Abstract
Peripheral blood progenitor cells (PBPCs) are increasingly being used to replace bone marrow cells (BMCs) as a source of hematopoietic stem cells also in the field of allogeneic transplantation. Whereas it is well known that PBPC grafts and BM differ significantly in progenitor cell content and lymphocyte dose, the clinical consequences of these differences with respect to engraftment, graft-versus-host disease (GVHD), and the graft-versus-leukemia (GVL) effect are more difficult to assess. We present a murine model that allows us to evaluate engraftment, GVHD, and GVL effect of allogeneic PBPC transplantation (PBPCT). Balb/c mice (H-2d) served as recipients. Donors were major histocompatibility complex-matched DBA/2 mice or syngeneic Balb/c mice, respectively. Experiments with increasing numbers of BMCs or Filgastrim-mobilized PBPCs showed that the number of progenitor cells in the graft was correlated with the probability to engraft, irrespective of the graft type. With identically high cell numbers transferred (1 × 109 nucleated cells/kg body weight [BW]), the mortality rates due to GVHD (25%) were about the same after allogeneic BM transplantation (BMT) and allogeneic PBPCT, although PBPC grafts contained four times more CD3+ T cells as compared with BM grafts (6.2 × 108v 1.4 × 108/kg BW). For investigation of GVL activity, Balb/c recipients were injected with syngeneic cells of the B-lymphocytic leukemia cell line A20 2 days before transplantation. After total body irradiation to a dose of 7.5 Gy, 1 × 109/kg BW Balb/c PBPCs, DBA BMCs, or DBA PBPCs were infused. The relapse rates observed were 80% after syngeneic PBPCT (n = 22), 60% after allogeneic BMT (n = 23), and 34% after allogeneic PBPCT (n = 26) (allogeneic BMT v PBPCT, P = .032). We conclude that transplantation of allogeneic PBPCs instead of BM may enhance the GVL effect without an increase of GVHD.
ALLOGENEIC bone marrow transplantation (BMT) is the treatment of choice for a variety of hematologic malignancies. Animal experiments performed as early as 19571 suggested that the strong antileukemic effect of BMT resulted not only from the high-dose chemoradiotherapy preceding the transplantation but also was a consequence of adoptive immunotherapy exerted by the graft itself. Numerous experimental and clinical reports2-8 have confirmed these findings and underscored our view that the superior antitumor effect of BMT is primarily due to the destruction of leukemic target cells by an allogeneic immune response mediated by T lymphocytes and natural killer (NK) cells contained in the transplant.
Recently, granulocyte colony-stimulating factor (G-CSF )–mobilized peripheral blood progenitor cells (PBPCs) have been introduced as an alternative source of hematopoietic stem cells for allogeneic transplantation.9-11 Harvesting PBPCs instead of BM avoids the discomfort of marrow harvesting and the risks of general anesthesia in the donor while the high numbers of progenitor cells contained in such a graft lead to rapid and reliable engraftment in the recipient. The large numbers of progenitor cells that can be harvested from the PB12,13 also allow for extensive ex vivo manipulations and make PBPC attractive as target cells for gene therapeutical maneuvers and immunotherapy.
Important questions related to PBPCT remain unanswered at this time. Whereas reports from single institutions14 and the European Group for Blood and Marrow Transplantation15 suggest that the transplantation of allogeneic PBPC does not cause devastating graft-versus-host disease (GVHD), a more precise comparison of the incidence and severity of GVHD after PBPCT as opposed to BMT must await the results of prospective clinical trials. There is even more uncertainty if the high numbers of T cells and NK cells contained in a typical PBPC collection product exert a more vigorous graft-versus-leukemia (GVL) effect, reduce the risk of relapse, and can thus improve disease-free survival after allogeneic PBPCT.
To address these latter questions, we developed a murine model that allows us to investigate the engraftment potential, graft-versus-host reactivity, and GVL activity of allogeneic PBPCs. Experiments presented here show a superior antileukemic activity of allogeneic, major histocompatibility complex (MHC)-matched PBPCs over BM cells (BMCs) in leukemia-bearing mice.
