Abstract
To elucidate how pyridoxine-refractory X-linked sideroblastic anemia (XLSA) develops, we analyzed the erythroid-specific 5-aminolevulinate synthase (ALAS-E) gene of a patient with the anemia. The activity and amount of the enzyme in bone marrow cells of the patient were found to be approximately 5% of the normal control. We identified a point mutation, which introduces an amino acid substitution from Asp 190 to Val. In transient transfection analyses using quail fibroblasts, accumulation of aberrantly processed proteins, the sizes of which were larger than that of mature ALAS-E, was found in mitochondria. The proteins were reproducibly detected in assays combining in vitro transcription/translation of ALAS-E precursor and import of the precursor into isolated mouse mitochondria. These results suggest that the mutation causing pyridoxine-refractory XLSA affects the processing of the ALAS-E precursor, thus provoking instability of the ALAS-E protein.
SIDEROBLASTIC ANEMIA comprises a heterogeneous group of disorders,1,2 which encompass hereditary types, characterized by common features including large numbers of ring sideroblasts in bone marrow, hypochromic erythrocytes in peripheral blood, ineffective erythropoiesis, and increased tissue iron levels. Hereditary sideroblastic anemia can be either X chromosome-linked (XLSA) or autosomal. XLSA has been further subdivided into pyridoxine-responsive and pyridoxine-refractory types based on response to pyridoxine treatment.1
Reduced levels of 5-aminolevulinate (ALA) synthase [EC2.3.1.37] activity in the mitochondria of bone marrow cells are found in most congenital sideroblastic anemia patients.3-5 In the initial regulatory step of heme biosynthesis, which requires pyridoxal 5′-phosphate (PLP),6,7 ALA synthase catalyzes the condensation of succinyl-CoA and glycine to form ALA in mitochondrial matrices. Recent evidence shows that erythroid-specific (ALAS-E) and nonspecific (ALAS-N) isozymes of ALA synthase exist in vertebrates8-10; ALAS-E is restricted to erythroid cells,11 but ALAS-N is expressed in all tissues examined including bone marrow cells. Human genes encoding ALAS-E (ALAS2) and ALAS-N (ALAS1) have been mapped to chromosomal regions Xp11.21 and 3p21.1, respectively.12-15
The X chromosomal assignment of the ALAS2 gene suggested that mutations in this locus might cause XLSA. In 1992, Cotter et al16 identified a point mutation in the exon 9 region of the ALAS2 gene of a patient with suspected XLSA, where a lysine residue (K391) critical for PLP binding7 is also encoded, causing an amino acid substitution (I471 to N) that resulted in an enzyme with weaker activity. Because the patient recovered after pyridoxine administration, this amino acid substitution possibly affected the affinity of the enzyme for the cofactor. Shortly after, the first pyridoxine-responsive XLSA kindred was reported, having a point mutation in the ALAS2 gene at a position (T388 to S).17 Recently, several kindred with pyridoxine-responsive XLSA due to ALAS2 mutations were reported.18-20
However, no link between ALAS2 and pyridoxine-refractory type XLSA has been shown and available data is still rather limited. Although some ALAS2 mutations were reported in pyridoxine-refractory XLSA patients,21,22 characterization of the mutant proteins has not yet been described. In two other XLSA cases (one pyridoxine-refractory and the other partially pyridoxine-responsive), ALA synthase activity was increased in whole lysate from bone marrow cells,23 but no mention was made of whether these cases had mutation(s) in the ALAS2 gene.
To elucidate the mechanisms of pyridoxine-refractory XLSA, we analyzed the ALAS2 gene of a patient and found that a nucleotide change (A621 to T) was present, which introduced an amino acid substitution of Asp190 to Val (V190 mutation). This V190 mutation appears to affect processing of the ALAS-E precursor during or after import into mitochondria, decreasing its stability, and resulting in a reduction of ALAS-E protein and activity in the patient's bone marrow cells. These results show that reduction of the level of ALAS-E protein in bone marrow cells may be an important determinant of pyridoxine refractory XLSA.
MATERIALS AND METHODS
Isolation and characterization of human ALAS2 gene.An ALAS2 cDNA clone was isolated independently from a human bone marrow λgt10 cDNA library using a rat ALAS2 cDNA24 fragment as a probe. As we could not isolate a full-length cDNA clone in the initial screening, the remaining portion was cloned by polymerase chain reaction (PCR) using reverse-transcribed ALAS-E cDNA. A full-length human ALAS2 cDNA was then constructed using the cDNA clone and PCR amplified fragment. The full-length cDNA plasmid contains EcoRI sites in both 5′ and 3′ ends, and these sites were used for the construction of expression plasmids (see below). Authenticity of the PCR fragment was verified by sequence analysis. Genomic clones were obtained by screening λEMBL3 human leukocyte and TE1 (human esophageal cancer cell line25 ) cell libraries with human ALAS2 cDNA fragments. PCR amplification of high molecular weight DNA isolated from normal human peripheral leukocytes was performed to obtain genomic DNA fragments spanning exon 4, and two sets of primers were prepared to allow isolation of fragments covering introns 3 (hAEi3) and 4 (hAEi4) (Table 1).
