Abstract
The objective of the study was to evaluate the diagnostic efficiency of laboratory tests, including serum transferrin receptor (TfR) measurements, in the diagnosis of iron depletion. The patient population consisted of 129 consecutive anemic patients at the University Hospital of Turku who were given a bone marrow examination. Of these patients, 48 had iron deficiency anemia (IDA), 64 anemia of chronic disease (ACD), and 17 patients had depleted iron stores and an infectious or an inflammatory condition (COMBI). Depletion of iron stores was defined as a complete absence of stainable iron in the bone marrow examination. Serum TfR concentrations were elevated in the vast majority of the IDA and COMBI patients, while in the ACD patients, the levels were within the reference limits reported earlier for healthy subjects. TfR measurement thus provided a reliable diagnosis of iron deficiency anemia (AUCROC 0.98). Serum ferritin measurement also distinguished between IDA patients and ACD patients. However, the optimal decision limit for evaluation of ferritin measurements was considerably above the conventional lower reference limits, complicating the interpretation of this parameter. Calculation of the ratio TfR/log ferritin (TfR-F Index) is a way of combining TfR and ferritin results. This ratio provided an outstanding parameter for the identification of patients with depleted iron stores (AUCROC 1.00). In anemic patients, TfR measurement is a valuable noninvasive tool for the diagnosis of iron depletion, and offers an attractive alternative to more conventional laboratory tests in the detection of depleted iron stores.
THE LEVEL of body iron stores is affected both by dietary intake and by the physiological need of iron for erythropoiesis. Consequently, iron deficiency anemia (IDA) can be caused by dietary deprivation of iron or by iron malabsorbtion; importantly, it may be the first clinical sign of increased blood loss. Unexplained iron deficiency anemia warrants extensive investigations of the gastrointestinal tract, since the probability of ulcers or malignant tumors as the cause of excessive blood loss is relatively high.1 Using laboratory tests, distinguishing between iron deficiency anemia and the anemia that accompanies infection, inflammation, or malignancy is difficult, as the commonly used laboratory parameters do not necessarily distinguish between these common causes of anemia.2-5 The conventional laboratory tests of iron status, serum iron, transferrin/total iron-binding capacity (TIBC), transferrin saturation, and ferritin are widely used in clinical practice, although they are considerably influenced by acute phase responses, which complicates the clinical interpretation of the test results.3-5 High sensitivity, as well as specificity, is of special importance for a test of iron status, as the further identification of the cause of the depletion of iron stores is bound to result in tedious clinical and laboratory investigations. Because the absence of stainable iron in bone marrow examination is generally regarded as the definitive marker of iron deficiency, marrow examinations are generally requested to confirm iron deficiency. There is an evident clinical need for noninvasive and sensitive means for the detection of iron deficiency, and in recent years, the serum transferrin receptor (TfR) level has been introduced as a promising new tool for the diagnosis of iron depletion.3 6-11
The TfR receptor is a transmembrane protein with two identical polypeptide chains, each weighing 95 kD; iron delivery to erythroblasts is mediated by the interaction of plasma transferrin with cell surface transferrin receptors.11,12 From the cell membrane the TfR-transferrin-iron complex is internalized via an endocytic vesicle, and in the intracellular compartment iron dissociates from TfR-transferrin complex.3,11,13 The iron remains in the cytosol, while the TfR-transferrin complex is recycled back to the cell surface. Virtually all cells have transferrin receptors on their surface, but in the normal adult, about 80% of them are in the erythroid marrow.12 Soluble TfR present in human plasma is a truncated form of the tissue receptor and exists as a transferrin-receptor complex.13-15 The TfR number on the cell surface reflects the iron requirement, and iron deprivation has been shown to result in the prompt induction of transferrin receptor synthesis.16
We have evaluated the clinical efficiency of TfR measurements in the identification of iron deficiency in an extensive, consecutive patient population. The diagnostic classification of all patients was based on an examination of bone marrow using iron staining as the gold standard for iron depletion.
