Abstract
Type 2B von Willebrand disease (vWD) is characterized by the absence of the very high molecular weight von Willebrand factor (vWF ) multimers from plasma, which is caused by spontaneous binding to platelet receptor glycoprotein Ib (GPIb). We studied two mutations in the A1 domain at position 543 in which arginine (R) was replaced by glutamine (Q) or tryptophan (W), respectively. Both mutations were previously identified in vWD type 2B patients. The mutations R543Q and R543W were cloned into a eukaryotic expression vector and subsequently transfected in baby hamster kidney cells overexpressing furin (fur-BHK). Stable cell lines were established by which the mutants were secreted in the cell culture supernatant. The subunit composition and multimeric structure of R543Q and R543W were similar to wild-type (WT) vWF. The mutants showed a spontaneous binding to GPIb. R543Q and R543W showed normal binding to collagen type III or heparin. Both mutants supported platelet adhesion under conditions of flow, usually when preincubated on a collagen type III surface. A low dose (2.5% of the concentration present in normal pooled plasma) of recombinant R543Q or R543W added to normal whole blood inhibited platelet adhesion to collagen type III. No inhibition was found when vWF was used as an adhesive surface. These results indicate that point mutations identified in vWD type 2B cause bleeding symptoms by two mechanisms: (1) the mutants cause platelet aggregation, which in vivo is followed by removal of the aggregates leading to the loss of high molecular weight multimers and thrombocytopenia, (2) on binding to circulating platelets the mutants block platelet adhesion. Relatively few molecules are required for the latter effect.
VON WILLEBRAND FACTOR (vWF ) is a multimeric plasma glycoprotein that has two essential functions. It acts as a carrier for coagulation factor VIII, and it functions as a mediator of initial platelet adhesion to the subendothelium following damage of the vascular endothelium. At high shear rates, platelet adhesion depends on binding of vWF to subendothelial components, inducing a conformational change in vWF.1 This conformational change is reproduced in vitro by action of nonphysiologic modulators: the antibiotic ristocetin2 or the snake venom protein botrocetin,3 exposing the platelet glycoprotein (GP) Ib binding site (see Roth4 ).
von Willebrand disease (vWD) is classified into three major categories. Type 1 and 3 are characterized by quantitative defects, whereas type 2 is characterized by qualitative defects.5 Subtype 2B vWD refers to variants with an increased and often spontaneous vWF binding to platelet GPIb and subsequent clearance of the highest multimers. Mutations belonging to this subtype produce the specific type 2B “gain-of-function” phenotype.6 In fact, vWD type 2B patients synthesize the full range of vWF multimers, as shown by the normal multimeric pattern of their platelet, and endothelial vWF.7 Most of the type 2B vWD mutations have been identified within the disulfide loop in the A1 domain between amino acid residues 509-695.6 In this loop, binding sites are localized not only for GPIb,8 but also for heparin,9 and sulfatides.10 Three vWD type 2B mutations have been found outside the A1 loop: at the N-terminus residue 505, and at the C-terminus residues 697 and 698.6,11 Expression of mutant recombinant (r) vWF in COS cells reproduced the characteristic functional abnormality of type 2B vWD: spontaneous binding to platelets.11-18
In the present report, we study two recombinant vWF type 2B mutants that have a point mutation at position 543 in which Arg (R) was substituted by Gln (Q) or Trp (W). The mutants and wild-type (WT) vWF were transfected into baby hamster kidney cells overexpressing furin (fur-BHK). The rvWFs were purified from the culture supernatant. This study confirms earlier data using R543W under static conditions,14 and extends these data using flow conditions. Addition of small quantities of the mutants to whole blood inhibits platelet adhesion to collagen type III, which may partly explain the bleeding symptoms observed in vWD type 2B patients.
