Abstract
Conversion by α-thrombin of the zymogen human protein C (HPC) to activated protein C (aPC) is an important physiologic feedback control mechanism for the coagulation cascade. Although activation of HPC by thrombomodulin-bound thrombin is relatively rapid, activation by free thrombin occurs at a significantly slower rate. Previously, we generated a “hyper-activatable” derivative of HPC (FLIN-Q3) with an increased activation rate by free α-thrombin in vitro. In this study, the antithrombotic efficacy of FLIN-Q3 was compared with both native zymogen and aPC in an arteriovenous shunt model of thrombosis in the guinea pig. Recombinant proteins were infused 15 minutes before and throughout a 15-minute period while blood was circulated from carotid to jugular through tubing that enclosed a thread on which fibrin was deposited. Parallel dose-dependent antithrombotic responses were observed. Under these non–steady-state conditions, the calculated infusion doses associated with a 50% reduction of thrombus mass were 2.7, 24, and 250 mg/kg/h for aPC, FLIN-Q3, and HPC, respectively. Thrombus weight correlated inversely with plasma concentration of aPC, measured amidolytically, from either direct infusion of aPC or that generated from the zymogens in the animal, and similarly correlated inversely with anticoagulant activity measured by whole blood aPTT. Neither zymogen form showed significant aPC activity before shunt circulation, suggesting a requirement for exposure to thrombin. After the infusion was discontinued for 15 minutes, a second period of thrombus formation in the shunt demonstrated the ability of zymogen forms of PC, unlike aPC, to provide “on-demand” anticoagulant responses to repeated thrombotic stimuli. Thus, a “hyper-activatable” PC molecule such as FLIN-Q3 may represent a superior form of anticoagulant therapy than either the native zymogen or aPC.
THROMBOSIS REPRESENTS a hemostatic disequilibrium between pro- and antithrombotic mechanisms. One of the critical factors in maintaining normal hemostasis is protein C (PC). This zymogen is the proenzyme for the anticoagulant enzyme activated PC (aPC), which is proteolytically activated at the endothelial cell surface by thrombin1,2 in complex with the cofactor thrombomodulin.3-7 The rate of aPC generation by free thrombin is approximately 1,000-fold less than that of thrombin in complex with thrombomodulin, suggesting that in vivo, little aPC is normally generated by free thrombin. The anticoagulant effect of aPC occurs in complex with the cofactor protein S on the membrane surface of platelets and endothelium,7 where factors Va and VIIIa, cofactors involved in the amplification of thrombin generation, are proteolytically inactivated.8-10
Both PC zymogen and aPC are effective antithrombotics in animal models and in human thrombotic diseases.11-13 However, the zymogen is used primarily for acquired PC deficiencies and microvascular thrombosis, such as disseminated intravascular coagulation (DIC) associated with sepsis. Because the ratio of endothelial surface area to vessel diameter has been estimated to be approximately 1,000 times greater in microvessels than in conduit vessels,7,14 the local availability of endothelial thrombomodulin may limit activation of the zymogen primarily to the microvasculature. As a thrombus enlarges into the stenotic lumen of a large vessel, thrombin generated in the thrombus binds to fibrin in the thrombus and retains enzymatic activity.15-24 Endothelial thrombomodulin, even in the immediate vicinity, may be too remote to complex with thrombin in sufficient quantity to activate PC and, thereby, stop the explosive generation of thrombin before the narrowed vessel occludes. To escape this limitation, aPC has been infused directly and produced antithrombotic efficacy.22-30 However, aPC has a short biological half-life because it is inhibited by the plasma inhibitors PC inhibitor and α1-antitrypsin.31-33 In humans, the reported half-life of aPC is approximately 23 minutes34 compared with 7 to 10 hours for zymogen human PC (HPC).35 36
In a previous study, we described a derivative of PC that was activated more efficiently by thrombin in the absence of thrombomodulin.37 In this derivative, designated FLIN-Q3, two inhibitory acidic residues near the thrombin cleavage site were changed (D167F and D172N) and combined with N313Q, which reduced the calcium-mediated physiologic inhibition of HPC activation by free thrombin in the absence of thrombomodulin. The rate of thrombin-catalyzed activation of FLIN-Q3 in vitro was 60-fold greater than that of HPC. Furthermore, unlike native HPC, FLIN-Q3 was activated by thrombin generated in clotting human plasma.
The purpose of the present study was to compare in an animal model the antithrombotic efficacy and potency of aPC, FLIN-Q3, and HPC. Using a guinea pig model of thrombosis in an extracorporeal arteriovenous (AV) shunt, we demonstrate that the antithrombotic activity of each PC correlated with both anticoagulant activity (activated partial thromboplastin time [aPTT]) as well as aPC blood level. Measurement of aPC concentration in the plasma demonstrated that the zymogens were activated only after production of thrombin during the thrombotic process. Further, the FLIN-Q3 derivative was activated and produced an antithrombotic effect at infusion rates at which HPC had no effect. Finally, a second period of thrombus formation that occurred 15 minutes after the end of the infusion demonstrated the ability of zymogen forms of PC, unlike aPC, to provide “on-demand” anticoagulant responses to repeated thrombotic stimuli.