MATERIALS AND METHODS
Patients.The patient population consisted of 129 consecutive anemic adult patients at the University Hospital of Turku who underwent a bone marrow examination because of anemia. The purpose of the examinations was to define the type of anemia and to determine iron stores. Anemia was defined as a hemoglobin concentration of less than 128 g/L in men and 117 g/L in women, which constitutes the lower 2.5% reference limits in our hospital. All blood samples were obtained before any blood transfusions, and patients on oral iron therapy were excluded from the study population. Patients with hematological malignancies were also excluded from this study, as certain hematological malignancies have been reported to be associated with an elevated serum TfR regardless of the iron status of the patients.14,17 Additionally, patients who had hemolytic anemia or defined deficiency of vitamin B12 or folic acid were excluded from the study population, as these conditions may be associated with elevated TfR levels irrespective of iron status.18 The patients were assigned to one of three groups on the basis of the bone marrow examination and clinical data. Forty-eight patients (32 women and 16 men) who fulfilled the morphologic criteria of iron deficiency and who had no stainable iron in the bone marrow were classified as having IDA. Sixty-four anemic patients (37 women and 27 men) were classified as having anemia of chronic disease (ACD), and these patients all had stainable iron in the bone marrow. Of these 64 patients, 34 had recurrent or chronic infections, while the remaining 30 had other chronic diseases (ie, nonhematological malignancies and inflammatory diseases such as rheumatoid arthritis). Those patients who had no iron in the bone marrow together with an infectious disease, a chronic inflammatory disease (eg, rheumatoid arthritis or colitis ulcerosa) or a nonhematological malignancy were placed in a COMBI group (n = 17). In addition, two iron-deficient patients who had a C-reactive protein (CRP) value above 20 mg/L were regarded as having an accompanying inflammatory or infectious condition and were included in the COMBI group. Preliminary results concerning a small subgroup of the patients (n = 36) have been reported earlier.19
Samples and analytical methods.Bone marrow was aspirated from the sternal bone or iliac crest. The smears were stained using the May-Grünwald-Giemsa method (Orion Diagnostica, Helsinki, Finland), and the iron stores were stained by the Prussian blue method. Blood counts were measured with an automated analyzer (Technicon H*2, Technicon Instruments Corp, Tarrytown, NY). Serum transferrin receptor assays were performed using a commercially available kit based on a polyclonal antibody in a sandwich enzyme immunoassay (EIA) format (Clinigen; R&D Systems, Minneapolis, MN). According to the assay kit from the manufacturer, the central 95th percentile of the reference distribution of TfR concentration is 0.85 to 3.05 mg/L (n = 1,000). Ferritin (reference range, 15 to 306 mg/L for men, 5 to 103 mg/L for women, according to the manufacturer) was measured using a radioimmunoassay (Spectria, Orion Diagnostics). Transferrin (reference range, 2.1 to 3.4 g/L for men, 2.0 to 3.1 g/L for women20 ) was measured with a Behring Nephelometer (Behringwerke AG, Marburg, Germany) together with antibodies provided by Dakopatts (Dakopatts, Glostrup, Denmark). Serum iron (reference range, 10 to 40 mmol/L) was measured using an Iron FZ assay (Hoffmann-LaRoche, Basel, Switzerland) based on a guanidine hydrochloride/Ferrozine reaction. The transferrin index (TI) was calculated as iron (mmol/L)/transferrin (g/L), as recently suggested by Beilby et al.21 Percent transferrin saturation was calculated as [iron/(transferrin × 23)] × 100.
Statistical analysis.Receiver operating characteristics (ROC) curves were visualized and the corresponding areas under the curves were calculated using the GraphROC for Windows software package.22 The areas under the ROC curves (AUCROC) were compared with each other using a software mathematically based on a method described earlier.23-25 The P values (one-tailed test) corresponding to the calculated z scores were drawn from a table of normal distributions.
RESULTS
The results for blood counts and iron status markers for the 129 anemic patients are summarized in Table 1. Serum iron was not a reliable indicator of iron depletion (Table 2). When the study population was analyzed as a whole (n = 129), serum transferrin distinguished fairly well between iron-deficient and ACD patients (Table 2). However, in a considerable proportion of the iron-deficient patients, the transferrin concentration was within the reference limits of a healthy population (see Materials and Methods), making the clinical interpretation of the transferrin concentrations difficult. The area under the ROC curve (AUCROC) provided by Transferrin-Index (TI, iron/transferrin),21 or the percentage transferrin saturation [iron/(transferrin × 23) × 100] in the identification of iron-deficient patients was 0.82. For comparison, the corresponding AUCROC for red cell mean corpuscular volume (MCV) was 0.88.