MATERIALS AND METHODS
Materials.Restriction enzymes and DNA modifying enzymes were from New England Biolabs (Beverly, MA) or Pharmacia (Uppsala, Sweden). Pfu DNA polymerase was obtained from Stratagene (La Jolla, CA). MAX Efficiency DH10B Competent Cells were from Gibco BRL (Paisley, UK). Synthetic oligonucleotides were prepared on an Applied Biosystems synthesizer model 3811A (Foster City, CA). Cell culture plastics were obtained from Nunc (Roskilde, Denmark) and Costar (Cambridge, MA). All culture media and supplements were from Gibco BRL. Ristocetin was purchased from DiaMed (Cressier sur Morat, Switzerland), and botrocetin from Pentapharm (Basel, Switzerland).
Site-directed mutagenesis.The preparation of the plasmids WT vWF-pNUT19 and pSV-vWFcas11 has been described previously. The construction of the mutants was performed with a polymerase chain reaction (PCR) by using mismatched primers.20 The method consists in the combination of two separate PCR products with overlapping sequence into one longer product, the overlapping primers contain the mismatched sequence. The first round of PCR, consisting of two simultaneous reactions, was performed with primers 1 (ATGATGGAGCAGCTTCGCATC (3907-3927)) or 3 (ATGAATGGAGTGGCTTCGCATC (3907-3927)) (R543Q or R543W mutant, respectively) and primer 6 (CAAAATAGCTAGCTGGGAAA (5122-5103)). The parallel reaction was performed with primer 2 (GATGCGAAGCTGCTCCATCAT (3927-3907)) or 4 (GATGCGAAGCCACTCCATCAT (3927-3907)) (R543Q or R543W mutants) and primer 5 (AAGACTGTCCAGTGTGTGAG (3581-3600)). The PCR was carried out with Pfu DNA polymerase (Stratagene) and the buffer provided by the manufacturer in the presence of 200 μmol/L dNTPs, 10 pmol of each primer, 6 mmol/L MgCl2 , 2% glycerol, 20 ng of pSV-vWFcassette11 as a template, and 2.5 U of enzyme in a final volume of 100 μL for 30 cycles (94°C 1 min, 50°C 1 min, 72°C 1 min). Amplified products were separated on a 1% agarose gel and purified from the agarose using QIAEX Gel extraction kit (QIAGEN GmbH, Germany). After purification, the amplified fragments from each PCR were mixed and subjected to another round of PCR with primers 5 and 6. This second round of PCR was performed using the same conditions as in the first two simultaneous reactions. The new product was electrophoresed and the DNA was purified, digested with SaII/Nhe I, and ligated into pSV-vWFcas. For expression in BHK cells pSV-R543Q-vWF and pSV-R543W-vWF were digested with BamHI and Nhe I and ligated into pNUT-vWF. The mutants were confirmed by restriction enzyme digests.
Transfection and selection of stable transformants.For transfection of WT-vWF, R543Q-vWF, and R543W-vWF, a BHK cell line overexpressing furin was used. The presence of furin is necessary for a proper processing of vWF.21 The construction of this BHK cell line is as follows. Furin cDNA cloned in the EcoRI site of PUC18, was kindly provided by Dr W.J.M. van de Ven (University of Leuven, Leuven, Belgium). For our purpose we cloned the furin cDNA in the EcoRI site of the eukaryotic pCDNA1 expression vector containing a neomycin resistance gene (Invitrogen, Leek, The Netherlands). BHK cells were transfected using the calcium phosphate method as described previously.19,22 After transfection, 1 mg/mL neomycin (G418) (GIBCO) was used for selection of stable cell lines. The expression of intracellular furin was checked by immunofluorescence microscopy using a monoclonal antibody (MoAb) directed against furin, MON148.23 One cell line was used for further studies, indicated as fur-BHK. The next step was the transfection of the pNUT constructs containing the type 2A mutations into this fur-BHK cell line, and stable cell lines were established. The culture supernatant of these cell lines was harvested every 3 to 4 days, and the amount of rvWF was estimated using an enzyme linked immunosorbent assay (ELISA).24 The subunit composition was checked by polyacrylamide gel electrophoresis (PAGE) under reducing conditions followed by Western blot using a horseradish peroxidase conjugated rabbit polyclonal antibody to human vWF (Dakopatts, Glostrup, Denmark; Fig 1A). The subunit contained no pro-vWF, indicating that the enzyme furin was biologically active, cleaving off the propeptide. The multimeric pattern of both mutants showed high molecular weight molecules identical to WT-vWF (Fig 1B).
