Abstract
We have previously reported that the serine protease plasmin generated during contact activation of human plasma triggers biosynthesis of leukotrienes (LTs) in human peripheral monocytes (PMs), but not in polymorphonuclear neutrophils (PMNs). We now show that purified plasmin acts as a potent chemoattractant on human monocytes, but not on PMNs. Human plasmin or plasminogen activated with urokinase, but not active site-blocked plasmin or plasminogen, elicited monocyte migration across polycarbonate membranes. Similarly, stimulation of monocytes with plasmin, but not with active site-blocked plasmin or plasminogen, induced actin polymerization. As assessed by checkerboard analysis, the plasmin-mediated monocyte locomotion was a true chemotaxis. The plasmin-induced chemotactic response was inhibited by the lysine analog trans-4-(aminomethyl)cyclohexane-1-carboxylic acid (t-AMCA), which prevents binding of plasmin/ogen to the appropriate membrane binding sites. In addition, active site-blocked plasmin inhibited monocyte migration triggered by active plasmin. Further, plasmin-induced monocyte chemotaxis was inhibited by pertussis toxin (PTX) and 1-O-hexadecyl-2-O-methyl-rac-glycerol (HMG) and chelerythrine, two structurally unrelated inhibitors of protein kinase C (PKC). Plasmin, but not active site-blocked plasmin or plasminogen, triggered formation of cyclic guanosine monophosphate (cGMP) in monocytes. LY83583, an inhibitor of soluble guanylyl cyclase, inhibited both plasmin-induced cGMP formation and the chemotactic response. The latter effect could be antagonized by 8-bromo-cGMP. In addition, KT5823 and (Rp)-8-(p-chlorophenylthio)guanosine-3′,5′-cyclic monophosphorothioate [(Rp)-8-pCPT-cGMPs], two structurally unrelated inhibitors of cGMP-dependent protein kinase, inhibited plasmin-mediated monocyte chemotaxis. Thus, beyond being a stimulus for lipid mediator release, plasmin is a potent and specific chemoattractant for human monocytes acting via a cGMP-dependent mechanism. Therefore, plasmin represents a proinflammatory activator for human monocytes.
THE ROLE OF THE serine protease plasmin in the lysis of fibrin clots is well characterized. However, plasmin may have additional physiologic and pathophysiologic functions. On the basis of its broad substrate specificity, plasmin is able to degrade extracellular proteins. Circumstantial and direct evidence from transgenic animals suggests that this activity might be important for various processes such as ovulation,1 tumoral2 and inflammatory cell migration,3-5 and wound healing.6
Most blood cells bind plasmin and plasminogen with similar affinity.7,8 Despite some variability, the number of binding sites per cell, in general, is high. A variety of membrane-associated macromolecules including annexin II, gangliosides, and also the enzyme α-enolase are able to bind plasmin/ogen, reflecting the heterogeneous nature of these binding sites.7-9 The functional consequences of plasmin/ogen receptor occupancy have been primarily regarded in terms of enhanced plasminogen activation, amplification loops, and protection, as well as enhancement of plasmin activity.8,10 However, it has also been reported that in some cell types such as polymorphonuclear neutrophils (PMNs),11 platelets,12,13 and endothelial cells,14 plasmin might affect various cell functions, pointing to some agonist activity.
Clinical studies have shown that rheumatoid arthritis is accompanied by enhanced levels of circulating α2 -antiplasmin–plasmin complexes.15,16 Moreover, α2 -antiplasmin–plasmin complexes have also been detected in synovial fluid from patients with both rheumatoid and nonrheumatoid arthritis.17 It has therefore been suggested that plasmin may play a role in the local inflammatory reaction.17
There is indeed substantial evidence that inflammatory processes such as arthritis are associated with contact activation,18 which triggers generation of plasmin via the so-called intrinsic fibrinolytic pathway.19 We have previously demonstrated that contact activation of human whole blood stimulates biosynthesis of 5-lipoxygenase–derived lipid mediators such as chemotactic leukotriene B4 (LTB4 ), but also of vasoconstrictory cysteinyl-LT.20-22 Under such experimental conditions, the 5-lipoxygenase pathway was selectively activated in monocytes, but not in PMNs.23 Various experimental approaches suggested that contact-mediated stimulation of the monocyte 5-lipoxygenase pathway was due to plasmin.24 In fact, purified human plasmin was shown to stimulate LTB4 production in human monocytes in a concentration-dependent manner.24 Since activation of the 5-lipoxygenase pathway reflects a proinflammatory stimulation of monocytes, we further characterized plasmin-induced proinflammatory reactions in human monocytes. Here, we show that plasmin is a potent chemoattractant for human monocytes. Thus, plasmin generated during the contact activation known to occur at the site of inflammatory reactions18 19 might contribute to monocyte recruitment into the inflamed tissue.
MATERIALS AND METHODS
Materials.Pertussis toxin (PTX), FMLP, 8-bromo-cyclic guanosine monophosphate (cGMP), trans-4-(aminomethyl)cyclohexane-1-carboxylic acid (t-AMCA), lysophosphatidylcholine, and essentially fatty acid–free bovine serum albumin (BSA) were purchased from Sigma Chemical Co (St Louis, MO). (Rp)-8-(p-chlorophenylthio)guanosine-3′,5′-cyclic monophosphorothioate ((Rp)-8-pCPT-cGMPS) was from Biolog Life Science Institute (Bremen, Germany). Human plasmin (13.83 Committee on Thrombolytic Agents [CTA] U/mg, lot no. 329 081) was obtained from Fluka (Buchs, Switzerland), and plasmin standard solution was from Boehringer (Mannheim, Germany). Human plasminogen, urokinase (87% high–molecular-weight form), and streptokinase were gifts from Behring (Marburg, Germany). D-Valyl-L-phenylalanyl-L-lysine chloromethyl ketone, 1-O-hexadecyl-2-O-methyl-rac-glycerol (HMG), LY83583 (6-anilino-5,8-quinolinedione), and KT5823 were from Calbiochem (San Diego, CA). The plasmin substrate S-2251 (H-D-valyl-leucyl-L-lysine-p-nitroanilide dihydrochloride) was supplied by Chromogenix (Mölndal, Sweden). Percoll was obtained from Pharmacia (Uppsala, Sweden) and chelerythrine chloride from Biomol (Plymouth Meeting, PA). N-(7-nitrobenz-2-oxa-1,3-diazol-4yl)-phallacidin (NBDphallacidin) was from Molecular Probes (Eugene, OR), and fluorescein isothiocyanate (FITC)-conjugated mouse antihuman monoclonal antibodies against CD14 were from Immunotech (Hamburg, Germany). The radioimmunoassay kit for guanosine 3′,5′-cyclic monophosphate was from Amersham (Braunschweig, Germany). Pyrogen-free water was from standard clinical supplies. Other chemicals of analytic grade were received from Merck (Darmstadt, Germany).
