600 stem cell equivalents) and 4 × 105 competitor BM cells (4 mice/culture). Peripheral blood cells from mice were analyzed for the presence of Ly5.2+ cells at 15 weeks after transplant. FL, 1.0 ± 0.57; FL/SL, 0.75 ± 0.25; FL/IL-6, 1.8 ± 0.14; SL/IL-6, 3.2 ± 0.46; FL/IL-6/SL, 1.7 ± 0.21; AFT024 5 days, 2.8 ± 0.11; AFT024 5 weeks, 32.2 ± 7.4. *P < .004, Student's t test. Error bars represent the SEM.
LTC on AFT024 maintains greater levels of repopulating stem cell activity than short-term cytokine- or short-term AFT024-supported cultures. Purified fetal liver cells were cultured for 5 days in suspension with cytokines or on an AFT024 monolayer (3,000/well, 12-well tray). Additional cells from the same purification were seeded onto AFT024 monolayers (3,000/6-cm dish) and maintained in Dexter LTC for 5 weeks. At completion of both culture periods, the cells were harvested, mixed with Ly5.1 BM, and used to transplant mice. Each mouse received 20% of each culture (600 stem cell equivalents) and 4 × 105 competitor BM cells (4 mice/culture). Peripheral blood cells from mice were analyzed for the presence of Ly5.2+ cells at 15 weeks after transplant. FL, 1.0 ± 0.57; FL/SL, 0.75 ± 0.25; FL/IL-6, 1.8 ± 0.14; SL/IL-6, 3.2 ± 0.46; FL/IL-6/SL, 1.7 ± 0.21; AFT024 5 days, 2.8 ± 0.11; AFT024 5 weeks, 32.2 ± 7.4. *P < .004, Student's t test. Error bars represent the SEM.
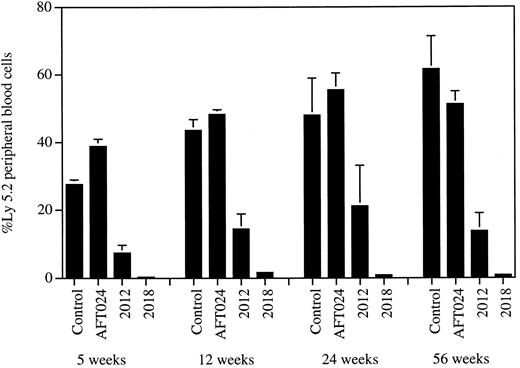
AFT024 maintains in vivo repopulating stem cells. The ability of 3 different stromal cell lines to support highly purified fetal liver stem cells was studied. Freshly purified day 14 fetal liver cells were transplanted directly (103 AA4.1+, lin−/lo, Sca-1+, c-kit+ cells plus 106 Ly5.1 competitor BM per mouse; n = 6) into Ly5.1 congenic mice (control). Additional stem cells from the same purification were also used to initiate Dexter LTC over irradiated AFT024, 2012, and 2018 stromal cell monolayers (104 cells/10-cm dish). After 4 weeks of culture, 10% aliquots of each culture (103 stem cell equivalents) were transplanted into Ly5.1 recipients (n = 8) together with 106 competitor Ly5.1 BM cells. Peripheral blood cells from mice were analyzed for the presence of Ly5.2+ cells at 5, 12, 24, and 56 weeks after transplant. Error bars represent the SEM.
AFT024 maintains in vivo repopulating stem cells. The ability of 3 different stromal cell lines to support highly purified fetal liver stem cells was studied. Freshly purified day 14 fetal liver cells were transplanted directly (103 AA4.1+, lin−/lo, Sca-1+, c-kit+ cells plus 106 Ly5.1 competitor BM per mouse; n = 6) into Ly5.1 congenic mice (control). Additional stem cells from the same purification were also used to initiate Dexter LTC over irradiated AFT024, 2012, and 2018 stromal cell monolayers (104 cells/10-cm dish). After 4 weeks of culture, 10% aliquots of each culture (103 stem cell equivalents) were transplanted into Ly5.1 recipients (n = 8) together with 106 competitor Ly5.1 BM cells. Peripheral blood cells from mice were analyzed for the presence of Ly5.2+ cells at 5, 12, 24, and 56 weeks after transplant. Error bars represent the SEM.
