Abstract
Chromosomal rearrangement of the HRX (MLL, ALL-1, Htrx) gene situated at chromosome band 11q23 is one of the most frequent genetic changes in infant leukemias of myeloid and lymphoid lineage and in treatment-induced secondary leukemias. The HRX gene codes for a predicted 431-kD protein that shows significant homology to the Drosophila trithorax protein, an Hox epigenetic regulator. Typically, the region encoding the HRX gene is rearranged, mostly in reciprocal translocations with a number of partners, resulting in a range of fusion genes. However, this is not the only abnormality affecting HRX because partial duplication of the gene, as well as interstitial deletions, can occur. Despite extensive studies of HRX at the genetic level, the protein products of the HRX gene and their patterns of expression in normal and leukemic cells remain uncharacterized. In this study we analyzed the distribution and localization of HRX proteins in cell lines and human tissues, using both polyclonal and monoclonal antibodies. The specificity of these reagents was confirmed using cells transfected with the HRX-ENL fusion gene. Western blot analyses of protein extracts from cells carrying the t(11; 19) and t(4; 11) translocations showed HRX chimeric proteins whose migrations corresponded to the sizes predicted from analyses of translocation-induced fusion mRNAs expressed by the derivative 11 chromosomes. Immunocytochemical analysis showed a punctate distribution of wild-type and chimeric HRX proteins within cell nuclei, suggesting that HRX localizes to nuclear structures in cells with and without 11q23 translocations. Nuclear staining was found in the majority of tissues studied with the strongest reactivity in cerebral cortex, kidney, thyroid, and lymphoid tissues. Thus, HRX is widely expressed in most cell types including hematopoietic cells, a finding that precludes an immunocytochemical approach for diagnosis of leukemias bearing 11q23 structural abnormalities.
REARRANGEMENTS in the 11q23 region in hematologic malignancies have been recognized for several years.1,2 The gene involved in these rearrangements encodes an unusually large protein with a predicted molecular weight of 431 kD. Based on its limited similarity to Drosophila trithorax, which is involved in segmental determination,3 it was named HRX for “homologue of trithorax.”4 Other laboratories independently cloned the same gene, which accounts for its other names: MLL, ALL-1, and Htrx.
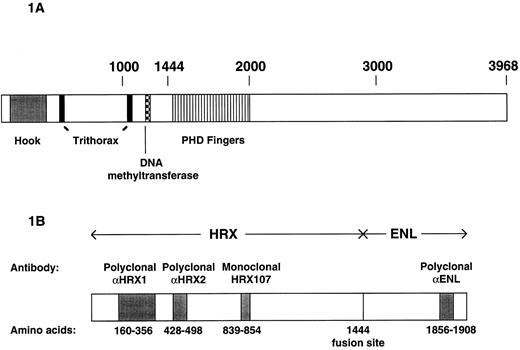
The similarity of HRX to trx extends over four specialized zinc finger domains with features of PHD fingers (Fig 1A).5 Two potential DNA binding domains are also present in HRX. The first consists of three AT hook motifs similar to those originally discovered in high mobility group I(Y) [HMG-I(Y)] proteins and implicated in minor groove DNA binding. The second is a region of DNA methyltransferase homology.6
Schematic representations of wild-type and chimeric HRX proteins. (A) Distinctive motifs or areas of similarity shared between wild-type HRX and D trithorax are indicated. (B) Schematic representation of the HRX-ENL fusion protein and the regions identified by various antibodies employed for these studies. The amino acids are numbered according to Tkachuk et al, 1992.4
Schematic representations of wild-type and chimeric HRX proteins. (A) Distinctive motifs or areas of similarity shared between wild-type HRX and D trithorax are indicated. (B) Schematic representation of the HRX-ENL fusion protein and the regions identified by various antibodies employed for these studies. The amino acids are numbered according to Tkachuk et al, 1992.4
The HRX gene has been shown to be involved in reciprocal translocations with at least 25 chromosomal loci to date,7 many of which result in in-frame fusions between HRX and a partner gene. Sequence analysis of these partner genes has identified some common protein motifs. For example, serine/proline motifs are found in AF-4, AF-6, AF-9, and ENL, suggesting a role in transcriptional activation. Indeed, ENL can influence the transcriptional activity of a subset of promoters in yeast and mammalian cells.8 ENL is a nuclear protein and several other HRX fusion partners contain nuclear targeting motifs. Another HRX fusion partner, ELL,9 has recently been shown to function as a transcription elongation factor. AF-10 and AF-17 both contain leucine zippers and PHD fingers. Both motifs are conserved in sequence and position with those of a human bromodomain-containing protein, BR140, which is homologous to the TAF250 subunit of TFIID.10 The weight of evidence strongly suggests that most and perhaps all HRX fusion proteins are likely to be involved in some aspect of activated or basal transcription.11
Cytogenetic studies indicate that 11q23 abnormalities are found in approximately 75% of infants with acute leukemia but in only 5% of adults.12 Moreover, the childhood cases are frequently in infants under the age of 1 year, including neonatal cases. Typically the infants have a high tumor burden of early B-cell phenotype, although biphenotypic lymphoid/myeloid cases are also common.13,14 Central nervous system (CNS) involvement is frequently found. These infants have a poorer prognosis compared with older children with acute lymphoblastic leukemia (ALL) even if the same translocation is present.15,16 Identical twins harboring the same translocation have also been documented, suggesting that the leukemia arises in utero in a single clone which spreads to the other twin via the placental circulation.17 This, together with the fact that the majority of infant leukemias harbor HRX rearrangements, indicates that the latency of HRX-associated leukemias may be short.18
Although the prevalence of 11q23 rearrangements is high in infant leukemia, 11q23 rearrangements are also associated with treatment-induced secondary leukemias, particularly where topoisomerase II-reactive drugs have been used.19 Indeed, it is now believed that 11q23 abnormalities may be more widespread in malignant disease after the identification of a complex rearrangement in a gastric carcinoma cell line.20
An important step in understanding the role of HRX in leukemogenesis would be to identify its pattern of expression in cell lines and tissues. A number of laboratories7 21 have reported that HRX mRNA is present at high levels in a wide range of tissues and that by implication, HRX protein is ubiquitously expressed. In this article we report an immunocytochemical study of HRX expression in cell lines and tissues using polyclonal and monoclonal antibodies (MoAbs) against human HRX protein.
