Abstract
The clinical results, cellular immune reconstitution, and hematopoietic chimerism obtained after transplantation of recombinant human granulocyte colony-stimulating factor mobilized allogeneic peripheral blood stem cells (PBSCs) from genotypically human leukocyte antigen (HLA)-identical sibling (n = 36) or alternative family donors (n = 24) were prospectively compared in patients with hematologic malignancies. Thirty-two of 34 evaluable patients with HLA-identical sibling donors and all patients with alternative family donors achieved trilineage engraftment. The median time intervals to reach peripheral neutrophil counts <500/μL (13 v 17 days) or <1,000/μL (16 v 19 days) and unsupported platelet counts <20,000/μL (11 v 15 days) or <50,000/μL (19 v 24 days) as well as red blood cell and platelet transfusion requirements were not significantly different between both patient subsets. The cumulative probability of grades II through IV acute graft-versus-host disease (GVHD) for the 60 study patients was 48% ± 10% but ranged between 86% ± 12% in patients whose donors had at least one HLA-A,B,DR,DQ,DP antigen disparity in direction to acute GVHD, and 25% ± 9% in recipients of GVHD-matched transplants (P < .003). The 2-year survival estimates were 54% ± 10% for patients with alternative family donors and 65% ± 9% for patients with HLA-identical sibling donors. Multivariate analysis identified the pretransplantation disease stage, patient age, and acute GVHD as independent predictors of overall and disease-free survival, whereas alternative family donors alone had no adverse effect on these clinical endpoints. Monthly monitoring of peripheral blood T-helper cell subsets, B cells, and monocytes during the first year posttransplantation showed a nearly identical course of immune cell reconstitution in both patient subsets. In addition, no differences in the proportions of complete chimeric patients were detectable between the two patient subsets by sex chromosome and variable number of tandem repeats analysis up to 12 months posttransplantation. In conclusion, PBSCs from alternative family donors represent an attractive source for allogeneic transplantation in patients lacking HLA-identical sibling donors and should be further evaluated in comparison with marrow transplants from alternative family donors.
TRANSFUSION OF recombinant human granulocyte colony-stimulating factor (rhG-CSF ) mobilized autologous peripheral blood stem cells (PBSCs) after high-dose chemotherapy for solid tumors and hematologic malignancies has been shown to accelerate hematopoietic recovery and immune reconstitution,1-3 thereby leading to a reduction in therapy-related complications when compared with autologous bone marrow support.4 Preliminary reports on the use of rhG-CSF for the mobilization and collection of PBSCs from syngeneic or allogeneic donors further support that this drug has tolerable, predominantly minor toxicities in healthy individuals.5-7 As shown by the comparative studies in the setting of autologous stem cell support, transplantation of allogeneic PBSCs may also provide the advantages of a more rapid hematologic and immune reconstitution compared with allogeneic bone marrow transplantation (BMT), and this expectation is supported by recent preliminary retrospective clinical comparisons and one prospective analysis.8-10 However, these potential advantages still need to be balanced against the potential hazards of the donor rhG-CSF pretreatment and the currently undefined risk of acute or chronic graft-versus-host disease (GVHD), which may be associated with the transfusion of 10- to 15-fold higher donor T lymphocyte numbers in unmanipulated allogeneic PBSC transplants as compared with conventional marrow grafts.10-13 The observation that allogeneic PBSC transfusions from the primary donor are capable of achieving durable engraftment in patients with primary or secondary marrow graft failures further argues for an increased transplantation potential of PBSC grafts14 that may rely on higher yields of marrow stem cells and/or graft-facilitating properties of the transfused donor T lymphocytes and accessory cells.
This is the first study that prospectively compared the clinical results of allogeneic PBSC transplantations in patients with human leukocyte antigen (HLA) genotypically sibling (n = 36) or alternative family donors (n = 24) over a 2-year period. The study was further aimed to compare the hematologic and immune reconstitution as well as the hematopoietic chimerism after PBSC transplantations from HLA-identical sibling donors or from alternative family donors.