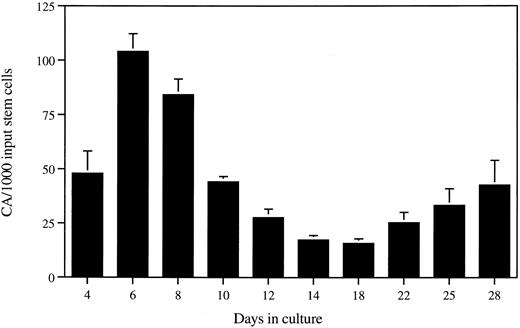
Time course of CA formation on AFT024. The formation of stromal-dependent CA derived from purified fetal liver stem cells was studied in AFT024 cocultures. Characteristic clusters of ≥50 cells were scored as CA over 28 days of culture. Results are expressed as the mean number of CAs/1,000 input stem cells from 3 separate fetal liver purifications (300 to 600 cells/well in 12-well trays). Error bars represent the SEM. The frequency of CA after 28 days is approximately 1 for every 20 input stem cells.
Time course of CA formation on AFT024. The formation of stromal-dependent CA derived from purified fetal liver stem cells was studied in AFT024 cocultures. Characteristic clusters of ≥50 cells were scored as CA over 28 days of culture. Results are expressed as the mean number of CAs/1,000 input stem cells from 3 separate fetal liver purifications (300 to 600 cells/well in 12-well trays). Error bars represent the SEM. The frequency of CA after 28 days is approximately 1 for every 20 input stem cells.
Retransplantation potential of stem cells was assessed by secondary transplantation. Mice from the experiment presented in Fig 1 were killed 60 weeks after transplantation, and the BM was harvested and stained with antibody to Ly5.2 followed by streptavidin TR. Ly5.2+ cells were collected by cell sorting (Coulter Epics 753) and used to transplant lethally irradiated secondary Ly5.1 recipients. Marrow from mice in the control and AFT024 groups was used to transplant mice in both radioprotection and competitive repopulation assays. Ly5.2+ BM from the primary 2012 transplants was used only in radioprotection assays. Primary 2018 mice did not contain sufficient Ly5.2+ cells for secondary transplantation. Transplanted mice were bled and analyzed by four-color flow cytometry for the presence of Ly5.2+ cells and multilineage reconstitution.
In vitro hematopoietic progenitor cell assays.The progenitor content of freshly purified hematopoietic stem cell populations and AFT024/stem cell cocultures was assessed using a variety of in vitro assays. All of the following assays were accomplished with fetal liver stem cells enriched as described earlier. To determine the time course of CA development, enriched stem cells were seeded onto irradiated AFT024 monolayers (300 to 600 cells/well in 12-well trays). CA development was evaluated over time, and characteristic clusters were quantified as described earlier. At different time points of the stem cell/AFT024 cultures, individual wells were harvested and replated into cytokine-supplemented semisolid clonogenic progenitor assays (CFU-C). The cytokine-enriched (rmIL-3 10 ng/mL, rhIL-6 10 ng/mL, rmSL 50 ng/mL, and erythropoietin 3 U/mL) methylcellulose mixture was purchased from Stem Cell Technologies Inc (Vancouver, BC, Canada). Colonies were scored after 8 to 14 days of culture at 37°C, 5% CO2 , and 100% humidity according to established criteria.60 Colonies that reached greater than 1 mm in size after 8 days and contained erythroid bursts and multiple myeloid cell lineages including megakaryocytes were scored as high–proliferative potential mixed-lineage colonies (CFU-HPP-Mix).61 Lineage content of typical colonies was determined by Wright/Giemsa staining of a cytospin slide preparation from individual colonies. Colony assays were also performed with 103 freshly purified cells. CFU progenitor contents of AFT024 cocultures were normalized to an initial input of 103 stem cells. To assess the ability of the AFT024 cell line to maintain primitive lymphoid progenitors, 4-week cocultures were plated into LD-WW assay on 2018 cells as described earlier. Resulting pro–B-cell colonies were scored after 7 days. The cell number in individual wells (96-well trays, 8 wells) was normalized from the original number of purified stem cells that initiated the coculture, ie, stem cell equivalents per well. As calculated from the line of best fit, the cell number at 37% negative wells