Subunit composition and multimeric pattern of the vWFs. The rvWFs were reduced and after SDS-PAGE (4% to 15%) the proteins were blotted. Detection was carried out using peroxidase-labeled rabbit anti-vWF Igs. These antibodies were detected by incubation with diamino-benzidine-tetrahydrochloride: lane 1, WT-vWF; lane 2, R543Q-vWF; lane 3, R543W-vWF. (B) For multimeric pattern analysis the proteins were blotted after electrophoresis using 1.7% agarose gel and detected as described above: lane 1, WT-vWF; lane 2, R543Q-vWF; lane 3, R543W-vWF.
Subunit composition and multimeric pattern of the vWFs. The rvWFs were reduced and after SDS-PAGE (4% to 15%) the proteins were blotted. Detection was carried out using peroxidase-labeled rabbit anti-vWF Igs. These antibodies were detected by incubation with diamino-benzidine-tetrahydrochloride: lane 1, WT-vWF; lane 2, R543Q-vWF; lane 3, R543W-vWF. (B) For multimeric pattern analysis the proteins were blotted after electrophoresis using 1.7% agarose gel and detected as described above: lane 1, WT-vWF; lane 2, R543Q-vWF; lane 3, R543W-vWF.
Purification of recombinant vWF.WT-vWF, R543Q-vWF, and R543W-vWF were purified using affinity chromatography. Purified IgG of MoAb RU9, which recognizes an epitope on the D4 domain, was used. The MoAb was coupled to CNBr activated Sepharose 4B (Pharmacia) according to the manufacturer's instructions. The RU9 Sepharose column was equilibrated with 50 mmol/L Tris/500 mmol/L NaCl, pH 7.4. The expression medium was applied to the column and after overnight recirculation the aspecific proteins were washed away using 5 column volumes of equilibration buffer. After rinsing the column with 50 mmol/L Tris/100 mmol/L NaCl (TBS), the bound rvWF was eluted in 1 mL fractions using 50 mmol/L triethylamine (TEA; Aldrich-Chemie, Bornem, Belgium) pH 11-12. Fractions containing TEA were immediately neutralized with 2 mol/L glycine, pH 3. Fractions containing rvWF were stored at 4°C and used within 10 days, or stored at −20°C.
Ristocetin or botrocetin induced binding of rvWF to platelets.rvWF was diluted in phosphate buffered saline (PBS) containing 3% bovine serum albumin (BSA) and 0.1% Tween-20 (PBS/T/BSA) to a concentration of 2 μg/mL and assayed for binding to platelets in the presence of ristocetin or botrocetin. Of this dilution, 50 μL was added to the incubation mixture containing 325 μL PBS/T/BSA, 100 μL fixed platelets, and 25 μL ristocetin (Diamed) or botrocetin (Pentapharm) giving a final incubation concentration of 200 ng/mL for rvWF. Formaldehyde fixed platelets were used at a final concentration of 1 × 108/mL and ristocetin or botrocetin was used at final concentrations as indicated. After incubation, while rotating, for 1 hour at room temperature, the platelets were spun down for 1 minute at 14,000g. The remaining vWF in the supernatant was measured using an ELISA. As a control, mixtures of rvWF and ristocetin or botrocetin were incubated in the absence of platelets.
Heparin binding studies.Fifty microliters of a 2 μg/ml dilution of rvWF cell culture supernatant in PBS/T/BSA was added to 300 μL PBS/T/BSA containing final concentrations of NaCl as indicated. After addition of 50 μL of a 50% suspension of heparin-Sepharose (CL-6B, Pharmacia), the mixture was incubated for 1 hour at room temperature. The beads were spun down for 1 minute at 14,000g and the remaining vWF present in the supernatant was measured using an ELISA. Incubation of rvWF with Sepharose CL-4B beads was used as a negative control.