PATIENTS AND METHODS
Patients and donor accrual. Between October 1994 and November 1996, 60 patients with hematologic malignancies (adult patients, n = 56; pediatric patients, n = 4) were enrolled in a study of first allogeneic PBSC transplantations. Patients and their donors were eligible for the study protocol if at least one of the following inclusion criteria was fulfilled: (1) HLA-A, B, DR, DQ, DP disparities between recipient and family donor (ie, a maximum of two class I and II antigen disparities in graft-versus-host [GVH] direction irrespective of the number of disparities in host-versus-graft direction),15-17 (2) prolonged exposure to interferon-α in patients with chronic myelogenous leukemia (CML),18 (3) a history of documented serious infectious complications within 4 months pretransplantation, and (4) organ functional impairment of the donor precluding general anesthesia and marrow harvest. These inclusion criteria were fulfilled by 65 of 158 patients with family donors (41%), who were referred for an allogeneic transplantation to the University Hospital of Essen (Essen, Germany) between October 1994 and November 1996. One of these patients, who was primarily diagnosed as having severe aplastic anemia and, therefore, received a nonmyeloablative conditioning regimen consisting of cyclophosphamide and antithymocyte globulin before a PBSC transplantation from her HLA-identical sister, developed acute myeloid leukemia at 1 year posttransplantation. Meticulous review of her pretransplantation marrow histological records then showed sparse focal infiltrates of myeloid blasts within a severely hypoplastic marrow architecture. This patient was considered not evaluable for the present study. The remaining four patients or their donors refused to participate in the study.
Study protocol. The administration of rhG-CSF (Neupogen; Amgen-Roche Inc, München, Germany) to donors for the purpose of PBSC mobilization, the use of leukapheresis for PBSC collection, and the transplantation of rhG-CSF–mobilized allogeneic PBSCs in patients with hematologic malignancies had been approved by the Ethics Committee of the University Hospital of Essen. Patients and donors were allowed to participate in this study after written informed consent for all aspects of the study had been obtained from patients or their legal guardians as well as from the donors.
Donor rhG-CSF pretreatment was initiated 4 days before the prospective first day of leukapheresis (designated day 0). Doses of rhG-CSF ranged between 5 and 16 μg/kg of the donor lean body weight per day applicated in one to two daily subcutaneous fractions. With one exception, all apheresis procedures were performed using a continuous flow blood cell separator (Cobe Spectra; Cobe Inc, München, Germany) equipped with software for the semiautomated collection of mononuclear cells. A minimum yield of 2.5 × 106 CD34+ cells/kg of the recipient body weight was accepted for PBSC transplantation in this study and could be obtained by 1 to 3 aphereses in all donor/recipient pairs. Details of the apheresis procedures performed in this study are given in Table 1. With two exceptions, in which cryopreservation of the PBSC harvest was necessary because of logistic reasons, all apheresis products were immediately transfused after collection without further manipulation.
Patient and donor demographic and treatment characteristics are included in Table 2. All 24 patients with alternative family donors were genotypically HLA-A, B, DR, DQ, DP–identical for one HLA haplotype but differed in at least one HLA-A, B, DR, DQ, DP antigen of the unshared haplotype (Table 2). All patients were treated under identical conditions of strict reverse isolation in high-efficiency air-filtered rooms with intestinal bacterial and mycotic decontamination using oral ciprofloxacin, metronidazole, and amphotericin-B. These conditions were generally maintained over a 5-week period posttransplantation. Details of the different regimens for myeloablative conditioning used in this study have been previously published.19,20 With eight exceptions, prevention of acute GVHD was attempted by the combination of a short course of methotrexate (sMTX) and continuous IV ciclosporin (CSP).21 In patients receiving more than one apheresis product, MTX was administered at days 1, 3, 6, and 11 after the last PBSC transfusion. CSP alone was administered in six patients, of whom five patients received transplants from HLA-identical sibling donors. Two patients, whose myeloablative regimen included the combination of total body irradiation, cyclophosphamide, and etoposide received an immunoprophylactic regimen consisting of CSP and prednisolone to spare mucosal toxicity.
Posttransplantation rhG-CSF at a continuous IV dose of 5 μg/kg/d was administered until absolute blood neutrophil counts exceeded 1,000/μL on 3 consecutive days. This was restricted to 44 patients whose pretransplantation marrow examinations showed less than 10% of clonal blasts as identified by multiparameter flow cytometry and no myelodysplastic marrow features.
Clinical study endpoints. The duration to neutrophil recovery was defined by the time intervals between the first day, on which absolute blood neutrophil counts declined below 500/μL and 1,000/μL, and the first of 3 consecutive days, on which these counts exceeded 500/μL and 1,000/μL, respectively. The duration of platelet recovery was similarly defined by the time intervals to reach untransfused platelet counts greater than 20,000/μL and 50,000/μL, respectively. Engraftment was assumed if self-sustaining blood neutrophil counts greater than 1,000/μL together with untransfused platelet counts were reached by day 30 posttransplantation. If applicable, this had to be further confirmed by the evaluations of chimerism performed in this study.