Epstein-Barr virus (EBV)-transformed cell lines.Lymphocytes from the peripheral blood of proband, his mother, and sister were isolated and transformed with EBV. They were cultured and harvested as described previously.26
Sequence analysis of ALAS2 gene.DNA was prepared from cultured EBV-transformed lymphoblastoid cells with the genomic DNA purification system (TurboGen, Invitrogen, San Diego, CA) and used for amplification to obtain the ALAS2 exons and 250 bp of the promoter region with a slight modification of the method in Saiki et al.27 The PCR mixture (100 μL) contained 0.5 μg of genomic DNA, dNTPs (20 μmol each), 100 pmol primers (see Table 1), 1× Tth DNA polymerase buffer and 2.5 U Tth DNA polymerase (Toyobo, Osaka, Japan). Samples were denatured at 94°C for 5 minutes followed by 30 cycles of amplification in a DNA Thermal Cycler (Perkin Elmer Cetus, Foster City, CA) with denaturation at 94°C for 1 minute, annealing for 1 minute at 0°C to 5°C below the melting temperature of the primers, and extension at 72°C for 1 minute. PCR products were purified by QIAEX agarose gel extraction system (QIAGEN Inc, Chatsworth, CA) and directly sequenced with the Taq Dye-deoxy Terminator Cycle Sequencing system and DNA sequencer (model 373A; Perkin Elmer Cetus). PCR amplified genomic DNA fragments of the patient and his family were subcloned into a ddT-tailed pBluescript KS(-) cloning vector and sequenced by the dideoxynucleotide method28 using Sequenase version 2.0 (Amersham International, Buckinghamshire, UK).
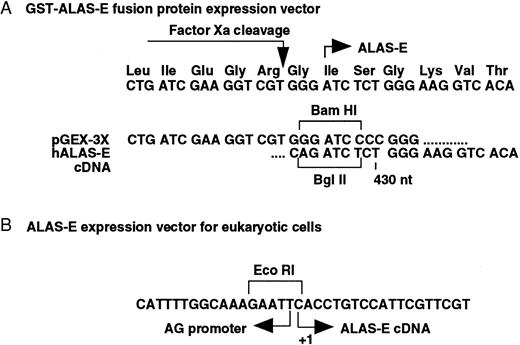
Expression of wild type and mutant ALAS-E as GST fusion proteins in E coli. An A621 to T mutation was introduced into human ALAS2 cDNA by site directed mutagenesis.29 Wild type and mutant cDNA fragments (amino acids 125 to 587 encoding the papain resistant core catalytic domain of ALAS-E30 ) were subcloned into the BamHI site of a pGEX-3X prokaryotic gene fusion vector (Pharmacia Biotech, Uppsala, Sweden), producing N-terminal GST fusions with Factor Xa-cleavable linkages, and confirmed by sequencing (Fig 1A). These GST fusion proteins were introduced into E coli BL21 pLysS (Novagen Inc, Madison, WI) as previously described31 and purified by a slight modification of the method described previously.18 Cells were harvested by centrifugation, resuspended in Buffer A (50 mmol/L HEPES [pH 7.5], 1 mmol/L EDTA, and 5 mmol/L dithiothreitol [DTT]) containing 0.1% Triton X100 and frozen at −80°C. Samples were thawed on ice, sonicated, and centrifuged, and the supernatant fractions applied to a Glutathione Sepharose 4B affinity column (Pharmacia Biotech). The fusion proteins were eluted with Buffer A containing 5 mmol/L glutathione, dialyzed, cleaved with Factor Xa (1 μg/50 μg protein), and reapplied to a Glutathione Sepharose 4B affinity column. The unbound proteins were used in the ALA synthase activity assays as described previously.32
Construction of human ALAS-E expression vectors. Junction sequences of both prokaryotic (A) and eukaryotic (B) ALAS-E expression vectors were determined by sequencing. (A) wild type and mutant ALAS-E cDNA fragments were subcloned into the BamHI site of a pGEX-3X prokaryotic gene fusion vector, producing N-terminal GST fusions with Factor Xa-cleavable linkages. The papain resistant core catalytic domain of ALAS-E30 (corresponding to amino acids 125 to 587) was used in this analysis. (B) Eukaryotic expression plasmids of human wild type and mutant ALAS-E were made by inserting full-length (1.9 kb) cDNAs into the EcoRI site of pCAGGS.37 +1 indicates the cap site of ALAS-E mRNA.
Construction of human ALAS-E expression vectors. Junction sequences of both prokaryotic (A) and eukaryotic (B) ALAS-E expression vectors were determined by sequencing. (A) wild type and mutant ALAS-E cDNA fragments were subcloned into the BamHI site of a pGEX-3X prokaryotic gene fusion vector, producing N-terminal GST fusions with Factor Xa-cleavable linkages. The papain resistant core catalytic domain of ALAS-E30 (corresponding to amino acids 125 to 587) was used in this analysis. (B) Eukaryotic expression plasmids of human wild type and mutant ALAS-E were made by inserting full-length (1.9 kb) cDNAs into the EcoRI site of pCAGGS.37 +1 indicates the cap site of ALAS-E mRNA.
Immunoblot analysis of ALAS-E.Bone marrow samples obtained from sideroblastic and iron deficiency anemia patients with their informed consent were used in ALA synthase activity assays.33 Nucleated cells and erythroblasts (EBLs) were counted. The cells were collected in the presence of heparin and washed twice with 1.15% KCl in 10 mmol/L Na phosphate buffer (pH 7.5). Red blood cells were disrupted by a 10-minute incubation on ice after addition of 10-fold volume of cold H2O. Samples were made isotonic with 11.5% KCl, centrifuged at 44,800g for 30 minutes, washed twice with 1.15% KCl, homogenized in 0.25 mol/L sucrose with a microscale Potter type homogenizer and stored at −80°C until use.
For immunoblot analysis, bone marrow samples containing 1 × 105 EBLs were homogenized in sodium dodecyl sulfate (SDS) sample buffer (2% SDS, 50 mmol/L Tris HCl [pH 6.8], 100 mmol/L DTT, and 10% glycerol), boiled for 3 minutes, and centrifuged at 12,000g for 1 minute. Supernatants were applied to a SDS/8% polyacrylamide-Laemmli gel,34 transferred to a polyvinylidene difluoride membrane, blocked overnight at 4°C with 5% skim milk in Tris-buffered saline (TBS), and incubated with a rabbit antirat ALAS-N antibody35 in TBS/0.05% Tween 20 followed by a secondary goat antirabbit IgG conjugated with horseradish peroxidase. Immune complexes were visualized by the enhanced chemiluminescence (ECL) method (Amersham International).