Preparation of human peripheral monocytes and PMNs.Blood was obtained from apparently healthy male volunteers. Peripheral monocytes (PMs) were isolated on iso-osmotic Percoll gradients basically as previously described.23,24 In brief, peripheral blood mononuclear cells (PBMCs) were obtained by centrifugation of EDTA-anticoagulated blood at 460g for 20 minutes at 20°C against 61% Percoll-NaCl 150 mmol/L (vol/vol) cushions. After washing twice with 150 mmol/L NaCl, PBMCs were resuspended in autologous platelet-poor plasma (PPP) anticoagulated with 5 mmol/L EDTA. The PBMC suspension was layered over discontinuous EDTA (5 mmol/L)-PPP-Percoll gradients consisting of 25% and 39% Percoll (vol/vol), respectively. After centrifugation at 800g for 45 minutes at 4°C, monocytes were collected at the 25%/39% interphase. The cells were washed twice in 150 mmol/L NaCl and resuspended in Hank's balanced salt solution (HBSS) containing 0.4% (wt/vol) BSA. Preparations with purity greater than 92% were used. Two −8% lymphocytes were found. Additional washings of PBMCs with 150 mmol/L NaCl improved our previously published method23,24 in such a way that no platelets or platelet satellitism, ie, platelets associated with the PM membrane, could be detected per 100 PMs by routine microscopic examination. PMNs were isolated from heparin-anticoagulated blood by centrifugation (300g for 20 minutes at 20°C) through a two-step Percoll-NaCl 150 mmol/L gradient consisting of 61% and 75% Percoll (vol/vol), respectively. Mononuclear cells and PMNs collected atop the 61% and 75% Percoll phase, respectively. PMNs were washed twice in 150 mmol/L NaCl, and any remaining red blood cells were removed by hypotonic lysis as described elsewhere.22 The cells were washed once more in 150 mmol/L NaCl, and purity was greater than 98%, with less than 2% lymphocytes. Cell purity was generally checked by phase-contrast microscopy and after staining with Türk's solution or Wright's stain. Viability was greater than 98% as judged by Trypan blue exclusion. The identity of monocytes was further confirmed by staining for α-naphthyl-acetate esterase (>92% positive staining). In addition, scanning and transmission electron microscopy and flow cytometric analysis of CD14+ cells has been used for characterization of monocytes.
Migration assays.Cell migration was evaluated in triplicate using tissue culture-treated 24-well Transwell plates (Costar, Cambridge, MA) with polycarbonate membranes of pore size 5 and 3 μm for monocytes and neutrophils, respectively.25,26 Monocytes and PMNs were suspended in HBSS containing 0.4% (wt/vol) BSA at a concentration of 2 × 106 cells/mL, and 100 μL was added to the upper compartment of each well. Chemoattractants or solvents were added to the lower compartments, and monocytes or PMNs were permitted to migrate at 37°C for 90 and 60 minutes, respectively.26 Polycarbonate membranes were removed, fixed, and stained with hematoxylin (Accustain; Sigma), and seven high-power oil-immersion fields (100×) were counted. The checkerboard assay was used to distinguish between true chemotaxis and chemokinesis.26 The results are expressed as the mean ± SEM for cells that migrated across the membranes.
Chemoattractants.FMLP at a concentration of 10 nmol/L was used as standard chemoattractant for monocytes and PMNs.25 26 When monocyte migration was tested in the presence of human plasmin, active site-blocked plasmin, human plasminogen, urokinase, or plasminogen plus urokinase, these compounds were dissolved in HBSS-BSA 0.4%.
Compounds affecting signal transduction.In experiments with PTX, monocytes were preincubated for 90 minutes at 37°C in the presence of PTX 1 μg/mL.27,28 Afterward, the cells were washed twice in HBSS-BSA 0.4%. Toxin treatment did not significantly affect viability of the cells as assessed by Trypan blue exclusion. The protein kinase (PKC) inhibitors HMG29,30 and chelerythrine chloride31 initially dissolved in dimethylsulfoxide (DMSO) or 20% DMSO, respectively, and diluted in HBSS-BSA 0.4% were added to the upper and lower compartment of the chemotaxis chamber at the concentrations indicated. In experiments with LY83583 (6-anilino-5,8-quinolinodione), an inhibitor of soluble guanylyl cyclase,32 dissolved in HBSS-BSA 0.4%, or with KT5823, an inhibitor of cGMP-dependent protein kinase,33 initially dissolved in DMSO and further diluted in HBSS-BSA 0.4%, the compounds added to both upper and lower compartments were preincubated for 15 minutes at 37°C before addition of plasmin. Similarly, (Rp)-8-pCPT-cGMPS, another inhibitor of cGMP-dependent protein kinase,34 dissolved in HBSS-BSA 0.4% was preincubated for 15 minutes at 37°C before addition of plasmin. When the stable cGMP analog 8-bromo-cGMP was used, this compound dissolved in HBSS-BSA 0.4% was added together with the chemoattractant plasmin to the lower compartment only.
Analysis of cellular F-actin content.Cellular content of polymerized actin was analyzed by NBD-phallacidin staining of fixed monocytes using a modification of the method of Howard and Meyer.35 In brief, 100-μL portions of monocyte suspensions (107 cells/mL) incubated at 37°C were treated with either plasmin, inactivated plasmin, plasminogen, or FMLP, and the reaction was stopped at designated time points by addition of fixing-staining buffer (137 mmol/L NaCl, 2.7 mmol/L KCl, 8.1 mmol/L Na2HPO4 , and 1.5 mmol/L KH2PO4 ), pH 7.0, containing 8% (wt/vol) paraformaldehyde, 0.2 mg lysophosphatidylcholine/mL, and 0.3 μmol/L NBD-phallacidin.36 The cells were stained for at least 30 minutes at room temperature, sedimented and washed, and finally resuspended in 500 μL phosphate-buffered saline (PBS). Monocytes were labeled with FITC-tagged mouse antihuman monoclonal antibodies against CD14. The cells were analyzed by flow cytometry using a FACScan (Becton Dickinson, San Jose, CA) with excitation at 488 nm and emission recorded at 530 nm. A minimum of 10,000 cells per sample were counted. The mean fluorescence intensity per cell was used as a measure of F-actin content per cell. Results are given as the relative fluorescence index representing the ratio of mean fluorescence intensity of agonist-treated cells at the designated time point to the mean fluorescence intensity of control cells.
Analysis of cGMP.Monocytes (107 cells/mL) incubated in HBSS-BSA 0.4% were adapted to 37°C for 6 minutes, plasmin was added, and the incubation was continued. At designated time points, incubation of 100 μL cell suspension was terminated by addition of 25 μL perchloric acid 35% (wt/vol). After 15 minutes at 4°C, the tubes were centrifuged at 3,000g for 15 minutes at 0°C, and the supernatants were collected and neutralized with 5.82 mol/L KOH. After 60 minutes at 4°C, the tubes were centrifuged again, the supernatants were acetylated, and cGMP was determined radioimmunologically according to the instructions of the manufacturer (Amersham). Standard curves were processed the same way as samples.
Plasmin activity and active-site blockade.Human plasmin was determined as activity against the substrate S-2251.37 It was assayed in a reaction buffer of 50 mmol/L Tris hydrochloride, pH 7.4, 110 mmol/L NaCl in a volume of 1.35 mL at 37°C. After addition of 250 μL prewarmed S-2251 (600 μmol/mL final concentration), the change in absorbance at 405 nm due to hydrolysis of the substrate was measured every 30 seconds. Slopes were calculated by least-squares linear regression analysis and compared with rates of substrate hydrolysis by known amounts of standard plasmin in the same system. Plasminogen was determined in the same amidolytic assay after activation with streptokinase 10,000 U/mL at 37°C for 10 minutes.37 Active-site–blocked plasmin was obtained by incubation of 4 μmol/L human plasmin in 100 mmol/L phosphate buffer, pH 7.4, with 4 mmol/L D-Val-L-Phe-L-Lys CH2Cl at 37°C for 15 minutes.38 39 The incubation mixture was dialyzed exhaustively against PBS. Active-site–blocked plasmin had no residual activity using S-2251 as substrate. Considering the sensitivity of the assay, greater than 99% of plasmin activity had been inactivated.