Our data confirm the prediction from RNA studies that HRX is a widely expressed protein, but we further show that its level of expression varies substantially among different cell types. HRX displays a punctate subnuclear distribution similar to other homeotic regulators of Drosophila consistent with its putative role as a Hox gene epigenetic regulatory factor. The widespread expression of HRX in most cell types including hematopoietic cells precludes an immunocytochemical approach for diagnosis of leukemias bearing 11q23 structural abnormalities.
MATERIALS AND METHODS
Production of anti-HRX antibodies.Synthetic peptides corresponding to amino acids 840-854 (peptide 1), 2370-2384 (peptide 2), and 2830-2845 (peptide 3) of the HRX protein (numbered according to Tkachuk et al,4 Fig 1B) were synthesized (Zinsser Analytical, Maidenhead, UK) and coupled to bovine serum albumin (BSA). Balb/c mice were immunized at weekly intervals, intraperitoneally, with a cocktail of the three peptide-BSA conjugates (30 μg of each conjugate/immunization) emulsified in Freund's adjuvant, using complete adjuvant for the first immunization and incomplete adjuvant subsequently. Following a test bleed after three immunizations, a booster (50 μg of each peptide without adjuvant) was administered 3 days before fusion that was performed as described by Pulford et al.22 Antibodies were screened against unconjugated peptide using enzyme-linked immunosorbent assay (ELISA) (see below). Subsequent immunohistochemical analysis on cell lines and tissues was then performed with supernatants from wells that were positive on ELISA. Cloning of the selected hybridoma was performed by a limiting dilution technique in flat-bottom, 96-well plates.
Immunogens for raising rabbit antisera consisted of GST-HRX or GST-ENL fusion proteins. Using polymerase chain reaction (PCR), regions encoding the amino acids 160 to 356 (HRX1), 428 to 498 (HRX2), and 1856 to 1908 (ENL) were amplified from the DNA sequence encoding the HRX-ENL fusion protein using the cloned HRX-ENL cDNA4 as a template. The resulting DNA fragments were inserted into the expression vector pGex-2T (Pharmacia, Uppsala, Sweden). GST-fusion proteins were expressed and purified according to the manufacturers instructions. Polyclonal sera directed against these recombinant proteins were affinity purified according to Ausubel et al23 using the respective GST fusion proteins. This resulted in the polyclonal sera αHRX1, αHRX2, and αENL.
ELISA.Fifty microliters of a 5-μg/mL solution of unconjugated peptide solution was added to each well of a Maxistrip microtiter plate (GIBCO Biocult Ltd, Paisley, Scotland). After overnight incubation at 4°C, the plates were washed in TBS pH 7.6, containing 0.1% BSA and 0.1% Tween 20 (Sigma, St Louis, MO). Free protein-binding sites on the plates were blocked overnight at 4°C with TBS containing 0.5% BSA and 0.1% Tween 20. After a wash in TBS pH 7.6, containing 0.1% BSA and 0.1% Tween 20, 50 μL of hybridoma supernatant was added to each well for 30 minutes. Following a wash in TBS pH 7.6, containing 0.1% BSA and 0.1% Tween 20, 50 μL of peroxidase-conjugated goat-antimouse Ig (Dako a/s, Glostrup, Denmark) at a dilution of 1/50 in TBS was then added. After 30 minutes and a final wash in distilled water, the reaction was developed using the soluble substrate 2,2′-azino-di-(3-ethylbenzthiazoline sulfonic acid) (Sigma). Supernatants that gave a strong reaction were then selected for further study.