Positive controls were obtained by administering allylisopropylacetamide to rats to induce liver expression of ALAS-N. The liver cytosolic fraction containing a precursor form of rat ALAS-N was prepared. A mitochondrial fraction from K562H cells, a subline of a human leukemia cell line36 with elevated ALAS-E mRNA levels (unpublished observations, May 1997) and rat reticulocyte lysate were also prepared and used as size standards.
Transient expression of ALAS-E in fibroblasts.Eukaryotic expression plasmids of human wild type (pCAGG-AE) and mutant ALAS-E (pCAGG-mutTA and -mutAG; see below) were made by inserting full-length (1.9 kb) cDNAs into the EcoRI site of pCAGGS37 (Fig 1B). QT6 cells38 were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 20 μmol/L pyridoxine hydrochloride. Transfection was performed in 10 cm dishes by Ca2PO4 precipitation39 with 15 μg of pCAGG plasmid, along with 5 μg of pGL control plasmid (Promega Co, Madison, WI) for an internal control, for 12 hours. Cells were washed twice, fresh media added, and incubation continued for another 36 hours. Cells were harvested and mitochondrial fractions prepared as described previously.32 The mitochondrial fraction from each 10-cm petri dish was usually resuspended in 50 μL of Buffer B (0.25 mol/L sucrose, 50 mmol/L Tris HCl [pH 7.4], 0.5 mmol/L EDTA, 0.1 mmol/L DTT and 10 μg/mL of antipain, leupeptin, pepstatin and chymostatin). Suspension aliquots were added to SDS sample buffer and boiled for 3 minutes before loading on a SDS/8% polyacrylamide Laemmli gel system. Loading volumes were adjusted by luciferase activities.
For the porphyrin analysis, supernatants from separate transfections were first collected. Cells were then harvested and homogenized in 200 μL (per 10-cm culture dish) of Buffer B containing 0.2 mmol/L PLP. Supernatants and homogenates were examined under an ultraviolet (UV) lamp (365 nm).
In vitro transcription/translation of ALAS-E precursor and import into mitochondria.cDNAs encoding V190 mutant and wild type ALAS-E precursors were subcloned into pBluescript plasmids. RNAs were transcribed with the Riboprobe system (Promega) using T3 RNA polymerase and translated in vitro with a reticulocyte lysate system (Promega) containing [35S]-labeled methionine. The products were mixed with a mouse liver mitochondria fraction as described,40 and incubated for 60 minutes at 25°C. The mitochondrial fractions were separated from supernatants by centrifugation, dissolved in SDS sample buffer containing 1 mmol/L phenylmethylsulfonyl fluoride (PMSF ), and analyzed by SDS-polyacrylamide gel electrophoresis (PAGE). Signals were visualized with a Fuji BAS1500 Bioimage Analyzer and quantified with MacBAS software (Fuji Film, Tokyo, Japan).
RESULTS
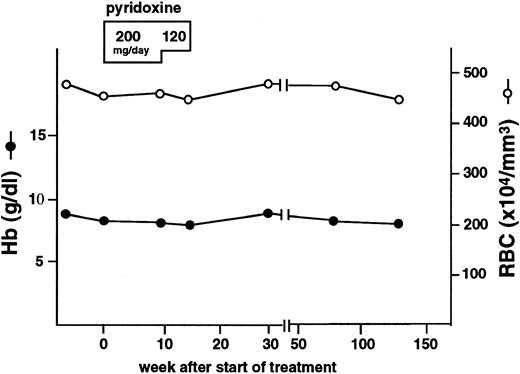
Pyridoxine-refractory XLSA patient.A Japanese male patient, indicated as proband, was admitted to the Research Institute for Radiation Biology and Medicine Hospital in Hiroshima University, at the age of 18, for anemia. Laboratory tests showed the presence of microcytic and hypochromic anemia (Table 2), indicated by normocellular bone marrow (31.0 × 104 cells/μL), erythroid hyperplasia (granulocyte/erythroid ratio is 0.6) and normal myelo- and megakaryopoiesis. Prussian blue staining showed that approximately 30% of bone marrow cells were ring sideroblasts. ALA synthase activity in bone marrow cells was 0.841 nmol ALA/108 EBL/30 minutes, which was markedly reduced as compared with normal control levels of 20.2 ± 7.59 (mean ± standard deviation [SD]) nmol ALA/108 EBL/30 minutes. The patient's mother was also found to have mild anemia with slightly increased serum iron concentration (Table 2) and increased red blood cell size distribution (red blood cell distribution width, 28.7%; normal, 10.5% to 15.3%). The patient was diagnosed with sideroblastic anemia, most likely XLSA, and received oral pyridoxine hydrochloride administration at daily doses of 200 mg for 10 weeks and 120 mg for an additional 4 weeks, but the therapy was ineffective (Fig 2 and Table 2). A markedly elevated concentration of serum ferritin was also found in this patient (Table 2), suggesting that this patient is severely iron overloaded.
Hematologic profile of the proband of pyridoxine refractory XLSA. Closed circles and open circles indicate hemoglobin concentration and numbers of red blood cells, respectively. Duration of the pyridoxine treatment and doses of pyridoxine administered are shown at the top of the figure. Note that the pyridoxine treatment did not affect the anemia.
Hematologic profile of the proband of pyridoxine refractory XLSA. Closed circles and open circles indicate hemoglobin concentration and numbers of red blood cells, respectively. Duration of the pyridoxine treatment and doses of pyridoxine administered are shown at the top of the figure. Note that the pyridoxine treatment did not affect the anemia.
Structure of human ALAS2 gene.Although the structure of the human ALAS2 gene has been reported, the sequence information provided was insufficient to design primers for genetic analysis of the human ALAS2 locus.41 We, therefore, obtained two lambda phage clones (λHEAL1a and λHEAL2a), which spanned the entire ALAS2 locus, with the exception of the region between intron 3 and exon 5. Regions covering intron 3, exon 4, and intron 4 were isolated by genomic PCR and three independent subclones for each fragment were sequenced. The sequence corresponding to exon 4 obtained from these genomic fragments was identical to that of the cDNA sequence42 (data not shown), thus confirming the authenticity of these clones.