Statistics.Values are expressed as the mean ± SEM. Statistical differences were assessed by analysis of variance combined with the Newman-Keuls test. A threshold P value less than .05 was considered significant.
RESULTS
Chemotactic activity of plasmin on monocytes and PMNs.Addition of purified human plasmin between 0.01 and 2.86 CTA U/mL to the lower compartments of the chemotaxis chambers triggered a significant (n = 9, P < .01 v HBSS control) and concentration-dependent stimulation of monocyte migration across the polycarbonate membranes (Table 1). At plasmin levels of 0.43 and 1.43 CTA U/mL, respectively, monocyte locomotion was not significantly different, indicating that the maximum effect of the concentration-response curve had been reached. A further increase of plasmin concentration to 2.86 CTA U/mL led to a significant decrease (n = 9, P < .05 v cell migration at a plasmin concentration of 0.43 CTA U/mL) in monocyte locomotion, indicating a bell-shaped concentration-response curve. At maximum effective plasmin concentrations, monocyte migration was not significantly different from that observed with the standard chemoattractant FMLP used at the chemotactic optimal concentration of 10 nmol/L (Table 1).
When PMNs were used instead of monocytes, cells migrated, as expected, toward the standard chemoattractant FMLP 10 nmol/L (n = 3, P < .01 v basal HBSS control), but did not react with plasmin as potential chemoattractant (Table 1).
Checkerboard analysis showed that plasmin-induced monocyte migration required a positive concentration gradient between the lower and upper compartment of the chemotaxis chamber (Table 2). Monocyte locomotion was therefore mainly due to chemotactic activity, with only a minor component attributable to chemokinetic effects (Table 2). The small chemokinetic effect of plasmin became apparent when plasmin 0.43 CTA U/mL was added to both the upper and lower compartments of the chemotaxis chamber. Under these circumstances, a tendency for an increase (n = 4, NS) in cell migration from 4.8 ± 0.6 to 6.8 ± 0.8 cells/high-power field was observed (Table 2).
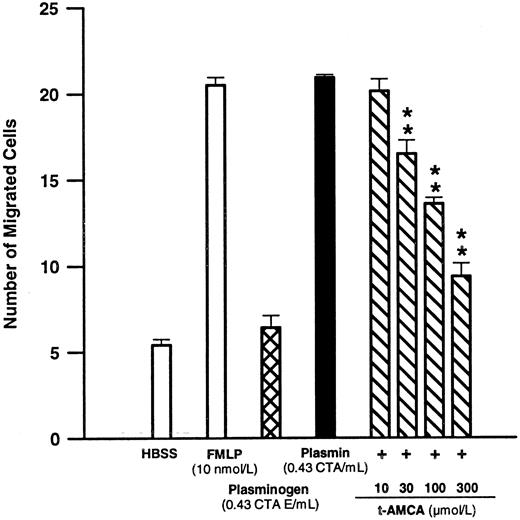
Significance of the intact catalytic center and lysine binding sites for plasmin-mediated chemotaxis.Plasmin at 0.43 CTA U/mL elicited a chemotactic response similar to 10 nmol/L FMLP (n = 4, NS; Fig 1). However, addition of the inactive zymogen plasminogen at 0.43 CTA U equivalents/mL to the lower compartment did not induce any significant monocyte locomotion indicating that the catalytic center might be important for chemotactic activation (Fig 1). However, when plasminogen 0.43 CTA U equivalents/mL was added together with urokinase 2,000 CTA U/mL to the lower compartment, the monocytes responded with chemotaxis (19.7 ± 0.4 cells/high-power field, n = 5) comparable to that seen with plasmin 0.43 CTA U/mL (18.6 ± 1.8 cells/high-power field, n = 5). Compared with basal conditions without chemoattractant, urokinase 2,000 CTA U/mL alone did not induce any significant monocyte migration (5.8 ± 0.4 v 7.2 ± 0.7 cells/high-power field, respectively, n = 5 each).
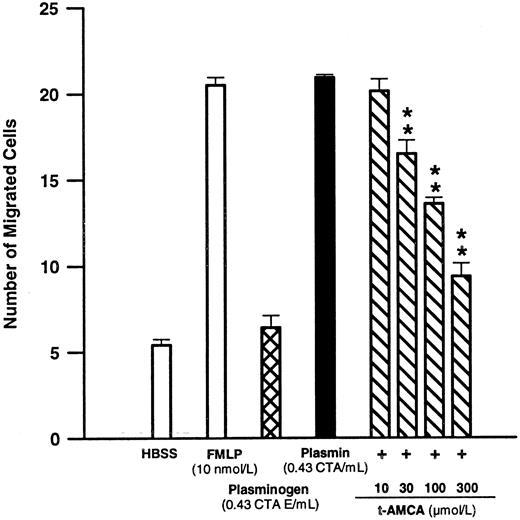
Effects of FMLP 10 nmol/L, plasminogen 0.43 CTA U equivalents/mL, or plasmin 0.43 CTA U/mL and the modulatory effect of t-AMCA on plasmin-induced monocyte migration. Chemotactic activity was evaluated for 90 minutes with polycarbonate membranes (pore size, 5 μm). **P < .01 v plasmin control. Results are the mean ± SEM of 4 experiments each performed in triplicate.
Effects of FMLP 10 nmol/L, plasminogen 0.43 CTA U equivalents/mL, or plasmin 0.43 CTA U/mL and the modulatory effect of t-AMCA on plasmin-induced monocyte migration. Chemotactic activity was evaluated for 90 minutes with polycarbonate membranes (pore size, 5 μm). **P < .01 v plasmin control. Results are the mean ± SEM of 4 experiments each performed in triplicate.
By interaction with the lysine binding sites located in the Kringle structures of the plasmin/ogen molecule, lysine analogs such as t-AMCA are able to inhibit the binding of plasmin/ogen to appropriate binding sites on the monocyte membrane.8 9 t-AMCA 10 to 300 μmol/L led to a concentration-dependent inhibition of plasmin-induced monocyte migration (n = 4; Fig 1).
