Abstract
A 42-year-old patient with mild hemophilia A developed spontaneous muscle hematomas 1 month after intense therapy with factor VIII concentrates. Factor VIII clotting activity was less than 1% and his factor VIII inhibitor was 10 Bethesda units (BU)/mL. The titer peaked at 128 BU despite daily infusions of factor VIII; 1 year later, the titer was 13 BU with no spontaneous bleeding for 4 months. The plasma inhibitor was 95% neutralized by factor VIII A2 domain but less than 15% neutralized by light-chain or C2 domain. His inhibitor did not cross-react with porcine factor VIII and was at least 10-fold less reactive to a series of hybrid factor VIII proteins in which human residues 484-508 are replaced by the homologous porcine sequence (Healey et al, J Biol Chem 270:14505, 1995). The inhibitor patient's DNA encoding his A2 domain and flanking sequences showed a C-T transition predicting Arg593 to Cys. Thirteen patients from 5 unrelated families with Cys593 have not developed inhibitors. Factor VIII clotting activity from one of them was inhibited similarly to diluted normal plasma by inhibitor patient plasma. In an homologous structure, ceruloplasmin (Zaitseva et al, J Biol Inorgan Chem 1:15,1996), the residue equivalent to Arg593, is in a loop distinct from residues 484-508. On solution phase immunoprecipitation with labeled factor VIII fragments, A2, light chain, and C2 domains bound. In contrast to typical immune responses to factor VIII in patients with severe hemophilia A, this patient's inhibitor was almost entirely reactive with common epitopes within the A2 domain whereas by more sensitive immunoprecipitation testing antibodies to light chain epitopes were also present. Accordingly, immune responsiveness to exogenous factor VIII (antigen burden) appears to be more critical than his endogenous, hemophilic factor VIII to his developing high-titer anti–factor VIII antibodies and loss of tolerance to both native and hemophilic factor VIII proteins.
HIGH-TITER ANTIBODIES that inhibit factor VIII clotting activity occur in about 20% of patients with severe hemophilia A,1 usually within the first few years of exposure to factor VIII concentrates. Patients developing these inhibitors most often have gross gene deletions, a common gene inversion, or specific premature termination codons and only rarely have had frameshift or missense mutations.2-4 Thus, lack of tolerance is an important predisposing factor. Even within families, not all patients with the same mutation develop inhibitors, suggesting that factors other than the mutation are also important. Inhibitors are rarely encountered in patients with moderately severe or mild hemophilia A; most are of low titer, <10 Bethesda units (BU)/mL and 14 of 23 occurred after a period of intense therapy.5 It is not known if the immune responses in milder patients (who circulate readily detectable quantities of at least partially active factor VIII) differ from those in severely affected patients, although patients with milder forms should circulate sufficient factor VIII protein to be at least partially tolerant. Where normal levels of factor VIII protein circulate, one explanation is that the mutation itself creates a neo-antigenic determinant. Alternatively, the extent to which the immune response shares common epitopes with those found in patients with complete gene deletions, for example, argues for a more generalized response.
Inhibitor antibodies bind to a limited number of epitopes within the A1-A2-B-A3-C1-C2 factor VIII domain sequence. Thrombin cleaves factor VIII at Arg372, Arg740, and Arg1689 producing a heterotrimer, A1/A2/A3-C1-C2, and the B domain. The A domains are homologous to ceruloplasmin and provide catalytic cofactor activity for the active site of factor IXa. The C2 domain contains a site that is essential for phospholipid binding. Distinct antibody populations are present in most inhibitor patients' plasmas and bind to epitopes within the A2 and C2 domains; some bind to epitopes within the A3-C1 region as well.6 Immunoprecipitation with purified factor VIII or factor VIII fragments offers a more sensitive method to detect antibodies that are either at too low a concentration to be detectable in neutralization assays or, less frequently, to detect binding antibodies that do not inhibit.7
A previously transfused, adult patient with mild hemophilia A presented with spontaneous bleeding and a high-titer factor VIII inhibitor after a period of intense therapy with factor VIII concentrates. Because his circulating level of functional factor VIII was previously about one third of the minimum for normal hemostasis, it can be assumed that he was at least partially tolerant to factor VIII and that characterization of his immune response could provide insight into the extent to which tolerance to a neo-antigen involving a putative missense mutation existed. The degree of restriction in the Ig epitope or epitopes involved and the relationship of these epitopes to his hemophilic mutation were examined. Data suggest that (1) his alloimmune responsiveness was triggered by severe trauma and/or a heavy allo-antigen load, and (2) his specific missense mutation was not detectably involved.