MATERIALS AND METHODS
Animals.Balb/c (H-2d) and DBA/2 (H-2d) mice were bred and kept at the animal facilities of our institution. All animals were housed in conventional cages, 7 to 10 animals to a cage, and received nonsterilized food and water ad libitum. Cotrimoxazole was added to the water for 40 days after BMT or PBPCT.
Hematopoietic stem cell transplantation.A Cs137 source was used for total body irradiation (TBI) of the recipients. A lethal dose of 7.5 Gy TBI was chosen for conditioning.16 BM grafts were prepared according to standard procedures as previously described.17 Mice intended to serve as donors of PBPCs had their spleens removed under general anesthesia at least 14 days before the donation of PBPCs. Starting 5 days before the collection of PBPC, 5 μg of rhu-met-G-CSF (Filgastrim; Amgen, Thousand Oaks, CA) was injected twice daily subcutaneously. The last injection of Filgastrim was administered 2 hours before harvesting of PBPC. The mice were then anticoagulated with heparin, anesthetized, and killed by cervical dislocation. The PB was collected under sterile conditions by dissection of both Aa carotidea. Erythrocytes were lysed by incubation of PB in 0.15 mol/L ammoniumchloride buffer at 20°C for 5 minutes. The cell number was adjusted to 6 × 107 nucleated cells (NC)/mL. For smaller grafts, a cell concentration of 1 × 107 NC/mL was used. BM or PBPC grafts were injected intravenously 2 hours after conditioning of the recipients by TBI.
T-cell depletion of PBPC grafts.Immunomagnetic depletion of T cells from the PBPC grafts was used in some experiments. Immunomagnetic beads (Dynal, Oslo, Norway), coated with goat-antimouse Ig, were coupled to an antimurine CD3 monoclonal antibody (MoAb; KT-3; Serotec, Wiesbaden, Germany). The CD3 MoAb was used at a concentration of 11 μg per 4 × 108 beads. Coupling of MoAb and magnetic beads was performed at 4°C for 30 minutes. The ratio of beads/target cell was 6:1. The magnetic beads were coincubated with the PBPC graft for 1 hour at 21°C, and the cells were then removed with a strong permanent-magnetic device (Dynal). The T-depleted grafts were examined for their content of residual T cells by fluorescence-activated cell sorting (FACS) analysis. The content of T cells after depletion was at the threshold of detection by FACS analysis (1% of nucleated cells; range, 0.2% to 2.3%).
Progenitor cell colony assays.For determining the progenitor cell content of the grafts, the Methocult GF M3434 kit was used (Stemcell Technologies, Vancouver, British Columbia, Canada). The assays were performed according to the protocol of the manufacturer. Colony-forming units–granulocyte-macrophage (CFU-GM) were counted 12 days after the plating of 1.5 × 104 nucleated cells per dish. The cell concentration was 1.5 × 105 NC/mL. Experiments were performed in parallel to the transplantation experiments with graded numbers of PBPCs and BMCs.
Leukemia cells.A20 is a B-cell leukemia/lymphoma of Balb/c origin that occurred spontaneously in a 15-month-old mouse.18 The cells were maintained in culture with RPMI 1640 + 5% fetal calf serum at 37°C and 5% CO2. We performed in vivo passages of these cells by intravenous injection into Balb/c animals. After the mice had developed hepatosplenomegaly, they were killed and their spleens were removed. Spleen cell suspensions containing close to 100% of these in vivo passaged leukemia/lymphoma cells were stored in liquid nitrogen and used for further experiments.