Cell lines.A number of cell lines of different origin were tested for their reactivity with MoAb HRX 107 and polyclonal antibody HRX2 on cytospins from exponentially growing cells.24 The cells were maintained in RPMI 1640 containing 10% fetal calf serum (GIBCO Biocult Ltd) at 37°C in 5% CO2 . The following cell lines were obtained from the Sir William Dunn School of Pathology (Oxford, UK): K562 (erythroleukemia), U937 (malignant histiocytosis), HL60 (myeloid leukemia), Raji (Burkitt's lymphoma), Molt-4 and Jurkat (T-cell acute lymphoblastic leukemia), A431 (vulval carcinoma), and HT29 (colon carcinoma). The cell lines SU-DHL-1 (T-cell line containing a 2; 5 translocation), ML2 (myeloid line containing a 6; 11 translocation), and RS4; 11 (B-cell line containing a 4; 11 translocation) were obtained from the American Type Culture Collection (ATCC, Rockville, MD). The NB4 (promyelocytic leukemia) cell line was obtained from M. Lanotte (INSERM, Paris, France); HB11; 19 (B-cell line containing a 11; 19 translocation) was described previously in Tkachuk et al.4
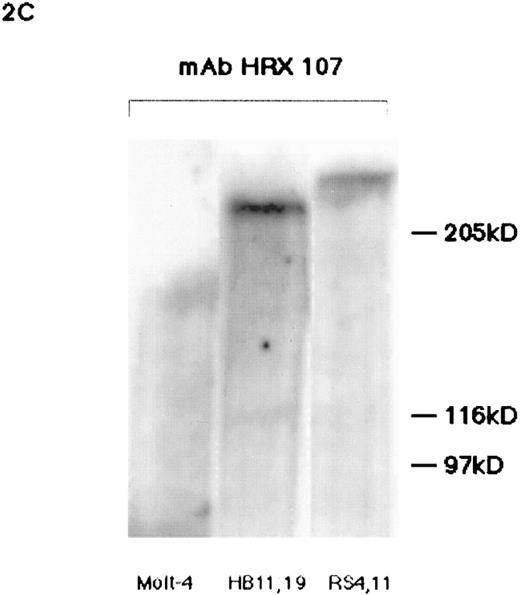
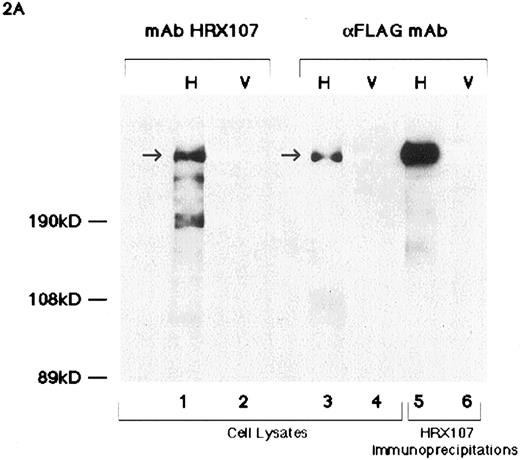
Western blot analyses of HRX proteins using various antibodies. (A) Western blot analysis of flag-tagged HRX-ENL expression. Lanes 1 through 4 contain a nuclear extract of Bosc 293 cells transfected with either the N-terminal flag-tagged HRX-ENL (H) or vector alone (V) (2.5 μg protein per lane). Lanes 1 and 2 were probed with MoAb HRX 107 whereas lanes 3 and 4 were probed with the antiflag antibody. Arrow indicates the protein detected by both MoAb HRX 107 and the antiflag antibody. For lanes 5 and 6, extracts of Bosc 293 cells (75 μg) that had been transfected with N-terminal flag-tagged HRX-ENL (H) or vector alone (V) were immunoprecipitated with MoAb HRX 107, with subsequent Western blotting with the antiflag antibody. (B) Comparison of MoAb HRX 107 with polyclonal anti-HRX and anti-ENL antibodies. Each lane contains extracts of Bosc 293 cells transfected with an HRX-ENL expression construct (H) or vector alone (V) as indicated above the gel lanes. Protein amounts applied to the gel were as follows: 50 μg in lanes 1, 2, and 6; 10 μg in lane 5; 20 μg in lanes 3, 4, 7, and 8. Note that the negative control lane 6 in the Western blot probed with MoAb HRX 107 contained five times as much protein as lane 5. Arrow indicates the protein detected by MoAb HRX 107 and the polyclonal antibodies αHRX1, αHRX2, and αENL. Arrowhead indicates the smaller isoforms of HRX-ENL detected by the polyclonal antibodies αHRX1 and αENL. (C) Western blot analysis of whole-cell lysates from Molt-4 (T-cell acute lymphoblastic leukemia), and two cell lines (HB 11; 19 and RS 4; 11) expressing chimeric HRX fusion proteins using HRX 107 on a 5% SDS-PAGE gel.
Western blot analyses of HRX proteins using various antibodies. (A) Western blot analysis of flag-tagged HRX-ENL expression. Lanes 1 through 4 contain a nuclear extract of Bosc 293 cells transfected with either the N-terminal flag-tagged HRX-ENL (H) or vector alone (V) (2.5 μg protein per lane). Lanes 1 and 2 were probed with MoAb HRX 107 whereas lanes 3 and 4 were probed with the antiflag antibody. Arrow indicates the protein detected by both MoAb HRX 107 and the antiflag antibody. For lanes 5 and 6, extracts of Bosc 293 cells (75 μg) that had been transfected with N-terminal flag-tagged HRX-ENL (H) or vector alone (V) were immunoprecipitated with MoAb HRX 107, with subsequent Western blotting with the antiflag antibody. (B) Comparison of MoAb HRX 107 with polyclonal anti-HRX and anti-ENL antibodies. Each lane contains extracts of Bosc 293 cells transfected with an HRX-ENL expression construct (H) or vector alone (V) as indicated above the gel lanes. Protein amounts applied to the gel were as follows: 50 μg in lanes 1, 2, and 6; 10 μg in lane 5; 20 μg in lanes 3, 4, 7, and 8. Note that the negative control lane 6 in the Western blot probed with MoAb HRX 107 contained five times as much protein as lane 5. Arrow indicates the protein detected by MoAb HRX 107 and the polyclonal antibodies αHRX1, αHRX2, and αENL. Arrowhead indicates the smaller isoforms of HRX-ENL detected by the polyclonal antibodies αHRX1 and αENL. (C) Western blot analysis of whole-cell lysates from Molt-4 (T-cell acute lymphoblastic leukemia), and two cell lines (HB 11; 19 and RS 4; 11) expressing chimeric HRX fusion proteins using HRX 107 on a 5% SDS-PAGE gel.