MATERIALS AND METHODS
Materials.The following reagents were used: bovine insulin hygromycin B from Eli Lilly and Company (Indianapolis, IN); Dulbecco's modified Eagle's medium (DMEM)/Ham's F12 medium (3:1 mixture, 93-0152PK), and gentamicin (600-5750AD) from GIBCO BRL (Grand Island, NY); fetal bovine serum (A-1111-L) from HyClone (Logan, UT); vitamin K1 (Aquamephyton, 7880) from Merck, Sharp, and Dohme (West Point, PA); Pentex human transferrin (82-316) and FAF bovine serum albumin (BSA; 82-002) from Miles Scientific (Kankakee, IL); Ultrafree-CL 30,000 NMWL filtration units (UCF4-LTK-25) from Millipore (Bedford, MA); Fast Flow Q Sepharose (17-0510-01) from Pharmacia (Piscataway, NJ); chromogenic substrate S-2366 (82-10-90) from Pharmacia Hepar (Franklin, OH); bicinchoninic acid (BCA) protein assay kit (23225-B) from Pierce (Rockford, IL); Centricell units (30,000 NMWL, Y18674-8) from Polysciences (Warrington, PA); benzamidine-HCl (B-6506) from Sigma (St Louis, MO); and HEPES (16926) from United States Biochemical (Cleveland, OH). Thrombin-Sepharose 4B was generously provided by J. Secnik and Dr W. Prouty of Eli Lilly and Co. Monoclonal antibody HPC-3 was provided by B. Chao and Dr B. Yan, also of Eli Lilly and Co. All other chemicals used were of the highest quality commercially available.
Preparation and purification of recombinant wild-type and mutant PC.Recombinant cell lines producing HPC and the FLIN-Q3 derivative were described previously.37,38 To obtain material for purification, recombinant cell lines were grown in serum-free medium (DMEM:Ham's F12, 3:1) containing 20 mmol/L HEPES (pH 7.4), 50 μg/mL gentamicin, 1 μg/mL bovine insulin, 1 μg/mL human transferrin, and 10 μg/mL vitamin K1. Conditioned medium was collected, filtered, and adjusted to a final concentration of 5 mmol/L benzamidine-HCl and 4 mmol/L EDTA. Purification was performed by the anion-exchange pseudo-affinity purification method described previously.39 Eluted protein was desalted/concentrated by centrifugation (30,000 NMWL retention filter), and dialyzed against normal saline. The concentration of protein was determined by BCA assay using BSA as a reference standard. Purity of recombinant PC preparations was greater than 95% as assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (data not shown).
Activation of HPC and FLIN-Q3 zymogens in vitro.Thrombin-Sepharose 4B (coupled at 2 mg thrombin/mL resin) was washed extensively in Buffer A-EDTA (150 mmol/L NaCl, 20 mmol/L Tris-HCl [pH, 7.40], and 1 mmol/L EDTA). Then, 800 μL of a solution containing 300 μg of zymogen PC (HPC or FLIN-Q3) in Buffer A-EDTA was incubated at 37°C with 200 μL packed/washed thrombin-Sepharose 4B on a rotating platform. During this incubation, the degree of zymogen PC activation was monitored by briefly pelleting the thrombin-Sepharose 4B and assaying an aliquot of the supernate for amidolytic activity using the chromogenic substrate S-2366. Following complete activation, the thrombin-Sepharose 4B was pelleted, the supernate collected, its protein concentration verified by BCA assay and then either assayed directly or frozen in aliquots at −20°C. Larger-scale preparations were similarly activated using a thrombin-Sepharose 4B column.
Thrombosis model.The extracorporeal AV shunt model for the rat40 41 was adapted for use in the guinea pig. Male Hartley guinea pigs (400 to 600g; Charles River, Wilmington, MA) were anesthetized with xylazine (Rompun, 30 mg/kg subcutaneously [sc]; Miles, Inc, Shawnee Mission, KS) and ketamine (Ketaset, 150 mg/kg sc; Fort Dodge Laboratories, Fort Dodge, IA) and were maintained on a heated water pad at 37°C. Shunts were constructed from two lengths (20 cm) of PE60 tubing (0.76 mm ID × 1.22 mm OD; Becton Dickinson, Sparks, MD) joined in the middle by a segment of PE190 tubing (6 cm, 1.19 mm ID × 1.70 mm OD). Within the lumen of the shorter segment, a piece of cotton thread (7 cm) was anchored on the arterial inflow between the friction-fit of the two pieces of tubing with 6 cm of thread exposed to the lumenal fluid. The tips of the longer sections, at the inflow of the arterial blood and the outflow of the venous blood, were beveled slightly (∼20°) to compensate for the lengthwise curvature of the tubing as it comes off the roll. The bevels were oriented laterally during insertion in the vessels to ensure unrestricted flow into and out of the tubing. The entire length of the shunt was filled with isotonic saline (Baxter, Chicago, IL) before implantation. The carotid and jugular vessels were approached through a ventral midline cervical incision from just below the mandible to just above the sternum. The right carotid (2 cm) and left jugular (1 cm) were carefully exposed with blunt dissection. The outflow end of the shunt was inserted 4 cm into the left jugular vein and tied in place using a 4-0 silk suture. The venous side of the shunt was clamped with silastic-shielded forceps to prevent backflow from the jugular vein whenever blood was not circulated through the shunt. The cephalic end of the isolated arterial segment was ligated and a Schwartz smooth vascular microclip (Roboz Surgical, Washington, DC) was clamped at the caudal end of the segment. A small incision was made in the artery near the ligature with a Vannus micro dissecting scissors (Roboz Surgical). The end of the shunt was inserted