The diagnosis of acute and chronic GVHD was based on the characteristic clinical appearance of the symptoms of organ involvement. In cases of doubt, the clinical diagnosis had to be confirmed by histological examinations of the suspected organ involvement whenever possible. Grading of acute and chronic GVHD followed the commonly accepted criteria.22 23
Transplant-related death was assumed if patients died in the posttransplantation course without hematologic evidence of disease recurrence. Causes of death were evaluated in all but two of the deceased patients by necropsy.
Patients who survived without clinical and hematologic evidence of recurrence of their malignancy posttransplantation were regarded as event-free survivors. Thus, isolated findings of disease persistence or recurrence by molecular analyses were not considered for the diagnosis of relapsing disease.
Laboratory studies. For the quantification of CD34+ cells, T-cell subsets, and B cells, PBSC transplants or patient peripheral EDTA blood samples were analyzed by multiparameter flow cytometry using methods previously published.10 24 Prospective monitoring of the peripheral blood cellular immune reconstitution was performed on days 20 and 30 posttransplantation and in monthly intervals during the first posttransplantation year thereafter.
In 43 informative donor/recipient pairs, chimerism was analyzed by polymerase chain reaction (PCR) amplification of variable numbers of tandem repeat (VNTR) polymorphisms using the published method for the human minisatellite region D1S80.25 In 20 of 31 opposite-sex donor/recipient pairs, evaluation of hematopoietic chimerism was additionally based on two-color fluorescence in situ hybridization (FISH) using probes containing specific sequences to the α satellite region of the X chromosome and to the satellite III sequence of the Y chromosome (XY CEP; Vysis Inc, Stuttgart, Germany).26 A total of 1,000 interphase nuclei per blood and marrow sample was enumerated in XY chromosome FISH analyses. The limit for the detection of residual recipient cells by XY chromosome FISH analysis was previously found to be ≥1 × 103. The corresponding value for the PCR amplification of D1S80 polymorphisms is in the range of ≥1 × 104 to 1 × 105. Hematopoietic chimerism studies were performed at 3, 6, 9, and 12 months during the first posttransplantation year. Patient samples were included in these evaluations before the clinical diagnosis of relapse or until death.
Statistical analysis. Comparisons between classified variables were performed by the two-tailed Fisher's exact test. Continuous variables were compared by the Wilcoxon rank-order statistics. For the comparisons of timely repeated continuous measures between strata, repeated measures analysis of variance was applied.27 Time-to-event estimates were calculated by the product-limit method with right censoring of subjects at the last time point at which they were at risk for a given event.28 Time-to-event distribution functions between strata were compared by the log-rank test. To evaluate the influence of different explanatory variables on the times to achieve the analytical endpoints of treatment outcome (survival, event-free survival, relapse, and transplant-related mortality), stepwise proportional hazard general linear model (PHGLM) analysis was performed.29 PHGLM analysis was further used to identify explanatory variables that influenced the times to reach the endpoints of hematopoietic reconstitution. To account for the multiple test situation, only those explanatory variables that have been identified by PHGLM analysis with a level of significance ≤1% were regarded as significant.
For the purpose of the analyses on treatment outcome, patients were stratified into two prognostic categories according to the risk of posttransplantation relapse. An early disease stage was assumed if patients were treated in the first remission of acute leukemia or in the first chronic phase of CML. This category further included two previously untreated patients with myelodysplastic syndromes, whose marrow blast cell content was below 10% at the time of transplantation. Accordingly, all other disease stages were categorized as advanced disease. The date of the final analysis was July 1, 1997.
Because no death occurred within the first 10 posttransplantation days in this study, all patients were regarded evaluable for acute GVHD. Estimates of chronic GVHD were based on patients surviving longer than 70 days posttransplantation.
RESULTS
PBSC procurement. In 55 of the 60 donors (92%), rhG-CSF administration was performed on an ambulatory basis. Mild to moderate adverse reactions to rhG-CSF were noted in the majority of donors and consisted predominantly of bone pain, headaches, or flu-like symptoms. These reactions were completely reversible within 1 to 3 days after cessation of rhG-CSF administration in all donors. With the exception of two moderate reactions to the infusion of the anticoagulant acid citrate dextrose during PBSC apheresis, which were immediately reversible after intravenous administration of calcium salt, no significant adverse events were associated with PBSC procurement.