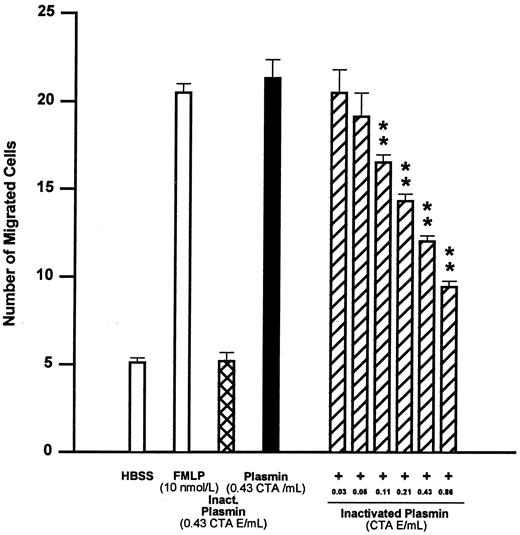
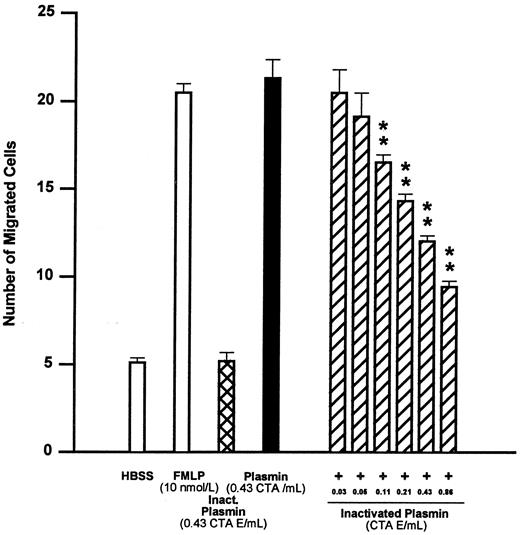
Similar to plasminogen, active-site–blocked plasmin at 0.43 CTA U equivalents/mL was unable to elicit monocyte locomotion (Fig 2). However, active-site–blocked plasmin from 0.03 to 0.86 CTA U equivalents/mL antagonized the chemotactic effect of active plasmin 0.43 CTA U/mL in a concentration-dependent manner (Fig 2). The concentration of active-site–blocked plasmin inducing 50% inhibition of monocyte chemotaxis (IC50 ) was calculated to be 0.34 CTA U equivalents/mL, with 95% confidence limits of 0.23 to 0.40 CTA U equivalents/mL (n = 4).
Effects of FMLP 10 nmol/L, active-center–blocked plasmin (inactivated plasmin), or plasmin and the inhibitory effect of active-center–blocked plasmin on plasmin-induced monocyte migration. **P < .01 v plasmin control. Results are the mean ± SEM of 4 experiments each performed in triplicate.
Effects of FMLP 10 nmol/L, active-center–blocked plasmin (inactivated plasmin), or plasmin and the inhibitory effect of active-center–blocked plasmin on plasmin-induced monocyte migration. **P < .01 v plasmin control. Results are the mean ± SEM of 4 experiments each performed in triplicate.
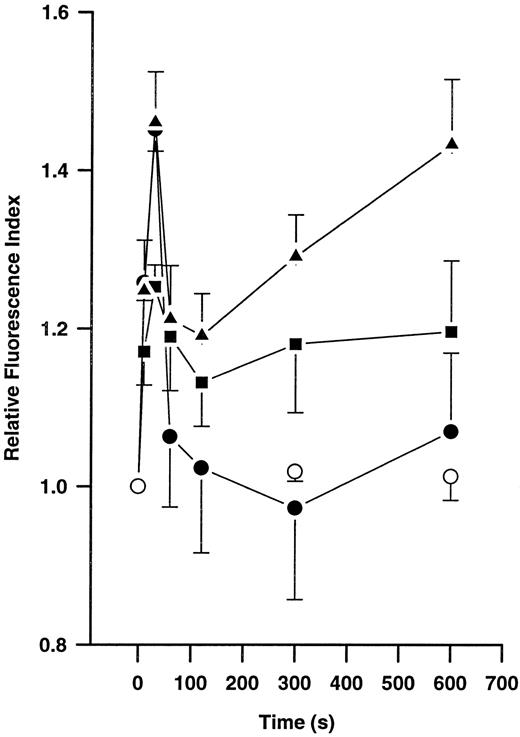
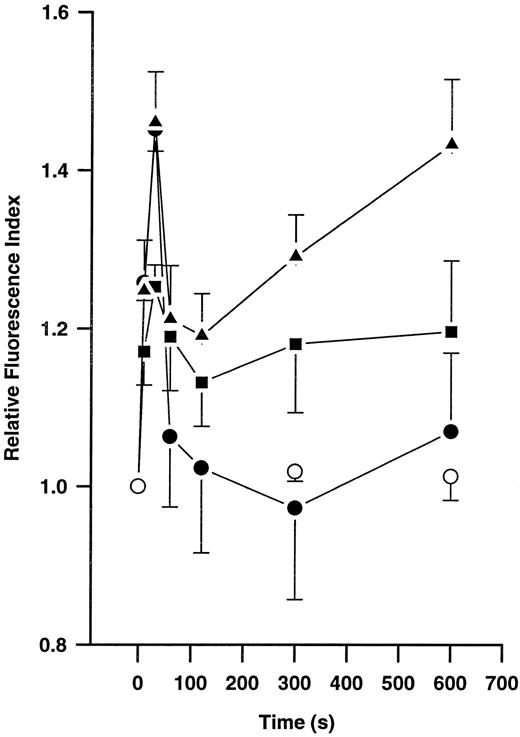
Effects of plasmin on actin polymerization.Incubation of monocytes without plasmin did not lead to any significant changes in F-actin content during a 10-minute incubation period (Fig 3). However, stimulation with plasmin 0.14 and 0.43 CTA U/mL led to a time- and concentration-dependent increase in F-actin formation (n = 4 each). Similarly, 10 nmol/L FMLP induced time-dependent changes in monocyte F-actin content (n = 4). With any amount of either stimuli, a biphasic response was observed. An early peak occurred at 30 seconds, followed by a decrease lasting from about 1 to 5 minutes that was followed by a repeated increase in F-actin content thereafter (Fig 3). During the first peak, the same extent of F-actin formation was observed with both stimuli, FMLP 10 nmol/L and plasmin 0.43 CTA U/mL. In contrast, the inactive zymogen plasminogen and active-site–blocked plasmin, both used at concentrations equivalent to 0.43 CTA U active plasmin, were unable to stimulate F-actin formation (n = 2 each, data not shown).
Time-dependent formation of polymerized actin in human monocytes (5 × 106/mL) without chemoattractant (○) and after stimulation with 10 nmol/L FMLP (•) or 0.14 (▪) or 0.43 (▴) CTA U/mL plasmin. Polymerized actin was quantified by flow cytometric analysis after staining with NBD-phallacidin. Results are the mean ± SEM of 4 experiments.
Time-dependent formation of polymerized actin in human monocytes (5 × 106/mL) without chemoattractant (○) and after stimulation with 10 nmol/L FMLP (•) or 0.14 (▪) or 0.43 (▴) CTA U/mL plasmin. Polymerized actin was quantified by flow cytometric analysis after staining with NBD-phallacidin. Results are the mean ± SEM of 4 experiments.
