Abstract
Spontaneous growth of myeloid colonies (colony-forming unit–granulocyte-macrophage [CFU-GM]) can be observed in methylcellulose cultures containing peripheral blood mononuclear cells (PB-MNCs) and is supposedly caused by the release of colony-stimulating factors (CSF ) by accessory cells. Because of its cytokine synthesis-inhibiting effects on T lymphocytes and monocytes, interleukin-10 (IL-10) may be a potential candidate for indirect modulation of hematopoiesis. We studied the effect of recombinant human IL-10 (rhIL-10) on spontaneous growth of myeloid colonies derived from human PB-MNCs. A total of 10 ng/mL of IL-10 almost completely inhibited spontaneous CFU-GM proliferation (by 95.1%; P < .001, n = 7) in unseparated PB-MNCs. This effect was dose-dependent and specific, because a neutralizing anti–IL-10 antibody was able to prevent IL-10–induced suppression of CFU-GM growth. Spontaneous CFU-GM growth, which required the presence of both monocytes (CD14+ cells) and T lymphocytes (CD3+ cells), was also greatly suppressed by a neutralizing anti–granulocyte-macrophage CSF (GM-CSF ) antibody but was only slightly or not at all inhibited by antibodies against G-CSF or IL-3. Moreover, IL-10–suppressed colony growth could be completely restored by the addition of exogenous GM-CSF. Using semiquantitative polymerase chain reaction, we were able to show that GM-CSF transcripts that spontaneously increased in PB-MNCs within 48 hours of culture were markedly reduced by the addition of IL-10. Inhibiton of GM-CSF production in PB-MNCs by IL-10 was also confirmed at the protein level by measuring GM-CSF levels in suspension cultures. Our findings suggest that autonomous CFU-GM growth, resulting from an interaction of monocytes and T lymphocytes, is mainly caused by endogenous GM-CSF release and can be profoundly suppressed by the addition of exogenous IL-10. Considering the strong inhibitory action of IL-10 on GM-CSF production and spontaneous cell growth in vitro, this cytokine may be useful in myeloid malignancies in which autocrine and/or paracrine mechanisms involving GM-CSF are likely to play a pathogenetic role.
ENDOGENOUS RELEASE of hematopoietic growth factors by accessory cells is supposed to play a crucial role in the proliferation, differentiation, and survival of hematopoietic cells, both in vitro and in vivo.1-3 In vitro, hematopoietic progenitor cells from peripheral blood (PB) or bone marrow (BM) can be stimulated by the addition of exogenous growth factors to form colonies of mature blood cells.4 When using unseparated PB mononuclear cells (PB-MNCs), substantial growth of myeloid colonies (colony-forming unit–granulocyte-macrophage [CFU-GM]) can still be observed in methylcellulose cultures in the absence of exogenous hematopoietins. This spontaneous growth is apparently linked to the presence of accessory cells, that mainly consist of monocytes/macrophages and T lymphocytes.5,6 In contrast, highly enriched hematopoietic progenitors from adult human donors fail to undergo spontaneous clonogenic maturation,7 probably because of the lack of accessory cells.
Because endogenous release of growth factors may play a role not only in the regulation of normal hematopoiesis, but also in the pathogenesis of some myeloid malignancies such as chronic myelomonocytic leukemia8 and juvenile chronic myeloid leukemia9 and in some cases of acute myeloblastic leukemia,10 it is of interest to look for molecules that modulate hematopoiesis through the release of growth factors. Because of its known ability to suppress gene expression and production of numerous cytokines including granulocyte colony-stimulating factor (G-CSF ), granulocytemacrophage CSF (GM-CSF ), and interleukin-3 (IL-3) in monocytes and T lymphocytes,11 12 IL-10 may be a potential candidate for indirectly affecting hematopoiesis. To test this hypothesis, we studied the effect of IL-10 on spontaneous hematopoietic colony formation in normal human PB-MNCs using a clonal stem cell assay.
MATERIALS AND METHODS
Reagents.Recombinant human IL-10 (rhIL-10; specific activity, 1 to 2 × 106 U/mg) and recombinant human stem cell factor (rhSCF ) were purchased from Genzyme (Cambridge, MA). A neutralizing IL-10 antibody was obtained from R&D Systems Europe Ltd (Abington, UK); antibodies directed against G-CSF, GM-CSF, and IL-3 were obtained from Genzyme; rhGM-CSF and rhIL-3 were kindly provided by Sandoz (Basel, Switzerland); and rhG-CSF was purchased from British Biotechnology (Oxan, UK).
Preparation of cells.PB-MNCs were isolated from PB of normal human donors by Ficoll-Hypaque density gradient centrifugation (density, 1.077 g/mL; 400g for 40 minutes). The low-density cells were collected from the interface between density solution and plasma, washed twice, and resuspended in Iscove's modified Dulbecco's medium (IMDM; GIBCO, Paisley, Scotland).
Depletion of monocytes or T lymphocytes was performed by magnetic-activated cell sorting (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany) using superparamagnetic MACS microbeads conjugated to monoclonal antibodies against the CD14 and CD3 antigens, respectively, as described previously.13 The efficacy of the depletion procedure was determined by flow cytometry, and the samples contained less than 2% (range, 0.3% to 2.0%) CD14+ and CD3+ cells, respectively.