Experimental design and definitions.Engraftment and GVHD were investigated in leukemia-free mice of the Balb/c strain. Donors were either animals of Balb/c or of DBA/2 origin. Increasing numbers of BMCs or PBPCs were transferred to the recipients after TBI (7.5 Gy). The animals were examined at least once daily afterwards. Signs of GVHD (weight loss, rough fur, and gibbus) were documented. PB counts were performed every 3 days from day 7 to complete recovery of hematopoiesis or death. In a number of surviving animals, long-term chimerism was investigated by FACS analysis determining the presence/absence of the Lyt 1.1 antigen (CD5, clone H11 86.1; Pharmingen, San Diego, CA) on spleen cells. This antigen exists in two different forms: Balb/c mice express the isoform Lyt 1.2, whereas DBA/2 mice are known to express Lyt 1.1. Animals were killed on day 100 posttransplantation, their spleens were removed, and a single-cell suspension was produced. Untreated animals of Balb/c and DBA/2 origin were used as negative and positive controls, respectively. If the recipient mouse showed the same fraction of splenocytes (±6%) being positive for Lyt 1.1 as for the DBA control, it was named a long-term chimera. Investigation of the GVL effect was performed in Balb/c recipient animals injected intravenously with 1 × 105 A20 cells 2 days before transplantation of either 2 × 107 Balb/c PBPCs, DBA PBPCs, or DBA BMCs, respectively. The animals were examined daily and necropsied after death. The cause of death was determined according to the following definitions. Graft failure or graft rejection was defined as death between day 6 and 30 after transplantation with leukocytes less than 1/nL and granulocytes greater than 0.5/nL. Death due to GVHD was defined as death with recovery of hematopoiesis (leukocytes >3/nL and thrombocytes >50/nL) and macroscopic signs of GVHD such as weight loss, rough fur, hair loss, and gibbus. In some mice with macroscopic GVHD, histologic examination of skin and liver was performed that showed changes compatible with acute GVHD.19 20 Leukemic relapse was defined as death with macroscopic tumor and liver weight more than 1.5 g and spleen weight more than 0.15 g. Histologic examination of liver and spleen was performed for two animals in each group. Animals with hepatosplenomegaly were found to harbor leukemic cells without exception. Healthy mice of the same age have liver weights of 1.3 ± 0.2 g and spleen weights of 0.1 ± 0.02 g, respectively.
Statistics.Survival data and freedom from leukemia were calculated according to the method of Kaplan and Meier. The experimental groups were compared using the log-rank test. The calculations were performed on a PC with Statistica statistical software (StatSoft, Tulsa, OK).
RESULTS
Mobilization of PB progenitor cells and composition of the transplants.To avoid pooling of PBPCs in the spleen, mice were splenectomized before G-CSF administration. This led to an increase of white blood cells from 6.4/nL to 9.9/nL (range, 9.6 to 10.8 cells/nL) in DBA/2 mice. Differential counts were not altered after splenectomy. G-CSF administration induced a substantial increase of the leukocyte count up to 59.6/nL (range, 42.9 to 73.3/nL) in Balb/c mice or 72.9/nL (range, 51.8 to 85.8/nL) in DBA mice. In steady state, blood of DBA mice contained 0.80 CFU-GM/104 NC. After mobilization, the PBPC collection product contained 1.13 CFU-GM/104 NC, resulting in a substantially higher concentration of progenitor cells in PB (8.23 CFU-GM/μL after and 0.79 CFU-GM/μL blood before G-CSF administration). BM of DBA mice gave rise to 4.83 CFU-GM/104 NC. Balb/c mice were shown to have lower concentrations of progenitor cells in BM or PB, but the relationship between the PBPC graft and BM was identical (Table 1). A total of 2 × 107 NC/animal was the standard dose transferred to each animal in GVHD and GVL experiments. With the average weight of a Balb/c mouse being 20 g, this would correspond to a dose of 1 × 109 NC/kg body weight (BW) and 11 × 104 CFU-GM/kg BW administered with an allogeneic PBPC graft. The progenitor cell dose administered with the standard BMT procedure was fourfold higher. The percentage of lymphocytes was significantly higher in the PB as compared with the BM transplants. Allogeneic PBPC grafts contained 62% (range, 50% to 71%) of CD3+ cells; BM grafts contained 14% (range, 6% to 29.8%; Table 1). This corresponded to 6.2 × 108 T cells/kg BW for allogeneic PBPCT and 1.4 × 108 T cells/kg BW for allogeneic BMT, respectively, with the standard transplant.