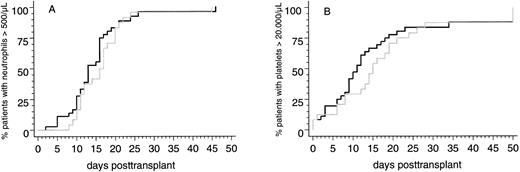
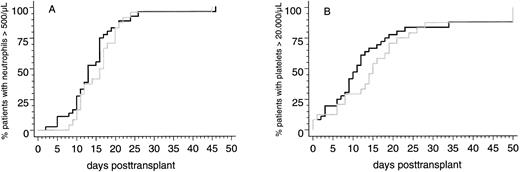
Engraftment. Two of 58 patients (3%) surviving more than 30 days posttransplantation did not reach the endpoints of peripheral blood neutrophil and platelet recovery and showed hypocellularity on day-30 marrow biopsy specimens. Both patients received transplants from HLA-identical sibling donors and developed severe multiorgan functional impairment during the immediate posttransplantation course. Therefore, it cannot be ruled out that a disturbed clearance of methotrexate, which was applicated to both patients after transplantation, contributed to poor graft function. Parameters of hematopoietic reconstitution and transfusion requirements of the two patient subsets are summarized in Table 3. Notably, the probabilities of reaching the endpoints of neutrophil and platelet recovery after PBSC transplants from HLA-identical sibling donors or from alternative family donors were not significantly different (Fig 1). This was confirmed by multivariate analysis in which the categorized number of transfused CD34+ cells and the use of MTX posttransplantation were identified as the only independent predictors for the time intervals to reach the endpoints of neutrophil and platelet recovery (CD34+ cell dose [n × 106/kg]: neutrophils >500/μL, P < .008; platelets >20,000/μL, P < .0006; MTX posttransplantation: neutrophils >500/μL, P < .0001; platelets >20,000/μL, P < .03).
Cumulative probabilities of reaching blood neutrophil counts <500/μL (A) or unsupported platelet counts <20,000/μL (B) in patients with genotypically HLA-identical sibling donors (black line) or alternative family donors (grey line) after allogeneic PBSC transplantation.
Cumulative probabilities of reaching blood neutrophil counts <500/μL (A) or unsupported platelet counts <20,000/μL (B) in patients with genotypically HLA-identical sibling donors (black line) or alternative family donors (grey line) after allogeneic PBSC transplantation.
Acute and chronic GVHD. Eighteen of the 60 patients (30%) in this study contracted grades II through IV acute GVHD. The cumulative probabilities of grades II through IV acute GVHD ranged between 25% ± 9% in patients who received transplants from HLA-genotypically or phenotypically identical donors and 86% ± 12% in patients with GVH-mismatched donors (P < .003; Table 4). Multivariate analysis showed that a disparity of major histocompatibility complex (MHC) class I or II antigens in GVH direction was the only independent predictor of grades II through IV acute GVHD in this study (P < .005).
At the time of this writing, 33 of 53 evaluable patients (62%; ie, patients surviving free of relapse for more than 70 days posttransplantation) had developed chronic GVHD, which was graded as limited disease in 23 patients (43%) and as extensive disease in 10 patients (19%). In 29 of the affected patients (88%) chronic GVHD developed from preceding acute GVHD. Aside from the evident influence of preceding acute GVHD, none of the analyzed factors (as given in Table 4) had a significant influence on the occurrence of chronic GVHD in univariate and multivariate analyses (data not shown).
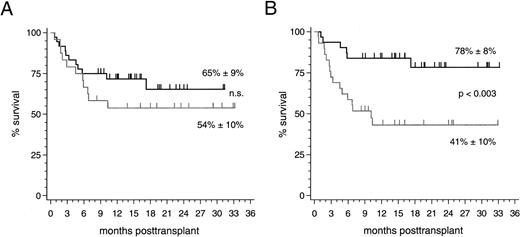
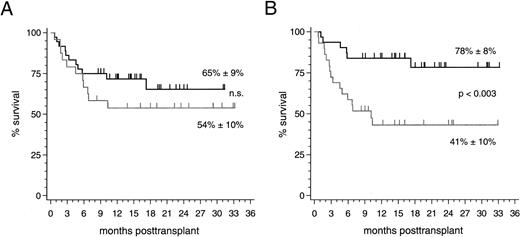
Clinical outcome. With a median follow-up of 16 (range 8 to 33) months, 37 of 60 patients (62%) are currently alive resulting in an overall survival estimate of 61% ± 7% at 2 years posttransplantation. No significant difference of this estimate was found between the 36 patients with HLA-identical sibling donors (65% ± 9%) and the 24 patients with alternative family donors (54% ± 10%; Fig 2). The prevailing factor of survival was the stage of disease at the time of transplantation: Twenty-five of 31 patients (81%; median follow-up 19 [range 8 to 33] months) who were treated in early disease stages remain presently alive compared with 12 of 29 patients (41%; median follow-up 15 [range 8 to 33] months) transplanted in advanced stages (P < .003; Fig 2). Nine of 10 patients (90%) with alternative family donors and 16 of 21 patients (76%) with HLA-identical sibling donors, who underwent PBSC transplantation in an early disease stage, are presently alive resulting in 2-year survival estimates of 90% ± 9% and 71% ± 12% (not significant), respectively. The 2-year survival estimates for patients treated in advanced disease stages were 27% ± 12% after transplantations from alternative family donors (4 of 14 surviving patients [29%]) and 52% ± 13% after HLA-identical sibling donor transplants (8 of 15 surviving patients [53%]; not significant). Multivariate analyses of factors influencing survival, event-free survival, transplant-related mortality, and relapse in this study are summarized in Table 5. The causes of death for the two patient subsets are listed in Table 6.