CD34+ RA− cells.Flow cytometric cell sorting was performed on a FACStar Plus (Becton Dickinson [BD], Mountain View, CA) using an argon ion laser (Coherent, Palo Alto, CA) adjusted to a wavelength of 488 nm. The samples originated from PB of patients recovering from the chemotherapeutical treatment of solid tumors or from BM of human healthy donors who had given informed consent. Data acquisition was performed with the FACStar Plus software (BD). Forward light scattering, orthogonal light scattering, and fluorescence signals (FL1 and FL2) were acquired and stored in listmode data files; each measurement contained 10,000 to 20,000 cells. The data were analyzed using the PAINT-A-Gate or FACStar Plus software (BD). The sort windows for the CD34+ cell population that were either RA− or RA++ were set arbitrarily in the RA+ region. Between 60 and 120 of 1,024 channels were allowed to separate the two populations. The purity of each sort usually ranged between 96% and 100%.14
Colony assay.Unseparated PB-MNCs (2 × 105), CD3-depleted PB-MNCs (2 × 105), and CD14-depleted PB-MNCs (2 × 105) were cultured in triplicates in either the presence or absence of IL-10 (0.01 to 10 ng/mL). These cultures contained 0.9% methylcellulose, 30% fetal calf serum (FCS; INLIFE, Wiener Neudorf, Austria) and IMDM without exogenous growth factors. To some cultures, either GM-CSF (100 U/mL) or G-CSF (100 U/mL) was added. CD34+ RA− cells (0.5 × 103) were cultivated in both the presence and absence of SCF (20 ng/mL), IL-3 (10 U/mL), and either G-CSF or GM-CSF with and without IL-10 (10 ng/mL). In some experiments, a neutralizing antibody against IL-10 was preincubated with IL-10 for 2 hours at room temperature. Neutralizing antibodies against G-CSF, GM-CSF, or IL-3 were used as recommended by the manufacturer. Plates were incubated at 37°C in 5% CO2 and full humidity. After a culture period of 14 days, cultures were examined under an inverted microscope. Aggregates with at least 50 translucent, compact, or dispersed cells were counted as CFU-GM.
Semiquantitative reverse transcriptase-polymerase chain reaction (RT-PCR) analysis of GM-CSF transcripts.PB-MNCs (1 × 106) were cultured in suspension both with and without IL-10 (10 ng/mL) for 48 hours. After incubation, cells were washed twice in diethylpyrocarbonate-treated water, and 107 cell aliquots were lysed by the addition of 1.6 mL RNAzol B (Biotecx, Houston, TX). Total RNA was extracted as described.15 The integrity of RNA was controlled by electrophoresis through formaldehyde agarose gels. High-quality RNA was quantitated by measuring absorbance at 260 nm, and 1 g of total RNA was subjected to cDNA synthesis as recently described.16
For semiquantitative analysis of GM-CSF mRNA, an RT-PCR technique that allows measurements of relative transcript levels was applied.17,18 The oligonucleotide primer sequences for amplification of GM-CSF were 5′-CTGCTGCTGAGATGAATGAAACAG-3′ and 5′-TGGACTGGCTCCCAGCAGTCAAAG-3′, which bracketed a GM-CSF fragment of 286 bp.19 PCR amplification of ABL transcripts was used as a reference to assess variation of total RNA or cDNA between samples. The primer sequences for amplification of ABL were as follows: 5′-CAGCGGCCAGTAGCATCTGACTTTG-3′ and 5′-CCATTTTTGGTTTGGGCATCACACCATTCC-3′ resulting in the production of a PCR fragment of 228 bp.16 The linear ranges of PCR amplifications of GM-CSF and ABL were established as a function of the cycle number and the cDNA concentration as described.17 18 Reaction conditions included 3 μL cDNA, 20 pmol of each primer, 1.5 mmol/L MgCL2 , 200 μmol/L of each deoxynucleotide triphosphate, 2.5 U Ampli Taq DNA Polymerase (Perkin Elmer-Cetus, Norwalk, CT), and [32P]-deoxycytidine triphosphate (dCTP; 150,000 cpm) in a 50-μL reaction volume. The thermal cycling conditions were denaturation at 94°C (1 minute), annealing at 60°C (1 minute), and extension at 72°C (2 minutes), preceded by an initial denaturation step at 94°C for 5 minutes and followed by a terminal extension of 10 minutes at 72°C. The number of PCR cycles for amplification of GM-CSF and ABL transcripts was 32 and 25 cycles, respectively. Reaction products were subjected to 6% polyacrylamide gels (Novex, San Diego, CA), and dried gels were exposed to Kodak XAR-5 films (Eastman-Kodak, Rochester, NY) at −70°C for 12 hours.
For quantification of PCR products, incorporated [32P]-dCTP was measured on autoradiograms using the BioRad 670 Imaging Densitometer (BioRad, Richmond, CA) and the system's volume integration program (BioRad Gel DOC 1000 system; Molecular Analyst/PC software). Potential differences of total cellular RNA/cDNA in PCR analyses were corrected by dividing GM-CSF values by the mean of the ABL value obtained for that cDNA from three PCR analyses. The relative level of GM-CSF transcripts was measured in samples of three healthy individuals in three PCR analyses in duplicate using freshly synthesized cDNA.