is the frequency of pro–B-cell colony-initiating cells in the starting population.62 In a similar manner, the frequency of CA initiating cells in week 4 stem cell/AFT024 cocultures was also determined by replating them in limiting dilution onto fresh, irradiated AFT024 monolayers in 96-well trays. CAs were scored as described earlier at 1, 2, 3, 4, and 5 weeks after replating in Dexter LTC. The resulting frequencies were calculated as described already for the LD-WW assays and are also expressed in relation to the number of stem cells that seeded the initial cultures (stem cell equivalents).
RESULTS
In vivo and in vitro assays for stem cell activity maintained by stromal cell lines.We chose to use highly enriched stem cell populations to initiate cultures supported by single stromal cell lines. We focused on one cell line, AFT024, which in preliminary experiments was a particularly potent stem/progenitor cell supporter. Two other stromal cell lines, 2012 and 2018, which were analyzed in a previous study,45 were included in some experiments. To more rigorously establish the clonality of these lines, they were subcloned by limiting dilution. All subclones obtained from a given cell line contained the same proviral integrant position as the parental cell line (data not shown). The AFT024 cell line was evaluated for its ability to maintain both in vivo competitive repopulating stem cells and a broad spectrum of stem/progenitor cells defined by a variety of in vitro assays. The in vivo assays focused on the ability of stem cells cultured for extended periods (4 to 7 weeks) to permanently reconstitute multilineage hematopoiesis in transplanted hosts. The in vitro assays included enumeration of CA appearing over time in the initial cultures and quantitation of stem/progenitor cells that can form colonies in cytokine-supplemented replating assays. Cells from 4-week AFT024/stem cell cocultures were also assayed by limiting dilution for the content of progenitors capable of initiating secondary CAs on AFT024 or B-lymphopoiesis in W-W cultures supported by 2018.
AFT024 maintains quantitative levels of long-term in vivo repopulating stem cell activity.In our first line of investigation, we asked if and at what levels in vivo transplantable stem cell activity was present in 4- to 7-week-old cultures initiated with highly enriched stem cells and supported by AFT024, 2012, or 2018. We used purified day 14 fetal liver cells (AA4.1+, lin−/lo, Sca-1+, c-kit+) and adult BM cells (lin−/lo, Sca-1+, c-kit+) as sources of stem cell activity. Both of these populations are about 1,000- to 1,500-fold enriched for stem cell activity, as measured by competitive repopulation.56 The Ly5.1/Ly5.2 congenic system was used for all competitive repopulation studies.57 The data presented in Fig 1 demonstrate that the cultures supported by AFT024 contain stem cell activity at levels quantitatively identical to those present in the uncultured purified populations. In this experiment, individual Ly5.1 mice received 103 freshly purified Ly5.2 cells or the cultured equivalent of 103 purified Ly5.2 cells. Each mouse also received 106 Ly5.1 competitor BM cells. The percentage of Ly5.2+ peripheral blood cells was approximately equal in both groups of recipient animals. Moreover, the cultured stem cell activity is as effective as freshly purified activity for in vivo periods of greater than 1 year. The data in Fig 1 also show that the 2018 cell line is completely ineffective in maintaining highly purified stem cell activity, whereas the 2012 cell line supports intermediate levels of repopulating activity. The data presented in Table 1 provide quantitative CRU calculations and results of multiparameter lineage analyses. The extremely low levels of reconstitution by 2018-cultured stem cells precluded lineage analysis. CRU values for AFT024-cultured and freshly purified populations are nearly identical. Moreover, both fresh and AFT024-cultured stem cells reconstitute myeloid