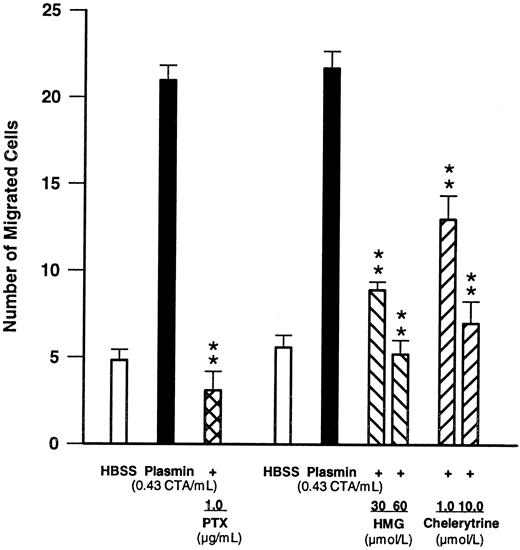
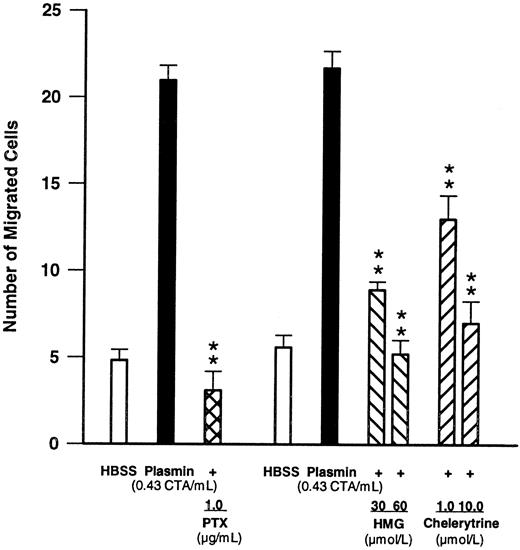
Effects of compounds modulating signal transduction.To obtain insight into the signal transduction mechanisms activated by plasmin, monocytes were pretreated for 90 minutes with PTX (1 μg/mL), a concentration known to inhibit G protein–mediated responses.27 28 Such pretreatment led to a complete blockade (n = 4, P < .01 v plasmin control) of plasmin-induced monocyte migration (Fig 4), indicating involvement of a PTX-sensitive G protein. Furthermore, when chemotaxis experiments were performed in the presence of two structurally unrelated PKC inhibitors such as HMG at 30 and 60 μmol/L or chelerythrine at 1 and 10 μmol/L, monocyte locomotion elicited by plasmin 0.43 CTA U/mL was significantly (n = 4, P < .01 v plasmin control) and concentration-dependently inhibited (Fig 4).
Effects of PTX (1 μg/mL, 90-minute preincubation) and of the PKC inhibitors HMG and chelerythrine on plasmin-induced monocyte migration. **P < .01 v the appropriate plasmin control. Results are the mean ± SEM of 4 experiments each.
Effects of PTX (1 μg/mL, 90-minute preincubation) and of the PKC inhibitors HMG and chelerythrine on plasmin-induced monocyte migration. **P < .01 v the appropriate plasmin control. Results are the mean ± SEM of 4 experiments each.
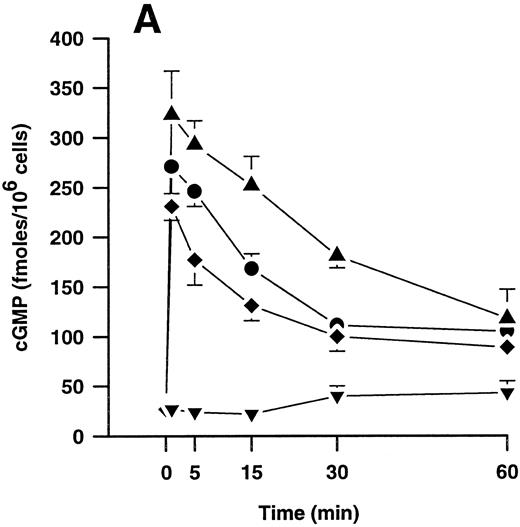
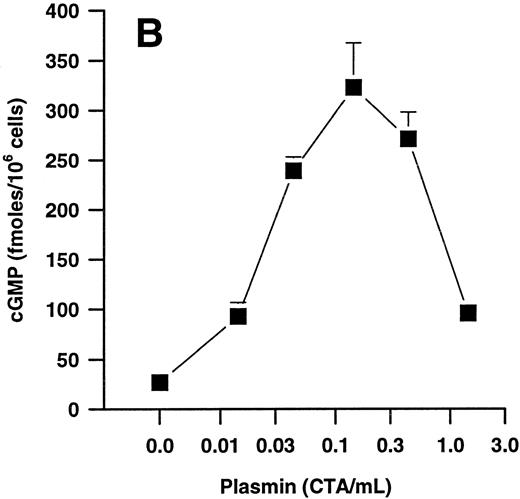
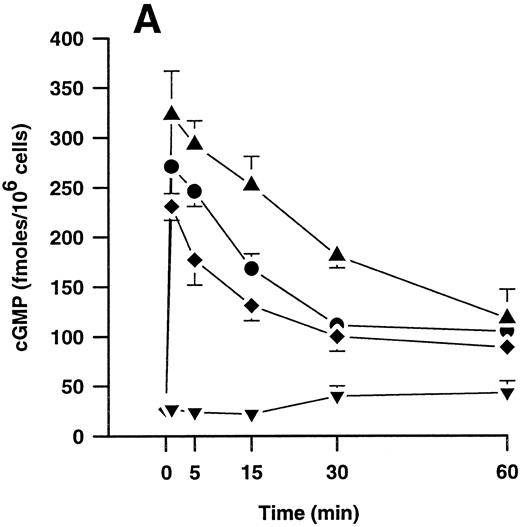
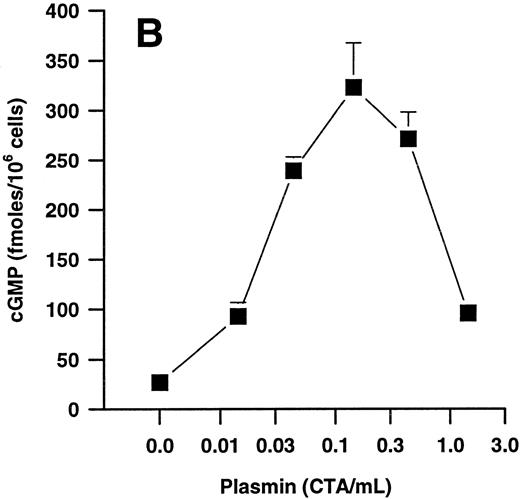
Effects of plasmin and related compounds on monocyte cGMP levels.cGMP seems to play a major role in morphologic alterations required for the monocyte response to chemoattractants such as FMLP.40-43 We therefore investigated whether plasmin might be able to elicit formation of cGMP in human PMs. Indeed, plasmin stimulated a time- and concentration-dependent formation of small amounts of cGMP in monocytes (Fig 5). Peak levels were found at 1 minute after plasmin stimulation, with a decrease up to 60 minutes. At 60 minutes, the levels were still significantly (n = 4, P < .05 v basal control) elevated with all concentrations tested (Fig 5A). Elevation of cGMP levels was slightly higher by plasmin 0.14 CTA U/mL versus 0.43 CTA U/mL, suggesting a bell-shaped concentration-response curve. This was confirmed when we analyzed the effect of a wider range of plasmin concentrations on cGMP formation by monocytes stimulated for 1 minute (Fig 5B). The most effective concentration of plasmin with respect to generation of cGMP was 0.143 CTA U/mL, followed by 0.43 and 0.043 CTA U/mL. The stimulatory effect of plasmin at 1.43 CTA U/mL was comparable to that of 0.014 CTA U/mL (Fig 5B).