GM-CSF assay.PB-MNCs from four independent samples were cultured at a concentration of 1 × 106 /mL in IMDM supplemented with 30% FCS with and without IL-10, and supernatants were collected at days 3 and 5. GM-CSF contents in supernatants were measured by an immunoenzymetric assay (EASIA; Medgenix Diagnostics, Fleurus, Belgium) as recommended by the manufacturer.
Statistical analysis.The paired t-test was used to determine the significance of differences. A P value of less than .05 was considered statistically significant.
RESULTS
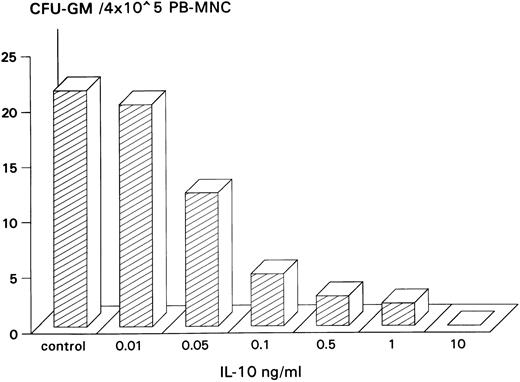
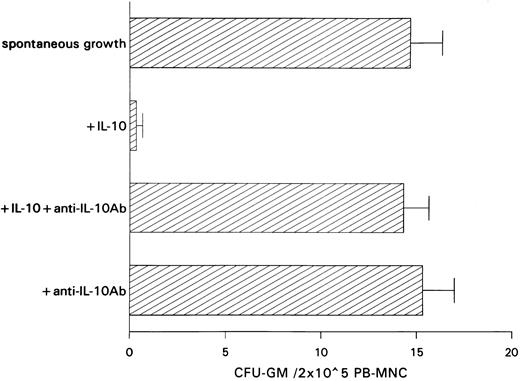
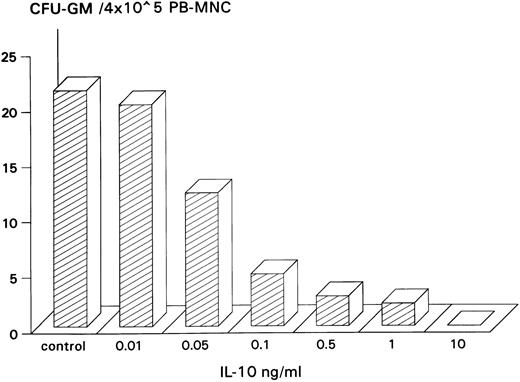
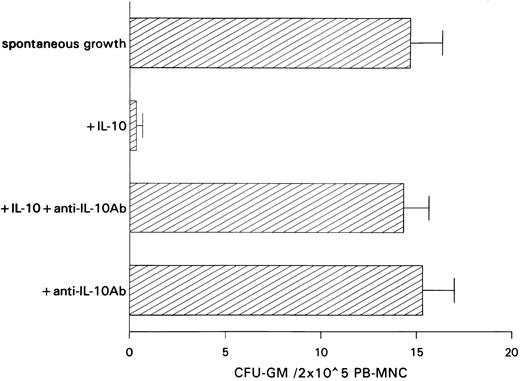
Inhibitory effect of IL-10 on spontaneous CFU-GM growth.IL-10 inhibited spontaneous growth of CFU-GM from unseparated PB-MNCs in a dose-dependent manner from 0.01 to 10 ng/mL. The maximal inhibition was observed at 10 ng/mL (Fig 1). Therefore, this concentration was chosen for further experiments. In 7 experiments, 10 ng/mL IL-10 reduced the mean number of myeloid colonies from 10.3 to 0.5/2 × 105 PB-MNCs (Table 1). On average, exogenous IL-10 decreased spontaneous CFU-GM proliferation by 95.1% (P < .001, paired t-test). Inhibition of autonomous CFU-GM growth by IL-10 was not observed in the presence of a neutralizing antibody against IL-10, excluding an unspecific suppression by this cytokine (Fig 2). On the other hand, the anti–IL-10 antibody had no effect on spontaneous colony formation, thereby excluding a potential inhibitory effect of endogenously released IL-10 in this system (Fig 2).
Dose-dependent inhibitory effect of IL-10 on spontaneous CFU-GM growth from PB-MNCs. PB-MNCs (4 × 105) were cultured in methylcellulose containing 30% FCS and IMDM and with increasing concentrations of IL-10 (0.01 to 10 ng/mL). Colony growth was assessed after 14 days. The mean colony number of triplicates is given from one representative experiment.
Dose-dependent inhibitory effect of IL-10 on spontaneous CFU-GM growth from PB-MNCs. PB-MNCs (4 × 105) were cultured in methylcellulose containing 30% FCS and IMDM and with increasing concentrations of IL-10 (0.01 to 10 ng/mL). Colony growth was assessed after 14 days. The mean colony number of triplicates is given from one representative experiment.