and lymphoid cell populations to a similar degree. To further access the supporting activities of AFT024 and 2012, we used a 10-fold lower number of fetal liver stem cells from two separate purifications to initiate the cocultures. The cultures were continued for 4 to 7 weeks, harvested, and used in competitive repopulation studies. Each recipient received the cultured equivalent of 100 purified Ly5.2 stem cells plus 4 × 105 Ly5.1 competitor BM cells. A total of 12 mice were transplanted with AFT024 cocultures (four each after 4, 5, and 7 weeks of LTC). The parental AFT024 line was used in the 4-week group, and two different subclones were used to support the 5- and 7-week cultures. AFT024-cultured Ly5.2 stem cells contributed 20% to 30% of peripheral blood cells in these recipients, whereas cells cultured on 2012 demonstrated more limited in vivo function (Table 1). The 2012 cultures were made with two subclones of the parental line and were maintained for 4 weeks before harvest and transplant (four mice per subclone). The data using different culture times or subclones did not vary significantly and are presented together in Table 1. An additional experiment was undertaken using enriched BM cultured on AFT024 and 2018 for 6 weeks. In this experiment, BM was purified from Ly5.1 congenic mice. Each Ly5.2 recipient mouse in this study received 100 freshly purified cells or the cultured equivalent of 100 purified cells. Both groups received 105 Ly5.1 competitor BM cells per mouse. Data analysis for the presence of Ly5.1+ cells at 4 months after transplant is presented in Table 1. For 6 weeks of culture, AFT024 cells maintained quantitative levels of reconstituting activity present in 100 purified BM stem cells. The 2018 cell line failed to maintain stem cell activity.
We extended these studies to include secondary transplantation as an additional assay for primitive stem cells. BM cells were harvested from the primary recipients of fresh and cultured fetal liver stem cells (Fig 1 and Table 1), and the Ly5.2+ fetal liver–derived fraction was collected by cell sorting. Secondary radioprotection and competitive repopulation transplants were performed. The data are presented in Table 2. The secondary-recipient repopulating activities are nearly identical for AFT024-cultured stem cells and noncultured controls. Lineage analysis of Ly5.2 cells in the secondary recipients showed similar numbers of myeloid and lymphoid cells derived from both AFT024-cultured and noncultured stem cells (data not shown). Due to the low cell numbers collected from recipients of 2012-cultured cells, only a radioprotection assay was feasible. Some level of secondary reconstituting cell activity was observed. Marrow from mice receiving 2018-cultured cells did not contain Ly5.2 cells, and therefore no secondary transplants were performed.
We also undertook an additional experiment where the levels of stem cell activity present in long-term AFT024 cocultures were compared with those in short-term cytokine-stimulated cultures or short-term AFT024-supported cultures (Fig 2). Purified fetal liver cells were seeded onto an AFT024 monolayer and maintained in Dexter LTC conditions for 5 weeks. Simultaneously, the same number of purified cells was cultured for 5 days (1) with different cytokine combinations or (2) on AFT024. Transplantable activity in the cultured cells was then assayed by competitive repopulation. Each mouse received the cultured equivalent of 600 stem cells together with 4 × 105 Ly5.1 competitor BM cells. It is evident from the data that the levels of in vivo repopulating activity present in long-term AFT024-supported cultures are much greater than those remaining after a short-term cytokine-supported culture period. Of interest also is that short-term AFT024 stem cell cocultures do not maintain significant in vivo reconstituting activity. In fact, these levels of stem cell activity are identical to the levels seen in cytokine-supported cultures.