Tissue specimens.Normal tonsil was obtained from the Ear, Nose and Throat Department of the Radcliffe Infirmary (Oxford, UK). Samples of fresh normal tissue samples were obtained from the Departments of Histopathology and Paediatric Pathology of the John Radcliffe Hospital. Fresh tissues were snap frozen in liquid nitrogen, and cryostat sections (8 μm) were cut, fixed, and stored as previously described.25 Tonsil cell cytopsins were prepared using lymphoprep (Nycomed Pharma AS, Oslo, Norway) as previously described.26
Immunohistochemical staining.The immunoperoxidase technique was performed using either a two-stage27 or a modified streptavidin-biotin three-stage28 technique. For the two-stage procedure, the slide preparations were incubated with an MoAb for 30 minutes. After washing in TBS (0.5 mol/L Tris HCL, pH 7.6, diluted 1:10 with 1.5 mol/L saline), the slides were incubated with a peroxidase-conjugated goat-antimouse Ig (Dako a/s) diluted 1/50 in TBS. For the three-stage staining process, slides were incubated with an MoAb for 30 minutes. The slides were then washed in TBS and incubated with a biotin-conjugated goat-antimouse Ig (Dako a/s), diluted 1/200 in TBS before being washed and incubated for a further 30 minutes with a streptavidin/HRP solution (Dako a/s). The peroxidase reaction was developed using diaminobenzidine tetrahydrochloride (DAB; Sigma) and hydrogen peroxide. The slides were then counterstained with hematoxylin before being mounted in Apathy's mountant (BDH, Lutterworth, UK).
The immunofluorescence technique was performed using a two-stage method. Slide preparations of transfected cells were incubated with a primary antibody cocktail for 30 minutes. Primary cocktails consisted of affinity purified αHRX2 diluted in MoAb HRX 107 (αHRX2+HRX 107), polyclonal antibody αHRX2 mixed with anti-flag M2 MoAb (IBI) (αHRX+M2), or MoAB HRX 107 mixed with anti-flag M2 MoAb (HRX 107+M2). After washing in phosphate-buffered saline (PBS), slides were incubated with appropriate secondary antibody combinations for 30 minutes. For αHRX2+HRX 107 and αHRX2+M2, a cocktail of fluorescein isothiocyanate (FITC)-conjugated goat-antirabbit (Southern Biotechnology) at 1/50 with Texas Red–conjugated goat-antimouse Ig (Southern Biotechnology, Birmingham, AL) at 1/25 was applied. For HRX107+M2, a cocktail of Texas Red–conjugated goat-antimouse IgM at 1/50 with FITC-conjugated goat-antimouse IgG1 (Southern Biotechnology) at 1/25 was applied. After a further wash in PBS, slides were mounted in fluorescent mounting medium (Dako a/s) containing 2% 4,6-diamidine-2-phenylindole dihydrochloride (DAPI) (Sigma).
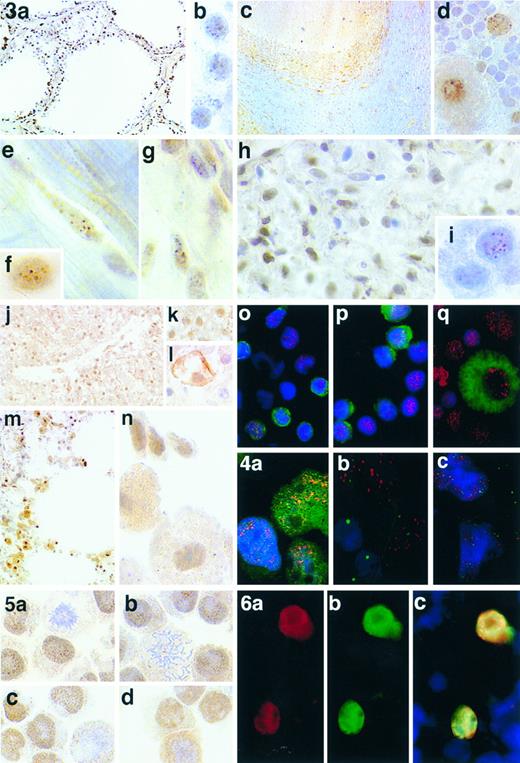
Immunostaining of human tissues and tonsil cell preparations using anti-HRX antibodies. (a) Antibody HRX 107 labels all nuclei in the thyroid gland in a punctate nuclear pattern. The pattern of labeling can be seen more clearly at high power (b). (c) MoAb HRX 107 labels most nuclei in the cerebellum in a punctate nuclear pattern, although the intensity of labeling varies from cell to cell. Again the pattern of labeling can be seen more clearly at high power, particularly the nuclear labeling of Purkinje cells (d). (e and f ) The punctate distribution of HRX in nuclei of cardiac myocytes found with MoAb HRX 107 which is comparable to the pattern of labeling with polyclonal antibody αHRX2 (g). (h) Labeling of nuclei in the glomerulus of kidney with MoAb HRX 107; the punctate pattern of distribution being clearly visible at high power (i). (j) Labeling of liver with polyclonal antibody αHRX2 and at high power (k), compared with MoAb HRX 107 (l). (m and n) Labeling of lung with polyclonal antibody αHRX2. (o) MoAb HRX 107 (red) labeling T cells (green). (p) MoAb HRX 107 (red) labeling B cells (green). (q) MoAb HRX 107 (red) labeling a macrophage cell (green).
Fig 4. Comparison of HRX with other known transcription factors that exhibit punctate nuclear staining. (a) MoAb HRX107 (red) and anti-TAL-1 (green) on the Jurkat cell line. (b) MoAb HRX107 (red) and anti-PML (green) on tonsil cell cytospins. (c) MoAb HRX107 (red) and anti-PML (green) on the NB4 cell line.