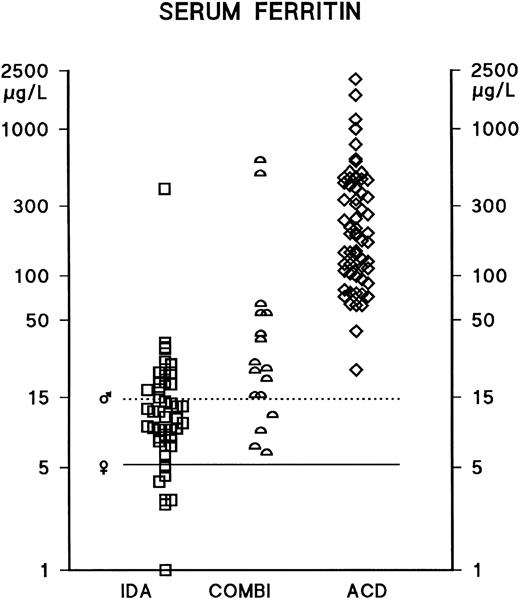
In male and female IDA patients, the median serum ferritin concentrations were 13 mg/L (mean ± standard deviation [SD], 37 ± 94) and 9 mg/L (mean ± SD, 12 ± 9), respectively. In male and female ACD patients, the median ferritin concentrations were 195 mg/L (mean ± SD, 364 ± 310) and 169 mg/L (mean ± SD, 326 ± 440), respectively. There have been no previous reports of any significant differences in TfR concentrations between male and female patients; the present findings are consistent with this, as for instance, in male and female ACD patients, the median TfR concentrations were 1.9 g/L (mean ± SD, 1.9 ± 0.7) and 1.7 g/L (mean ± SD, 1.8 ± 0.5), respectively. As neither ferritin nor TfR concentrations differed significantly between male and female patients, the results have been analyzed and presented without distinction between male and female patients. Serum ferritin measurements distinguished between IDA and ACD patients (AUCROC 0.98); however, the optimal decision limit for the interpretation of ferritin measurements was considerably above the conventional lower reference limit, which is based on evaluations of apparently healthy populations (Tables 1-3 and Fig 1). TfR concentrations were elevated in the vast majority of IDA and COMBI patients, and the serum TfR concentration was found to be a good indicator of iron deficiency, as demonstrated by the scattergram in Fig 2 and by the ROC curve in Fig 3 (AUCROC 0.98). In the ACD patients, the levels were within the reference limits reported earlier for healthy subjects.19 TfR measurements effectively identified iron deficiency, even in the presence of an accompanying inflammatory or infectious condition (Tables 2 and 3, Fig 2); furthermore, the statistical comparison of the ROC curves indicates that TfR measurements distinguished between the COMBI and the ACD patients more effectively than ferritin measurements (P = .033).
Serum ferritin concentrations in anemic patients. IDA (iron-deficiency anemia) (n = 48) and ACD (anemia of chronic disease) (n = 64). Those patients who had depleted iron stores together with an infectious disease, a chronic inflammatory disease, or a nonhematological malignancy were assigned to the COMBI group (n = 17). The lower reference limits of the serum ferritin assay are indicated separately for male (♂) and female (♀) subjects by horizontal bars.
Serum ferritin concentrations in anemic patients. IDA (iron-deficiency anemia) (n = 48) and ACD (anemia of chronic disease) (n = 64). Those patients who had depleted iron stores together with an infectious disease, a chronic inflammatory disease, or a nonhematological malignancy were assigned to the COMBI group (n = 17). The lower reference limits of the serum ferritin assay are indicated separately for male (♂) and female (♀) subjects by horizontal bars.
Serum TfR concentrations in anemic patients. For abbreviations, see text to Fig 1. The central 95th percentile of the reference distribution for the TfR assay is shown with horizontal bars.
Serum TfR concentrations in anemic patients. For abbreviations, see text to Fig 1. The central 95th percentile of the reference distribution for the TfR assay is shown with horizontal bars.