MATERIALS AND METHODS
Patient history.The patient is a 43-year-old Iranian-American man, diagnosed with mild hemophilia A 30 years ago after prolonged mouth bleeding from a bicycle accident. Whole blood was transfused at that time. He had muscle hematomas that required bed rest for 2 to 3 weeks. He received some factor VIII concentrate 26 years ago before tooth extraction and again 8 years later for a scalp laceration. He received cryoprecipitate for a tooth extraction 15 years ago and heat-treated factor VIII concentrate on two other occasions in the past 6 years. The patient's baseline factor VIII clotting activities in 1991, 1992, and 1993 were 8%, 10%, and 11%, consistent with his clinically mild bleeding disorder. He remains anti-human immunodeficiency virus (a-HIV)–negative. In August 1995 he sustained nasal and depressed frontal skull fractures after a bicycle accident, requiring prolonged therapy and surgical repairs. He received recombinant factor VIII twice daily for 4 weeks and daily (at home) for a fifth week. One month after stopping therapy, he developed spontaneous painful swelling of a forearm. His factor VIII clotting activity was less than 1%. His anti-human factor VIII inhibitor titer was 10 BU/mL; there was no inhibition when porcine factor VIII was substituted for human factor VIII in a Bethesda inhibitor assay. Samples for study were drawn with Human Subjects Institutional Review Board (IRB) approved consent. Among over 300 other mild to moderately severe hemophilia A patients followed by the Puget Sound Blood Center's Hemophilia Treatment Center for up to 25 years, none has developed a high-titer inhibitor.
Factor VIII assays.Factor VIII clotting activity was determined as described using a one-stage, kaolin-activated assay8 in a semi-automated system (CoaScreener; American Labour, Raleigh, NC). Inhibitor titers were determined after 2-hour incubations, 37°C, according to the Bethesda assay.9 Where indicated, individual plasma from a patient with mild hemophilia A with Cys593 and no detectable inhibitor against normal plasma was substituted for normal plasma.
An enzyme-linked immunosorbent assay (ELISA) for plasma factor VIII antigen levels was as described by Donath et al10 with minor modifications. Each of at least four dilutions were tested in duplicate. Factor VIII concentrations are expressed as percent of a normal curve (dilutions of a plasma pool from normal donors; numerically the same as units per deciliter). Monoclonal antibodies (MoAbs) against light-chain epitopes were provided kindly by Dr Jan A. Van Mourik (Central Laboratory of the Netherlands Red Cross Blood Transfusion Service, Amsterdam). Microtiter wells were coated with 150 μL of antibody CLB-CagA IgG (1 mg/mL diluted 1:800 in 0.1 mol/L NaHCO3 , pH 9.5), incubated overnight at 4°C, washed three times with 50 mmol/L Tris, 0.1 mol/L NaCl, 0.05%, Tween-20, pH 7.5 (TBST), and blocked with 5% nonfat milk in TBST (1 hour, 37°C). After three washes with TBST, 150 μL diluted standard or patient plasma samples diluted in blocking buffer were added to wells and incubated for 2 hours at 37°C. Plates were washed five times with TBST and incubated with peroxidase-conjugated antibody CLB-Cag 117 (1.16 mg/mL diluted 1:2,000 in blocking buffer) for 1.5 hour at 37°C. After five more washes with TBST, plates were stained with a 100-μL aliquot of a solution of one tablet 3-3′-5-5′-tetramethylbenzidine (Sigma T-3405, St Louis, MO) in 10 mL citrate/phosphate buffer (24 mL 0.1 mol/L citric acid, 26 mL 0.2 mol/L dibasic sodium phosphate, 56 mL ddH2O, pH 5) and, just before staining, 3 μL 30% H2O2 (Sigma H-1009) was added. After 10 minutes at 37°C, the reaction was stopped with 120μL 2N H2SO4. Absorbance was determined at 450 nm with a Bio-Rad model 450 plate reader (Bio-Rad, Hercules, CA) and dose responses were compared to the normal dilution curve by their Microplate Manager data analysis software (Bio-Rad).