into the artery and was advanced (∼1 cm) to the microclip and was secured with a 4-0 silk suture. The vascular clip remained in place until it was time to circulate blood through the shunt. The right external jugular vein was isolated and a blood sampling catheter (Microrenathane Mre-40, 0.025 in ID × 0.040 in OD; Braintree Scientific, Inc, Braintree, MA), filled with isotonic saline, was implanted immediately proximal to the junction with the cephalic vein. The tip was advanced 5 cm, to a predetermined mark, so it was distal to the heart, as determined from previous postmortem examination. A drug infusion catheter was also implanted in the right jugular vein through the same insertion site as the sampling catheter but was advanced only to a length of 1 cm, to a predetermined mark, to ensure the sampling site would not be exposed to PC derivatives in the immediate vicinity of the infusion site. A single 4-0 silk suture was used to ligate the vein and both catheters. The infusion catheter was filled with a solution of PC derivative before implantation. Test solutions were prepared in isotonic saline and were infused with a Harvard syringe infusion pump (Harvard Apparatus, South Natick, MA) through a syringe connected with extension tubing (Tygon, 0.01 in ID × 0.04 in OD; Norton Performance Plastics, Akron, OH) to the infusion catheter. The infusion volume was 3.3 mL/h (1.65 mL over the 30-minute infusion). The infusate concentrations ranged from 0.15 to 75.8 mg/mL for an average 500-g guinea pig infused with doses ranging from 1 mg/kg/h (aPC and FLIN-Q3) to 500 mg/kg/h (HPC).
Blood sampling.Blood samples (1.0 mL) were collected into a 1-mL syringe. Before each sample, 0.2 mL of blood was withdrawn to clear the dead space (0.04 mL) of the catheter. The syringe was then replaced with a sample syringe that was preloaded with 0.1 mL of 3.8% sodium citrate and 0.9 mL of blood was sampled for assay. The sample was expelled into a 1.5 mL Eppendorf tube and was inverted to ensure proper mixing. The initial volume was returned to the animal and was followed with isotonic saline (0.3 mL). aPTT of the whole blood was determined immediately after collection to minimize potential inhibition of aPC. The remaining blood was centrifuged (14,000 rpm for 5 minutes) as soon as possible after withdrawal of the aliquots for aPTT determination. The plasma was then immediately removed, placed on ice, and frozen at −20°C for later amidolytic assay.
Coagulation times.The aPTT of blood samples was measured with a fibrometer (Fibrosystems; Becton Dickinson, Cockeysville, MD). To conduct the assay, 0.1 mL of blood was incubated with 0.1 mL of aPTT Reagent (Organon Teknika, Durham, NC) for 5 minutes at 37°C in a fibrometer cup. Then, calcium chloride (0.1 mL, 0.025 mol/L) was added and the fibrometer was started immediately. The elapsed time until the fibrometer stopped was recorded and duplicates were averaged. The ratio of experimental to corresponding control aPTT values was reported as aPTT ratio. Values are expressed as means ± SEM.
Determination of inhibition rates in guinea pig plasma.Inhibition rates were determined essentially as described previously.42 Briefly, 100 nmol/L aPC or aFLIN-Q3 was incubated at 37°C in pooled citrated guinea pig plasma; the plasma concentration was 90% (vol/vol) with the remaining volume consisting of 150 mmol/L NaCl, 20 mmol/L Tris-HCl, pH 7.40 and 1 mg/mL BSA. At selected times, aliquots were removed and residual amidolytic activity was determined using S-2366. The t1/2 values for aPC and aFLIN-Q3 were determined from decay curves generated using Enzfitter software (Elsevier Biosoft, UK).
Determination of aPC and aFLIN-Q3 plasma levels.Plasma concentrations of aPC and aFLIN-Q3 were determined using the immunocapture assay of Gruber and Griffin43 with minor modifications. Costar 96-well radioimmunoassay plates were coated for 24 to 72 hours at 4°C with anti-PC light chain monoclonal antibody HPC-3 (150 μL coating solution/well, 100 μg IgG/mL) in 10 mmol/L Na2CO3 , pH 9.20, and 0.02% NaN3 . The antibody solution was removed and plates were blocked for 1 hour at 37°C with freshly prepared 5% nonfat dried milk in phosphate buffered saline (PBS, 200 μL blocking solution/well). Citrated guinea pig plasma samples were thawed and immediately benzamidine-HCl was added to a final concentration of 50 mmol/L. Then, samples were further diluted into PBS containing 0.5% nonfat dried milk, 50 mmol/L benzamidine-HCl and 0.05% Tween-20. Blocked plates were washed (3×) with PBS containing 0.5% nonfat dried milk and 50 mmol/L benzamidine-HCl, then incubated overnight at 4°C (or 2 hours at room temperature) with diluted plasma samples. Plates were then washed (6×) with IC wash buffer (150 mmol/L NaCl, 20 mmol/L Tris-HCl, pH 7.4, 20 mmol/L EDTA, 0.02% Tween-20 and 0.02% NaN3 ). After the addition of IC wash buffer containing 0.86 mmol/L S-2366, amidolytic activity was determined at 37°C in a Thermomax kinetic plate reader (Molecular Devices Corp, Sunnyvale, CA). Standard curves, using purified aPC diluted into citrated guinea pig plasma containing 50 mmol/L benzamidine-HCl, generated correlation coefficients of 0.99 or greater. Values are expressed as mean ± SEM. Correction was also made for the higher specific amidolytic activity of aFLIN-Q3 as compared with wild-type recombinant aPC.