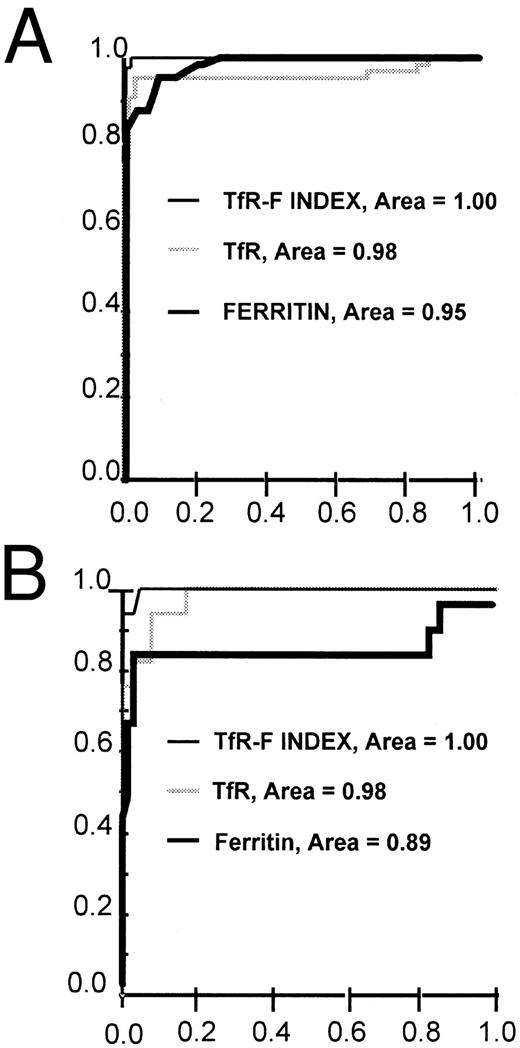
ROC curves for TfR, ferritin, and TfR-F Index (TfR/log ferritin ratio) in the identification of iron-deficient patients. The ROC curves are shown separately for the whole patient population (n = 129) (A) and for the distinction between the ACD (n = 64) and COMBI (n = 17) patients (B). TfR-F Index -, TfR ▩, ferritin ▪.
ROC curves for TfR, ferritin, and TfR-F Index (TfR/log ferritin ratio) in the identification of iron-deficient patients. The ROC curves are shown separately for the whole patient population (n = 129) (A) and for the distinction between the ACD (n = 64) and COMBI (n = 17) patients (B). TfR-F Index -, TfR ▩, ferritin ▪.
Earlier, the calculation of the TfR/ferritin ratio was reported to reflect the depletion of iron stores in response to phlebotomies.7 In the present patient material, the calculation of the TfR/ferritin ratio as such only slightly improved diagnostic sensitivity and specificity compared with the use of TfR or ferritin alone (Tables 2 and 3). However, both sensitivity and specificity were improved by logarithmic transformation of the ferritin value, and we suggest that this parameter be referred to the TfR-Ferritin Index (TfR-F Index). The calculation of the TfR/log ferritin ratio (TfR-F Index) provided an outstanding parameter for the identification of iron-deficient patients (Table 3, Figs 3 and 4). When the whole study population (n = 129) was analyzed, the TfR-F Index provided an AUCROC value of 1.00. Consequently, when subgroups were analyzed, the AUCROC values were 1.00 for the distinction between the ACD (n = 64) and IDA groups (n = 48), and 1.00 for the distinction between the ACD and COMBI groups (n = 17) (Tables 2 and 3, and Figs 3 and 4).
The TfR-F Index (TfR/log ferritin ratio) in anemic patients. For explanation of the abbreviations, see text in Fig 1. The median values for each patient group (IDA, 5.4; COMBI, 3.2; ACD, 0.8) are indicated by horizontal bars.
The TfR-F Index (TfR/log ferritin ratio) in anemic patients. For explanation of the abbreviations, see text in Fig 1. The median values for each patient group (IDA, 5.4; COMBI, 3.2; ACD, 0.8) are indicated by horizontal bars.
DISCUSSION
The clinical situation in which serum transferrin receptor (TfR) measurements have been suggested to be especially useful is the differentiation between IDA and ACD.2 11 The distinction between IDA and the anemia that accompanies infection, inflammation, or malignancy is difficult, as the laboratory parameters commonly used do not necessarily distinguish these common causes of anemia. In the present study, we have evaluated the clinical efficiency of both TfR measurements and a variety of more conventional laboratory tests in the identification of patients with iron deficiency. Every attempt was made to avoid any bias due to preselection of patients by using a clinically relevant study population consisting of consecutive anemia patients subjected to a bone marrow examination. Iron deficiency was defined as a complete absence of stainable iron in bone marrow.