Fig 5. Comparison of MoAb HRX 107 and polyclonal antibody αHRX2 on cell lines. MoAb on U937 (a) and RS4; 11 (c). Polyclonal antibody on U937 (b) and RS4; 11 (d).
Fig 6. (a) Immunofluorescent labeling of Bosc 293 cells transfected with a flag-tagged HRX-ENL expression construct under control of the early CMV promoter and enhancer using MoAb HRX 107. (b) Immunofluorescent labeling of the same cells illustrated in 4a with the antiflag antibody. (c) Colocalization of MoAb HRX 107 and the antiflag antibody on the same cells illustrated in 4a and 4b. The untransfected cells (blue) can clearly be seen.
Immunostaining of human tissues and tonsil cell preparations using anti-HRX antibodies. (a) Antibody HRX 107 labels all nuclei in the thyroid gland in a punctate nuclear pattern. The pattern of labeling can be seen more clearly at high power (b). (c) MoAb HRX 107 labels most nuclei in the cerebellum in a punctate nuclear pattern, although the intensity of labeling varies from cell to cell. Again the pattern of labeling can be seen more clearly at high power, particularly the nuclear labeling of Purkinje cells (d). (e and f ) The punctate distribution of HRX in nuclei of cardiac myocytes found with MoAb HRX 107 which is comparable to the pattern of labeling with polyclonal antibody αHRX2 (g). (h) Labeling of nuclei in the glomerulus of kidney with MoAb HRX 107; the punctate pattern of distribution being clearly visible at high power (i). (j) Labeling of liver with polyclonal antibody αHRX2 and at high power (k), compared with MoAb HRX 107 (l). (m and n) Labeling of lung with polyclonal antibody αHRX2. (o) MoAb HRX 107 (red) labeling T cells (green). (p) MoAb HRX 107 (red) labeling B cells (green). (q) MoAb HRX 107 (red) labeling a macrophage cell (green).
Fig 4. Comparison of HRX with other known transcription factors that exhibit punctate nuclear staining. (a) MoAb HRX107 (red) and anti-TAL-1 (green) on the Jurkat cell line. (b) MoAb HRX107 (red) and anti-PML (green) on tonsil cell cytospins. (c) MoAb HRX107 (red) and anti-PML (green) on the NB4 cell line.
Fig 5. Comparison of MoAb HRX 107 and polyclonal antibody αHRX2 on cell lines. MoAb on U937 (a) and RS4; 11 (c). Polyclonal antibody on U937 (b) and RS4; 11 (d).
Fig 6. (a) Immunofluorescent labeling of Bosc 293 cells transfected with a flag-tagged HRX-ENL expression construct under control of the early CMV promoter and enhancer using MoAb HRX 107. (b) Immunofluorescent labeling of the same cells illustrated in 4a with the antiflag antibody. (c) Colocalization of MoAb HRX 107 and the antiflag antibody on the same cells illustrated in 4a and 4b. The untransfected cells (blue) can clearly be seen.
Tonsil cell cytospins were also immunostained using the two-stage immunofluorescent technique. Primary antibody cocktails consisted of MoAb HRX 107 with either anti-CD3 (HRX107+CD3), anti-CD19 (HRX107+CD19), anti-CD68 (HRX 107+CD68), or anti-PML29 (HRX 107+PGM3) antibodies. The secondary antibody cocktail consisted of a Texas Red–conjugated goat-antimouse IgM at 1/50 with FITC-conjugated goat antimouse IgG1 at 1/25.
Anti-HRX and anti-TAL-130 (HRX 107+BTL 73) MoAbs were used as a primary antibody cocktail on the Jurkat cell line, and anti-HRX and anti-PML29 (HRX107+PGM3) MoAbs were used as a primary antibody cocktail on the NB4 cell line. The secondary antibody cocktail consisted of a Texas Red–conjugated goat-antimouse IgM at 1/50 with FITC-conjugated goat-antimouse IgG1 at 1/25.
Transfections.The cDNA sequence encoding the HRX-ENL protein4 was inserted into a mammalian expression vector (pCMV1) under the control of the immediate early human cytomegalovirus (CMV) enhancer/promoter region resulting in the plasmid pCMV-d11. A flag-tagged version was generated by introducing a linker oligonucleotide into pCMV-d11 resulting in addition of the aminoacids MDYKDDDDKGNSAN to the native amino terminus of the expressed HRX-ENL protein (pCMV-d11f ). The plasmids or vector alone were transfected into Bosc cells.31 Cell lysates were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and standard Western blotting on nitrocellulose or PVDF (see below). The blot was developed using ECL (Amersham, Little Chalfont, UK).
Western blotting.Cells or tissue samples were lysed in 1× Laemmli loading buffer32 containing 1 μmol/L leupeptin (Sigma), 1 μmol/L Pepstatin (Sigma), and 1 mmol/L Pefabloc (Boehringer Mannheim, Mannheim, Germany). Lysate proteins were fractionated using either 5% polyacrylamide, 10.5% to 4.5% gradient polyacrylamide, or 3% low-melting agarose gel in TBE (89 mmol/L Tris base; 89 mmol/L boric acid; 1 mmol/L EDTA, pH 8.0) containing 0.1% SDS. Proteins were transferred by semidry method to PVDF membranes from polyacrylamide gels or by capillary transfer from agarose gels. Membranes were blocked overnight in TBS-T (TBS with 0.05% Tween 20) buffer plus 5% nonfat powdered milk. Filters were then incubated with primary antibody for 30 minutes. After washing in TBS-T, a peroxidase-conjugated goat-antimouse Ig (Dako a/s) antibody or peroxidase-conjugated goat-antirabbit Ig (Dako a/s) antibody diluted 1/500 in TBS-T was applied for a further 30 minutes. After extensive washing in TBS-T, blots were developed using ECL (Amersham).