Experimental protocol.Control samples were collected immediately before the start of the 30-minute infusion. Subsequent blood samples were taken every 15 minutes for 60 minutes. After 15 minutes of infusion and immediately after the second blood sample was collected, the vascular clip was removed from the carotid artery and the shunt circulation was started. The shunt was closed after 15 minutes of blood circulation by applying a hemostatic clamp to the arterial side of the shunt. The middle segment, containing the thread, was disconnected from the arterial and venous segments. The segment was held vertically and the thread was carefully removed from the tubing and weighed. The average wet weight of ten equal lengths of thread (5 mg) was subtracted from the observed thread weight to arrive at the actual wet thrombus weight. Both 20-cm segments of the shunt were flushed with saline and a new center segment, containing the thread, was inserted. To assess whether residual PC zymogen was available for activation in some experiments, a second 15-minute circulation period was started 15 minutes after the first was completed. Values are expressed as means ± SEM.
RESULTS
A primary purpose of these studies was to determine in vivo if the zymogens HPC or the thrombin-sensitive FLIN-Q3 could be activated by thrombin formed in an animal, and subsequently have an antithrombotic effect. Limited availability of the mutant dictated the use of a small animal model of thrombosis. To select the most suitable species, the anticoagulant activity of recombinant human aPC was compared in vitro in human, guinea pig, and rat blood. Parallel dose-dependent increments in aPTT ratio were recorded in blood from each species. Relative to human blood, the concentration of aPC required to prolong aPTT by 50% was approximately fourfold higher in guinea pig blood and almost 12-fold higher in rat blood (data not shown); consequently, the guinea pig was selected to conserve FLIN-Q3. The average aPTT ratio recorded after 1 μg/mL of aPC added to guinea pig blood (assay concentration, 0.3 μg/mL) was 1.50 ± 0.04 (n = 3).
Preliminary studies addressed the optimal blood circulation time through the AV shunt in the guinea pig to permit detection of dose-dependent reduction of thrombus weight. The objective was to allow sufficient shunt circulation time for the thrombus to grow as large as possible without extension beyond the thread into the venous outflow segment of the shunt. Thrombus weight averaged 34 ± 4, 43 ± 3, and 45 ± 1 mg after periods of 10, 15, and 20 minutes of blood circulation, respectively. Because thrombus weight was not increased appreciably after 15 minutes and because thrombotic material was present in the venous outflow after 20 minutes of circulation, 15 minutes was selected as the standard shunt circulation time for subsequent experiments. To validate this model, heparin was infused following the same infusion protocol. Heparin dose-dependently reduced thrombus weight and the correlation was significant ( P < .05). An infusion dose of 50 U/kg/h reduced thrombus weight to 50% of control (data not shown).
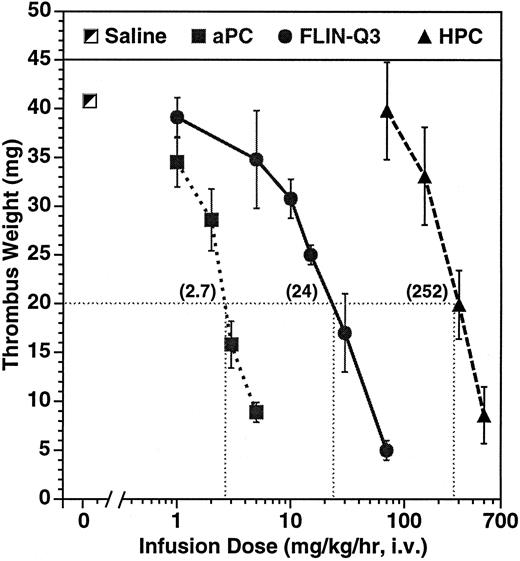
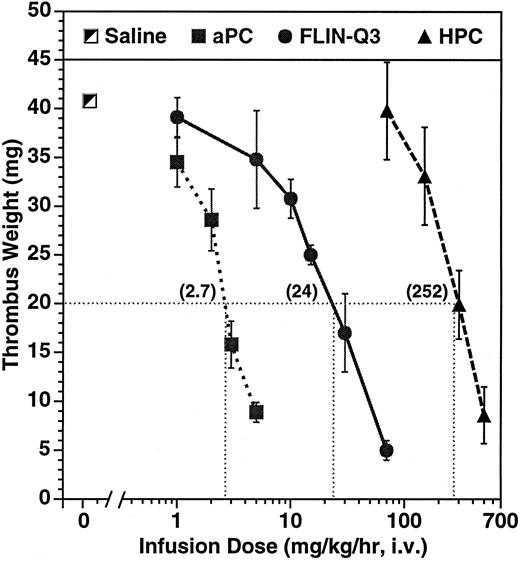
Dose-dependent effect on thrombus weight in the guinea pig AV shunt thrombosis model. PC variants were infused for 15 minutes before and throughout a 15-minute period of blood circulation through the shunt. Thrombi were removed and weighed after 30 minutes of infusion. The numbers in parentheses represent the calculated infusion dose (mg/kg/h) required to reduce thrombus weight to 20 mg under the conditions of the experiment. The number of animals per dose ranged from 4 to 11 for aPC and from 2 to 4 for the zymogens.