Effect of a neutralizing anti–IL-10 antibody on spontaneous CFU-GM growth and on IL-10–induced inhibition of CFU-GM growth from unseparated PB-MNCs. PB-MNCs (2 × 105) were cultured in methylcellulose containing 30% FCS and IMDM. Colony growth was assessed after 14 days. The mean colony numbers are given from three independent experiments.
Effect of a neutralizing anti–IL-10 antibody on spontaneous CFU-GM growth and on IL-10–induced inhibition of CFU-GM growth from unseparated PB-MNCs. PB-MNCs (2 × 105) were cultured in methylcellulose containing 30% FCS and IMDM. Colony growth was assessed after 14 days. The mean colony numbers are given from three independent experiments.
The role of accessory cells in autonomous CFU-GM growth.To elucidate the role of accessory cells in autonomous CFU-GM growth, we cultured in methylcellulose PB-MNCs that were depleted either of monocytes by CD14 antibodies or of T lymphocytes by CD3 antibodies using MACS. Table 2 shows that depletion of monocytes as well as of T lymphocytes almost completely prevented autonomous CFU-GM growth. However, this was not due to a loss of progenitor cells by the depletion procedure, because significant CFU-GM growth was observed in the presence of exogenous GM-CSF. Not unexpectedly, spontaneous CFU-GM growth was also not observed in cultures containing highly enriched CD34+ cells, thus reconfirming the necessity of accessory cells for autonomous CFU-GM growth (mean CFU-GM growth from 6 experiments cultivating 0.5 × 103 CD34+ cells/mL without exogenous CSF, 0 ± 0). When these cultures were stimulated with the combination of SCF, IL-3, and either G-CSF or GM-CSF, CFU-GM growth was observed with a cloning efficiency of up to 15% (data not shown).
The role of endogenously released CSFs in spontaneous CFU-GM growth.To identify the factor responsible for autonomous proliferation of myeloid colonies, we cultured PB-MNCs in the presence of neutralizing antibodies against G-CSF, GM-CSF, and IL-3. Among the antibodies tested, the anti–GM-CSF antibody had by far the greatest effect, resulting in a 91.1% inhibition of spontaneous CFU-GM growth. In contrast, the anti–G-CSF antibody inhibited autonomous proliferation by only 23.3% (not significant), whereas anti–IL-3 had no effect at all. A combination of anti–GM-CSF and anti–G-CSF antibodies was slightly but not significantly more suppressive than anti–GM-CSF alone (Table 3). These data suggest that GM-CSF is the main but possibly not the only factor responsible for spontaneous CFU-GM growth from PB-MNCs.
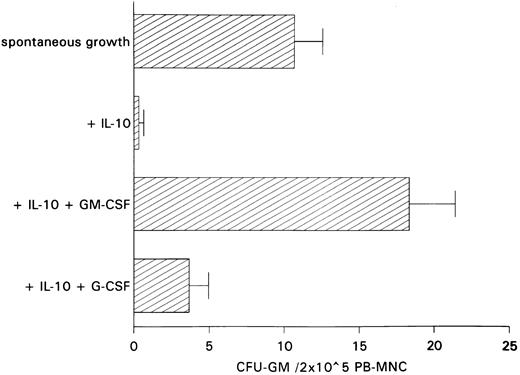
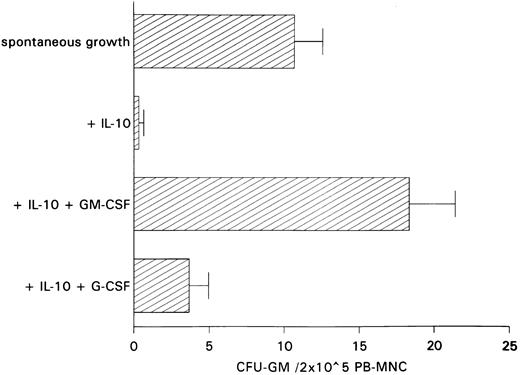
Restoration of IL-10–induced inhibition of spontaneous CFU-GM growth by exogenous GM-CSF.The antiproliferative action of anti–GM-CSF antibody in PB-MNCs and the fact that IL-10 has been shown to inhibit cytokine synthesis including that of GM-CSF in monocytes and T lymphocytes11 12 led us to hypothesize that inhibition of autonomous CFU-GM growth by IL-10 is secondary to IL-10–induced suppression of endogenous GM-CSF release. If this is the case, one would expect the addition of GM-CSF to largely reverse growth inhibition by IL-10. In contrast, restoration of colony growth by exogenous growth factors would not be observed if IL-10 has a direct cytotoxic effect on progenitor cells. In fact, exogenous GM-CSF was able to completely overcome IL-10–induced suppression, whereas exogenous G-CSF, as a control, was ineffective in overcoming this suppression (Fig 3).
Effect of GM-CSF and G-CSF on IL-10–induced inhibition of spontaneous CFU-GM growth. Unseparated PB-MNCs (2 × 105) were cultured in methylcellulose containing 30% FCS and IMDM. Either GM-CSF (100 U/mL) or G-CSF (100 U/mL) was added to cultures containing IL-10 (10 ng/mL). Colony growth was assessed after 14 days. The mean colony numbers (±SEM) are given from six independent experiments.