Immunoprecipitation.Fifteen milligrams of a crude preparation of ammonium sulfate–precipitated HRX 107 was coupled to cyanogen bromide–activated 4B (Sigma) according to the directions of the manufacturer. Precipitations and washings were performed under native conditions according to Ausubel et al.23
RESULTS
Antibodies to HRX.Cell fusion was performed with spleen cells from mice immunized with HRX peptides conjugated to BSA (see Materials and Methods). Initial screening by the ELISA technique identified a number of hybridoma supernatants with strong reactivity against peptide 1 comprised of amino acids 839-854 (Fig 1B) from a portion of HRX retained in all leukemia-associated fusion transcripts reported to date.
The reactivity of one antibody, HRX 107, appeared specific for HRX upon further evaluation. Bosc 293 cells were transfected with a construct that expresses a flag-tagged form of HRX-ENL (flagHRX-ENL) and then examined by Western blot analysis. HRX 107 detected a protein whose migration was identical to that detected by the anti-flag antibody (Fig 2A, lanes 1 through 4, arrow) present in HRX-ENL–transfected but not mock-transfected cells. Protein bands that represent N-terminal cleavage products were detected by MoAb HRX 107. This is deduced from the fact that the flag epitope was attached to the N-terminus of the flagHRX-ENL construct and the anti-flag antibody does not detect these proteins. Furthermore, a protein of the same size was detected in HRX 107 immunoprecipitates from flagHRX-ENL transfected Bosc cells analyzed by Western blotting with the antiflag and HRX 107 antibody (Fig 2A, lanes 5 and 6). Immunocytochemical analyses using both anti-flag and HRX 107 antibodies showed strong nuclear staining in Bosc cells transfected with the flagHRX-ENL construct and the staining patterns were coincident (Fig 6). These studies indicated that MoAb HRX 107 specifically recognized HRX in either a denatured (Western blot) or native conformation.
Rabbit antisera were raised against GST fusion proteins containing various portions of HRX or ENL. The polyclonal antibodies to HRX (αHRX1 and αHRX2) and the polyclonal antibody to ENL (αENL) also recognized a band of the same size as that recognized by MoAb HRX 107 in cell lines transfected with the HRX-ENL construct (Fig 2B, arrow). Smaller isoforms of HRX-ENL (the most predominant displaying a molecular weight of approximately 190 kD) were also detected with antisera αHRX1 and αENL but not αHRX2 (Fig 2B, arrowhead). These HRX-ENL isoforms appear to arise from differential splicing of the HRX-ENL transcript under conditions of hyper expression in Bosc cells (R.S. and M.L.C., unpublished observations, June 1995) and have not been observed in cells with a t(11; 19) that express endogenous HRX-ENL proteins (see below). Immunocytochemical analyses using both anti-flag and αHRX2 antibodies showed coincident nuclear staining in Bosc cells transfected with the flagHRX-ENL construct (data not shown) as found when immunostaining with antiflag and HRX 107 antibodies.
HRX chimeric proteins are expressed in cells bearing 11q23 translocations.HRX 107 was used to examine cells bearing 11q23 translocations to assess their expression of chimeric HRX proteins. HRX 107 identified bands of approximately 220 kD and 240 kD in the cell lines HB11; 19 and RS4; 11, respectively, bearing 11q23 translocations productive of HRX-ENL and HRX-FEL (AF-4) fusion proteins. No bands of this molecular weight were identified in Molt-4 (Fig 2C) and K562 (data not shown), cell lines that lack 11q23 abnormalities but express wild-type HRX transcripts. A protein of approximately 220 kD was identified in the cell line HB11; 19 using polyclonal antibodies αHRX2 and αENL (data not shown). These analyses further confirmed the specificity of HRX 107 and showed that the chimeric HRX proteins encoded by fusion transcripts from the derivative 11 translocated chromosomes are expressed in HB11; 19 and RS4; 11 cell lines.
To facilitate transfer of high-molecular-weight wild-type HRX protein (of predicted molecular weight 431 kD), electrophoresis was performed in a 3% low-melting point agarose gel. Although probable degradation products have been observed using MoAb HRX 107, the predicted wild-type HRX of 430 kD has thus far eluded detection possibly because of an increased lability and the low resolution and decreased transfer from agarose gels (data not shown). A further method was then used, a 10.5% to 4.5% gradient polyacrylamide gel which also failed to identify the wild-type HRX protein. To ensure this method was applicable to high-molecular-weight proteins, a control muscle sample was resolved on the gel and a protein of approximately 400 kD was detected using an anti-Dystrophin antibody (Novocastra; Novocastra Laboratories Ltd, Newcastle, UK). This is consistent with the predicted molecular weight for Dystrophin33 (data not shown).
HRX proteins display a punctate nuclear distribution. Immunostaining of a series of hematopoietic cell lines with MoAb HRX 107 and polyclonal antibody αHRX2 is summarized in Table 1. In all cell lines studied a punctate nuclear pattern was observed, with nucleoli and mitotic cells negative (Fig 5). The number of punctate nuclear structures varied depending on the cell line, irrespective of the presence of 11q23 translocations. Approximately 20 dots per nucleus were found in Molt-4 and HB11; 19 nuclei compared with very large numbers of dots giving a fine granular appearance in the nuclei of U937 and RS4; 11 cells.