In vitro stem/progenitor populations are expanded by AFT024.AFT024/stem cell cocultures have vigorous hematopoiesis throughout the entire in vitro culture period. This is reflected in the large number of relatively mature hematopoietic cells that are produced throughout the culture period (data not shown). In addition, CA colonies are observed throughout the culture period. Figure 3 shows a time course of CA appearance with purified fetal liver stem cells (three separate experiments). After 28 days in culture, approximately one in every 20 input stem cells is capable of proliferating into a CA. In addition, CA appearance over time follows a biphasic distribution, with many CAs observed early in the culture period. To enumerate the various classes of stem/progenitor cells present in AFT024 cocultures, we performed a series of in vitro replating experiments. These included quantitation of (1) progenitor cells capable of colony formation in cytokine-supplemented semisolid cultures (CFU assay), (2) progenitor cells capable of initiating secondary CA in limiting-dilution AFT024 cultures, and (3) progenitor cells that can initiate B-lymphopoiesis in LD-WW cultures. In all of these experiments, the primary cultures were initiated with purified fetal liver cells. All data presented are normalized to an initial input of 103 purified cells (CFU assay) or the actual number of initial-input stem cells (limiting-dilution assays).
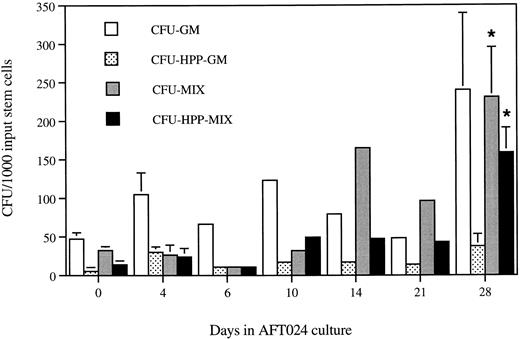
Shown in Fig 4 are the number and type of cytokine-responsive CFU progenitors present at various times in AFT024-supported cocultures. Production of CFU is evident at all time points. However, the content is especially high after 4 weeks, representing a fivefold to sevenfold increase/expansion compared with the content in freshly purified populations. The content of more primitive progenitors (CFU-HPP-Mix) is increased by 12-fold. These HPP-Mix colonies often reach a size of 2 mm in 8 days and contain large numbers of erythroid bursts and megakaryocytes. Interestingly, there does not appear to be a correlation between CA number and CFU content at different culture times. This is most apparent at day 6, when CA number is at the peak but progenitor content is similar to that observed in noncultured stem cells. Furthermore, there is no correlation between CFU content and the absolute number of maturing hematopoietic cells present in a given culture (data not shown).
HPP multilineage clonogenic progenitors are selectively expanded on AFT024. The clonogenic progenitor content of stem cells maintained in AFT024-supported Dexter LTC was determined. Enriched fetal liver stem cells were seeded onto AFT024 monolayers. At various time points, an individual well was harvested, and the cells were placed into semisolid clonogenic progenitor assay (CFU-C). Colonies were scored at 8 to 14 days. Colonies were designated as HPP upon reaching a size ≥1 mm after 8 days. CFU numbers at days 0, 4, and 28 are averaged from 3 to 5 individual stem cell purifications. Error bars represent the SEM for these experiments. Other time points are individual determinations. CFU are normalized to 1,000 input stem cells in the stromal cocultures for comparison to day 0 progenitors. *CFU-Mix (P = .01) and CFU-HPP-Mix (P = .001) are significantly expanded at day 28 compared with day 0 (Student's t test).
HPP multilineage clonogenic progenitors are selectively expanded on AFT024. The clonogenic progenitor content of stem cells maintained in AFT024-supported Dexter LTC was determined. Enriched fetal liver stem cells were seeded onto AFT024 monolayers. At various time points, an individual well was harvested, and the cells were placed into semisolid clonogenic progenitor assay (CFU-C). Colonies were scored at 8 to 14 days. Colonies were designated as HPP upon reaching a size ≥1 mm after 8 days. CFU numbers at days 0, 4, and 28 are averaged from 3 to 5 individual stem cell purifications. Error bars represent the SEM for these experiments. Other time points are individual determinations. CFU are normalized to 1,000 input stem cells in the stromal cocultures for comparison to day 0 progenitors. *CFU-Mix (P = .01) and CFU-HPP-Mix (P = .001) are significantly expanded at day 28 compared with day 0 (Student's t test).