(A) Survival probability after PBSC transplantation using genotypically HLA-identical sibling donors (black line) or alternative family donors (grey line) and (B) survival probability of all patients stratified by disease stage at transplant: early stages (black line) and advanced stages (grey line).
(A) Survival probability after PBSC transplantation using genotypically HLA-identical sibling donors (black line) or alternative family donors (grey line) and (B) survival probability of all patients stratified by disease stage at transplant: early stages (black line) and advanced stages (grey line).
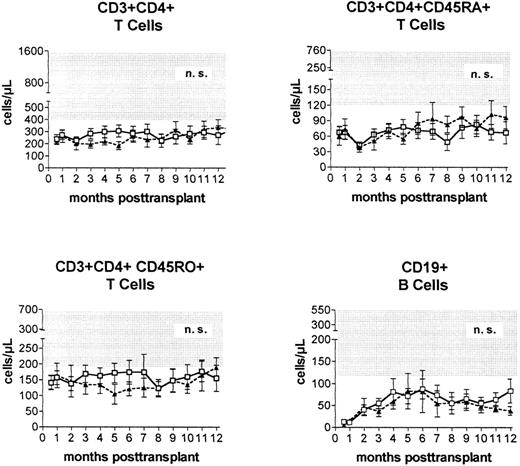
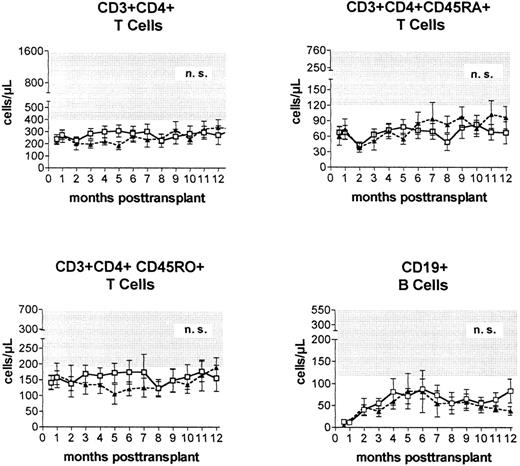
Immune reconstitution. Prospective monitoring of the phenotypic regeneration of peripheral blood mononuclear cells showed no significant differences between the numbers of CD3+CD4+ T cells, CD3+CD4+CD45RA+ T cells, CD3+CD4+CD45R0+ T cells, B cells, and monocytes (not shown) after transplantations from HLA-identical or alternative family donors (Fig 3).
Peripheral blood concentrations (mean ± standard error of the mean) of helper (CD3+CD4+) T cells, naive (CD3+CD4+CD45RA+) T cells, experienced (CD3+CD4+CD45R0+) T cells, and CD19+ B cells after PBSC transplantation from genotypically HLA-identical sibling donors (□) or alternative family donors (▴) during the first posttransplantation year. Shaded areas represent reference ranges (5th to 95th percentile) of 60 healthy individuals.
Peripheral blood concentrations (mean ± standard error of the mean) of helper (CD3+CD4+) T cells, naive (CD3+CD4+CD45RA+) T cells, experienced (CD3+CD4+CD45R0+) T cells, and CD19+ B cells after PBSC transplantation from genotypically HLA-identical sibling donors (□) or alternative family donors (▴) during the first posttransplantation year. Shaded areas represent reference ranges (5th to 95th percentile) of 60 healthy individuals.