Binding of rvWF to collagen type III.Human placenta collagen type III (Sigma) was solubilized in 50 mmol/L acetic acid (1 mg/mL), and subsequently dialyzed against PBS to obtain fibrillar collagen (48 hours at 4°C). Wells of a 96 well ELISA-tray were coated with 100 μL of fibrillar collagen (100 μg/mL) by centrifuging for 15 minutes at 250g. Nonadsorbed collagen was then removed by washing the wells with running tap water. After incubation with a blocking buffer (50 mmol/L Tris, 100 mmol/L NaCl, 3% BSA, 0.1% Tween-20, pH 7.4) for 1 hour at room temperature, 100 μL of the cell culture supernatant containing the rvWF was added. Incubations in duplicate were performed for 2 hours at room temperature. After incubation, the wells were washed three times, and the amount of vWF bound to the collagen was measured by ELISA.
Perfusion procedures.Coating of the coverslips with the purified rvWFs or spraying of the coverslips with collagen type III was performed as previously described.19 For platelet adhesion to the purified rvWFs, whole blood from healthy volunteer donors, who had taken no aspirin or other platelet function inhibitors in the preceding week, was anticoagulated with 1/10 volume of 110 mmol/L trisodium citrate or with 20 U/mL low molecular weight heparin (LMWH, Fragmin; Kabi Pharmacia, Stockholm, Sweden). Reconstituted perfusates in 4% human albumin solution (HAS; 4% human albumin in Krebs-Ringer buffer, 5 mmol/L a-D-glucose, pH 7.35) were prepared as described.19 vWF-depleted plasma was obtained by affinity chromatography. Briefly, citrated O+ plasma from a healthy volunteer was passed over a column of CNBr-activated Sepharose (Pharmacia), to which MoAb RU1 directed against the A2 domain of vWF was coupled according to the manufacturer's instructions. The flow-through is plasma depleted of vWF, which has a vWF:Ag less than 0.4 μg/mL (<4%). Washed platelets were resuspended to a platelet count of 190,000/μL in vWF-depleted plasma, and washed red blood cells from a normal O+ donor were added in a volume fraction of 40% of total volume, 5 minutes before perfusion.1 The flat chamber perfusion system as developed by Sakariassen et al25 was used for the adhesion experiments to purified rvWFs coated to glass. For adhesion studies to collagen the single passage perfusion system was used.26 This system has the advantage that the volume perfusate needed (vWF depleted plasma) is much less compared with the recirculated system. The perfusate was prewarmed for 5 minutes at 37°C and was subsequently drawn through the perfusion chamber using an infusion pump (Pump 22, model 2400-004; Harvard Apparatus, South Natick, MA) placed distally to the perfusion chamber for 5 minutes at a shear rate of 1,600 s.1 After perfusion, recirculated or single passage, the coverslips were rinsed with HEPES-buffered saline (10 mmol/L HEPES, 150 mmol/L NaCl, pH 7.35), fixated by 0.5% glutaraldehyde in PBS, dehydrated in methanol, and stained with May-Grünwald/Giemsa.25 Platelet adhesion was evaluated with a light microscope at 1,000× magnification, connected to an Image Analyzer (AMS 40-10; Saffron, Walden, UK). Evaluation of 30 fields was performed perpendicular to the flow-axis, in the center of each glass coverslip. In Tables 1 and 2, platelet adhesion was expressed as percentage of surface covered with platelets. The data shown in Table 3 are given as the percentage of the WT-vWF control value. Before and after perfusion samples were taken to measure single platelet disappearance as a check on platelet clumping due to addition of rvWF to the perfusate.
Statistical analysis.Mean ± SEM was calculated for the perfusion experiments based on three or more determinations. Comparison of the paired raw data (% platelet coverages) was performed using a repeated one-way analysis of variance (ANOVA) followed by a Tukey-Kramer multiple comparison test. Probability values less than P < .05 were considered significant.
