Abstract
Programmed cell death, or apoptosis, is well documented as a physiological means of eliminating activated lymphocytes and maintaining immune homeostasis. Apoptosis has also been implicated in the targeting of tumor cells by cytotoxic T lymphocytes and natural killer cells. One of the two primary mechanisms used in cell-mediated cytotoxicity is the Fas/FasLigand system. Activated or transformed cells expressing the Fas antigen on their surface are susceptible to killing by immune effector cells that express the Fas ligand. Many neoplastic cells, including those derived from patients with multiple myeloma, express Fas antigen on their surface, but do not undergo apoptosis in response to antigen crosslinking. One possibility for the lack of Fas-mediated apoptosis includes mutations in the Fas antigen. Loss of function mutations in the Fas antigen have been associated with congenital autoimmune disease in humans, and have been defined as the genetic defect the in lpr mice. Mutations in the Fas antigen have not been previously described in cancer patients. In this study, we show that mutations occur in the Fas antigen which may cause loss of function and contribute to the pathogenesis of the neoplastic disease, multiple myeloma. Using reverse transcriptase-polymerase chain reaction (RT-PCR), single-stranded conformation polymorphism (SSCP) analysis, and DNA sequencing, we examined the cDNA structure of the Fas antigen in 54 bone marrow (BM) specimens obtained from myeloma patients. Six patient specimens (11%) did not express detectable levels of Fas antigen mRNA. Of the 48 BM specimens which did express Fas antigen, 5 (10%) displayed point mutations. All of the mutations identified were located in the cytoplasmic region of the Fas antigen known to be involved in transduction of an apoptotic signal. Two separate individuals demonstrated an identical mutation at a site previously shown to be mutated in the congenital autoimmune syndrome, ALPS. One patient exhibited a point mutation at a site only two amino acids removed from the documented lesion of the lprcg mouse. Although the functional status of these point mutations remains to be determined, we propose that Fas antigen mutations may contribute to the pathogenesis and progression of myeloma in some patients.
THE FAS ANTIGEN (Apo-1/CD95) is a 45-kD transmembrane protein of the tumor necrosis factor receptor (TNF-R) family which can induce programmed cell death when crosslinked by ligand or specific antibodies.1,2 This family of proteins is characterized by a cysteine-rich extracellular domain, and a highly conserved cytoplasmic region of 70 to 80 amino acids known as the death domain, which has been shown to be necessary and sufficient for transduction of an apoptotic signal.3 Crosslinking by Fas ligand or agonistic antibody induces the association of the Fas death domain with cytoplasmic proteins, forming a death-inducing signaling complex (DISC).4 Site-directed mutagenesis studies of the Fas antigen have shown that deletion of the 15 carboxy-terminal amino acids enhance the response to Fas antibody-mediated cell death, indicating a negative regulatory role for this region, while deletions or mutations further into the death domain abrogate DISC formation and prevent apoptosis.3 4
The Fas/Fas ligand system of apoptosis plays a central role in the regulation of hemostasis by elimination of self-reactive lymphocytes during ontogeny, and activated lymphocytes following an immune response.5,6 Aberrant expression or function of the Fas antigen has been associated with both human and animal diseases, most particularly alterations that affect the signal transducing death domain. lpr Mice represent a model of autoimmune disease characterized by lymphadenopathy, hypergammaglobulinemia, and autoimmunity.7 8 The genetic defect in lprcg mice has been identified as a point mutation within the death domain of the Fas antigen which completely abolishes the apoptotic response of lpr cells. lpr Mice fail to eliminate reactive T cells, resulting in the accumulation of CD4−CD8− T cells and the production of autoantibodies with a Lupus-like syndrome.
Similar syndromes have been identified in humans and associated with Fas antigen mutations. Several recently published studies have identified function ablating mutations in patients with a congenital autoimmune lymphoproliferative syndrome (ALPS).9-12 This disease is identical to the lpr phenotype in the accumulation of activated lymphocytes resulting in lymphadenopathy, hypergammaglobulinemia, and autoimmune cytopenias. Peripheral blood mononuclear cells (PBMCs) isolated from ALPS patients show a diminished apoptotic response to crosslinking by anti-Fas monoclonal antibodies (MoAbs). The profile of ALPS patients is identical to an autoimmune disorder known as Canale-Smith syndrome, which was also associated with heterozygous mutations in the Fas antigen.13 In each of these disorders, Fas mutations have been identified in the signal-transducing death domain of the protein.