The surrounding sequences of all exon-intron boundaries were determined and 14 sets of primers were designed to allow PCR amplification of all exons, as well as the promoter region (Table 1). These primers gave reproducible results and their products could be used for direct sequence analysis of various DNA samples. To amplify exons 9 and 10, nested PCR was used as indicated in Table 1.
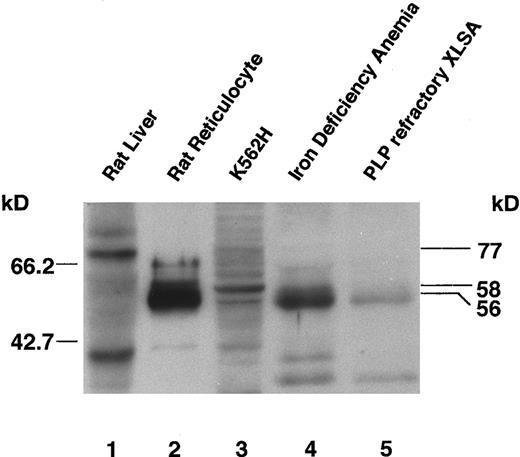
Decrease of ALAS-E in patient's bone marrow cell mitochondria.The amount of ALAS-E protein present in the patient's bone marrow cells was determined by immunoblot analysis using a rabbit antirat ALAS-N antibody. ALAS-E and ALAS-N are synthesized as a cytosolic precursor and subsequently transported into mitochondria, where proteolytic processing occurs to form the mature enzyme.43 The antibody used recognizes rat ALAS-N precursor, as well as ALAS-E mature protein (Fig 3, lanes 1 and 2) and cross-reacts with both GST-fused human ALAS-E (data not shown) and native human ALAS-E (see below).
Immunoblot analysis of ALAS-E protein in bone marrow cells of a pyridoxine-refractory XLSA patient. Expression of ALAS-E in rat reticulocytes and human bone marrow erythroid cells was analyzed with a rabbit antirat ALAS-N polyclonal antibody. Lanes 1 and 2 are loaded with a cytosolic fraction of rat liver and a whole homogenate of rat reticulocytes, respectively. A 77-kD precursor form of ALAS-N is detected in rat liver (lane 1, upper band), and the mature form of ALAS-E in rat reticulocytes (lane 2). Lane 3 contains a mitochondrial fraction of K562H cells and human ALAS-E can be detected (lane 3). Lanes 4 and 5 are loaded with a whole homogenate of bone marrow cells. Normal levels of ALAS-E are present in the bone marrow cells of an iron deficiency anemia patient (lane 4), but only about 5% of the level of normal controls are detectable in those of a pyridoxine-refractory XLSA patient (lane 5).
Immunoblot analysis of ALAS-E protein in bone marrow cells of a pyridoxine-refractory XLSA patient. Expression of ALAS-E in rat reticulocytes and human bone marrow erythroid cells was analyzed with a rabbit antirat ALAS-N polyclonal antibody. Lanes 1 and 2 are loaded with a cytosolic fraction of rat liver and a whole homogenate of rat reticulocytes, respectively. A 77-kD precursor form of ALAS-N is detected in rat liver (lane 1, upper band), and the mature form of ALAS-E in rat reticulocytes (lane 2). Lane 3 contains a mitochondrial fraction of K562H cells and human ALAS-E can be detected (lane 3). Lanes 4 and 5 are loaded with a whole homogenate of bone marrow cells. Normal levels of ALAS-E are present in the bone marrow cells of an iron deficiency anemia patient (lane 4), but only about 5% of the level of normal controls are detectable in those of a pyridoxine-refractory XLSA patient (lane 5).
Whereas the molecular mass of human ALAS-E precursor has been calculated to be 64.6 kD,42 that of the mature ALAS-E is not known, as the proteolytic cleavage site has not been experimentally determined. One report predicted the size of the signal sequence in human ALAS-E precursor to be 49 amino acids and the molecular mass of the mature protein as 59.5 kD,42 based on comparison with known cleavage sites for chicken and rat ALAS-N precursors. However, previous analysis of the mouse ALAS-E precursor44 suggests that in mouse (and human) ALAS-E, the leader sequence may be 78 amino acids in length, which would predict a molecular mass of approximately 56 kD.18 By SDS-PAGE, we have observed the sizes of rat ALAS-E precursor and mature forms to be 64 kD and 56 kD, respectively,30 supporting the likelihood of the human mature form ALAS-E to be also 56 kD.
In human bone marrow cells, we detected an ALAS-E band of approximately 56 kD (Fig 3, lanes 4 and 5), but the size appeared slightly smaller than the major ALAS-E band (58 kD) observed in the mitochondria fraction of K562H cells (compare lane 3 with lanes 4 and 5). However, both ALAS-E and ALAS-N are known to be easily degraded by papain and endogenous protease(s),30,35 40 and, therefore, the slight size discrepancy may be due to partial degradation during the preparation of sample. Despite all attempts to rapidly process the patient bone marrow aspirates, technical limitations in preserving the condition of clinical specimens still exist.
The intensity of the ALAS-E band from proband's bone marrow EBLs (lane 5) was approximately 5% of that observed from the same number of EBLs from a female iron deficiency anemia patient (lane 4), whose ALA synthase activity falls within a normal range (data not shown). The decreased intensity of proband's ALAS-E band appears to correlate well with the reduction seen in the enzyme activity (see above), indicating that the decrease in ALA synthase activity in the bone marrow cells may be due to the reduced amount of ALAS-E.