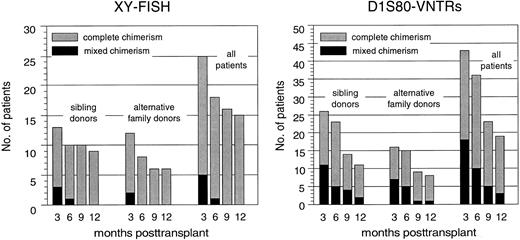
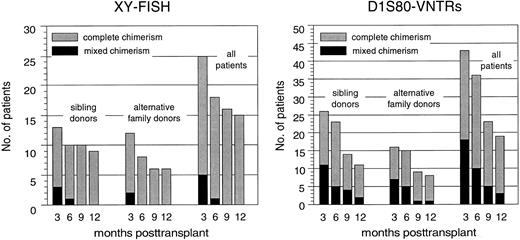
Hematopoietic chimerism. By using XY chromosome and D1S80 genomic markers, a total of 49 patients were initially evaluable for their chimerism status using at least one of both methods at 3 months posttransplantation. Figure 4 summarizes the results obtained by chimerism analyses during the first year after PBSC transplantation and illustrates that the proportion of complete chimeric patients remained nearly identical in both patient cohorts. Persistence or reappearance of a mixed chimeric status was significantly more frequent in those patients who underwent PBSC transplantation in advanced disease stages (data not shown).
Proportions of complete chimeric patients as defined by X and Y chromosome FISH or D1S80–VNTR-PCR analysis after PBSC transplantation from genotypically HLA-identical sibling donors or alternative family donors during the first posttransplantation year.
Proportions of complete chimeric patients as defined by X and Y chromosome FISH or D1S80–VNTR-PCR analysis after PBSC transplantation from genotypically HLA-identical sibling donors or alternative family donors during the first posttransplantation year.
DISCUSSION
This is the first study presenting prospective comparisons between results obtained after allogeneic transplantation of unmanipulated rhG-CSF–mobilized PBSCs from HLA-genotypically identical sibling donors or alternative family donors. Although this study is limited by a relatively short follow-up period and heterogeneous study inclusion criteria, it provides new and important information regarding the feasibility of allogeneic rhG-CSF–mobilized PBSC transplantations even in a patient population that is supposed to have otherwise increased hazards of transplant-associated complications.
The first important finding is that the kinetics of neutrophil and platelet reconstitution was not significantly different between the two patient subsets. This is indirectly confirmed by the nearly identical transfusion requirements after allogeneic PBSC transplantations from HLA-identical sibling or alternative family donors in this study. Twenty-three of the 24 patients with alternative family donors had at least one MHC class I or class II antigen disparity in host-versus-graft direction. From previous experience using marrow transplants from MHC-disparate family donors, we expected that the rate of graft failures in this patient subset would be in the range of 10% to 20%.15-17 Therefore, it was surprising that all of these patients had timely and sustained trilineage engraftment without episodes of primary or secondary graft failure. Together with the observations that allogeneic PBSCs are able to engraft in patients with previous marrow graft failure,14 this may point to a higher capability of PBSCs, compared with marrow cells, to restore hematopoiesis even in recipients who carry an increased risk of graft failure.
Two major differences between PBSCs and marrow cells might contribute to a putative higher transplantation potential of allogeneic PBSCs: First, as has already been shown by studies comparing autologous PBSC transplantation and BMT, allogeneic PBSC transplantations may contain higher numbers of hematopoietic progenitor cells.13 In our own unpublished prospective evaluations, CD34+ cell numbers of allogeneic PBSC transplants were 3 to 4 times higher compared with marrow grafts, and similar relationships have been described by other investigators.6,13 This phenotypically defined cell population consists predominantly of lineage-committed hematopoietic cells that are thought to mediate short-term hematologic regeneration posttransplantation. The question as to whether allogeneic PBSC transplants also encompass sufficient numbers of hematopoietic stem cells capable to ensure permanent multilineage engraftment still needs to be formally proven, although flow-cytometric analyses on CD34+ cell subsets in PBSC transplantations suggest that rhG-CSF also peripheralizes more primitive hematopoietic precursors with putative stem cell properties.13 The second explanation is that unmanipulated PBSC transplants contain 1 to 2 log10 higher numbers of donor T and accessory cells,11-13 and these higher numbers alone may potentiate the ability of PBSC transplants to overcome graft resistance. This hypothesis is indirectly supported by the experience with the use of T-cell–depleted marrow allografts, which generally showed that a 3- to 4-log10 reduction of donor T-cell numbers in the graft leads to an increased risk of marrow graft failure compared with unmanipulated marrow transplants.30 Accordingly, it appears reasonable to assume that a substantial increase in the transfused donor T-cell numbers by itself may facilitate engraftment of hematopoietic donor cells. At present, the exact role of functionally different T-cell subsets during the process of marrow cell engraftment remains obscure. Therefore, it further needs to be defined whether the higher numbers of transfused donor T and accessory cells alone or functional differences between blood- or marrow-derived donor T- and accessory-cell populations contribute to a putative higher engraftment capability of allogeneic PBSCs.