To date, diseases associated with mutations in either the Fas antigen or Fas ligand have primarily been congenital autoimmune disorders; however, no mutations have been described in cancer patients. Multiple myeloma is a neoplastic disease of plasma cells characterized by a latent accumulation of monoclonal cells, which typically display low proliferative activity. Several lines of evidence indicate that, although the original genetic insult occurs in the pre-B or early B-cell population, lymphocyte development occurs along normal lines before antigenic stimulation.14,15 Thus, it is likely that the transforming event is not a loss of growth control, but rather, a loss of death control. Previously published studies showed that although many myeloma cells express Fas antigen on their cell surface, only some respond to an apoptotic signal when treated with the apoptosis inducing anti-Fas antibody.16-18 We have examined the expression of the Fas antigen in bone marrow (BM) specimens obtained from 54 multiple myeloma patients to determine if mutations occur in the Fas antigen. If present, these mutations may contribute to the pathogenesis or progression of myeloma.
Using reverse transcriptase-polymerase chain reaction (RT-PCR) and single-stranded conformation polymorphism (SSCP) analysis, we have identified point mutations resulting in a single amino acid change in the Fas antigen of myeloma patients. Although the functional significance of these mutations remains to be defined, all mutations occur in the death domain where they are likely to effect the signal transducing capacity of the protein.
MATERIALS AND METHODS
Patient population. BM specimens were obtained during routine clinical assessment of multiple myeloma patients. PB lymphocytes (PBLs) were obtained from normal volunteers. BM mononuclear cells were isolated on a Ficoll gradient and cryopreserved in RPMI with 20% fetal bovine serum (FBS; Gemini, Calabasas, CA) and 10% dimethyl sulfoxide (DMSO) for up to 4 years before RNA extraction and analysis. Cultured cell lines used for control experiments were the human myeloma cell line 8226 and the human T-cell leukemia cell line, both originally obtained from American Type Culture Collection (ATCC; Rockville, MD). All cell culture reagents were from Sigma (St Louis MO) unless otherwise noted.
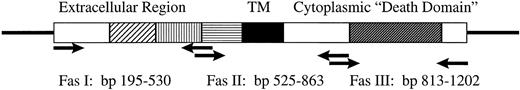
RT-PCR and SSCP analysis. Total RNA was isolated by lysis in guanidine isothiocyanate followed by cesium chloride density centrifugation and ethanol precipitation. Fifty to 100 ng of RNA was reverse transcribed by random hexamer priming, and the indicated fragments amplified by 35 cycles in a 9600 Perkin Elmer Thermocycler (Foster City, CA). Primers, shown in Figure 1 (Biosynthesis, Lewisville, TX), amplified products corresponding to bp 195-525, bp 520-863, and bp 813-1202, which covered the entire open reading frame of the Fas antigen.19 Radioisotope was incoporated into the PCR products for detection by autoradiogram. PCR conditions used for Fas II and Fas III amplification were: 60 seconds at 94°C; denature 15 seconds at 94°C; anneal 15 seconds at 52°C; extend 15 seconds at 72°C; final extension 60 seconds at 72°C. Due to the high GC content of the Fas I amplicon, the denaturation step was increased to 3 minutes, and Mg2+ concentraction in the PCR buffer was increased to 2.5 mmol/L for optimum amplification. Identity of the products was established by restriction digest and direct sequencing. A 215-bp fragment of Histone 3.3 was amplified as a control for RNA integrity.20 After amplification, PCR products were denatured 5 minutes at 80°C at a 1:10 dilution of sample buffer containing 98% formamide/5 mmol/L NaOH. SSCP gel conditions for each amplicon were optimized for strand separation and detection of a single base change.21-24 Fragments were analyzed under three electrophoretic conditions: (1) 5% to 6% acrylamide with 1% to 5% crosslinking at 4°C, (2) 6% acrylamide with 10% glycerol at room temperature, and (3) the FMC Mutation Detection Enhancement (MDE) system (Intermountain Scientific, Kaysville, UT). cDNAs showing mobility shifts were extracted from the gel, and re-amplified for 42 cycles using the same primer set, inserted into the TA cloning vector pCR2.1 (Invitrogen, La Jolla, CA), and sequenced either by Sequenase 2.0 (USB, Cleveland, OH) or ALF (Pharmacia, Piscataway, NJ). To control for potential PCR error, all patient samples displaying mobility shifts were analyzed by two independent PCR reactions. Three to five clones were selected for sequencing, and confirmation of point mutations was performed with both forward and reverse primers T7 and SP6 or M13. Fragment analysis and alignment was done using the programs Mac Vector and AssemblyLign (Oxford Molecular Group, Campbell, CA).