Sequence analysis of the proband's genomic DNA.The structure of the ALAS2 gene of proband, his mother, and sister was analyzed by PCR amplification of all the exons, using the primers described in Table 1, and sequence information was compiled. A single A to T transversion at nt 621 (nucleotide number corresponds to human ALAS2 mRNA sequence42 ), in exon 5 (Fig 4B), was the only difference between proband's sequence, that of reported cases16-20 45 and our normal controls (Fig 4A and also see below). This A621 to T transversion was found to be specific to one of the two alleles of the ALAS2 locus of the patient's mother (Fig 4C) and sister (not shown); they are heterogeneous for the A to T transversion, having both normal and mutated alleles at this locus (Fig 4C). Screening of nine unrelated Japanese ALAS2 genes (haploid) indicated that the A621 transversion is unique to this family. This mutation results in an amino acid substitution of Asp to Val at position 190 (V190 mutation).
A single A621 to T transversion is identified in the ALAS-E gene of the pyridoxine-refractory XLSA patient. Sequence analysis of DNA from peripheral lymphoid cells of proband, his mother, and sister showed a single A to T transversion at nt position 621 (compare [A] (normal control) with [B] (proband)). (C) shows sequence from his mother's ALAS-E genes. Note that the nt position 621 consists of compound wild type (A) and mutant nucleotides (T), indicating X chromosome-linked inheritance of this mutation.
A single A621 to T transversion is identified in the ALAS-E gene of the pyridoxine-refractory XLSA patient. Sequence analysis of DNA from peripheral lymphoid cells of proband, his mother, and sister showed a single A to T transversion at nt position 621 (compare [A] (normal control) with [B] (proband)). (C) shows sequence from his mother's ALAS-E genes. Note that the nt position 621 consists of compound wild type (A) and mutant nucleotides (T), indicating X chromosome-linked inheritance of this mutation.
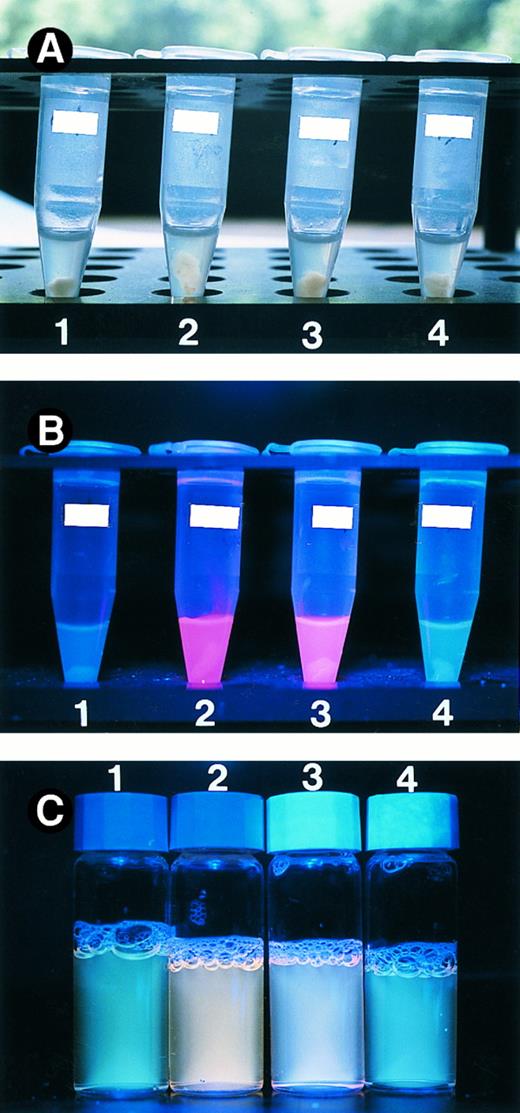
The V190 mutant can function in mitochondria. Wild type and mutant ALAS-E proteins were expressed in QT6 cells. Panel A shows cell homogenate under normal lamp. The porphyrin accumulation in the cells was measured by exposing the cell homogenate (B) and culture supernatant (C) to UV light. Tube 1 contains cells transfected with control plasmid. Cell homogenate and culture supernatant expressing wild type ALAS-E (tube 2) displays an intense red fluorescence under UV lamp, indicating the presence of porphyrins. Expression of the V190 mutant (tube 3) also gave rise to red fluorescence, albeit of weaker intensity than wild type, while cell homogenate and culture supernatant transfected with the V426 mutant plasmid (tube 4) showed only marginal red fluorescence.
The V190 mutant can function in mitochondria. Wild type and mutant ALAS-E proteins were expressed in QT6 cells. Panel A shows cell homogenate under normal lamp. The porphyrin accumulation in the cells was measured by exposing the cell homogenate (B) and culture supernatant (C) to UV light. Tube 1 contains cells transfected with control plasmid. Cell homogenate and culture supernatant expressing wild type ALAS-E (tube 2) displays an intense red fluorescence under UV lamp, indicating the presence of porphyrins. Expression of the V190 mutant (tube 3) also gave rise to red fluorescence, albeit of weaker intensity than wild type, while cell homogenate and culture supernatant transfected with the V426 mutant plasmid (tube 4) showed only marginal red fluorescence.
Activity of V190 ALAS-E expressed in E. coli is normal.To determine the effect of the V190 mutation on ALAS-E activity, the enzyme activities of both mutant and wild type proteins were examined by expressing the papain resistant core catalytic domains30 in E coli as fusion proteins with GST (GST-mut and GST-AE). These were purified by affinity columns and cleaved with Factor Xa, to obtain Xa-AE (wild-type) and Xa-mut (mutant). The specific activities of GST-AE determined in the presence or absence of excess PLP (200 μmol/L) were found to be 5,507 ± 100 and 3,817 ± 389 nmol/h/mg, and those of GST-mut were 5,426 ± 413 and 3,806 ± 23 nmol/h/mg, respectively. The activities of Xa-AE and Xa-mut were comparable and found to be twice the levels of their GST-fusion counterparts (data not shown). The significance of this finding is that the specific activities of the mutant and wild-type enzymes are similar in the presence of excess amounts of PLP and reduction of the PLP concentration affects both in a like manner, indicating that the V190 mutation is not critical for the enzyme activity of ALAS-E. This prompted us to look for other ways in which the mutation might influence the ALAS-E protein level and activity in the patient's bone marrow cells.