Effect of GM-CSF and G-CSF on IL-10–induced inhibition of spontaneous CFU-GM growth. Unseparated PB-MNCs (2 × 105) were cultured in methylcellulose containing 30% FCS and IMDM. Either GM-CSF (100 U/mL) or G-CSF (100 U/mL) was added to cultures containing IL-10 (10 ng/mL). Colony growth was assessed after 14 days. The mean colony numbers (±SEM) are given from six independent experiments.
Inhibitory effect of IL-10 on GM-CSF m-RNA expression in PB-MNCs.Analyses of the effect of IL-10 on GM-CSF expression in unseparated PB-MNCs were performed by the semiquantitative PCR technique.17 18 To prove that the PCR technique used by us allows at least semiquantitative measurements of GM-CSF transcript levels, we have established the linear ranges of amplifications of GM-CSF as a function of the cycle number and the cDNA concentration for each donor (Fig 4a). GM-CSF transcripts were already detectable in freshly isolated PB-MNCs and further increased in these cells after 48 hours of suspension in medium alone (Fig 4b, panel A). In comparison, ABL-transcripts, which served as a control, remained unchanged during time of culture (Fig 4b, panel B). In the presence of IL-10 (10 ng/mL), GM-CSF transcript levels after 48 hours were significantly lower than the transcript levels of PB-MNCs kept in suspension without IL-10 (P = .035). Fig 4b, panel C shows corrected GM-CSF mRNA levels in PB-MNCs at 48 hours. Comparison of corrected GM-CSF transcript levels between PB-MNCs cultured with and without IL-10 showed a mean decrease of 66.6% (range, 53.0% to 92.0%) in three independent experiments.
(a) Linear PCR amplification of GM-CSF transcripts. PCR amplification products were electrophoresed through 6% polyacrylamide gels. Incorporated [32P]-dCTP was measured in counts per square millimeter on autoradiograms using the BioRad Gel Doc 1000 system. The linear relationship between GM-CSF gene transcripts and the PCR cycle number (upper panel) and between GM-CSF transcripts and various concentrations of cDNA (lower panel), which represented a calculated amount of total RNA, are shown from one donor. (b) Semiquantitative RT-PCR analysis of GM-CSF transcript levels. (A) Autoradiograms showing incorporated radioactivity of amplification products obtained from unseparated PB-MNCs of three normal donors (AH, HV, and KG) cultured in suspension with or without IL-10 for 48 hours. (B) Autoradiograms showing ABL transcripts that served as a reference to correct for potential variations of RNA or cDNA samples. (C) Corrected mean GM-CSF transcript levels in cultured PB-MNCs. Each donor sample was analyzed in three radioactive PCR analyses in duplicate using freshly synthesized cDNA. The quantity of [32P] incorporated into the PCR product was determined by densitometric scanning of autoradiograms. Results were corrected by dividing GM-CSF values by the mean values obtained from six ABL transcripts of that cDNA.
(a) Linear PCR amplification of GM-CSF transcripts. PCR amplification products were electrophoresed through 6% polyacrylamide gels. Incorporated [32P]-dCTP was measured in counts per square millimeter on autoradiograms using the BioRad Gel Doc 1000 system. The linear relationship between GM-CSF gene transcripts and the PCR cycle number (upper panel) and between GM-CSF transcripts and various concentrations of cDNA (lower panel), which represented a calculated amount of total RNA, are shown from one donor. (b) Semiquantitative RT-PCR analysis of GM-CSF transcript levels. (A) Autoradiograms showing incorporated radioactivity of amplification products obtained from unseparated PB-MNCs of three normal donors (AH, HV, and KG) cultured in suspension with or without IL-10 for 48 hours. (B) Autoradiograms showing ABL transcripts that served as a reference to correct for potential variations of RNA or cDNA samples. (C) Corrected mean GM-CSF transcript levels in cultured PB-MNCs. Each donor sample was analyzed in three radioactive PCR analyses in duplicate using freshly synthesized cDNA. The quantity of [32P] incorporated into the PCR product was determined by densitometric scanning of autoradiograms. Results were corrected by dividing GM-CSF values by the mean values obtained from six ABL transcripts of that cDNA.