RESULTS
Expression and characterization of R543Q-vWF and R543W-vWF.To study the influence of two vWD type 2B point mutations in the A1 loop of vWF, we substituted Arg-543 by Gln (R543Q), and Trp (R543W). The pNUT constructs of R543Q-vWF and R543W-vWF, were transfected into a fur-BHK cell line, and compared with WT-vWF. The levels of expression in the cell culture supernatant varied from 5 to 10 μg/mL for WT-vWF, from 10 to 20 μg/mL for R543Q-vWF, and from 15 to 30 μg/mL for R543W-vWF. These levels indicated that the point mutations did not decrease the synthesis or secretion of the rvWFs.
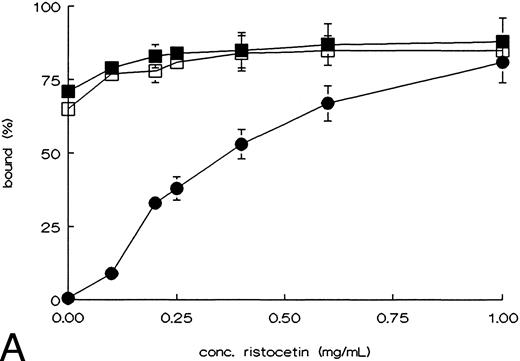
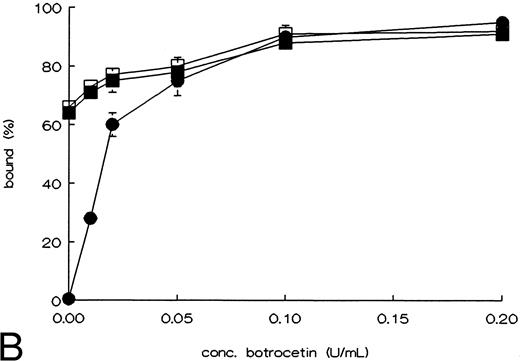
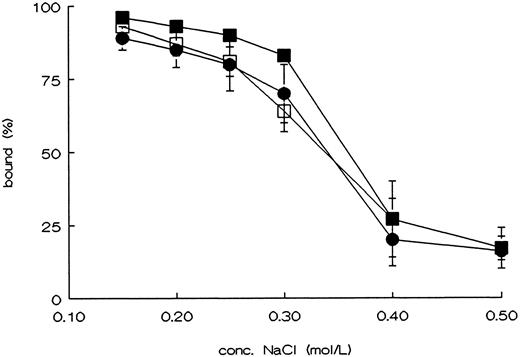
Binding of rvWF type 2B mutants to platelets, and to heparin.To determine whether the point mutations in the A1 domain affect the functional properties of this domain, we tested the ristocetin and botrocetin induced platelet binding, and binding to heparin. WT-vWF only showed binding to fixed platelets in the presence of ristocetin or botrocetin. R543Q-vWF and R543W-vWF showed an almost maximal binding to fixed platelets in the absence of ristocetin or botrocetin (Fig 2A and B).27 Binding of the rvWFs to heparin was studied using heparin-Sepharose beads. The affinity of the binding was investigated using increasing concentrations of NaCl to elute vWF from the beads. As shown in Fig 3, both mutants showed the same heparin binding capacity as compared with WT-vWF.28
Ristocetin, or botrocetin induced binding of rvWF mutants to fixed platelets. rvWF was incubated with formaldehyde fixed platelets in the presence of the indicated concentrations ristocetin (A) or botrocetin (B). Nonbound rvWF was measured in the supernatant, using an ELISA, after spinning down the platelets; WT-vWF (•), R543Q-vWF (▪), and R543W-vWF (□). The figures show the curves of the mean ± SEM of three independent experiments.
Ristocetin, or botrocetin induced binding of rvWF mutants to fixed platelets. rvWF was incubated with formaldehyde fixed platelets in the presence of the indicated concentrations ristocetin (A) or botrocetin (B). Nonbound rvWF was measured in the supernatant, using an ELISA, after spinning down the platelets; WT-vWF (•), R543Q-vWF (▪), and R543W-vWF (□). The figures show the curves of the mean ± SEM of three independent experiments.