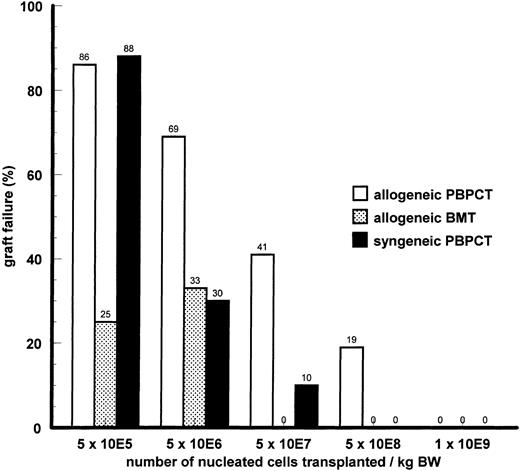
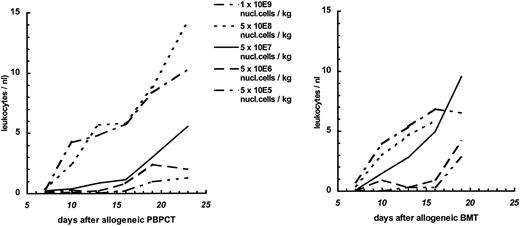
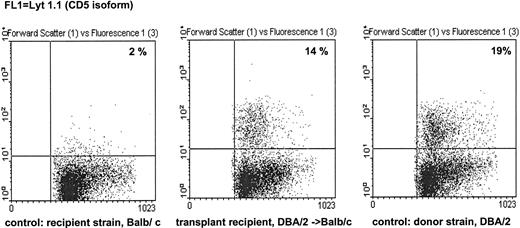
Engraftment.In experiments with nonleukemic animals, three parameters possibly determining engraftment were varied: the cell dose of the transplant, the immunogenetic difference between donor and recipient, and the source of hematopoietic stem cells. With PBPCs, the number of nucleated cells transferred had a major impact on engraftment as measured by the rate of graft failure (Fig 1) or the kinetics of hematopoietic recovery in the surviving animals (Fig 2). Transplantation of a higher cell dose was followed by a lower rejection rate and faster hematopoietic recovery after both syngeneic and allogeneic transplantation. Using the same fixed cell dose, the rate of graft failure was higher after allogeneic MHC-matched PBPCT than after syngeneic PBPCT. Complete engraftment was achieved with a minimum cell dose of 5 × 108 cells/kg BW after syngeneic PBPCT; the same cell dose caused a graft failure rate of 19% after allogeneic PBPCT. Thus, the immunogenetic difference was another parameter determining engraftment after PBPCT. On the basis of fixed numbers of nucleated cells transferred, allogeneic BMT was followed by lower rejection rates (Fig 1) than allogeneic PBPCT. The source of hematopoietic stem cells also influenced engraftment kinetics: a cell dose of 5 × 107 cells/kg BW gave a substantial slower engraftment after PBPCT (>2 leukocytes/nL on day 17) as compared with BMT (>2 leukocytes/nL on day 11). Long-term engraftment was investigated by immunofluorescence staining of recipient splenocytes with an anti-Lyt 1.1 antibody (CD5) and subsequent FACS analysis (Fig 3). Staining of the spleen cells of allo-PBPCT recipients 100 days after transplantation showed the majority of T lymphocytes to be of donor origin in all surviving recipients tested (n = 10). High cell numbers of either origin, PBPC and BM, uniformly resulted in complete and very fast engraftment (>2 leukocytes/nL on day 7, Fig 2).
Comparison of graft failure rates after syngeneic or allogeneic MHC-matched PBPCT. Recipients were Balb/c mice and donors were Balb/c or DBA/2 mice, respectively. Donors were pretreated by 2 × 5/μg Filgastrim subcutaneously for 5 consecutive days before stem cell harvest. Experiments were performed in a head-to-head manner with six repeated single experiments. The total number of animals in each group ranges from 8 to 27.
Comparison of graft failure rates after syngeneic or allogeneic MHC-matched PBPCT. Recipients were Balb/c mice and donors were Balb/c or DBA/2 mice, respectively. Donors were pretreated by 2 × 5/μg Filgastrim subcutaneously for 5 consecutive days before stem cell harvest. Experiments were performed in a head-to-head manner with six repeated single experiments. The total number of animals in each group ranges from 8 to 27.