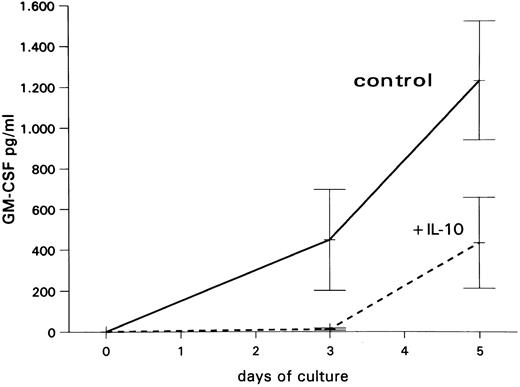
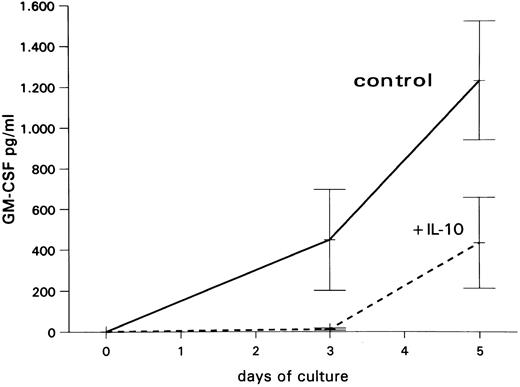
Inhibitory effect of IL-10 on GM-CSF production by PB-MNCs.To confirm the inhibitory effect of IL-10 on GM-CSF production in PB-MNCs at the protein level, supernatants from unseparated PB-MNC suspension cultures obtained at days 3 and 5 were analyzed for GM-CSF by an immunoenzymetric assay (EASIA). As shown in Fig 5, PB-MNCs released increasing amounts of GM-CSF when cultured in medium alone. In contrast, in the presence of IL-10, only very low GM-CSF levels were detected at day 3, and those were clearly lower than control levels at day 5.
Mean GM-CSF levels (±SEM) from three independent experiments in supernatants of unseparated PB-MNCs cultured in medium alone or with 10 ng/mL IL-10.
Mean GM-CSF levels (±SEM) from three independent experiments in supernatants of unseparated PB-MNCs cultured in medium alone or with 10 ng/mL IL-10.
DISCUSSION
Multiple roles in regulating immune response have been proposed for IL-10, including inhibition of the monocyte/macrophage and T-cell effector functions, that render this molecule a potent immunosuppressive cytokine.20-22 Because of its ability to inhibit the production of proinflammatory cytokines, IL-10 is now being evaluated in various clinical settings such as chronic arthritis, sepsis, and chronic inflammatory bowel disease.23 24 This potential clinical application of IL-10 makes the understanding of its possible role in the regulation of hematopoiesis of certain interest. The data presented in this study show that IL-10 markedly inhibits the growth of myeloid colonies that form from PB-MNCs in the absence of exogenous growth factors.
Spontaneous growth of myeloid colonies can be consistently detected when PB-MNCs are plated in the methylcellulose assay. In this system, accessory cells, mainly consisting of monocytes and T cells, seem to support growth of hematopoietic progenitors through production of various stimulatory cytokines and cell-to-cell interactions.25,26 PB-MNCs obtained by Ficoll-Hypaque centrifugation, as used in this study, almost exclusively contain lymphocytes, monocytes, and progenitor cells.27 We observed that depletion of monocytes or T lymphocytes from PB-MNCs almost completely abrogated spontaneous CFU-GM formation, indicating that the presence of both cell types seems to be essential for this in vitro phenomenon. Therefore, as with a number of immunoregulatory processes,28 production of colony-stimulating activity by MNCs seems to require the interaction of T lymphocytes and monocytes.
The production of blood cells is the result of the balance between positive and negative growth signals, both in vitro and in vivo.29,30 The fact that we have not observed any suppression of CSF-stimulated colony formation by IL-10 in cultures containing highly enriched CD34+ cells (data not shown) allows us to exclude a direct suppression of progenitor cells. Therefore, the inhibitory effect of IL-10 on spontaneous myeloid colony formation may be theoretically explained by an increased release of negative growth signals or, alternatively, by a decreased release of positive signals by accessory cells. There is no evidence that IL-10–induced suppression of CFU-GM formation is caused by an increased release of growth inhibitory cytokines. For example, it is known that IL-10 decreases the production of TNF-α and INF-γ, two cytokines that have been shown to suppress the in vitro growth of myeloid colonies.31 32 Thus, it is more likely that IL-10 decreased the release of positive growth signals. We have shown that IL-10 and an anti–GM-CSF antibody inhibited autonomous colony formation to a similiar extent and that the IL-10–induced suppression could be completely restored by the addition of exogenous GM-CSF. These findings suggest that the inhibitory effect of IL-10 is mainly caused by a decreased release of GM-CSF by accessory cells. Moreover, we were able to show a profound inhibition of GM-CSF synthesis in PB-MNCs both at the mRNA and the protein level. This is in agreement with a number of studies showing the inhibition of cytokine synthesis by IL-10 in T cells as well as in monocytes.
So far, only a few studies have investigated the effect of IL-10 on murine and human hematopoiesis in vitro. Rennick et al33 reported a growth-promoting activity of IL-10 on megakaryocytes, mast cells, and multilineage colonies in a murine system but found no effect on colony formation by nonadherent BM cells in methylcellulose cultures. These apparently contrary findings may be explained by differing culture systems and cell sources and by differences between human and murine hematopoiesis. For example, both human and murine IL-10 can inhibit T-cell proliferation and cytokine production by a monocyte/macrophage-dependent mechanism, but, in contrast to murine IL-10, human IL-10 can also directly inhibit T-cell proliferation through inhibition of IL-2 production.34,35 More recently, Schibler et al7 reported a 50% inhibition of autonomous CFU-GM growth from cord blood and BM-MNCs by IL-10 in the human system. These findings are in agreement with our results. In contrast to their observation that both anti–GM-CSF and anti–IL-3 antibodies inhibited autonomous CFU-GM growth from BM-MNCs, we found no inhibitory effect of a neutralizing anti–IL-3 antibody on myeloid colony formation from PB-MNCs.