(A) Time-dependent effects of plasmin 0.043 CTA U/mL (♦), 0.14 CTA U/mL (▴), and 0.43 CTA U/mL (•) on cGMP formation by human monocytes (107 cells/mL) compared with controls without plasmin (▾). (B) Concentration-dependent effect of plasmin on generation of cGMP by monocytes stimulated for 1 minute. Cell suspensions were prewarmed to 37°C for 6 minutes before addition of plasmin for the periods indicated. Results are the mean ± SEM of 4 experiments each.
(A) Time-dependent effects of plasmin 0.043 CTA U/mL (♦), 0.14 CTA U/mL (▴), and 0.43 CTA U/mL (•) on cGMP formation by human monocytes (107 cells/mL) compared with controls without plasmin (▾). (B) Concentration-dependent effect of plasmin on generation of cGMP by monocytes stimulated for 1 minute. Cell suspensions were prewarmed to 37°C for 6 minutes before addition of plasmin for the periods indicated. Results are the mean ± SEM of 4 experiments each.
During an observation period of 60 minutes, neither plasminogen nor active-site–blocked plasmin, both at concentrations up to 0.43 CTA U equivalents/mL, triggered any significant cGMP formation (n = 2, data not shown).
cGMP formation could be concentration-dependently inhibited by LY83583, an inhibitor of soluble guanylyl cyclase.32 Pretreatment of monocytes for 15 minutes with 1 to 100 μmol/L LY83583 before stimulation with plasmin 0.143 CTA U/mL for 2 minutes concentration-dependently inhibited cGMP formation with an IC50 value of 6.8 μmol/L and 95% confidence limits of 4.3 to 10.8 μmol/L (n = 4).
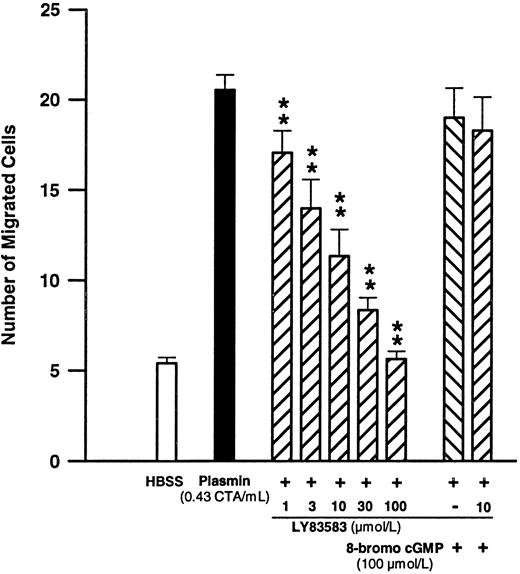
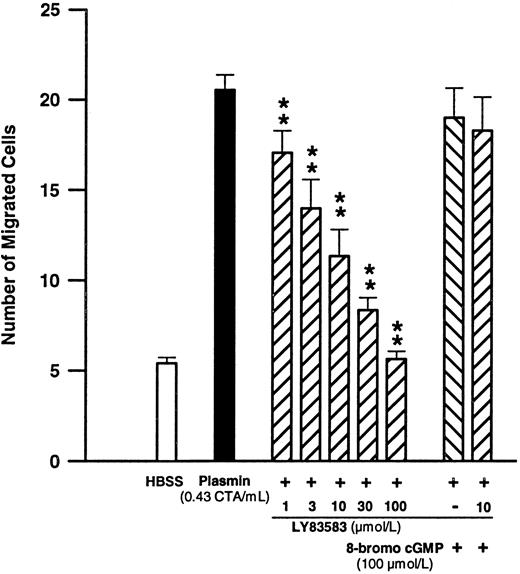
Significance of cGMP formation for plasmin-induced monocyte migration.Since cGMP is generated by monocytes upon plasmin stimulation and is purportedly important for their chemotactic response,40-42 we have investigated the effect of LY83583 on plasmin-induced monocyte locomotion. Pretreatment of monocytes with LY83583 concentration-dependently inhibited plasmin-mediated monocyte migration (Fig 6). The inhibitory effect was significant (n = 6, P < .01 v plasmin control) between 1 and 100 μmol/L LY83583 (Fig 6). The IC50 value for LY83583 was calculated to be 6.4 μmol/L, with 95% confidence limits of 4.4 to 9.4 μmol/L. LY83583 at a concentration of 100 μmol/L completely blocked monocyte migration triggered by plasmin 0.43 CTA U/mL. At 10 μmol/L, LY83583 reduced the plasmin-induced chemotactic response by 63.8% ± 9.7% (n = 6, P < .01 v plasmin control). The inhibitory effect of 10 μmol/L of the guanylyl cyclase inhibitor LY83583 could be antagonized by 100 μmol/L of the stable cGMP analog 8-bromo-cGMP. Addition of 8-bromo-cGMP 100 μmol/L in the absence of LY83583 did not significantly affect the plasmin-triggered chemotactic response (Fig 6).
Concentration-dependent effects of the soluble guanylyl cyclase inhibitor LY83583 on plasmin-induced monocyte migration and the effect of 100 μmol/L 8-bromo-cGMP. **P < .01 v plasmin control. Results are the mean ± SEM of 6 experiments.
Concentration-dependent effects of the soluble guanylyl cyclase inhibitor LY83583 on plasmin-induced monocyte migration and the effect of 100 μmol/L 8-bromo-cGMP. **P < .01 v plasmin control. Results are the mean ± SEM of 6 experiments.