Multivariate analysis on defined endpoints of neutrophil and platelet reconstitution confirmed that the transfused CD34+ cell number is an independent predictor of the time intervals to reach these endpoints. This association has, so far, not been shown by other single institutional studies on allogeneic PBSC transplantations with sufficient patient numbers and statistical consideration of interactions between factors affecting neutrophil and platelet recovery.31 The second independent predictor of neutrophil and platelet recovery times was the type of acute GVHD prophylaxis, and the use of a short course of methotrexate was significantly associated with a delay in attaining the analyzed reconstitution endpoints. The median recovery times of neutrophils and platelets in the present study are in good concordance with those of previous reports on allogeneic PBSC transplantations,8,9,31 32 in which the majority of patients had received the combination of short course methotrexate and ciclosporin to prevent acute GVHD. The statistical independence of the influence of these two factors on the analyzed recovery times suggests that the posttransplantation hematotoxic effects of methotrexate can at least partially be ameliorated by increasing the CD34+ cell numbers in PBSC grafts.
The use of rhG-CSF posttransplantation in 43 of the 60 study patients did not affect neutrophil and platelet recovery times. Only sparse information with regard to the effects of posttransplantation rhG-CSF on blood cell regeneration kinetics after allogeneic PBSC transplantation are currently available. In one recent retrospective survey including 44 patients from nine United Kingdom transplantation centers, of whom 20 patients had been treated with rhG-CSF posttransplantation, no significant influence of rhG-CSF on neutrophil engraftment was detectable.31 Together with the present analysis, the available data still do not allow valid conclusions as to whether posttransplantation rhG-CSF treatment may lead to a clinically meaningful acceleration of neutrophil and/or platelet recovery after allogeneic PBSC transplantation. This issue clearly needs to be further examined by prospective randomized clinical trials including more homogeneous patient populations.
One major concern about the clinical application of allogeneic PBSC transplantation is that the substantially higher donor T-cell numbers transfused along with unmanipulated PBSC transplantations may increase the frequency and/or severity of acute GVHD. However, the available results from pilot studies in patients who received PBSC transplants from HLA-identical sibling donors do not support the hypothesis that the risk of acute GVHD is substantially higher in PBSC recipients compared with this risk in marrow transplant recipients.8,9,13,31 32 The cumulative estimate of grades II through IV acute GVHD after HLA-identical sibling donor PBSC transplantation in the present analysis was approximately 30%, and this appears very similar to the estimates of this complication reported in the mentioned pilot studies. As expected, grades II through IV acute GVHD developed more frequently after PBSC transplantations from alternative family donors and was the highest in the subset of 19 patients who had MHC class I or II antigen disparate donors in GVH direction. Although this estimate was more than threefold higher than that for patients who had MHC class I and II antigen identical donors in respect to GVHD, only 5 of the 19 recipients of GVH-mismatched donor transplants died from causes directly related to acute GVHD.
Aside from the influence of MHC disparate donors, only the use of rhG-CSF posttransplantation was significantly associated with grades II through IV acute GVHD in univariate analysis. However, this effect was not confirmed after adjustment for MHC disparity between patients and donors. Because donor T cells in rhG-CSF–mobilized PBSC transplants might have different functional properties than in conventional marrow grafts, it presently remains to be evaluated whether rhG-CSF treatment of the recipient might modulate acute GVHD after allogeneic PBSC transplantation. The average number of TCRαβ+CD3+CD4−CD8− cells in rhG-CSF–mobilized allogeneic PBSC transplantations is much higher than in conventional marrow grafts,10 33 and in vitro studies have shown that these cells can mediate suppressor activity. If these cells were indirectly influenced by posttransplantation rhG-CSF treatment, they might contribute to the development of host-specific tolerance.
Chronic GVHD developed in 62% of evaluable patients and was strongly associated with preceding acute GVHD. This incidence appears similar to the magnitude of this complication reported in other series of allogeneic PBSC transplantations.8,9,13,31,32 Preliminary comparisons between PBSC and marrow transplant recipients presently do not support that the risk of chronic GVHD is substantially higher after allogeneic PBSC transplantation.8 9 However, patient numbers and follow-up are still too short to allow reliable estimates of the real risk of chronic GVHD after allogeneic PBSC transplantation. The crucial question as to whether the incidence, severity, and duration of chronic GVHD are different between PBSC and marrow transplant recipients, therefore, has to await results of prospective randomized trials, which are currently under way.