Serum iron and iron saturation of transferrin are of limited value in the diagnosis of IDA, as the corresponding AUCROC values, consistent with earlier studies,26 do not reflect proper diagnostic sensitivity and specificity. This is apparently due to the interference of the acute-phase response in serum iron and transferrin concentrations. Serum ferritin has been widely used to define iron depletion. In this study population, ferritin measurements (AUCROC 0.98) and even serum transferrin (AUCROC 0.98) distinguished effectively between patients with uncomplicated IDA and those with ACD, but the optimal decision limit for the interpretation of both ferritin and transferrin measurements was found to be considerably above the conventional reference limits, which are based on the evaluation of apparently healthy populations. It is evident that the ability of ferritin to distinguish between IDA and ACD is due not only to the decrease in serum ferritin level in IDA patients but, to a considerable extent, to the increase in serum ferritin caused by the acute phase responses associated with chronic inflammatory disease. The ROC curves produced on the basis of the present patient material (Table 2) are in good agreement with those generated earlier on the basis of meta-analysis for ferritin and iron saturation of transferrin.26 Based on the present data, it can be statistically estimated22 that in anemic patients who do not have an accompanying infection or inflammatory disease, a cut-off limit of 41 mg/L for serum ferritin provides optimal diagnostic efficiency. However, this estimation is apparently no longer valid if we assume that the iron-deficient patient has another disease accompanied by an acute phase reaction (ie, the COMBI group), so that the diagnostic usefulness of ferritin measurements is reduced (Tables 2 and 3). In the interpretation of ferritin results, the conventional population-based reference limits are not very useful, as for instance in female IDA patients (n = 32), the lower reference limit of ferritin concentrations provided only 22% sensitivity in the identification of iron deficiency. On the other hand, it is obvious that the need for disease-specific decision limits is difficult to meet. In conclusion, the measurement of serum ferritin may provide a rational basis for the identification of iron deficiency; the interpretation of the ferritin levels, however, requires careful diagnostic classification of the patients and knowledge of all the factors that may cause changes in serum ferritin levels.
The proinflammatory cytokines tumor necrosis factor-α and interleukin-6 have been suggested to reduce TfR expression under in vitro experimental conditions.27 On the basis of the present study, TfR measurements are able to distinguish between patients with IDA and those with ACD. The finding suggests that in ACD patients the potentially increased cytokine production does not greatly affect the TfR response caused by iron depletion; this is consistent with earlier reports.2 A major advantage of TfR measurements over serum ferritin is the apparent specificity of the biological response to changes in iron status and erythropoiesis. The present study suggests that when compared with ferritin, the clinical interpretation of TfR measurements is simpler. When hemolysis or megaloblastosis can be excluded, the TfR measurement should be an attractive alternative to more conventional tests of iron status.
The serum ferritin level varies with iron stores, while TfR is assumed to reflect reliably the degree of tissue iron supply.3,11 The TfR/ferritin ratio has been suggested to be a good estimate of body iron in individual subjects,7 but no studies of a representative patient population have so far been published. In the present study, we evaluated a variety of possibilities of combining the TfR and ferritin parameters; the results suggest that calculation of the TfR/ferritin ratio does not considerably improve diagnostic efficiency compared with TfR alone (Tables 2 and 3). However, logarithmic transformation of the ferritin values and calculation of the TfR/log ferritin ratio (the TfR-F Index) provided an outstanding indicator of iron depletion, as demonstrated by the scattergram and the corresponding ROC curves (Figs 3 and 4). This TfR-F Index takes advantage of the relationship between two phenomena, ie, an increase in TfR and a decrease in the ferritin concentration. This parameter consists of two variables, which in general, are influenced by the body iron stores, the availability of iron for erythropoiesis, and the total mass of erythroid bone marrow.
This study shows that, while serum TfR measurements are useful in the diagnosis of iron deficiency and in the differential diagnosis of various types of anemia, the combination of TfR and ferritin measurements provides the highest sensitivity and specificity. It may be predicted that these measurements are likely to replace the conventional parameters of iron status, ie, serum iron, transferrin, and ferritin alone, in clinical laboratories. They would be especially useful at outpatient clinics, where bone marrow examinations are often either not available or are regarded as an invasive means of identifying patients with depleted iron stores.
Address reprint requests to Kari Punnonen, MD, PhD, Department of Clinical Chemistry, University Hospital of Turku, Kiinamyllynkatu 4-8, 20520 Turku, Finland.