In conjunction to the punctate nuclear pattern, additional staining was observed on the cell lines K562 and A431 with the MoAb HRX 107. Although the K562 cell line exhibited a punctate nuclear staining pattern, cytoplasmic string-like structures were occasionally seen. Some cross-reactivity with the membranes of A431 cells was also found together with small rings in mitotic cells. Cross-reactivities were not observed with other cell lines and searches of data bases using the peptide sequence used for immunization have thus far not highlighted any matches other than HRX itself. On tissue staining with the HRX 107 monoclonal, membrane reactivity was observed only in hepatocytes (see below). However, staining of cell lines or hepatocytes with the HRX polyclonal did not show comparable reactivities, suggesting that HRX 107 may occasionally cross-react with an unknown cytoplasmic or membrane antigen, but this cross-reactivity can be easily distinguished from the HRX nuclear staining pattern.
HRX does not localize to those structures highlighted by TAL-1 or PML.Several other nuclear oncoproteins display a punctate subnuclear distribution, and we assessed the potential colocalization of HRX with TAL-1 or PML. When immunostaining the Jurkat (T-cell acute lymphoblastic leukemia) cell line with anti-HRX and anti TAL-1 antibodies (Fig 4a) the number of punctate structures highlighted by TAL-1 (green) is far in excess of those highlighted by HRX (red). On the tonsil cells (Fig 4b), HRX (red) and PML (green) are also clearly localized to different sites and it appears that there are fewer PML structures in comparison to HRX. The same phenomenon is also seen on the NB4 (promyelocytic leukemia) cell line (Fig 4c) where the sites of the PML-RARα fusion protein (green) are different from the sites of HRX localization illustrated by the anti-HRX MoAb HRX 107 (red).
Wild-type HRX is widely expressed but at varying levels in different cell types.The normal tissue distribution for HRX expression was assessed through a survey of human tissues using immunohistochemical techniques. Strong nuclear staining was evident in many tissues (Fig 3 and Table 2), particularly in neural and glial cells of the cerebral cortex and other areas of the CNS (Fig 3c and d). Strong punctate nuclear staining was also found in all the nuclei of thymus and testis and in the red and white pulp of spleen. Nuclear labeling was also present in the striated muscle cells of heart (Fig 3e and f ) and smooth muscle cells of colon. All nuclei of the thyroid gland were positive (Fig 3a) and the nuclei of macrophages in the lung were particularly strongly stained (Fig 3m and n).
Freshly prepared tonsil cell cytospins were immunostained and all nuclei were strongly positive as shown in (Fig 3o through q). This was performed as immunohistochemical staining of lymphoid tissue, particularly tonsil, frequently produced inconsistent results. Generally endothelial and epithelial cells were clearly labeled, but lymphoid cells often appeared negative or only very weakly stained. This was not always the case as several examples of tonsil immunostained exhibited ubiquitous labeling of all nuclei. This indicates that HRX may be a particularly labile protein, and care must be taken to use fresh material for immunohistochemical staining.
For most tissues, immunohistochemical staining results with HRX 107 were comparable to those obtained with αHRX2. One notable exception was the liver, where hepatocyte nuclei were clearly stained by the polyclonal antibody αHRX2 but only weakly, if at all, by the MoAb HRX 107 (Fig 3j through l). These differences suggested that hepatocytes may express an isoform of HRX that lacks the epitope recognized by HRX 107. HRX isoforms could arise by differential splicing of HRX transcripts, support for which derives from previously reported Northern blot and cDNA cloning studies of RNA from cultured cells.7 34
DISCUSSION
In this report we have used highly specific antibodies to characterize the tissue expression profile and subcellular localization of the proto-oncoprotein HRX, which is frequently fused in-frame to a variety of heterologous proteins by 11q23 chromosomal translocations in acute leukemias. Previously, the expression of human HRX has only been inferred from its pattern of mRNA expression in cultured cells or whole tissues.4 21 Our studies significantly extend previous observations that HRX is widely expressed by showing that there are tissue-specific differences in levels of its expression and in the presence of HRX isoforms. Furthermore, our studies show that HRX has a punctate subnuclear distribution, the significance of which is discussed below.
Of the many translocations in which HRX is involved the most common are t(4; 11)(q21; q23) and t(11; 19)(q23; p13), which result, respectively, in the fusion of HRX with AF-4 and ENL. By using two cell lines harboring these translocations, RS4; 11 and HB11; 19, we observed, via Western blotting with both monoclonal and polyclonal antibodies, immunoreactive proteins whose molecular weights corresponded to proteins encoded by the disrupted HRX gene contained on the derivative 11 chromosomes. Because our antibodies recognized HRX epitopes amino-terminal to the fusion site in HRX, we cannot rule out the expression of chimeric proteins containing carboxy-terminal portions of HRX as encoded by the derivative 19 and 4 chromosomes, respectively, in these cell lines as well. However, it is clear from our data that there is consistent expression of stable, abundant HRX chimeric proteins containing the amino-terminal portions of HRX in cells bearing 11q23 translocations and these findings support previous suggestions that products encoded by the derivative 11 chromosomes are the leukemogenic contributions from 11q23 translocations.