Patient outcome in this study was significantly influenced by the disease stage at transplantation, acute GVHD, and patient age. These three factors are generally recognized as the most important prognostic determinants of allogeneic BMT. In concordance with experiences from single centers with regard to the influence of MHC disparate related marrow donors on patient outcome,15-17 multivariate analysis confirmed that PBSC transplants from MHC partially matched related donors did not adversely affect overall and event-free survival. In considering the limited patient number and heterogeneous inclusion criteria, the present study results do not permit definitive conclusions regarding the general applicability of PBSC transplantation using MHC partially matched related donors. However, they clearly indicate that this procedure can be successfully performed in the majority of patients with partially matched related donors despite an increased risk of grades II through IV acute GVHD. Similar to the experience with marrow transplants using family donors other than HLA-identical siblings, it can be expected that this increased risk will be at least partially balanced by a lower rate of posttransplantation disease recurrence.
Monthly phenotypic monitoring of peripheral blood mononuclear cells in the two patient cohorts showed an identical course of the absolute numbers of CD3+CD4+ T cells, CD3+CD4+CD45RA+ T cells, CD3+CD4+CD45R0+ T cells, B cells, and monocytes during the first posttransplantation year. The combined results of both patient subsets are further comparable with those previously reported for the first 20 study patients,10 which showed an improved regeneration of T-helper–cell subsets and B cells as well as higher proliferative T-cell in vitro responses in a prospective comparison with 20 marrow transplant recipients.
Monitoring of hematopoietic chimerism using XY-FISH and VNTR-PCR analysis showed nearly identical proportions of complete chimeric patients after HLA-identical sibling or alternative family donor PBSC transplantation during the first posttransplantation year. With the exception of three patients, in whom residual recipient-type signals were detectable by the more sensitive D1S80-PCR assay, all evaluable patients in both subsets had achieved a complete chimeric status at 12 months posttransplantation. Thus, this analysis showed no evidence that the development of chimerism is adversely influenced by transplants from MHC disparate donors. Other studies of chimerism after allogeneic PBSC transplantation with similar follow-up periods and patient numbers have not been published thus far. In a recent comparison between patients with advanced hematologic malignancies who received either PBSC or marrow cells from HLA-identical sibling donors, chimerism was analyzed by Y-FISH or D1S80-PCR up to day 98 posttransplantation.8 This study described complete chimerisms in both the PBSC and marrow transplant recipients except in cases of relapse. Our own preliminary prospective comparison between PBSC and marrow transplant recipients using VNTR-PCR analysis showed that complete chimerism develops even earlier and more frequently after PBSC transplantation.25 Although this finding still awaits further confirmation by more comprehensive studies and larger patient numbers, the available analyses on chimerism support that PBSCs are at least as effective as marrow cells to induce complete chimerism.
In summary, this study for the first time indicates that PBSC transplantation using alternative family donors is feasible and can be successfully performed without compromising patient survival compared with PBSC transplantation using HLA-identical sibling donors. Evaluations of engraftment, immune reconstitution, and hematopoietic chimerism in this study further suggest that PBSCs from alternative family and HLA-identical sibling donors are equally effective in restoring donor-type lymphohematopoiesis. Thus, PBSCs from alternative family donors represent an attractive source for allogeneic transplantation in patients lacking HLA-identical sibling donors and should be further evaluated in comparison with marrow transplants from alternative family donors.
ACKNOWLEDGMENT
The authors thank Martina Franke and Brigitta Siegel for their excellent technical performance of the flow-cytometric and fluorescence in-situ hybridization studies. We acknowledge the skillful technical performance of the PCR analyses by Jitka Stockova and Susanne Trzensky. The study contains analyses performed by Birgit Scheulen as a partial fulfillment to obtain the doctor of medicine degree of the Faculty of Medicine at the University of Essen.
Supported by grants from Deutsche Forschungsgemeinschaft (DFG) Gr 608/5-1, Deutsche Krebshilfe 70-1669-El I, ‘Aktion Kampf dem Krebs’ der Deutschen Krebsgesellschaft, and Amgen-Roche Inc.
Address reprint requests to Dietrich W. Beelen, MD, Department of Bone Marrow Transplantation, University Hospital of Essen, Hufelandstr. 55, 45122 Essen, Germany.