Diagrammatic representation of the Fas antigen cDNA. Arrows indicate positions of primers used for RT-PCR. Hatched boxes represent cysteine-rich extracellular region. Solid box represents the transmembrane region, and the dark hatched box respresents the death domain.
Diagrammatic representation of the Fas antigen cDNA. Arrows indicate positions of primers used for RT-PCR. Hatched boxes represent cysteine-rich extracellular region. Solid box represents the transmembrane region, and the dark hatched box respresents the death domain.
RESULTS
Because programmed cell death through the Fas/Fas ligand system has been implicated in the elimination of activated B cells both during ontogeny and in immune physiology, we examined the Fas antigen in myeloma tumor cells for mutations which could contribute to a failure of immune surveillance in the progression of disease. The cDNA sequence of the Fas antigen was divided into three amplicons of 316 (Fas I), 330 (Fas II), and 392 bp (Fas III), spanning the entire coding region19 (Fig 1). Because myeloma patient BM samples typically display tumor cell content from 2% to 98%, control experiments were performed to determine the minimum percentage of cells with a single base change which could be detected in our SSCP system.25 For these experiments we took advantage of a previously identified polymorphism at bp 836.19 Sequence analysis showed that the T-cell leukemia cell line, CEM, expressed the alternative codon ACT, while the myeloma cell line 8226 contained the higher frequency codon, ACC. CEM cells were mixed with 8226 cells at ratios of 1:1, 1:5, 1:10, 1:20, and 1:50. Total RNA was isolated from the mixed cell population, and the RT-PCR products analyzed by SSCP. With this system, we were able to identify mobility shifts with as little as 5% heterogeneous mRNA (data not shown). Using this criteria, 54 myeloma patient BM samples displaying a minimum of 5% plasma cells on histopathology were selected for analysis. SSCP conditions for strand separation were further optimized using a PCR product which introduced a single base change in the upstream primer (data not shown.)
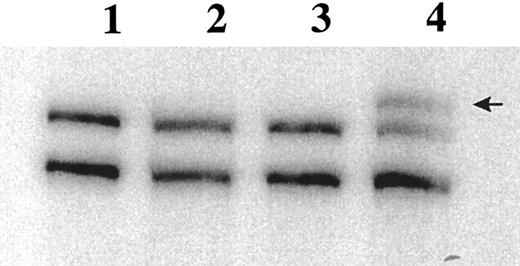
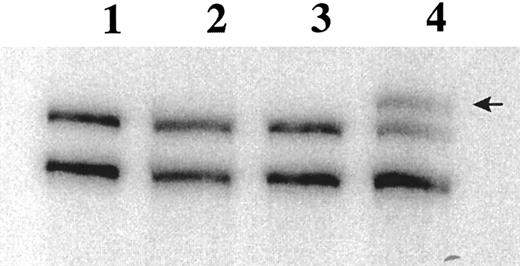
Of the 54 BM specimens analyzed, 48 expressed Fas antigen mRNA at levels detectable by RT-PCR. In the 6 patients who did not express Fas antigen mRNA, the integrity of the mRNA was confirmed by amplification of the housekeeping gene Histone 3.3. SSCP analysis identified mobility shifts in the Fas III amplicon in 15 of the 48 patient specimens (Fig 2). These bands were always seen in addition to the wild-type cDNA. Because whole mononuclear cell preparations were used for analysis, we are unable to distinguish homozygous versus heterozygous mutations in the myeloma cells. The Fas III fragment encompasses the cytoplasmic region, including the signal transducing death domain. Shifted bands were extracted from the SSCP gel, re-amplified by PCR, and ligated into the pCR2.1 cloning vector for bidirectional sequencing.
RT-PCR SSCP analysis of the Fas antigen in myeloma patient BM specimens. 32P-labeled PCR products are separated on a 6% acrylamide (49.5:0.5 bis) gel. Lane 1, PBLs; lane 2, 8226; lane 3, BAKA27 (normal); lane 4, FOSM32. Arrow indicates band excised for sequence analysis.
RT-PCR SSCP analysis of the Fas antigen in myeloma patient BM specimens. 32P-labeled PCR products are separated on a 6% acrylamide (49.5:0.5 bis) gel. Lane 1, PBLs; lane 2, 8226; lane 3, BAKA27 (normal); lane 4, FOSM32. Arrow indicates band excised for sequence analysis.