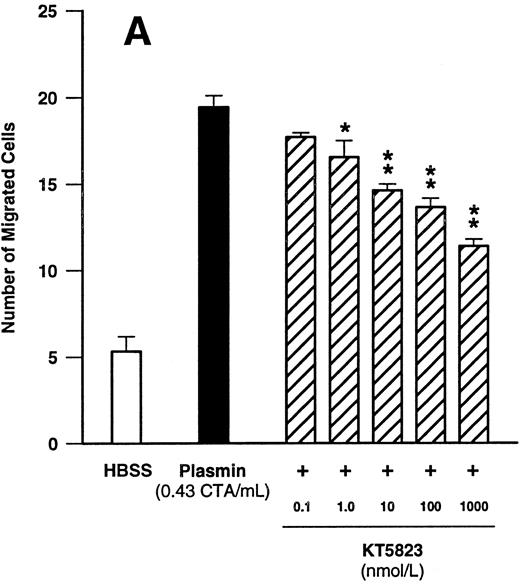
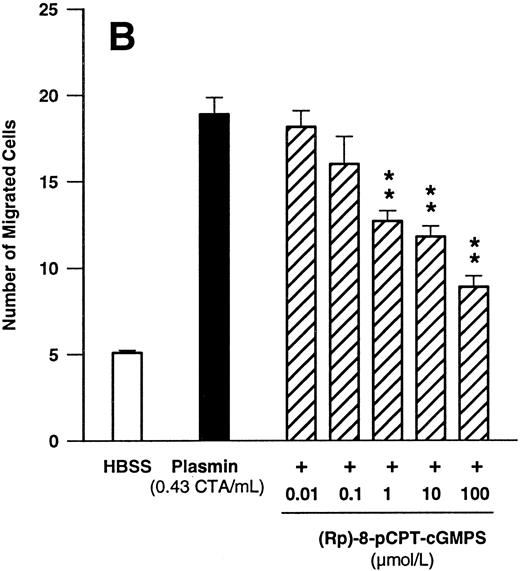
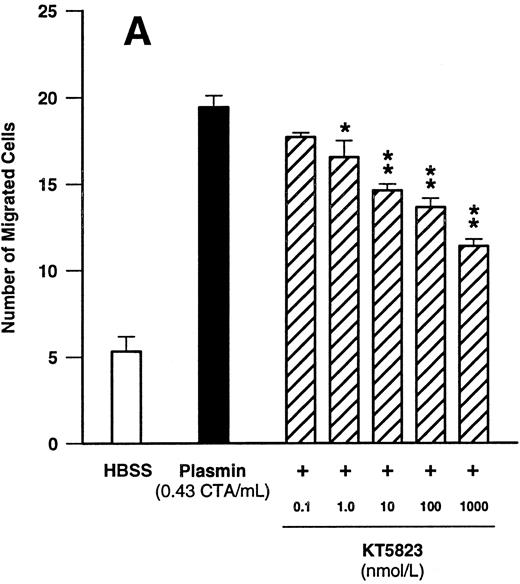
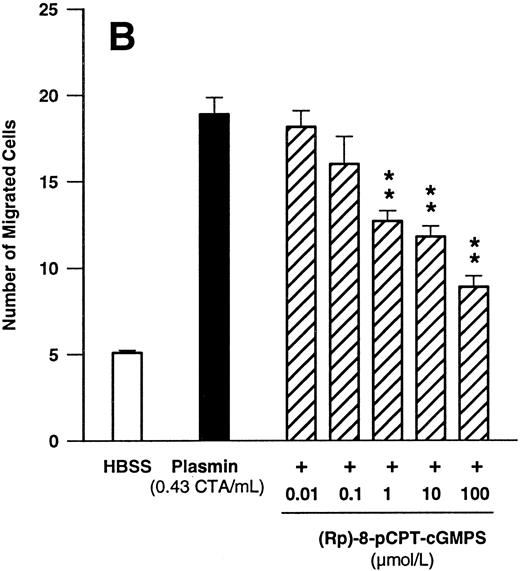
Since these data indicated that cGMP may play a significant role in plasmin-induced monocyte locomotion, we further tested the effect of KT5823, an inhibitor of G-kinase. KT5823 between 1 nmol/L and 1 μmol/L led to a significant (n = 4, P < .05 v plasmin control) and concentration-dependent inhibition of monocyte migration elicited by plasmin 0.43 CTA U/mL (Fig 7A). The IC50 value was 230 nmol/L, with 95% confidence limits of 70 to 761 nmol/L. In additional experiments with the structurally unrelated G-kinase inhibitor (Rp)-8-pCPT-cGMPS, a concentration-dependent inhibition of plasmin-induced monocyte chemotaxis was also observed (Fig 7B). With this compound, the IC50 value was 2.1 μmol/L, with 95% confidence limits of 0.6 to 7.6 μmol/L (n = 4).
Concentration-dependent effects of inhibitors of the cGMP-dependent kinase such as KT5823 (A) and (Rp)-8-pCPT-cGMPS (B) on plasmin-induced monocyte migration. *P < .05 and **P < .01 v appropriate plasmin controls. Results are the mean ± SEM of 4 experiments each.
Concentration-dependent effects of inhibitors of the cGMP-dependent kinase such as KT5823 (A) and (Rp)-8-pCPT-cGMPS (B) on plasmin-induced monocyte migration. *P < .05 and **P < .01 v appropriate plasmin controls. Results are the mean ± SEM of 4 experiments each.
DISCUSSION
We have previously shown that plasmin at low concentrations triggers release of lipid mediators of the 5-lipoxygenase pathway such as LTB4 from human PMs.23 24 To test whether plasmin could trigger a more general proinflammatory response in human monocytes, we have now investigated the effect of plasmin on monocyte locomotion.
Our data demonstrate that purified plasmin is a potent chemoattractant for human monocytes. At a concentration as low as 0.01 CTA U/mL, it triggered a significant chemotactic response. At 0.43 CTA U/mL, the potency of plasmin was comparable to that of the standard chemoattractant FMLP at its chemotactic optimal concentration of 10 nmol/L.25,26 Although there may be formation of approximately 2.2 CTA U plasmin/mL plasma,43 to our knowledge, there is only little information available regarding the local generation of plasmin in circumscript inflammatory reactions. Plasma levels of plasmin–α2 -antiplasmin complexes commonly used as an index for the amount of plasmin formed in vivo are drastically elevated only under severe pathogenetic circumstances, eg, disseminated intravascular coagulation,44 and do not reflect local concentrations. Thus, patients with rheumatoid arthritis have high levels of plasmin–α2 -antiplasmin complexes in the synovial fluid17 but only a moderate increase in plasma as compared with normal subjects.16 Local plasmin generation could occur on the monocyte membrane, where it would be protected from endogenous inhibitors.45,46 Indeed, plasmin generated during contact activation of plasma was sufficient for plasmin-induced LTB4 formation by human monocytes.24 The trace amounts of plasmin generated under such conditions are well below the detection limit of conventional analytic methods.47 The previous experiments on LT generation clearly show23 24 that even in the presence of plasminogen, which may compete for the same binding site, small amounts of plasmin are able to stimulate monocytes. Therefore, a plasmin-mediated monocyte chemotaxis should also occur under physiologic conditions.
The chemotactic effect of plasmin appears to be specific for monocytes, as it was not found with PMNs, which nevertheless responded to FMLP. This specificity for monocytes is in line with previous results on plasmin-mediated LTB4 biosynthesis that was also selectively stimulated in monocytes but not in PMNs.24
Moreover, the plasmin-mediated effect on monocytes is apparently specific for the molecule plasmin, since the lysine analog t-AMCA, which is able to prevent binding of plasmin/ogen to the appropriate membrane binding sites,8,9 concentration-dependently inhibited the chemotactic response. Interactions of plasmin with the surface of fibrin and the membranes of various cells are mediated by lysine binding sites associated with the Kringle domains of the plasmin/ogen molecule.7,8 In our experiments, an intact catalytic center was also required, since plasmin or plasminogen activated with urokinase induced a chemotactic response. In contrast, the inactive zymogen plasminogen or active-site–blocked plasmin were unable to trigger monocyte chemotaxis. On the other hand, active-site–blocked plasmin inhibited the effect of active plasmin in a concentration-dependent manner, suggesting that both compounds compete for the same binding site. The effects of plasminogen and of t-AMCA and catalytic-center–blocked plasmin on plasmin-induced monocyte locomotion are consistent with those observed for plasmin-stimulated LTB4 formation.48,49 These criteria might represent common principles for cellular stimulation by plasmin. Thus, the significance of binding via lysine binding sites and the importance of the active catalytic center have also been demonstrated for plasmin-induced PMN aggregation11 and the early phase of plasmin-stimulated arachidonate release from bovine endothelial cells.14
One could argue that LTB4 generated by plasmin-stimulated monocytes24 may contribute to the chemotactic response. However, LTB4 would be produced by the monocytes located in the upper compartment of the chemotaxis chamber. Therefore, no positive gradient of the chemoattractant known to be essential for chemotaxis25,26 would be formed across the membrane, precluding a chemotactic response. Moreover, we have performed experiments with monocytes pretreated with the 5-lipoxygenase inhibitor BAY X1005 at 50 μmol/L,50 a concentration that blocks the ionophore A23187-induced LT formation. Such pretreatment did not affect monocyte chemotaxis elicited by plasmin 0.43 CTA U/mL (Syrovets et al, unpublished results, August 1996), supporting the view that LTB4 is not relevant for the chemotaxis observed.