Dose-dependent effect on thrombus weight in the guinea pig AV shunt thrombosis model. PC variants were infused for 15 minutes before and throughout a 15-minute period of blood circulation through the shunt. Thrombi were removed and weighed after 30 minutes of infusion. The numbers in parentheses represent the calculated infusion dose (mg/kg/h) required to reduce thrombus weight to 20 mg under the conditions of the experiment. The number of animals per dose ranged from 4 to 11 for aPC and from 2 to 4 for the zymogens.
Parallel dose-dependent antithrombotic effects were observed during infusion of aPC and of the zymogens as presented in Fig 1. The calculated infusion dose required to reduce thrombus weight to 20 mg, about half of the average control of 41 ± 2 mg, was 2.7, 24, and 252 mg/kg/h infused over 30 minutes for aPC, FLIN-Q3, and HPC, respectively. The antithrombotic potency of aPC was approximately ninefold greater than zymogen FLIN-Q3 and approximately 93-fold greater than for native HPC.
To determine if the antithrombotic efficacy of the zymogens was due to activation by thrombin in vivo, the plasma concentration of aPC in each treatment group was monitored. However, because activated FLIN-Q3 differs from aPC by several amino acid residues, the rates of inhibition by plasma inhibitors in vitro were determined. The amidolytic activity of aPC and of activated FLIN-Q3 in guinea pig plasma decayed linearly at approximately the same rate with calculated plasma activity half-lives of 12.7 minutes for native aPC (r = 0.993, P < .01) and 15.5 minutes for aFLIN-Q3 (r = 0.974, P < .01). These data suggest that observed differences in vivo were not due to differences in plasma inhibitor interactions.
Parallel and dose-dependent increases in blood aPTT ratio and in plasma aPC concentration were recorded for aPC and for the zymogens FLIN-Q3 and HPC in samples collected after thrombus formation at the end of the 30-minute infusion. The results are summarized in Table 1 and are compared with the infusion rates required for 50% reduction of thrombus weight from the data in Fig 1. The 30-minute infusion dose of aPC required to prolong aPTT to 1.5 times control was 1.6 mg/kg/h. The relative potency, based on this comparator, indicated that aPC was approximately eightfold more potent than zymogen FLIN-Q3 and was roughly 138-fold more potent than native zymogen HPC. The calculated infusion dose of aPC that was required to achieve a plasma concentration of 5 μg/mL at the end of the 30-minute infusion was 2.5 mg/kg/h. To achieve the same plasma concentration, an approximate eightfold dose increment in FLIN-Q3 and almost 95-fold increase in HPC was required. Thus, the infusion rates required to obtain equivalent anticoagulant activity (aPTT) and aPC blood levels directly correlated with the infusion rates required for antithrombotic activity. Further, while the zymogen FLIN-Q3 was not as potent as infused aPC, it was substantially more potent than native zymogen.