The V190 mutant of ALAS-E can be incorporated into and functions in mitochondria. To elucidate how the V190 mutation might cause the severe reduction in ALAS-E activity, we designed a method to examine the enzyme activity in eukaryotic cells. We expressed wild type and mutant ALAS-E proteins in a quail fibroblast cell line QT6, which lacks endogenous ALAS-E, under control of a strong CAG promoter and measured the presence of porphyrins by simply exposing cell homogenates to UV light of 365 nm wavelength. Because ALA synthase catalyzes the first and rate-limiting step of heme biosynthesis, overproduction of ALAS-E in fibroblasts should result in the accumulation of large amounts of porphyrins, the intermediate metabolic products of this pathway. Indeed, in the homogenates of QT6 fibroblasts expressing wild type ALAS-E, an intense red fluorescence could be detected under UV lamp illumination (Fig 5B, tube 2), showing that porphyrins and hence ALAS-E, are overproduced in the cells. This fluorescence was not seen in cells transfected with control pCAGGS plasmid (tube 1). Expression of the V190 mutant also gave rise to a clearly recognizable red fluorescence in QT6 cells (tube 3), albeit of a weaker intensity than that of the wild type enzyme. As succinyl-CoA, a substrate of ALAS-E, is available exclusively in the mitochondrial matrix,46 this result indicates that the V190 mutant of ALAS-E is incorporated into mitochondria and can catalyze the formation of ALA from succinyl-CoA and glycine. Similar fluorescence patterns were observed with culture supernatants (Fig 5C) and cell pellets (data not shown) on exposure to UV light.
We then made an expression plasmid containing a mutation of A1328 to G, which we recently identified to be the cause of a reported case of pyridoxine-responsive sideroblastic anemia47 and introduced an amino acid substitution of M426 to Val (V426 mutation) (unpublished observation, May 1997). Cells transfected with mutant plasmid showed only marginal red fluorescence (Fig 5B, tube 4 and Fig 5C, vial 4), demonstrating that the V190 mutant enzyme present in pyridoxine-refractory patient retains almost intact enzyme activity compared with the severe impairment observed with the V426 mutant enzyme found in the pyridoxine-responsive case.
Proteolytic cleavage of ALAS-E precursor is affected by the V190 mutation.We next examined whether normal processing of the ALAS-E precursor V190 mutant occurs after import into mitochondria by analyzing the sizes of exogenously expressed ALAS-E in QT6 cells by immunoblotting. In the mitochondrial fraction of the QT6 cells transfected with wild type ALAS2 cDNA, we observed two major bands (Fig 6); the larger corresponds to the human ALAS-E precursor (64 kD), and the smaller band (58 kD) (lane 2) likely corresponds to the mature form of ALAS-E (see Fig 3, lane 3). Because the CAG promoter is capable of driving strong expression of ALAS-E, the rate of ALAS-E synthesis may surpass the rate of its mitochondrial import, therefore, enabling the recovery of precursor protein, probably attached to mitochondria, in the mitochondrial fraction. A similar phenomenon can be observed when ALAS-N expression is induced strongly in rat liver by porphyrinogenic drugs (unpublished observation, May 1997). Neither band was detected in nontransfected QT6 cells (Fig 6, lane 1). In the V190 mutant analysis (Fig 6, lane 3), three bands were detected whose sizes were larger than that of the mature ALAS-E. One band corresponds to the ALAS-E precursor, displaying a size similar to that detected in cells transfected with wild type plasmid. The other two bands, estimated to be 59.5 and 59 kD, are larger than the expected size of the mature form of ALAS-E (58 kD, compare lanes 2 and 3). These bands were not detectable in the cytosol fraction of the V190 mutant transfected QT6 cells (data not shown) or in the mitochondrial fraction of erythroid K562H cells (see Fig 3, lane 3), indicating that the V190 mutation may affect the processing of the ALAS-E precursor during or after import into mitochondria.
Immunoblot analysis of ALAS-E transfected into QT6 fibroblasts. Extracts of wild type ALAS-E and V190 mutant ALAS-E expressed in QT6 were analyzed. Lane 1 represents nontransfected QT6 cells. Two major bands were seen in the mitochondrial fraction of cells transfected with wild type ALAS2 cDNA (lane 2). The larger band (64 kD) corresponds to the human ALAS-E precursor and the smaller band (58 kD) corresponds to the mature ALAS-E. In the V190 mutant fraction (lane 3), two bands are detected whose sizes are estimated to be 59.5 and 59 kD.
Immunoblot analysis of ALAS-E transfected into QT6 fibroblasts. Extracts of wild type ALAS-E and V190 mutant ALAS-E expressed in QT6 were analyzed. Lane 1 represents nontransfected QT6 cells. Two major bands were seen in the mitochondrial fraction of cells transfected with wild type ALAS2 cDNA (lane 2). The larger band (64 kD) corresponds to the human ALAS-E precursor and the smaller band (58 kD) corresponds to the mature ALAS-E. In the V190 mutant fraction (lane 3), two bands are detected whose sizes are estimated to be 59.5 and 59 kD.