Factor VIII fragments and inhibitor neutralization assays.Factor VIII was purified from human plasma; thrombin activation and light-chain purification were as described.11 12 For purification of A1 and A2 domains, 625 μg of plasma-derived factor VIII was activated for 10 minutes at room temperature with 2.4 U/mL human thrombin (Sigma). The reaction was terminated by adding D-Phe-L-Pro-L-Arg chloromethylketone (PPACK; Calbiochem, San Diego, CA) to a final concentration of 0.2 μmol/L. After twofold dilution into 30 mmol/L histidine-0.01% Tween 80 (pH 6.0), fragments were loaded onto a Resource S column (Pharmacia, Piscataway, NJ) equilibrated with 10 mmol/L histidine-0.1 mol/L NaCl-0.01% Tween 80 (pH 6.0) and washed extensively with equilibration buffer. With a linear NaCl gradient, A2 and A1/light-chain fractions eluted at 0.3 and 0.5 mol/L salt, respectively. A2 fractions were pooled, diluted threefold in 20 mmol/L HEPES-0.15 mol/L NaCl-0.01% Tween 80 (pH 7.2, HEPES buffer) and applied to a Resource Q column (Pharmacia) developed in HEPES with a linear NaCl gradient. A total of 45 μg of A2 fragment was recovered for a 47% overall yield. Fractions containing A1/light chain were pooled and dialyzed against 20 mmol/L HEPES-40 mmol/L EDTA (pH7.4) for 4 hours at room temperature. After threefold dilution into HEPES buffer with 10 mmol/L EDTA, the sample was applied to a Resource Q column equilibrated with the HEPES-EDTA buffer and eluted with a linear NaCl gradient. The A1 fraction eluted before the light chain and was subsequently passed through immunoaffinity columns of MoAbs with anti-C2 and anti-A2 domain specificity to remove trace contaminants of light chain and A2, respectively; 45 μg of A1 was recovered, representing a 53% overall yield. Protein concentrations were determined by A280 for light chain with an extinction coefficient of 1.3413 or by Quantigold assay (Diversified Biotech, Newton Centre, MA).
For neutralization assays, dilutions of inhibitor plasma and partially purified recombinant A214 or C215 or plasma-derived light-chain fragments were preincubated 2 hours at 37°C and then 1 more hour with an equal volume of normal plasma. Residual inhibitor activity was determined in the Bethesda assay. Results are expressed as percent inhibitor neutralization (1 − Inhibitor Titer With the Factor VIII Fragment ÷ Titer Without the Fragment × 100).
Labeling of factor VIII fragments and immunoprecipitation assay.Peptide fragments were dialyzed against 0.2 mol/L sodium acetate, 5 mmol/L Ca(NO3 )2 (pH 6.8), and 6 to 10 μg in 35 μL were radiolabeled with 5 μL lactoperoxidase beads (50% suspension; Worthington, Freehold, NJ), 5 μL Na125I (100 mCi/mL; Amersham, Arlington Heights, IL), 4 μL 0.03% H2O2 in the acetate-nitrate buffer. Three minutes after addition of H2O2 , peptide-bound label was recovered by gel filtration on a PD-10 column (Pharmacia) blocked with 0.1 mol/L 2-[N-Morphilino]ethane sulfonic acid (MES), 0.15 mol/L NaCl, 5 mmol/L CaCl2 , and 1% bovine serum albumin (fatty-acid free) (pH 6.3) and washed with 30 mL buffer without albumin before addition of 125I-polypeptide. Specific radioactivities ranged from 5 to 13 μCi/μg.
Immunoprecipitation assays were performed as described.6 Briefly, duplicates of 50 μL inhibitor plasma dilutions in 0.02 mol/L Tris, 0.15 mol/L NaCl, pH 7.4 (TBS), and 1% bovine serum albumin were incubated with 10 μL 125I-labeled A1, A2, light-chain, or C2 fragments (0.75 nmol/L, final concentration) for 15 to 24 hours at 4°C with agitation and 2 more hours after addition of 100 μL dilution buffer and 50 μL of a suspension of protein G-Sepharose beads (Pharmacia). After washing the beads three times with TBS-0.05% Tween 20 (Bio-Rad), bound radioactivity was determined in a gamma counter (LKB, Fredsforsstigen, Sweden). Background radioactivity without antibody was 1% to 2% and maximal binding with antibody was 60% to 70%. Results are expressed as immunoprecipitation units per milliliter, being calculated as: 1 − (bound/total radioactivity − background) × Plasma Dilution × 16.7 (the latter to convert results from units/60 μL to units/mL).