We next determined the content of primitive B-lymphoid progenitors present in AFT024/stem cell cultures. We accomplished this by plating cells from the 4-week AFT024 cocultures into LD-WW assays over the 2018 stromal cell line. Two experiments with freshly purified stem cells and AFT024-cultured stem cells showed that the frequency of pro–B-cell progenitors is expanded 10-fold in AFT024 cultures compared with the freshly purified input population (day 0 frequency, one in 11.0, R2 = .98; day 28 AFT024-cultured frequency, one in 1.1, R2 = .97).
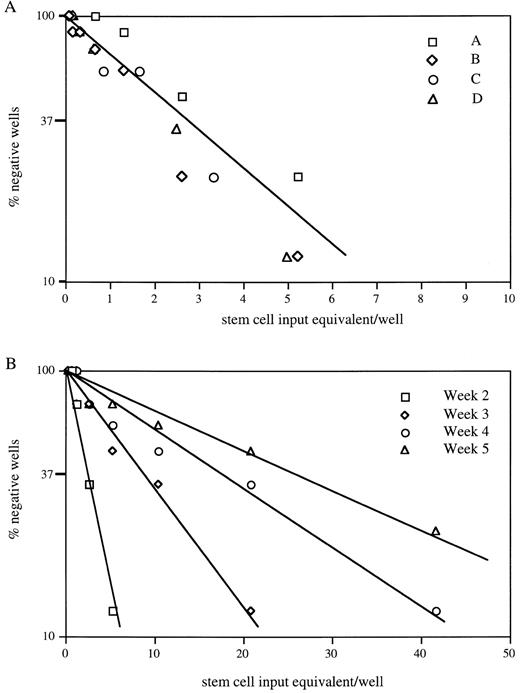
To measure the content or frequency of progenitor cells capable of initiating secondary CA, four separate 4-week AFT024/stem cell cocultures were replated in limiting dilution onto fresh AFT024 monolayers. CAs were scored after 1 week. The data are presented in Fig 5A. Large numbers of secondary CAs were observed. When normalized to the stem cell numbers used to initiate the primary cultures (stem cell equivalents), the frequency of these progenitors is one in three to four. Figure 5B shows data from one of four experiments presented in Fig 5A, where the quantitation of secondary CAs was extended for 4 more weeks. The frequency of CA decreases slowly over time (one in 19 after an additional 4 weeks), approximating the frequency seen in the primary cultures at 4 weeks. In summary, our in vitro replating assays collectively demonstrate a significant expansion of primitive progenitor populations in 4-week AFT024 cultures. In these same cultures, there is no decrease in the level of transplantable stem cell activity present in the total hematopoietic cell population.
CA-initiating cells are expanded on AFT024. (A) A quantitative estimate of the number of 28-day cultured stem cell equivalents required to form a CA after replating on secondary AFT024 monolayers was determined. Four different AFT024 cocultures from separate fetal liver purifications were studied (A, B, C, and D). In limiting-dilution assay, the frequency of stem cell equivalents required to form a CA in another 7 days was 1 in 4 (3.56 ± 0.64, R2 = .96). (B) CA maintenance in 1 of 4 cultures was followed for an additional 4 weeks. The frequency of stem cell equivalents maintaining CA was determined. At 37% negative wells, the frequencies were as follows: 2 weeks, 1 in 3; 3 weeks, 1 in 10; 4 weeks, 1 in 19; and 5 weeks, 1 in 29.