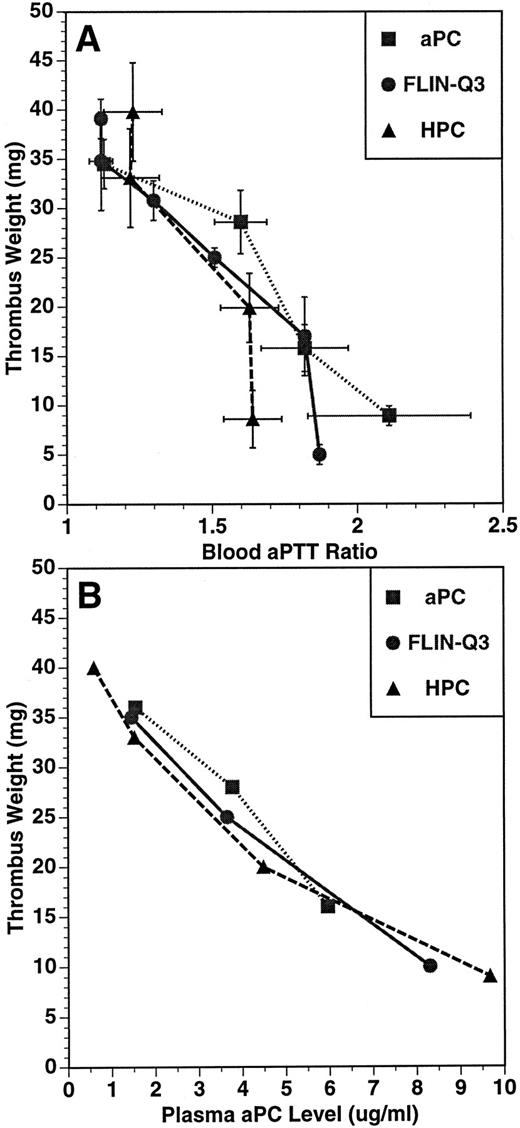
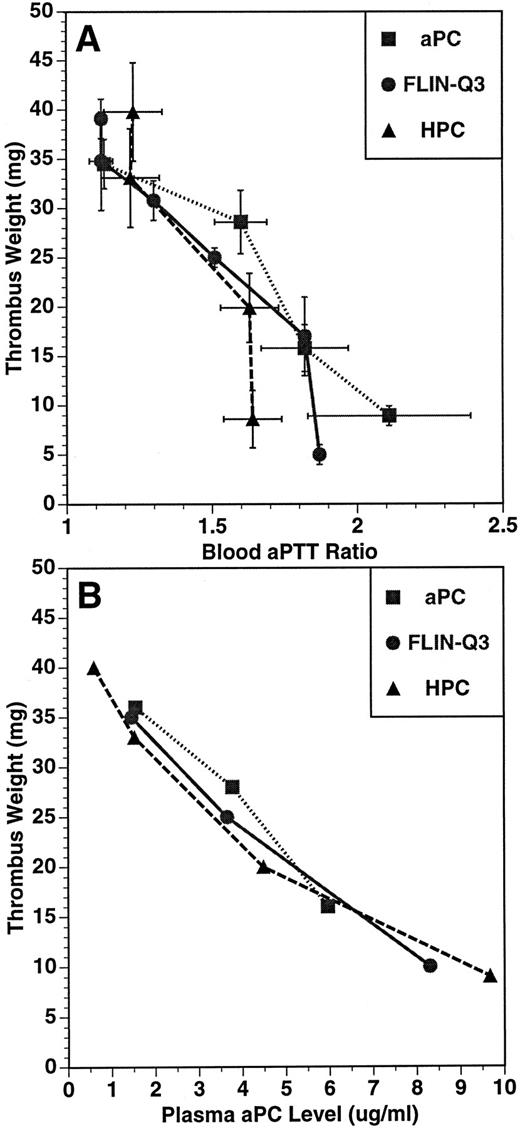
The relationship between thrombus weight and aPTT ratio (A) and plasma aPC concentration (B) in the guinea pig thrombosis model. PC variants were infused for 15 minutes before and throughout a 15-minute period of blood circulation through the shunt. Thrombus weight, blood aPTT, and plasma aPC concentration were determined at the end of the 30-minute infusion. Plasma concentration of aPC was determined using an immunocapture amidolytic assay.
The relationship between thrombus weight and aPTT ratio (A) and plasma aPC concentration (B) in the guinea pig thrombosis model. PC variants were infused for 15 minutes before and throughout a 15-minute period of blood circulation through the shunt. Thrombus weight, blood aPTT, and plasma aPC concentration were determined at the end of the 30-minute infusion. Plasma concentration of aPC was determined using an immunocapture amidolytic assay.
The relationships between the anticoagulant and antithrombotic activities, and between the plasma aPC concentration and the antithrombotic effects for aPC, FLIN-Q3 and HPC are presented in Fig 2A and B, respectively. The values represent the means of parameters measured at the end of the infusion after the formation of the thrombus. For each infused preparation, thrombus weight was inversely related to aPTT ratio and to plasma aPC concentration. These data suggest the antithrombotic effect of both FLIN-Q3 and HPC zymogens resulted from generation of aPC in vivo.