In conclusion, our findings indicate that IL-10 is a potent molecule in the suppression of spontaneous myeloid colony formation from PB-MNCs. Our results further suggest that endogenous GM-CSF mainly contributes to autonomous CFU-GM growth in our culture system and that inhibition of spontaneous CFU-GM proliferation by IL-10 is likely to be caused by suppression of endogenous GM-CSF release. This endogenous GM-CSF release from normal PB-MNCs seems to result from an interaction of accessory cells including monocytes and T lymphocytes. Malignant cells have also been shown to spontaneously release growth factors in patients with chronic myelomonocytic leukemia8 and juvenile chronic myeloid leukemia9 and in some cases of patients with acute myeloblastic leukemia,10 resulting in spontaneous colony formation. Considering the strong inhibitory action of IL-10 on spontaneous GM-CSF release and on autonomous CFU-GM growth from normal PB-MNCs and the fact that IL-10 has been recently shown to suppress GM-CSF production and in vitro colony formation from chronic myelomonocytic leukemia cells,36 this cytokine may be useful in the treatment of myeloid malignancies in which autocrine and/or paracrine mechanisms involving GM-CSF are suspected of playing a pathogenetic role.
Address reprint requests to Klaus Geissler, MD, Division of Hematology-Internal Medicine I, University of Vienna, Währinger Gürtel 18-20, A-1090 Vienna, Austria.

![Fig. 4. (a) Linear PCR amplification of GM-CSF transcripts. PCR amplification products were electrophoresed through 6% polyacrylamide gels. Incorporated [32P]-dCTP was measured in counts per square millimeter on autoradiograms using the BioRad Gel Doc 1000 system. The linear relationship between GM-CSF gene transcripts and the PCR cycle number (upper panel) and between GM-CSF transcripts and various concentrations of cDNA (lower panel), which represented a calculated amount of total RNA, are shown from one donor. (b) Semiquantitative RT-PCR analysis of GM-CSF transcript levels. (A) Autoradiograms showing incorporated radioactivity of amplification products obtained from unseparated PB-MNCs of three normal donors (AH, HV, and KG) cultured in suspension with or without IL-10 for 48 hours. (B) Autoradiograms showing ABL transcripts that served as a reference to correct for potential variations of RNA or cDNA samples. (C) Corrected mean GM-CSF transcript levels in cultured PB-MNCs. Each donor sample was analyzed in three radioactive PCR analyses in duplicate using freshly synthesized cDNA. The quantity of [32P] incorporated into the PCR product was determined by densitometric scanning of autoradiograms. Results were corrected by dividing GM-CSF values by the mean values obtained from six ABL transcripts of that cDNA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/4/10.1182_blood.v89.4.1147/4/m_bl_0033f4a.jpeg?Expires=1771110426&Signature=BoFTDKlMWsuG-4~MIdte4hzMMvNyRfI03fWlmnEDFEFxsm6h5V~qigVrf3zr5bDpWT63GzBR4fJA5HWCKY8cONMFJwBkowaqHC8oyMX91uLbwKWivrCRYw4BhDA3kJWB3O-bl1982qEngjqLASkKe8AbNgxlQSiBq0jls7R3dJ8H8ebIM~agqk68UtzmpDaIisiuikPvPQ3YiKFTYx-haPFhgG43ODGl5ddJagJJQr7kPPI-sMeQ~1TeD8pdty54-~EAzED5Oh7B3GZ8JFnHs7JLjCcpMDsyZPcV3EGpAJxdUDIPnl0JQSAqUdN~718c0eDORzYaUSXY07xTeMTStg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. (a) Linear PCR amplification of GM-CSF transcripts. PCR amplification products were electrophoresed through 6% polyacrylamide gels. Incorporated [32P]-dCTP was measured in counts per square millimeter on autoradiograms using the BioRad Gel Doc 1000 system. The linear relationship between GM-CSF gene transcripts and the PCR cycle number (upper panel) and between GM-CSF transcripts and various concentrations of cDNA (lower panel), which represented a calculated amount of total RNA, are shown from one donor. (b) Semiquantitative RT-PCR analysis of GM-CSF transcript levels. (A) Autoradiograms showing incorporated radioactivity of amplification products obtained from unseparated PB-MNCs of three normal donors (AH, HV, and KG) cultured in suspension with or without IL-10 for 48 hours. (B) Autoradiograms showing ABL transcripts that served as a reference to correct for potential variations of RNA or cDNA samples. (C) Corrected mean GM-CSF transcript levels in cultured PB-MNCs. Each donor sample was analyzed in three radioactive PCR analyses in duplicate using freshly synthesized cDNA. The quantity of [32P] incorporated