Binding of rvWF mutants to heparin. Cell culture supernatant of cell lines producing rvWF was incubated with heparin-Sepharose in the presence of the indicated concentrations of NaCl. Unbound rvWF was measured in the supernatant, using an ELISA, after spinning down the heparin-Sepharose; WT-vWF (•), R543Q-vWF (▪), and R543W-vWF (□). The figure shows the curves of the mean ± SEM of three independent experiments.
Binding of rvWF mutants to heparin. Cell culture supernatant of cell lines producing rvWF was incubated with heparin-Sepharose in the presence of the indicated concentrations of NaCl. Unbound rvWF was measured in the supernatant, using an ELISA, after spinning down the heparin-Sepharose; WT-vWF (•), R543Q-vWF (▪), and R543W-vWF (□). The figure shows the curves of the mean ± SEM of three independent experiments.
Binding of the type 2B mutants to collagen type III.WT-vWF and the mutants were assayed for binding to collagen type III immobilized on microtiter plates under static conditions. As shown in Fig 4, point mutations in the A1 domain did not affect the binding to collagen type III. Recently, Hilbert et al11 reported that the type 2B mutation L697V, located outside the disulfide loop, bound similar as WT-vWF to collagen type I or III.
Binding of rvWFs to collagen type III. Binding curves of WT-vWF (•), R543Q-vWF (▪), and R543W-vWF (□) to collagen type III coated microtiter wells. Incubations were performed in these wells for 2 hours at room temperature by adding increasing concentrations of rvWFs. The values shown represent the mean of a duplicate determination. The figures show a representative curve of three independent experiments (mean ± SEM).
Binding of rvWFs to collagen type III. Binding curves of WT-vWF (•), R543Q-vWF (▪), and R543W-vWF (□) to collagen type III coated microtiter wells. Incubations were performed in these wells for 2 hours at room temperature by adding increasing concentrations of rvWFs. The values shown represent the mean of a duplicate determination. The figures show a representative curve of three independent experiments (mean ± SEM).
Perfusion studies.For the purpose of perfusion studies, rvWFs were purified as described in the Materials and Methods and coated by adsorption to glass coverslips. Citrated whole blood was circulated for 5 minutes at shear rates of 300 s−1 and 1,600 s−1 over vWF coated coverslips. By blocking the noncoated glass with human albumin, vWF from the plasma cannot coat to this surface anymore; thus platelet adhesion to purified vWF can be performed using whole blood. The adhesion was evaluated by light microscopy and image analysis as described in the Materials and Methods. The results, given as the percentage of surface covered with platelets, are shown in Table 1. These data indicate that platelet adhesion to R543Q-vWF was similar to WT-vWF. The adhesion to R543W tends to be lower at both shear rates in comparison with WT-vWF, although the decrease was not significant.
In previous studies we showed that the main collagen binding site on vWF is located in the A3 domain,29,30 whereas the interaction with GPIb is located in the A1 domain.19 24 Platelet adhesion to collagen was studied by preincubation of vWF on collagen type III. In order to avoid interference of plasma vWF, reconstituted blood in which the normal plasma was replaced by vWF-depleted citrated plasma was used. The results of the perfusion experiments are summarized in Table 2. No significant difference in adhesion was seen between WT-vWF and the mutants. These results indicate that the point mutations R543Q and R543W have no influence on the functioning of the A1 and A3 domain of immobilized vWF in platelet adhesion under flow.