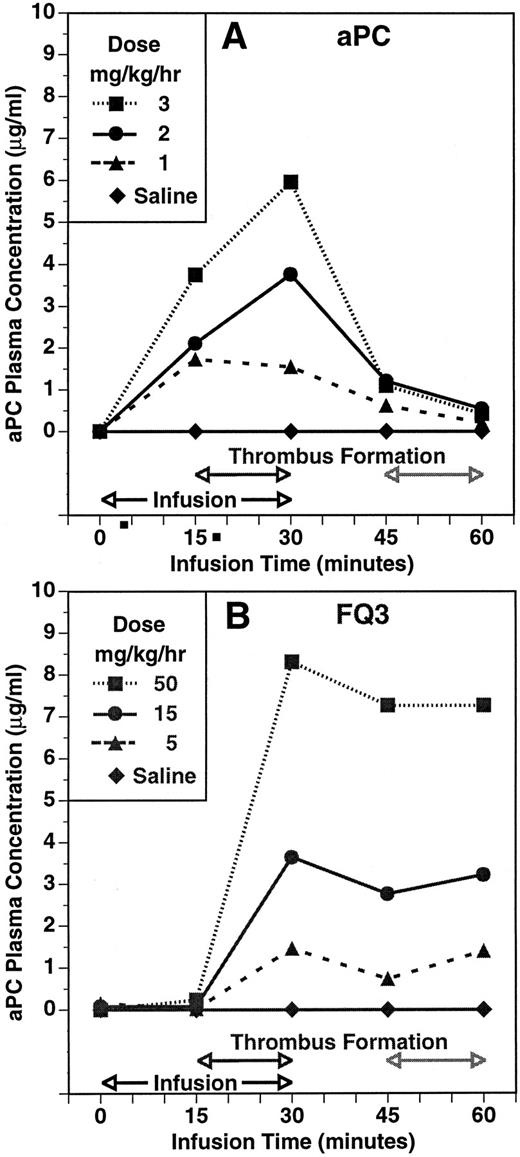
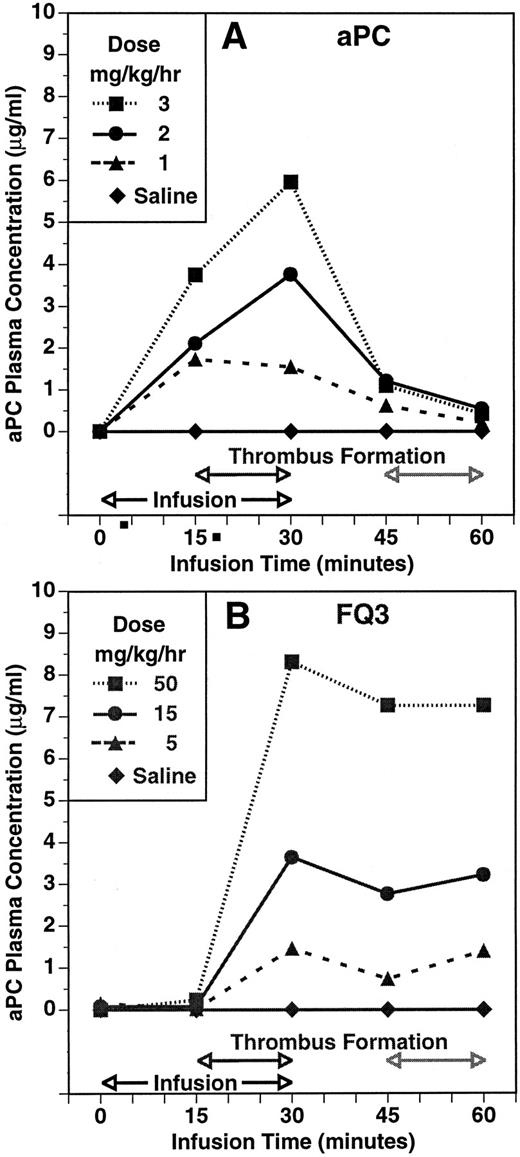
The experimental protocol allowed for a second period of thrombosis to assess whether residual zymogen was available for activation. The second thrombotic event occurred 15 minutes after the infusion was discontinued at the end of the initial period of blood circulation through the shunt. The time course and dose-dependency of plasma aPC concentration during and after infusions of aPC and FLIN-Q3 are summarized in Fig 3A and B, respectively. Plasma aPC concentration increased dose-dependently 15 minutes into the infusion and before the shunt was opened in the group infused with aPC. In contrast, aPC was not detected before thrombin formation after 15 minutes of infusion with FLIN-Q3. The aPC plasma concentration was increased dose-dependently in both groups in samples collected at the end of the initial thrombotic period. Plasma concentration decreased toward control in all the aPC and FLIN-Q3 groups 15 minutes after the infusions were stopped, although the amount of decay appeared to be less in the FLIN-Q3 groups. At the end of the second period of thrombin generation in the shunt, plasma aPC concentration continued to decline to near control in the aPC groups. However, aPC concentration tended to increase at this point in the FLIN-Q3 groups. Results similar to those obtained with FLIN-Q3 were observed with HPC zymogen.
The time course and dose-dependency of plasma aPC concentration in the guinea pig thrombosis model during infusion of aPC (A) and of the zymogen FLIN-Q3 (B). Plasma concentration was determined using an immunocapture amidolytic assay for aPC. Compare the presence of activity during infusion with aPC (A) with the absence of aPC activity after 15 minutes of infusion with FLIN-Q3 (B) before thrombin generation and thrombus formation during the first period of thrombosis. Note the increase in aPC concentration during the second period of thrombus formation even though the FLIN-Q3 infusions (B) had been stopped for 15 minutes and compared with the absence of activity during this period after infusion with aPC (A).
The time course and dose-dependency of plasma aPC concentration in the guinea pig thrombosis model during infusion of aPC (A) and of the zymogen FLIN-Q3 (B). Plasma concentration was determined using an immunocapture amidolytic assay for aPC. Compare the presence of activity during infusion with aPC (A) with the absence of aPC activity after 15 minutes of infusion with FLIN-Q3 (B) before thrombin generation and thrombus formation during the first period of thrombosis. Note the increase in aPC concentration during the second period of thrombus formation even though the FLIN-Q3 infusions (B) had been stopped for 15 minutes and compared with the absence of activity during this period after infusion with aPC (A).