into the PCR product was determined by densitometric scanning of autoradiograms. Results were corrected by dividing GM-CSF values by the mean values obtained from six ABL transcripts of that cDNA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/4/10.1182_blood.v89.4.1147/4/m_bl_0033f4b.jpeg?Expires=1771110426&Signature=wU3NZP2k2S27~5qhj8XrnYgspqn1FJL8q162eIWOEtvLllU9drlO8qn51FoRcjlXRKg46d8KKodjTBCzaFmUCtJsnvVjZsnGRcLAnGwbYAxiWL14VaFkVjCObXAT25ehUbSWbeQrLibmzJf0knK2qacH0vTncmCVQC0-3YOJFf0kzwoQ0YezCflBxo-v1qW3iBtJxxwZv5ApxYKFgjX4wsjBS5B7TsBtXcNn4YL5OkCfxtbdw5wTH3GfvCeq3J0aI5l8Y9vG4R5rOYPoALckAZPjXhvQopcLuQA5QSt7j0-dZHUOIZQkYHMxu6uKHM43uzmV1FMbHTsPZhpswX0gkA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 4. (a) Linear PCR amplification of GM-CSF transcripts. PCR amplification products were electrophoresed through 6% polyacrylamide gels. Incorporated [32P]-dCTP was measured in counts per square millimeter on autoradiograms using the BioRad Gel Doc 1000 system. The linear relationship between GM-CSF gene transcripts and the PCR cycle number (upper panel) and between GM-CSF transcripts and various concentrations of cDNA (lower panel), which represented a calculated amount of total RNA, are shown from one donor. (b) Semiquantitative RT-PCR analysis of GM-CSF transcript levels. (A) Autoradiograms showing incorporated radioactivity of amplification products obtained from unseparated PB-MNCs of three normal donors (AH, HV, and KG) cultured in suspension with or without IL-10 for 48 hours. (B) Autoradiograms showing ABL transcripts that served as a reference to correct for potential variations of RNA or cDNA samples. (C) Corrected mean GM-CSF transcript levels in cultured PB-MNCs. Each donor sample was analyzed in three radioactive PCR analyses in duplicate using freshly synthesized cDNA. The quantity of [32P] incorporated into the PCR product was determined by densitometric scanning of autoradiograms. Results were corrected by dividing GM-CSF values by the mean values obtained from six ABL transcripts of that cDNA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/4/10.1182_blood.v89.4.1147/4/m_bl_0033f4a.jpeg?Expires=1771288020&Signature=gpSKia2FG5faVP-qE-ZapGAYPj3KatOS0IS1QBkI2JSBFcphfzCciOFCVj0mPONEGwCrC-U9-8EB5ppfliutfI9mNmocGPPTPNufRb5Rk8neJMc1I7KIb71rY~226OEPCwrR-6XT155ZP9g5VkifgVpGe-9Od-z-nTlYs0q-Ei~zJeNTxhTe-9CfV6vLPT8yKlXOFzOCDEa82wDg2NFanroolLfPloHZC-YzDRTz18rF1R4s8L4w8ikpQIoCIvQRyqx2kjhwlzsTuHKosKUtBE3Wzx2IW7zqqW9E8Ll~znzejPYbOZfsvZjuq4mnbpxHJf7t-Qm08chI2SVMluSsxw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. (a) Linear PCR amplification of GM-CSF transcripts. PCR amplification products were electrophoresed through 6% polyacrylamide gels. Incorporated [32P]-dCTP was measured in counts per square millimeter on autoradiograms using the BioRad Gel Doc 1000 system. The linear relationship between GM-CSF gene transcripts and the PCR cycle number (upper panel) and between GM-CSF transcripts and various concentrations of cDNA (lower panel), which represented a calculated amount of total RNA, are shown from one donor. (b) Semiquantitative RT-PCR analysis of GM-CSF transcript levels. (A) Autoradiograms showing incorporated radioactivity of amplification products obtained from unseparated PB-MNCs of three normal donors (AH, HV, and KG) cultured in suspension with or without IL-10 for 48 hours. (B) Autoradiograms showing ABL transcripts that served as a reference to correct for potential variations of RNA or cDNA samples. (C) Corrected mean GM-CSF transcript levels in cultured PB-MNCs. Each donor sample was analyzed in three radioactive PCR analyses in duplicate using freshly synthesized cDNA. The quantity of [32P] incorporated into the PCR product was determined by densitometric scanning of autoradiograms. Results were corrected by dividing GM-CSF values by the mean values obtained from six ABL transcripts of that cDNA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/89/4/10.1182_blood.v89.4.1147/4/m_bl_0033f4b.jpeg?Expires=1771288020&Signature=TtOnOk8MUkIF8YrEegr9XqsaHWlUFfPyDxVn08-eI38nr~l8P1Oc4N3swq~Sv7wPmTs5O36q0PvkVzm~k7aU4DZ4zQrWKEOCcksW3OYK-8yyeUlECcKLmbA~PJa0BSB0Yfru7u7B-gAKW-GQbXddlzZLTEm861EQrwRO5vg-eJ-qbTunT3IFMCq3kV1krZ4a0cEpmXzmfiHpbnwPdNfhgC6NyFDPoNrrvHJ~amvMP0j6TV1FhGbrsWXhu3XUG8oTNFl~LH8yD2X0euhp3PpnPAQTVVf~a7rz3XL1EwKXJZmGL890BtyvOn7afRFKejW0uwbf05dG0Q7HCVcEoqpXzQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)