Leukocyte recovery in recipients of allogeneic MHC-matched PBPC or BM graft. Data are from the same experiments as described in Fig 1.
Leukocyte recovery in recipients of allogeneic MHC-matched PBPC or BM graft. Data are from the same experiments as described in Fig 1.
Example for analysis of long-term chimerism. Spleen cells of surviving animals from the experiment described in Figs 1 and 2 were collected 100 days after transfer of 1 × 109 DBA PBPC/kg BW in Balb/c mice. The mononuclear cells were prepared by density gradient centrifugation and stained with MoAb anti-Lyt 1.1. Lyt 1.1 is an isoform of murine CD5 found in DBA but not in Balb/c mice. Untreated Balb/c and DBA mice served as negative and positive controls, respectively. The transplant recipients showed the same percentage of Lyt 1.1+ splenocytes as DBA mice, demonstrating long-term chimerism of T cells after transplantation.
Example for analysis of long-term chimerism. Spleen cells of surviving animals from the experiment described in Figs 1 and 2 were collected 100 days after transfer of 1 × 109 DBA PBPC/kg BW in Balb/c mice. The mononuclear cells were prepared by density gradient centrifugation and stained with MoAb anti-Lyt 1.1. Lyt 1.1 is an isoform of murine CD5 found in DBA but not in Balb/c mice. Untreated Balb/c and DBA mice served as negative and positive controls, respectively. The transplant recipients showed the same percentage of Lyt 1.1+ splenocytes as DBA mice, demonstrating long-term chimerism of T cells after transplantation.
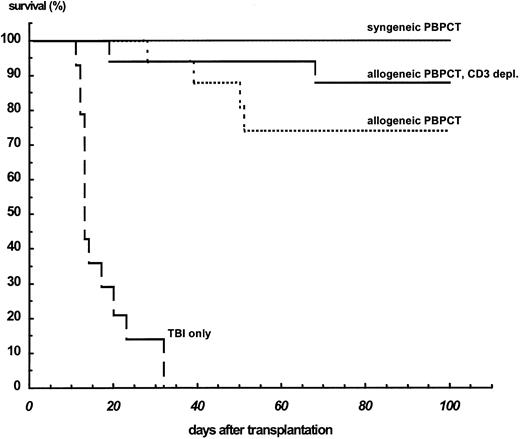
GVHD.The transplantation of 1 × 109 cells/kg BW allogeneic MHC-matched PBPCs resulted in stable engraftment, but 4 of 16 (25%) nonleukemic animals died with signs of GVHD. Histological examination showed GVHD-typical infiltration in the liver and skin in these animals. After allogeneic BMT with 1 × 109 cells/kg BW, 2 of 8 animals died on days 43 and 93 with signs of GVHD (Fig 4). This type of mortality was not observed after syngeneic PBPCT. After depletion of the CD3+ cells from allogeneic PBPC transplants, only 1 of 17 animals (6%) died with macroscopic signs of GVHD. One of the animals died during the period of TBI-induced aplasia.
Survival of nonleukemic Balb/c mice after TBI only (n = 12), transfer of 1 × 109 syngeneic PBPCs/kg BW (n = 10), and transfer of the same dose of unmanipulated allogeneic PBPC (n = 16) or CD3-depleted allogeneic PBPCs (n = 16). T-cell depletion was performed by immunomagnetic removal of these cells by anti-CD3–coated magnetic beads, resulting in less than 1% CD3+ cells as shown by flow cytometry.
Survival of nonleukemic Balb/c mice after TBI only (n = 12), transfer of 1 × 109 syngeneic PBPCs/kg BW (n = 10), and transfer of the same dose of unmanipulated allogeneic PBPC (n = 16) or CD3-depleted allogeneic PBPCs (n = 16). T-cell depletion was performed by immunomagnetic removal of these cells by anti-CD3–coated magnetic beads, resulting in less than 1% CD3+ cells as shown by flow cytometry.