In our studies, cell lines believed to express wild-type HRX (based on RNA and immunocytochemical analyses) were negative by conventional Western blot analysis. This discrepancy reflects the fact that the predicted 430-kD wild-type protein is too large to enter 5% polyacrylamide gels. Using alternative fractionation methods (ie, agarose gel electrophoresis and gradient gel electrophoresis) we have not yet detected wild-type HRX. In the case of agarose gel electrophoresis this may be due to the lower resolution and poor transfer efficiency from agarose. However, using gradient gel electrophoresis, where the detection of a high-molecular protein was demonstrated, wild-type HRX again eluded detection. On occasion, by whatever method used, a band of approximately 200 kD was observed that may represent the N-terminal portion of HRX resulting from proteolytic cleavage, suggesting that HRX may be labile in vivo or under conditions of our analysis. By comparison, work by Kuzin et al35 has shown that D trithorax is also highly unstable and generally succumbed to specific proteolytic cleavage producing proteins of around 200 kD when examined by Western blotting. Clearly, additional studies are required to further characterize wild-type HRX and the potential significance of its cleavage products, both in mammals and flies.
HRX mainly localizes to the nucleus producing a punctate nuclear staining pattern. Nuclear localization is consistent with the presence of several motifs contained in HRX that are indicative of a potential role as a transcriptional protein.4,36,37 The subnuclear distribution of HRX closely resembles the distribution of Drosophila polycomb,38 which functions antagonistically to trx in the epigenetic control of Hox gene expression. Several of the polycomb group and trithorax group proteins in Drosophila are known to form multiprotein complexes presumed to function in development in part through their effects on chromatin structure. Given that polycomb and trx colocalize on a subset of bands in Drosophila polytene chromosomes, the punctate nuclear distribution of HRX in mammalian cells may reflect a similar organization into multiprotein complexes that localize at genomic sites containing subordinate target genes. Consistent with this possibility, genetic analyses in both Drosophila and knockout mice indicate that Hox genes are likely subordinate targets for both trx and HRX.39
Several nuclear oncoproteins display a punctate subnuclear distribution. Based on our initial studies, we postulated that the punctate nuclear structures as shown in Figs 3 and 4 possibly represented a distinct subnuclear domain such as the PML organizational domain (POD),40 41 a structure associated with the PML protein implicated in the t(15; 17) translocation. However, this was disproved by applying anti-HRX and anti-PML antibodies to the same cells, which clearly illustrated that the structures highlighted by each antibody were not the same. It was also shown that using anti-HRX and anti-TAL-1 antibodies that HRX and TAL-1 do not colocalize.
No differences in HRX subnuclear localization were observed in cell lines bearing 11q23 translocations indicating that HRX-ENL, like wild-type HRX, shares a punctate nuclear distribution, a conclusion supported by immunolocalization of HRX-ENL hyper-expressed in transiently transfected cells (R.S. and M.L.C., unpublished observations, June 1995). This raises the likely possibility that chimeric HRX proteins target the same genomic sites occupied by wild-type HRX. The amino terminal portions of HRX, which are consistently present in all chimeric proteins reported to date, contain several motifs implicated in DNA recognition4,42-45 and capable of effecting the similar subnuclear distributions of wild-type and mutant proteins. It would then follow that chimeric HRX proteins may perturb the regulated expression of genes normally subordinate to wild-type HRX. Under this scenario, oncogenic activation of HRX would result from a gain-of-function through fusion with various partner proteins consistent with the recent induction of experimentally induced myeloid malignancies by HRX-AF9 but not truncated HRX in genetically altered “knock-in” mice.46
Our immunocytochemical analyses indicate that there is widespread expression of HRX protein, a finding consistent with recent studies of lacZ reporter gene expression in MLL knockout mice.39,46 Immunoreactive HRX was observed with both polyclonal and monoclonal antibodies in cell lines of different hematopoietic origin including K562, Molt-4, HB11; 19, RS4; 11 and Jurkat which in previous studies were shown to express HRX mRNA.4 In the majority of tissues, results obtained with the polyclonal antibodies and MoAbs were in agreement. One exception was the liver where HRX was detected by the αHRX2 polyclonal antibody but not by the MoAb HRX 107. We attribute the latter observations to the possible expression of HRX isoforms that may arise by differential splicing of HRX RNA. Domer et al34 have reported variant forms of HRX (MLL) cDNAs that lack exonic sequences encoding the epitopes detected by the HRX 107 and αHRX2 antibodies but with preservation of sequences encoding the epitopes recognized by αHRX1. Mbangkollo et al7 have described a different splice variant containing exon 8 but lacking a 9-bp extension 3′ of exon 12. More splice variants cannot be ruled out and could account for the differences in staining we observed using antibodies against different portions of HRX. Isoforms of trx that result from differential processing of its large transcript have also been reported.47
From the data presented here it appears that the most practical application of the anti-HRX antibody described here is the detection of aberrant fusion proteins via Western blotting. Because HRX does indeed appear to be ubiquitously expressed, the only clear indication that a translocation is present is the appearance of fusion proteins of considerably reduced molecular weight in comparison with wild-type HRX. It is clear that HRX is present in high-grade lymphomas (L.H.B. and D.Y.M., unpublished observations, December 1995), but Westerns are required to ascertain whether translocations are present. We are currently analyzing a series of such cases to examine this possibility further.
ACKNOWLEDGMENT
We thank Doug Tkackuk for preparation of GST-HRX fusion constructs. We also thank Dr K. Pulford for the gift of the anti-TAL-1 antibody and Dr B. Falini for the gift of the anti-PML antibody.
Supported in part by a grant from the National Institutes of Health (CA55029) and by grants from the Leukaemia Research Fund. R.S. was supported by DFG grant SL27/1-1. M.L.C. is a scholar of the Leukemia Society of America.
Address reprint requests to Lisa H. Butler, MRC Haematology Unit, Institute of Molecular Medicine, John Radcliffe Hospital, Headington, Oxford, OX3 9DU, UK.