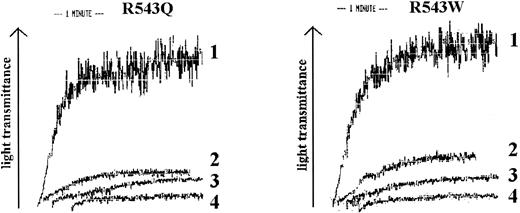
R543Q and R543W were added to citrated whole blood and the maximum concentration that could be added without inducing spontaneous platelet aggregation was determined (Fig 5). These experiments showed that at a concentration of 0.5 μg/mL no spontaneous aggregation was measured. In the perfusion experiments we therefore added 0.5 μg/mL R543Q or R543W to citrated whole blood using collagen type III as the adhesive surface (Table 3). Both R543Q and R543W strongly inhibited platelet adhesion to collagen type III. After addition of 0.5 μg/mL type 2B mutants the platelet number in the perfusate before and after perfusion showed a difference of less than 10%. The results indicate that these recombinant homozygous mutants are able to inhibit platelet adhesion considerably at a level of only 5% in the presence of normal vWF (10 μg/mL). The inhibition of platelet adhesion to collagen type III was less pronounced when these experiments were repeated with whole blood anticoagulated with LMWH. Addition of 0.5 μg/mL R543Q and R543W to whole blood did not inhibit platelet adhesion when WT-vWF coated on a glass coverslip was used as the adhesive surface.
Spontaneous platelet aggregation analysis of R543Q-vWF and R543W-vWF. The recombinant purified type 2B vWFs, R543Q and R543W, were incubated with fixated platelets in an aggregometer cuvette. 1, 5 μg/mL; 2, 1 μg/mL; 3, 0.5 μg/mL; 4, 0.1 μg/mL.
Spontaneous platelet aggregation analysis of R543Q-vWF and R543W-vWF. The recombinant purified type 2B vWFs, R543Q and R543W, were incubated with fixated platelets in an aggregometer cuvette. 1, 5 μg/mL; 2, 1 μg/mL; 3, 0.5 μg/mL; 4, 0.1 μg/mL.
DISCUSSION
In the present study we describe the expression and characterization of two mutations in the A1 domain of vWF, R543Q and R543W, that have been identified in vWD type 2B patients. These substitutions occur at a CpG dinucleotide, which is a hotspot for mutations.14 31 The mutated cDNAs are transfected into fur-BHK cells. After stable cell lines have been established the mutants are normally processed and secreted showing the full range of multimers. They show a spontaneous binding to platelet GPIb. Under flow conditions, platelet adhesion to immobilized R543Q and R543W was comparable with WT-vWF. Soluble R543Q and R543W added at 0.5 μg/mL to the perfusate (normal whole blood) inhibited platelet adhesion to collagen type III considerably. This observation may indicate that in vWD type 2B patients the mutated vWF can inhibit normal vWF to support platelet adhesion.
To study the effect of soluble R543Q and R543W added to the perfusate, we have to use very low levels of these mutants, since spontaneous platelet aggregation will disturb the adhesion process in two ways. First, a decrease in platelet count below 100,000/μL decreases platelet adhesion, and second, aggregates will influence the shear rate by occluding the perfusion chamber. At a dose of 0.5 μg/mL these type 2B mutants did not influence platelet number during the perfusion experiments. The same amount added to citrated or LMWH anticoagulated blood inhibits platelet adhesion to collagen type III. We can only speculate about the explanation of this observation. Presuming that these mutants bind to GPIb, only 40% of the GPIb receptors on platelets (based on 20,000 GPIb/platelet, and a measured platelet count of 150,000/μL) can be maximally occupied. Carriers for Bernard-Soulier Syndrome, expressing only 50% of GPIb on their platelet membrane, do not show any bleeding complications, indicating that 50% GPIb on the membrane is sufficient for normal platelet function. However, occupancy of 40% of GPIb binding sites available by type 2B mutant vWF is sufficient to inhibit platelet adhesion to collagen. No inhibitions of platelet adhesion is found when vWF is used as adhesive surface indicating that there is no competition between WT-vWF and type 2B vWF for GPIb on the platelet membrane. Two possible explanations remain for the observed effects: (i) vWF bound to a platelet is unable to bind subsequently to collagen. vWF has to bind first to the surface to mediate platelet adhesion or (ii) after binding of vWF the platelets might become activated and the expression of collagen receptors on their surface is downregulated.