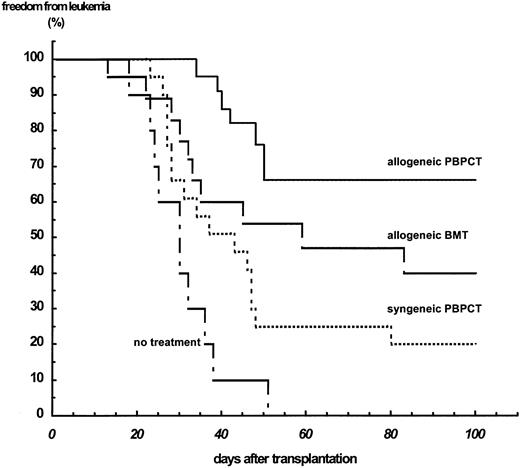
GVL effect.To evaluate the GVL effects after transplanting PBPCs or BM cells, the relapse risk of Balb/c mice bearing the lymphoid leukemia A20 and transplanted with PBPCs or BM cells was assessed. After intravenous injection of 1 × 105 A20 cells, all untreated animals died after a median of 28 days. TBI with a dose of 7.5 Gy and subsequent syngeneic PBPCT with 1 × 109 cells/kg BW 2 days after leukemia cell injection resulted in a prolongation of the time to relapse to a median of 43 days; 20% of the animals remained free from leukemia. Transfer of 1 × 109 cells/kg BW allogeneic MHC-matched PBPCs after identical pretreatment of the recipients caused a significantly lower relapse rate with freedom from leukemia at 66% (P = .0045). Freedom from leukemia is 40% (median time to relapse, 58 days) after allogeneic BMT and thus significantly lower than after allogeneic PBPCT (P = .032; Fig 5).
Freedom from leukemia of Balb/c mice injected with 1 × 105 cells of the B-lymphocytic leukemia cell line A20. Recipients received no further treatment (n = 10) or received TBI with a dose of 7.5 Gy followed by transplantation of hematopoietic stem cells 2 days after leukemia cell injection. The transplants were either syngeneic PBPCs (n = 22), allogeneic BM cells (n = 23), or allogeneic PBPCs (n = 26).
Freedom from leukemia of Balb/c mice injected with 1 × 105 cells of the B-lymphocytic leukemia cell line A20. Recipients received no further treatment (n = 10) or received TBI with a dose of 7.5 Gy followed by transplantation of hematopoietic stem cells 2 days after leukemia cell injection. The transplants were either syngeneic PBPCs (n = 22), allogeneic BM cells (n = 23), or allogeneic PBPCs (n = 26).
DISCUSSION
The animal data presented here address some of the important questions related to transplantation of allogeneic PBPCs. In particular, and in contrast to other murine21-23 and dog experiments,24 our model allows the investigation of the GVL activity of PBPCs as opposed to BM grafts. Results of animal experiments can seldom be directly translated into the clinic because of inherent differences between the experimental setting and the clinical situation. In our model, recipients (Balb/c) and donors (DBA/2) of hematopoietic stem cells share the MHC complex but differ with respect to the rest of their genetic background. This situation most closely resembles the transplantation of hematopoietic stem cells between HLA-identical donor/recipient pairs, more so in the matched unrelated than in the HLA-identical sibling setting. There are other limitations of the model pertaining to the composition of both marrow and PBPC grafts in mice and humans and their clinical implications. Although other parameters may be more important with respect to certain experimental or clinical endpoints, we chose to primarily characterize marrow and PBPC grafts by their content of nucleated cells and relate comparisons between BMT and PBPCT to fixed numbers of nucleated cells present in either type of graft. This is practical and matches the way how clinical BM and PBPC grafts have been characterized in many instances.10,12 13 We additionally determined the progenitor cell and lymphocyte content of the grafts to enable a comparison of these parameters and their possible relevance with respect to engraftment potential, graft-versus-host reactivity, and GVL effect observed after BMT or PBPCT.