Polymerization of actin is essential for cell motility and consequently for the chemotactic response.35,51 Using NBD-phallacidin as a specific label for F-actin,35 plasmin was shown to induce a concentration-dependent polymerization of actin. The extent of F-actin formation does not necessarily correlate with the chemotactic response.52 Nevertheless, it is of interest that with respect to the chemotactic response and F-actin formation, both FMLP 10 nmol/L and plasmin 0.43 CTA U/mL yielded quantitatively similar results. Since cell movement requires a dynamic polymerization and depolymerization of actin,51 the failure of complete depolymerization and the apparent repolymerization at 300 and 600 seconds in the presence of higher concentrations of plasmin could partially explain the bell shape of the plasmin concentration-response curve.
Receptors for chemotactic agents are generally thought to be coupled to G proteins.53 This has been concluded on the basis of experiments using specific bacterial toxins such as PTX. PTX has been shown to inhibit FMLP-, C5a-, and MCP-1–induced signal transduction and chemotaxis in human monocytes.27,28 In our experiments, a concentration of PTX known to block monocyte chemotaxis toward the aforementioned chemoattractants27,28 was equally effective in blocking the plasmin-induced chemotactic response. Thus, consistent with results from plasmin-stimulated bovine endothelial cells,14 the signaling pathway activated by plasmin involves a PTX-sensitive G protein.
Another important component of chemoattractant signal transduction is PKC.53 HMG, an ether lipid believed to interact with the diacylglycerol binding site of PKC, has been shown to inhibit the FMLP-stimulated respiratory burst in human PMNs.29 This compound, claimed to be specific,29,30 concentration-dependently inhibited plasmin-induced monocyte locomotion. In addition, chelerythrine, another inhibitor of PKC known to interact with the catalytic domain of PKC,31 likewise led to a concentration-dependent inhibition of plasmin-induced monocyte migration. Thus, two structurally but also functionally different inhibitors of PKC exerted significant inhibitory effects suggesting a crucial role of PKC in plasmin-induced monocyte locomotion.
Apart from phosphatidylinositol hydrolysis, which does not seem to be activated by plasmin stimulation in monocytes,49 cyclic nucleotides might also play a major role as second messengers in chemoattractant signal transduction. Particularly cGMP has been implicated in monocyte chemotaxis, since agents that elevate cGMP levels, in contrast to those that increase cAMP levels, enhanced monocyte chemotactic responses.40,41 We therefore tested whether plasmin was to induce cGMP formation in human monocytes. Our data demonstrate that plasmin induces moderate but long-lasting elevations of monocyte cGMP content. The plasmin-mediated cGMP formation is governed by a bell-shaped concentration-response curve. The optimal concentration for cGMP formation was 0.143 CTA U/mL, which is identical to the optimal concentration for stimulation of LTB4 biosynthesis.49 Although the monocyte chemotactic response to plasmin also follows a bell-shaped concentration-response curve, the optimal concentration for chemotaxis is much higher (0.43 CTA U/mL). However, concentrations in chemotaxis experiments do not reflect the true concentrations the cells are exposed to. In fact, plasmin is largely diluted during chemotaxis experiments, since the chemoattractant diffuses through the polycarbonate membrane into the upper compartment, leading to formation of a concentration gradient.
Bell-shaped concentration-response curves for chemoattractants are not unusual. FMLP is known to induce bell-shaped chemotactic responses,25 and thrombin also elicits monocyte chemotaxis with a bell-shaped concentration-response curve.54 Since plasmin/ogen binding sites represent a heterogeneous group of molecules,7-9 such receptors could be linked to different second-messenger systems evoking opposite results.55 On the other hand, autodigestion of the plasmin molecule56 could lead to fragment binding to the membrane receptor without the catalytic center. Such fragments, similar to catalytic-center–blocked plasmin, might then act as antagonists.
Even opposite effects have been observed in platelets at different plasmin concentrations.12,13 Thus, plasmin triggers both platelet activation and inhibition. The inhibitory effect on agonist-induced platelet activation was observed at low concentrations of plasmin,12 whereas platelet activation and aggregation was observed at higher concentrations.13
cGMP formation by monocytes is critical for the plasmin-mediated chemotactic response, since both parameters were concentration-dependently inhibited by LY83583, an inhibitor of the soluble guanylyl cyclase.32 Indeed, at approximately 6.5 μmol/L, the IC50 values for both effects were identical. Moreover, these data are similar to the reported Ki of approximately 10 μmol/L for isolated soluble guanylyl cyclase.32 The inhibitory effect of 10 μmol/L LY83583 on monocyte chemotaxis was antagonized with 100 μmol/L of the stable cGMP analog, 8-bromo-cGMP, implying that the effect of LY83583 ought to be due to inhibition of cGMP formation. Several cellular receptor proteins exist for cGMP, such as cGMP-dependent phosphodiesterase, cGMP-gated ion channels, and G-kinase.57 The effects of two specific, structurally different inhibitors of G-kinase, KT5823 and (Rp)-8-pCPT-cGMPS,33,34 indicate that protein phosphorylation is crucial. The IC50 values obtained for inhibition of monocyte chemotaxis (230 nmol/L and 2.1 μmol/L, respectively) are in accordance with the reported Ki values of both compounds for G-kinase (234 nmol/L and 1.5 μmol/L, respectively).33 34
In conclusion, our data show that plasmin is a specific and potent chemoattractant for human monocytes acting via a cGMP-dependent pathway. Considering the ubiquitous distribution of plasminogen throughout the human body6,8 and its transformation to active plasmin at sites of wound healing,6 thrombus organization, and inflammatory and allergic reactions,18,19 it is feasible that plasmin-mediated chemotaxis significantly contributes to monocyte recruitment during such processes. This effect may be particularly important for later phases of inflammatory reactions, which are known to depend on the influx and recruitment of monocytes.58,59 Combining our present results on plasmin-mediated monocyte chemotaxis with our previous findings on plasmin-induced lipid mediator release,24,49 it appears that plasmin represents a fundamental proinflammatory activator for human monocytes. Indeed, this notion is further supported by our recent findings showing that plasmin additionally induces cytokine gene expression and cytokine release in human monocytes.60 Therefore, plasmin might be a pathophysiologically important proinflammatory stimulus acting on monocytes but not on PMNs.
ACKNOWLEDGMENT
The authors thank Dr S. Philippou for scanning and transmission electron microscopy. Human Glu-plasminogen, urokinase, and streptokinase were gifts from Dr J. Römisch (Behring, Marburg, Germany).
Supported in part by the Deutsche Forschungsgemeinschaft.
Address reprint requests to Thomas Simmet, MD, Department of Pharmacology and Toxicology, Ruhr University, Universitätstrasse 150, D-44780 Bochum, Germany.