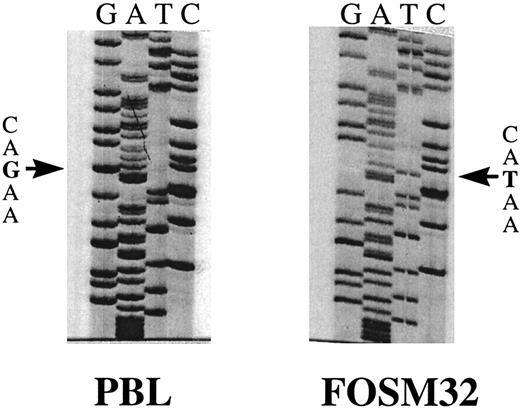
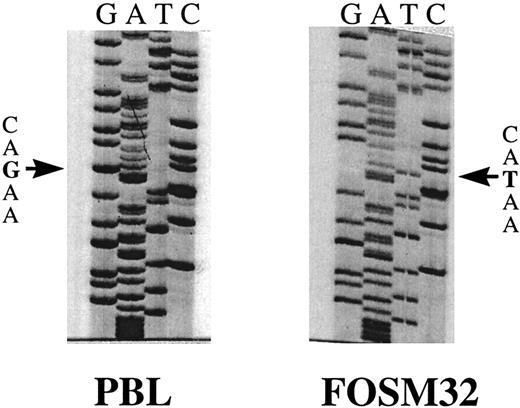
Sequence analysis showed point mutations in the Fas antigen cytoplasmic domain of 7 myeloma patients (Table 1). Two of these patients, FOSM32 and NYSR35, showed an identical G to T transversion at bp 999 (Fig 3). Translation of the open reading frame of the Fas antigen indicated this mutation would result in the substitution of ASP to TYR at amino acid 253. A G to T transversion in patient BRYR24 was identified at bp 1066, resulting in the substitution of TYR to SER of amino acid 275. Patient SUTH23 showed an A to G transition at bp 946, resulting in LYS to ARG at 235. Although this is a conservative substitution, it is only two amino acids away from the ILE to ASN mutation at residue 238 in lprcg mice which completely abolishes the signal transduction activity of the Fas antigen. Patient WEBW41 was found to have an A to C transversion at bp 1032, which resulted in ASN to HIS change at amino acid 264. Two patient specimens, CLAA48 and CONH24, were found to have silent mutations effecting no change in the amino acid sequence. The remaining 8 patient specimens which demonstrated mobility shifts in the Fas III amplicon were found to be heterozygous for the previously described polymorphism at bp 836.19
Sequence analysis of FasIII RT-PCR products extracted from SSCP gels. Sequence shown is bp 966 to 1011, including the point mutation G to T at bp 999 in patient FOSM32. The normal PB sequence is shown on the left (PBL).
Sequence analysis of FasIII RT-PCR products extracted from SSCP gels. Sequence shown is bp 966 to 1011, including the point mutation G to T at bp 999 in patient FOSM32. The normal PB sequence is shown on the left (PBL).
Analysis of the Fas II amplicon, which encompasses the transmembrane domain, showed the expression of alternative splice products in nearly all patient specimens, as has been previously described.26 27 Extraction and sequence analysis of the lower molecular weight amplicon identified a deletion of 73 bp corresponding to exon 6. In the majority of these patients, expression of the truncated mRNA appeared equivalent to that of the full-length mRNA. These protein products are predicted to be secreted as soluble factors due to the absence of a transmembrane domain, and have been identified in normal subjects as well as patients with autoimmune disease such as systemic lupus erythematosis. In vitro studies have shown an inhibitory effect of Fas antigen crosslinking by the transmembrane-deleted soluble factors; however, their clinical significance has not been established.
Only 1 of the 48 patient specimens that were positive for Fas antigen expression showed a mobility shift on SSCP analysis of the Fas II domain. Sequence analysis revealed a C to T transversion that did not affect the amino acid sequence. No mutations were detected in the extracellular region designated Fas I; however, minor products representing previously reported alternative splice variants were seen in 6 of 48 patients.27 28 The significance of these variants is not yet known.