The inhibition of platelet adhesion to collagen type III is more pronounced in citrated blood compared with LMWH-blood. Platelet adhesion at higher shear rates is sensitive for the presence of Mg2+. Optimal adhesion is reached at Mg2+ concentrations above the physiologic concentration of 1.1 mmol/L.32 It is possible that, in the presence of physiologic concentrations of Mg2+, higher concentrations of type 2B-vWF are necessary to reach the same inhibition. Unfortunately, we could not test this due to platelet clumping at higher type 2B-vWF concentrations. With blood anticoagulated with 40 μmol/L PPACK (p-phenylalanyl-L-prolyl-L-arginine chloromethyl ketone dihydrochloride) similar results were found as with LMWH-blood (not shown).
The effect of the type 2B mutations, R543Q and R543W, on platelet adhesion under flow conditions using immobilized vWF was studied either coated to glass or bound to collagen type III. Our results show that under low and high shear rate conditions (300 s−1 and 1,600 s−1), platelet adhesion to purified recombinant R543Q-vWF coated on glass, and to R543Q-vWF or R543W-vWF preincubated on a collagen type III surface is comparable with WT-vWF. Probably, due to an induced conformational change, WT-vWF adsorbed to glass or collagen expresses the optimal exposed GPIb binding site, and supports adhesion similar to the type 2B mutants.4,33 Previously, De Groot et al7 used the extracellular matrix of cultured endothelial cells isolated from the umbilical cord of a vWD type 2B baby. They reported that vWF present in the extracellular matrix of vWD type 2B endothelial cells had the same ability to support platelet adhesion as vWF in the matrix of normal endothelial cells. Our in vitro perfusion results using rvWF type 2B mutants confirm these observations.
In contrast to the spontaneous platelet binding, the binding of R543Q and R543W to collagen type III was comparable with WT-vWF, an observation earlier reported by Randi et al34 using the type 2B mutant L697V. In type 2B vWD plasma, vWF binding to collagen is usually decreased due to the loss of high-molecular-weight multimers.35 Introduction of a type 2B point mutation in rvWF leads to an enhanced and often spontaneous GPIb binding but an enhanced binding to collagen type III or heparin has not been reported. Apparently, this enhanced GPIb binding property of type 2B vWF is mediated by a relatively small part of the A1 domain without interfering with other parts of this domain involved in binding to other ligands. The elucidation of the three-dimensional structure of the A1 domain will improve the understanding of the mechanism and localization of these binding sites.
In vWD type 2B the loss of high molecular weight multimers is explained by their removal from the circulation by binding to platelets caused by an increased affinity of vWF for GPIb.5,7 The native conformation of vWF prevents its binding to GPIb in the circulation. A native A1 domain loop located between the Cys residues 509 and 695 may be necessary to maintain a hidden conformation of the peptide segment 514-542 involved in the interaction to GPIb.8 Conformational changes in the A1 domain, induced by type 2B mutations, increase the affinity for GPIb by exposure of the GPIb binding site in this domain.6,11,12 14
For the first time we describe here functional studies of type 2B mutants performed under flow conditions. To summarize, the mutations R543Q and R543W located in the A1 domain are reproduced and rvWF molecules are expressed in heterologous eukaryotic cells. The mutants show a spontaneous binding to platelet GPIb. At a low dose, R543Q and R543W are able to block platelet adhesion in the presence of excess normal vWF. We hypothesize that bleeding symptoms occurring in vWD type 2B patients are not only due to the lack of the higher molecular weight vWF multimers, but also due to a blocking of the GPIb receptors on platelets, preventing platelets to adhere. In conclusion, we show that vWD type 2B mutants are a useful tool to study the mechanism of vWF-dependent platelet adhesion.
Supported by the Netherlands Organization for Scientific Research (N.W.O.) (Grant No. 900-526-123).
Address reprint requests to Philip G. de Groot, PhD, University Hospital Utrecht, Department of Haematology G03.647, PO Box 85.500, 3508 GA Utrecht, The Netherlands.