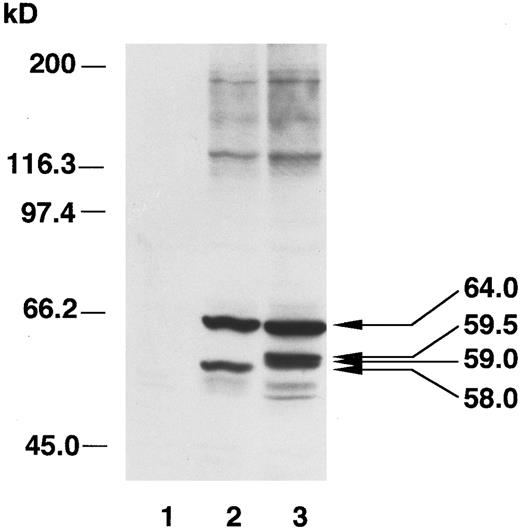
These results were further supported by analyses using an in vitro transcription/translation system to synthesize V190 mutant and wild type precursors and in vitro transfer of the precursors into isolated mitochondria. While mature ALAS-E of a normal size could be detected in the mitochondrial fractions mixed with wild type precursor (Fig 7, lane 5), in the fractions with the V190 mutant precursor, again the larger size band could be seen (Fig 7, lane 6). In the in vitro analysis, only one aberrantly sized band was detected, in contrast to the observation of two larger sized bands from the transfection analysis. This may be a reflection of the different experimental conditions (eg, in vivo ν in vitro, quail cells ν mouse mitochondria). The consistent result of these analyses is that a larger form of ALAS-E occurs during processing of the V190 mutant precursor, indicating that it may undergo aberrant proteolytic processing during or after transfer into mitochondria. We also noticed that the intensity of the processed band of the mutant ALAS-E (59.5 kD) is 42% of the precursor (64 kD band), while that of the normal mature ALAS-E protein (58 kD) is 87% of the precursor. Smearing of the mutant band is observed, suggesting the relative instability of the aberrantly processed protein in mitochondria as compared with the mature wild type protein.
In vitro transcription, translation, and transport of ALAS-E precursor into mitochondria. Wild type (lanes 2 and 5) and V190 mutant ALAS-E (lanes 3 and 6) precursors were synthesized in the presence of [35S]-labeled methionine. Lanes 1 and 4 are controls in which the RNA was omitted. The translation products were incubated with mouse liver mitochondria and then centrifuged to collect the mitochondria. Supernatant (lanes 1 to 3) and mitochondrial (lanes 4 to 6) fractions were analyzed by SDS-PAGE. Note that the sizes of the precursors are similar, but the processed mutant proteins are larger than the wild type.
In vitro transcription, translation, and transport of ALAS-E precursor into mitochondria. Wild type (lanes 2 and 5) and V190 mutant ALAS-E (lanes 3 and 6) precursors were synthesized in the presence of [35S]-labeled methionine. Lanes 1 and 4 are controls in which the RNA was omitted. The translation products were incubated with mouse liver mitochondria and then centrifuged to collect the mitochondria. Supernatant (lanes 1 to 3) and mitochondrial (lanes 4 to 6) fractions were analyzed by SDS-PAGE. Note that the sizes of the precursors are similar, but the processed mutant proteins are larger than the wild type.
DISCUSSION
Analysis of the ALAS2 locus of a pyridoxine-refractory XLSA patient showed an A621 to T transversion. This mutation showed X-linked inheritance and resulted in an Asp to Val change at position 190, where strong conservation of Asp (or Glu) residues is seen among ALAS-E proteins in various species.30 Despite marked decrease in both the activity and protein level of ALAS-E in the patient's bone marrow cells, this hydrophilic to hydrophobic, acidic to neutral substitution did not seem to significantly affect the enzyme activity in vitro. However, on overexpression of wild type and mutant ALAS-E proteins in QT6 fibroblasts, two proteins with sizes larger than that of the wild type were observed in the mitochondrial fraction of V190 mutant transfected cells. These bands were reproducibly detected in assays combining in vitro transcription/translation and import of the precursor. Therefore, it appears that the V190 mutation affects the proteolytic processing of ALAS-E precursor during or after import into mitochondria. This abnormal processing may result in proteins, which are unstable in bone marrow cells in vivo, causing a decreased level of ALAS-E protein and hence, reduced ALAS-E activity in mitochondria.
The processing of ALAS-E should be catalyzed by a mitochondrial processing peptidase that cleaves the signal sequences of various precursor proteins.48 Evidence exists, which suggests that secondary or tertiary structural features of the region surrounding the processing site may be important for the recognition and cleavage of signal sequences.49,50 In line with this, a mutation downstream of the cleavage site in ornithine aminotransferase has been reported to affect its processing.51 Additionally, Graf et al52 reported that incorrectly processed preornithine transcarbamylase, which is larger in size than the normal mature enzyme, has been shown to be catalytically active, but more sensitive to proteolytic degradation. Similarly, we could not detect the abnormally processed ALAS-E protein in the bone marrow cells of the patient. This finding suggests that the abnormally processed ALAS-E precursor has probably undergone extensive proteolysis after import into mitochondria, although we could not exclude the possibility completely that the mutant ALAS-E precursor may have degraded either before the import into mitochondria or after normally processed in mitochondria in human bone marrow cells. Further analyses of the mitochondrial proteolytic degradation pathway is required to elucidate the mechanisms by which the increased degradation of mutant ALAS-E protein occurs.
Molecular analyses of the ALAS2 gene in one pyridoxine-responsive type XLSA case and five kindred have been reported,16-20 and in four of the six cases, amino acid substitutions were found near the binding site of the PLP cofactor, in addition to impaired ALAS-E activity. In this study, we showed that the expression of a pyridoxine-responsive type ALAS-E mutant in QT6 fibroblasts resulted in low porphyrin production. In the remaining two cases, mutations were found in exon 5, resulting in amino acid substitutions at positions far from the PLP-binding lysine.18,20 However, response to pyridoxine treatment was seen in one case,20 implicating decreased thermolability of mutant ALAS-E at low or normal PLP concentrations as the cause of the low ALA synthase activity. These results suggest that pyridoxine-responsive XLSA is caused by mutations in ALAS2, which reduce the affinity for its coenzyme. The reduction in PLP-binding affinity results in impairment of activity and/or stability of the mutant enzyme.