DISCUSSION
Multiple myeloma is a neoplastic disease of plasma cells characterized by the latent accumulation of monoclonal plasma cells which typically display a low proliferative index. The disease progression of myeloma is characterized by (1) cell proliferation, often regulated by autocrine growth factors; (2) dissemination and vascular circulation of plasma cells; and (3) loss of programmed cell death leading to the accumulation of tumor cells.29,30 Programmed cell death mediated by the Fas/Fas ligand system is one of two primary mechanisms used in the elimination of activated lymphocytes and maintainance of homeostasis. It has also been implicated in cell-mediated cytotoxicity of tumor cells by cytotoxic T lymphocytes and natural killer (NK) cells.31,32 Loss of function of the Fas antigen may contribute to the pathogenesis and progression of neoplasias by allowing susceptible cells to evade immune surveillance. The prolonged survival would allow the cell to accumulate mutations leading to malignancy.6
Several recent reports have identified Fas antigen expression on primary myeloma cells as well as cultured myeloma cell lines.33-35 However, not all myeloma cells are capable of undergoing apoptosis in response to crosslinking with anti-Fas antibody.16-18 We have examined the Fas antigen cDNA in BM samples obtained from 54 patients with multiple myeloma, and have identified mutations in the signal transducing region of the Fas antigen that may account for the functional deficiencies identified in those studies.
Loss of function of the Fas antigen is associated with hyperplasia and lymphoproliferation. A congenital autoimmune disorder known as autoimmune lymphoproliferative syndrome (ALPS) has been attributed to Fas antigen mutations. In three separate studies, function ablating mutations in the fas antigen were identified in patients with ALPS.9-11 This disease is similar to the phenotype of the lpr mouse which is characterized by an accumulation of activated lymphocytes resulting in lymphadenopathy, hypergammaglobulinemia, and autoimmune cytopenias. PBMCs isolated from ALPS patients show a diminished apoptotic response to crosslinking by anti-Fas MoAbs. Molecular analysis of the Fas antigen in these patients showed unique mutations. Five of the 11 combined patients analyzed in these studies demonstrated mutations within exon 9 of the Fas antigen. In the study by Rieux-Laucat et al,9 two siblings demonstrated a 2-bp deletion which generates an unrelated amino acid sequence sequence beginning at residue 254, and a premature stop codon 9 amino acids downstream. The site of this maternally inherited deletion is immediately adjacent to the point mutation that we identified in two unrelated myeloma patient specimens, indicating a potential hot spot in the Fas coding sequence. Functional analysis of lymphocytes isolated from siblings with ALPS showed normal surface expression of the protein, with a 50% reduction in the apoptotic response of the cells when crosslinked by an anti-Fas antibody. We would anticipate a similar loss of function in the myeloma cells of patients FOSM32 and NYSR35, and these studies are currently underway in our laboratory.
More recently, a similar disorder has been described in pediatric patients who display a generalized autoimmune disease without the accumulation of CD4−CD8− T cells.36 This disease, called ALDS, has been associated with a decrease in Fas antigen function. However, no alterations in Fas gene expression were identified, implicating a lesion distal to the Fas antigen in the apoptotic signal transduction pathway of mature B cells. Because only 10% of the multiple myeloma patients in our study showed mutations of the Fas antigen, it is possible that aberrations in the Fas signal transduction pathway may also contribute to resistance to apoptosis in myeloma and other hematologic malignancies.
Several recent reports have provided evidence that apoptosis induced by anticancer drugs share a common pathway with physiological mechanisms of cell death including the Fas/Fas ligand system.37,38 We have recently reported that cell lines which are selected for resistance to chemotherapeutic agents are also resistant to Fas-mediated apoptosis.18 Conversely, Friesen et al39 reported that cell lines selected for resistance to Fas-mediated apoptosis are also resistant to chemotherapeutic agents. These data suggest that aberrant function of the Fas antigen may contribute to enhanced survival of tumors under treatment with chemotherapeutic agents, and promote the emergence of drug-resistant disease. Further definition of the mechanisms involved in apoptosis may contribute to the development of more effective therapeutic strategies.
Genetic defects in the ability of a cell to undergo apoptosis has defined a new class of proto-oncogenes, those which effect cell death rather than cell proliferation.6,40,41 Although Fas antigen has been proposed as a tumor-suppressor gene, this study is the first to show mutations in the coding sequence of Fas associated with human neoplasia. We propose that Fas antigen mutations may contribute to the pathogenesis and progression of some hematologic malignancies. In this study, we report point mutations effecting the signal transduction domain of the Fas antigen in 10% of patients analyzed. Two unrelated patients showed an identical point mutation, located immediately adjacent to a 2-bp deletion which displays a dominant negative effect of Fas function in ALPS patients. None of the mutations we identified have been reported to occur in normal tissues.10,13 19 Functional analysis of the mutations is currently underway in our laboratory.
Supported in part by the Molecular Biology Core Facility at H. Lee Moffitt Cancer Center and Research Institute.
Submitted June 17, 1997; accepted August 26, 1997.
Address reprint requests to William S. Dalton, MD, PhD, H. Lee Moffitt Cancer Center, University of South Florida, 12902 Magnolia Dr, Tampa, FL 33612.