CA-initiating cells are expanded on AFT024. (A) A quantitative estimate of the number of 28-day cultured stem cell equivalents required to form a CA after replating on secondary AFT024 monolayers was determined. Four different AFT024 cocultures from separate fetal liver purifications were studied (A, B, C, and D). In limiting-dilution assay, the frequency of stem cell equivalents required to form a CA in another 7 days was 1 in 4 (3.56 ± 0.64, R2 = .96). (B) CA maintenance in 1 of 4 cultures was followed for an additional 4 weeks. The frequency of stem cell equivalents maintaining CA was determined. At 37% negative wells, the frequencies were as follows: 2 weeks, 1 in 3; 3 weeks, 1 in 10; 4 weeks, 1 in 19; and 5 weeks, 1 in 29.
DISCUSSION
In the experiments presented, we have demonstrated that the AFT024 stromal cell line can maintain quantitative levels of in vivo repopulating stem cells for at least 7 weeks of in vitro culture. We used highly enriched stem cell populations in low numbers (100 cells) and measured stem cell activity in a stringent, competitive-repopulation assay system. The cultured stem cell activity satisfies all in vivo criteria normally ascribed to the most primitive stem cell compartment: (1) long-term engraftment ability, (2) multilineage potential, and (3) ability to repopulate secondary recipients. In addition, our studies with the low number of culture-initiating stem cells for both BM and fetal liver imply that AFT024 stromal cells exert their supportive effects in a direct manner. We believe that these studies represent a clear example of an in vitro system capable of directly supporting the most primitive stem cell compartment.
Our previous studies had shown that stromal cell lines isolated from a single tissue source are heterogeneous with respect to the ability to maintain long-term repopulating stem cells.45 We speculated that the rare cell lines that were effective in supporting in vivo reconstituting stem cells may represent immortalized components of in vivo stem cell niches. However, our present studies together with those of others46 argue for the necessity of using purified stem cell populations to support such a hypothesis. Specifically, in the previous experiments, the 2018 cell line maintained transiently reconstituting activity present in unfractionated BM. In the current studies, 2018 fails to maintain measurable repopulating activity when cultured for 4 to 6 weeks with highly purified BM or fetal liver stem cells. Similarly, in preliminary experiments using purified cells, we have failed to show robust levels of reconstituting stem cell activity in cultures supported by CFC034, the most effective cell line in the whole-BM studies45 (data not shown). The 2012 cell line that in one previously reported experiment45 was effective in maintaining long-term repopulating whole-BM–derived stem cells is only partially effective in the present studies. Moreover, only some subclones of 2012 display such activity (despite identical proviral integration positions in all subclones). Recently, studies have shown that the S17 cell line that consistently supports stem cell activity present in whole BM39 is not similarly effective in the maintenance of purified-BM stem cells.46 Taken together with these current data, the previously observed stem cell–supporting stromal cell activities may reflect the action(s) of indirect mechanisms and therefore do not permit identification of cellular stem cell niche components. One previous study has shown that the Sys1 stromal cell line can maintain high levels of transplantable activity present in purified BM.46 The competitor cells in this study were compromised by prior serial transplantation. Moreover, in these studies, the culture period was extended for only 2 weeks and effective maintenance required the addition of exogenous leukemia inhibitory factor. In contrast, our current studies identify AFT024 as a cell line that provides a direct-acting long-term stem cell–supporting environment without the addition of exogenous factors. A rigorous confirmation of this must await the development of a serum-free AFT024/stem cell coculture system. These experiments have been initiated.