DISCUSSION
These studies demonstrate the antithrombotic activity of the HPC derivative FLIN-Q3, which was designed specifically to be activated more efficiently than native HPC by free thrombin.37 We show that this derivative, as well as HPC zymogen, could be activated to aPC in vivo and have an antithrombotic effect. While it required ∼100 times the dose of zymogen PC to obtain an antithrombotic effect equivalent to preactivated aPC, it required only ∼9 times the amount of the thrombin-sensitive FLIN-Q3 zymogen. These data support the interpretation that FLIN-Q3 was activated more efficiently by thrombin formed during thrombosis, and are consistent with the in vitro results reported previously.37
That the antithrombotic efficacy of FLIN-Q3 was mediated by formation of aPC subsequent to thrombin activation is supported by several lines of evidence. Blood aPTT ratio and plasma aPC concentration were increased dose-dependently in samples collected at the end of the infusion, which corresponded to the end of the initial thrombotic period. As summarized in Table 1, the relative infusion rates required for equivalent antithrombotic effects, aPTT ratio, and plasma aPC concentration were very similar. The separations between these dose response relationships for aPC and FLIN-Q3 were similar to the potency difference for the antithrombotic effect (∼10-fold). The virtually identical inverse relationships between the antithrombotic responses and both the aPTT ratio and the plasma aPC concentration for aPC, FLIN-Q3, and HPC (Fig 2) are consistent with common mediation by aPC. Additionally, no significant aPC could be detected in the plasma during infusion of the FLIN-Q3 zymogen until after thrombin production during the thrombotic period (Fig 3B). In contrast, aPC concentration was increased dose-dependently before shunt circulation in animals infused with aPC (Fig 3A).
Anticoagulant activity and plasma aPC concentration decreased after the end of infusion of the aPC, FLIN-Q3, and HPC. After the second thrombotic period which was 30 minutes after the infusions were stopped, aPC concentration tended to increase in animals previously infused with the zymogens but continued to decline toward control in the groups given aPC. Thus, sufficient zymogen persisted in the circulation to permit thrombin-mediated activation after a second period of thrombosis. From the data in Fig 3, it appears that the rate of decay of preformed and administered aPC was faster than that of the aPC generated following infusion of FLIN-Q3. As indicated in the results, the rate of decay of preformed aPC and activated FLIN-Q3 were not significantly different when incubated in guinea pig plasma. These data suggest that additional aPC continued to be formed after shunt closure. It is possible that thrombin, generated and released from the thrombus, may avoid natural inhibition by circulating antithrombin III and heparin cofactor II7,44-46 long enough to activate the zymogens in the circulation or to bind to microvascular thrombomodulin where activation could occur. Thus, we cannot rule out that some aPC generation occurred systemically and accounted for the maintenance of the plasma levels seen in Fig 3B.
In our previous in vitro analysis, we found that the rate of FLIN-Q3 activation by free human thrombin was 60-fold greater than that of native HPC,37 whereas in this in vivo study, the difference in the amount of aPC generated following infusion of HPC and FLIN-Q3 was found to be ∼10-fold. While one must be cautious about extrapolating rates from biochemical studies to those from in vivo experiments, the difference in magnitude may be due to differences in the rate of activation by human as opposed to guinea pig thrombin. The lack of availability of purified guinea pig thrombin has precluded addressing this question. Alternatively, this difference may reflect factors in addition to a faster activation rate by free thrombin in the animal, such as clearance, and systemic microvascular activation by the thrombin-thrombomodulin complex, which also more rapidly activates FLIN-Q3 than HPC.37 Future experiments will be required to compare these and other pharmacokinetic features.
In summary, our results demonstrate the antithrombotic efficacy and potency of the proenzyme FLIN-Q3. Esmon has estimated there are approximately 100,000 thrombomodulin molecules per endothelial cell; therefore, the concentration of thrombomodulin in conduit vessels (diameter ∼ 0.3 cm) would be approximately 0.15 nmol/L compared with around 500 nmol/L in the microcirculation. Given that thrombin is a very active coagulation enzyme at concentrations higher than approximately 10 nmol/L, the relatively low thrombomodulin concentration suggests that most of the thrombin generated in large vessels would be free to catalyze coagulation.7 Thus, the improved antithrombotic potency of a thrombin-sensitive zymogen, such as FLIN-Q3, may represent a more practical form of an “on-demand” antithrombotic for large vessels, particularly because of the longer circulating half-life for zymogen PC as compared with its activated form.32,34-36 Further, such a derivative may be useful in prothrombotic inflammatory disease states such as sepsis where thrombomodulin is down-regulated.47-49 Administration of a thrombin-sensitive zymogen may also allow a reduction in dose compared with aPC administration due to its longer half-life and the possibility of forming a pool of material that can be efficiently activated on demand. Overall, this approach may represent a promising strategy for effective antithrombotic therapy with improved safety.
ACKNOWLEDGMENT
We thank J. Secnik and Dr W. Prouty (Eli Lilly and Co) for preparation of portions of the recombinant protein C zymogen used in this study.
Address reprint requests to Ken Kurz, PhD, Lilly Corporate Center (0524), Indianapolis, IN 46285.