The other XLSA case with an exon 5 mutation was a substitution of Phe to Leu at position 165.18 When bacterially expressed as a fusion protein, its specific activity was approximately 13% of that of normal enzyme in the absence of PLP, and increased to 26% on addition of PLP, suggesting that this may also be a pyridoxine-responsive case. Pyridoxine treatment of the patient, however, resulted in only modest improvement. Therefore, the mutation was believed to perturb the conformation of the ALAS-E protein, resulting in decreased stability and/or reduction in the affinity for PLP. This is consistent with our hypothesis that reduction of the ALAS-E in bone marrow cells plays an important role in the pathogenesis of pyridoxine-refractory sideroblastic anemia. We suspect that the increased susceptibility to proteases and the reduction of affinity to PLP causes reduction of mutant enzyme activity in vivo.
To facilitate the identification of mutations that affect ALAS-E activity, we developed a simple assay in QT6 cells to measure the effect of various ALAS-E proteins on the accumulation of porphyrins. Expression of the wild type ALAS-E resulted in marked accumulation of porphyrins, whereas the pyridoxine-responsive (V426) mutation resulted in only marginal porphyrin accumulation (data not shown). This difference can be easily observed by exposing the samples to UV irradiation. With the V190 mutation, however, the magnitude of difference in accumulation of porphyrins between mutant and wild type enzymes was less than expected, based on the bone marrow cell analysis and may be due to the use of the strong CAG promoter to drive expression in QT6 cells. The resulting high level of protein expression in QT6 cells may mask the difference in the degradation rate between wild type and V190 mutant enzymes. Indeed, the intensity of the aberrant mature protein was always weaker than that of the wild type protein in in vitro analyses (see Fig 7), suggesting that it is not so stable. Alternatively, a bone marrow-specific protein degradation pathway which selectively destroys mutant ALAS-E proteins (eg, a mitochondrial protease in bone marrow53 ) may exist, which is not present in QT6 cells.
Our analysis of the ALAS2 locus in a pyridoxine-refractory XLSA patient suggests that the reduction of ALAS-E plays an important role in the pathogenesis of the anemia. The results of this study, in conjunction with recent progress in this field, strongly suggest that reduction of ALAS-E activity in mitochondria causes both pyridoxine-responsive and -refractory XLSA. The confirmation for this conjecture must await the results of ALAS2 gene disruption, which are presently underway, to create an animal model for XLSA. In addition, further investigation into the regulation of ALAS2 gene expression, as well as its mitochondrial import mechanisms and maintenance of stability within mitochondria are essential to better understand the pathogenesis of XLSA and other related disorders.
ACKNOWLEDGMENT
We thank Drs Kazuyasu Endo, Kazuhiko Igarashi, Hajime Ishihara, Sigeo Kure, Jun-ichi Miyazaki, and Ruth Yu for assistance with this work.
Supported in part by grants from the Ministry of Education, Science and Culture (to H.F., N.H., and M.Y.), the Japanese Foundation for Promotion of Science (to M.Y.), and the Uehara Memorial Foundation (to M.Y.). K.Y. is a Research Fellow of the Japanese Society for the Promotion of Science.
Address reprint requests to Masayuki Yamamoto, MD, Institute of Basic Medical Science, University of Tsukuba, 1-1-1 Tennoudai, Tsukuba 305, Japan.

![Fig. 4. A single A621 to T transversion is identified in the ALAS-E gene of the pyridoxine-refractory XLSA patient. Sequence analysis of DNA from peripheral lymphoid cells of proband, his mother, and sister showed a single A to T transversion at nt position 621 (compare [A] (normal control) with [B] (proband)). (C) shows sequence from his mother's ALAS-E genes. Note that the nt position 621 consists of compound wild type (A) and mutant nucleotides (T), indicating X chromosome-linked inheritance of this mutation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.822/4/m_bl_0035f4.jpeg?Expires=1763497983&Signature=vWPdAtAcdrYnwq6JpbHj3fdb0snh6cEENfp2qmO6OPhiLyzBARKaP~EmkBTBWmzSIrdHPYs0X55~KRFPtY9cFCdn5NOFgrcwJlG6Ka3UpEo44nX~naxcg-7i3hDaLQPevoRO0Z7dQI5j~vbBj00Uu7b~BI~RBr-mg~BrSF0XKoZtWljKKiKl708u3EebX65pHtDINFSHgsb61DieZkAHkjipN6-KIe7IVOVcOMLTGl2Qy1ALZC5Kvaxz1ZMQvJ0EkBHf2mzV-OE83YpLNE60Fa2~5zqaKuXzKWrt5Pr0m2RqJ6D37hNs2v1elIozka5WG4C7xQAZO4k0yuAm2dj4jQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 7. In vitro transcription, translation, and transport of ALAS-E precursor into mitochondria. Wild type (lanes 2 and 5) and V190 mutant ALAS-E (lanes 3 and 6) precursors were synthesized in the presence of [35S]-labeled methionine. Lanes 1 and 4 are controls in which the RNA was omitted. The translation products were incubated with mouse liver mitochondria and then centrifuged to collect the mitochondria. Supernatant (lanes 1 to 3) and mitochondrial (lanes 4 to 6) fractions were analyzed by SDS-PAGE. Note that the sizes of the precursors are similar, but the processed mutant proteins are larger than the wild type.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/90/2/10.1182_blood.v90.2.822/4/m_bl_0035f7.jpeg?Expires=1763497983&Signature=PtIVuJs26ypTnlSk3FXZ0dvgZZmwJxWRJCeY4hTm7AdfroUXZvclpk2UqwNt-iwC~zydkjazsiqUJFvGnmwJg0vk4LdLAg50qnMz9KpKoptJnKIxz1a33ktryqqffejExnxPiVqzS4qDvWfEybGyenC~vwrpX3ENmrQ79c6Tlt5qDzYpkDjTyLkqYXysC~1aZGg7xHnHpV3FVUJfJGRNuluFLk2iwEv~i~EokxaoO37M8KYhIET1ufl9d1NbdP6OAJzbkWjbiyZ1BhAZHdQKUP2DmmfX4xJX8FpmAbcf72TmqO6dfjJZzHckIiejeQJM41FIHTyWNp3CKJ99d36HTQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