We and others have previously shown that the progenitor cell dose in a graft is the decisive parameter to predict the engraftment potential of a hematopoietic stem cell graft if pretransplantation immunosuppression and the immunogenetic difference between donor and recipient remain unchanged.16,25,26 In this series of experiments we were able to confirme that the transfer of high numbers of marrow or PB cells improve engraftment and engraftment kinetics. However, when the engraftment potential of BM cells and PBPCs was compared with identical numbers of nucleated cells in the grafts, BM cells resulted in faster and more reliable engraftment, a situation contradictory to clinical experience.9 This seemingly surprising result can easily be explained if one keeps in mind that, in the clinic, we aim to transplant the highest possible number of cells and do not transfer fixed numbers of nucleated cells, CD34+ cells, or colony-forming cells. However, the average number of nucleated cells contained in a PBPC collection product is 3 to 4 times higher than in a typical BM graft13 and thus can more than compensate for the relative scarcity of colony-forming cells in PB.
Acute and chronic graft-versus-host disease are less well defined and may even have a partly different pathogenesis in mice27,28 as compared with humans29 or dogs.30 Therefore, sublethal forms of GVHD were not taken into account for this analysis. With these limitations in mind, we did not find substantial differences between BM or PBPC grafts with respect to their GVHD-inducing potential. Roughly the same rates of mortality due to GVHD (25%) were observed after allogeneic BMT and PBPCT. With approximately three times the number of T cells in a PBPC graft than in marrow, this is a surprising finding that is in line, however, with clinical observations reported so far.14,15 The model of the leukemia-bearing mouse allows to investigate the GVL effect conferred by allogeneic PBPCs as compared with allogeneic BMCs. This feature of the model is especially valuable because the heterogeneity of human recipients of allogeneic PBPCs in terms of their underlying disease and stage precludes any conclusive answer to the question if the relatively low relapse rates observed after PBPCT so far are the consequence of a more vigorous GVL effect. Reliable clinical data on the GVL effect after transplantation of unmanipulated or T-cell–depleted allogeneic PB stem cells will not be available for years because this will need long-term follow-up of a homogeneous patient population grafted with either PBPCs or BM. The results presented here suggest an advantage of PBPC over BM grafts with respect to their antileukemic activity. What are the possible explanations for this finding? First, there have been reports that priming with G-CSF can alter T-cell phenotype and function.31,32 It cannot be excluded that these differences not only affect graft-versus-host reactivity but also GVL activity. Second, it has repeatedly been reported that human PBPC grafts contain 10-fold more T cells and 19- to 25-fold more NK cells as compared with BM grafts.11-13 In this experimental system, even with a fixed number of nucleated cells administered, we also transferred more T cells and NK cells with PBPCs than with BM grafts. Both cell types have been shown to be involved in GVL activity.33-39 In the murine model presented here, the increase in GVL reactivity was not accompanied by a substantial increase of GVHD. One possible explanation for this finding could be that GVL and GVHD are stochastic processes. If the frequency of graft-versus-host–reactive cells in the donor is higher than the frequency of GVL-reactive cells, the relatively small lymphocyte dose transferred with a BM graft may already contain the near complete GVHD potential of the donor but may be too small to confer all of the GVL potential. An increase of the lymphocyte dose as found in a PBPC harvest will not add significantly more GVHD-reactive cells but could result in a substantial increase of GVL-reactive cells. Further experiments using the experimental model reported here are underway to further elucidate the relative roles of T cells and NK cells triggering graft-versus-host reactivity and GVL activity. These experiments will hopefully shed further light on the basic biology of graft-versus-host reactivity and GVL effects and give some indication how allogeneic PBPC grafts should best be manipulated to avoid GVHD but preserve their antileukemic potential. The experiments reported here have encouraged us to continue with the clinical evaluation of allogeneic PBPCT.
ACKNOWLEDGMENT
The authors thank S. Schlemminger for expert technical assistance.
Supported by a grant from Deutsche Krebshilfe (1-0958-Uh I).
Address reprint requests to Bertram Glass, MD, 2nd Department of Internal Medicine, Christian-Albrechts-University of Kiel, Chemnitzstrasse 33, D-24116 Kiel, Germany.