Our studies also show that in addition to recovering net input levels of transplantable activity from AFT024-supported cultures, we also obtain significantly expanded populations of primitive progenitor cells. CFU-HPP-Mix progenitors are expanded by 12-fold after 4 weeks of culture, and the number of stromal-dependent pro–B-lymphoid progenitors is similarly amplified. We suggest that the AFT024-mediated process of stem cell maintenance is in reality a dynamic phenomenon. Specifically, during the first portion of the culture period, the majority of transplantable stem cell activity may be lost through differentiation or cell death. The remaining primitive stem cells may expand to yield input levels of transplantable activity and increases in the number of more committed progenitors. An obvious prediction is that short-term AFT024-supported cultures should contain reduced levels of transplantable stem cell activity. In the experiment presented in Fig 2, this appears to be the case. The standard Dexter-type media used in parallel long-term AFT024 cocultures was not used in these short-term cultures. However, in another short-term experiment using Dexter LTC media, we have observed a similarly dramatic reduction in stem cell activity after 4 days of culture on AFT024.63 These observations are intriguing because they suggest that the AFT024 cell line is able to facilitate some degree of ex vivo transplantable stem cell proliferation and expansion. Indeed, in other studies, we have shown that AFT024 can support colony formation initiated by single purified stem cells with B- and T-lymphoid, myeloid, and erythroid potentials (H. Ema and I.R. Lemischka, manuscript in preparation). Moreover, preliminary experiments suggest that it is possible to efficiently introduce retroviral markers into transplantable stem cells at various times during AFT024 coculture (K.A. Moore, unpublished observations, January 1995). Extension of such marking experiments and analysis of proviral integration patterns will be necessary to rigorously ascertain if self-renewal replication is occurring during these coculture periods.
The ability of AFT024 to maintain the most primitive stem cell compartment while generating and expanding at least some less primitive members of the stem/progenitor cell hierarchy raises interesting issues regarding the nature of stem cell niches. Our studies suggest that microenvironmental niche models that postulate distinct cellular entities responsible for stem cell self-renewal and other cellular entities that support generation of committed progenitor cells may be overly simplified.64 Clearly, a single microenvironmental cell type represented by AFT024 is sufficient for keeping stem cells in an undifferentiated state and for allowing commitment and progenitor expansion to take place. We suggest that a hallmark feature of a stem cell niche is the ability to facilitate generation of the entire stem/progenitor cell hierarchy from very primitive cells. Therefore, the main functional role of such niches may be to provide an environment that permits production of the correct numerical balance of more and less primitive stem/progenitor cell entities. This model contains several testable hypotheses. The most important is that in vitro stem cell maintenance should not be interpreted literally as the maintenance of quiescent cells, but rather as a phenomenon that results from a balance of self-renewal and commitment decisions that occur during stem cell division.
What is the molecular nature of the mechanisms responsible for the AFT024 supportive phenotype? Although far from exhaustive both in terms of cytokine combinations and in the choice of media formulations, our experiments (Fig 2) do suggest that a cytokine cocktail of IL-6, SL, and FL is not effective in maintaining fetal liver stem cell activity. We have shown that RNA transcripts for these and 10 other cytokines are present in AFT024 (data not shown), but they are also detected at similar levels in nonsupporting lines such as 2018.45 These observations suggest the existence of novel AFT024-derived molecules that may act on stem cells. Indeed, using a subtractive hybridization molecular cloning strategy, we have identified a number of candidate molecules (K.A. Moore et al, manuscript in preparation). Two of these molecules contain epidermal growth factor (EGF)-like repeat motifs that are related to those found in the Notch/Notch-ligand family. Interestingly, one of these molecules appears to have activity on primitive stem cell populations.63
ACKNOWLEDGMENT
The authors thank Chris Karras, Darren Hasara, and the Princeton Animal Facility staff for the excellent animal care that makes these experiments possible; Jerome Zawadzki for expert flow cytometry assistance; Dr Ruth E. Wager for help with some of the stem cell purifications and analyses; and Dr Craig Jordan for critically reading the manuscript.
K.A.M. and H.E. contributed equally to this work.
Supported in part by grants from the American Cancer Society (DHP-144/01) and the National Institutes of Health (RO1 CA45339-09).
Address reprint requests to Ihor R. Lemischka, PhD, Princeton University, Department of Molecular Biology, Lewis Thomas Laboratory, Washington Road, Princeton, NJ 08544.