Porcine/human factor VIII hybrids.A series of six recombinant, hybrid factor VIII proteins were used. These were constructed by substituting the porcine factor VIII cDNA sequence for the A2 domain or portions thereof into the homologous portion of a B-domainless human factor VIII cDNA as described by Healey et al.16
DNA studies.Patient DNA samples and polymorphism analyses were as described.4 Sense and antisense primers for the 5′ end of exon 14 and the antisense primers for exons 9 and 12 and amplification were as described.16a The other primers were as follows: for exon 8 sense, 5′-TAGCAAGACACTCTGACATTGT-3′ and antisense, 5′-TGAATAACTGGTAAGAACTT-3′; for exon 9 sense, 5′-ATTTGAGCCTACCTAGAATT-3′; for exon 10 sense, 5′-TTCTTGTTGATCCTAGTCGT-3′ and antisense, 5′-GAGCTATAAACGAGGGAATA-3′; for exon 11 sense, 5′-ACTCTAATTGAGCTATTTAT-3′ and antisense, 5′-GGACATACACTGAGAATGAA-3′; for exon 12 sense, 5′-CTACCTGACAACATCAGTAG-3′; for exon 13 sense, 5′-GTATCATGACAATCACAATC-3′ and antisense, 5′-ATATAATAACTAACCTGGGT-3′. BstUI screening of exon 12 was by amplification mismatch, with sense primer, digest, and electrophoresis as described.17 Automated sequence determination was on an Applied Biosystems model 373 sequencer (Foster City, CA) and both directions of amplified fragments from exons 8 through the 5′ end of exon 14 (including the second base of codon 320 through codon 806, and intervening splice junctions) were examined using the amplification primers for sequencing.
RESULTS
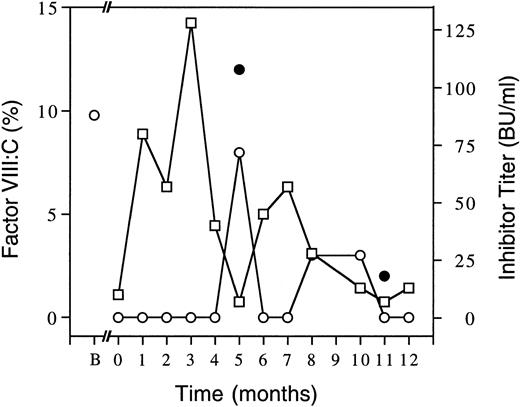
Course of the inhibitor in a patient with mild hemophilia A.Figure 1 shows factor VIII clotting activity and inhibitor levels from this patient's first episode of spontaneous bleeding, when it was less than 1%, for the ensuing year. Inhibitor level peaked at 2 months after detection. As an attempt to induce immune tolerance, he was treated with intermediate purity factor VIII concentrate infusions (50 U/kg) daily for the first 4 months and then twice daily for the next 8 months. He averaged two spontaneous muscle bleeds per month until he developed recurrent bouts of gross hematuria beginning 4 months from the inhibitor's onset. Hematuria recurred but responded to twice-daily infusions. Since 8 months from onset he has had no spontaneous hematomas, has resumed playing recreational soccer, and at 10 to 12 months after onset had detectable trough factor VIII:C levels at 12 (but not 24) hours postinfusion. Trials of intravenous DDAVP (3 μg per kg of desamino-D-arginyl-vasopressin, desmopressin) at 6 and 11 months from the onset of his inhibitor resulted in suboptimal responses (Fig 1) that were comparable to low 1-hour postinfusion recoveries of infused factor VIII concentrate (not shown). At 13 months the inhibitor titer was 5 BU/mL and his factor VIII clotting activity was 5% on a sample drawn 14 hours after 2,000 factor VIII units (30 U/kg) was infused and at 14 months the inhibitor titer was 3 BU/mL (not shown). From 15 through 18 months post onset, the inhibitor level was <1 BU/mL and 24-hour trough factor VIII levels were 3% despite decreasing the treatment to alternate days of 15 U of intermediate purity factor VIII per kilogram and, on odd days, two sprays of Stimate (a nasal DDAVP formulation).
Time course of factor VIII clotting activities and inhibitor levels. Factor VIII clotting activities (○) are shown as average baseline (B) or trough levels 24 hours after factor VIII concentrate (except for month 5 at 2 hours and month 8 at 12 hours postinfusion) or as clotting activities 1 hour post DDAVP (•, at 5 and 11 months). Inhibitor titers (□) are also indicated. Zero time is taken as first detection of the inhibitor with subsequent follow-up for 1 year shown. Factor VIII doses were 50 U/kg per day until month 4 when it was increased to twice daily then, after 6 weeks, reduced to 25 U/kg twice daily.
Time course of factor VIII clotting activities and inhibitor levels. Factor VIII clotting activities (○) are shown as average baseline (B) or trough levels 24 hours after factor VIII concentrate (except for month 5 at 2 hours and month 8 at 12 hours postinfusion) or as clotting activities 1 hour post DDAVP (•, at 5 and 11 months). Inhibitor titers (□) are also indicated. Zero time is taken as first detection of the inhibitor with subsequent follow-up for 1 year shown. Factor VIII doses were 50 U/kg per day until month 4 when it was increased to twice daily then, after 6 weeks, reduced to 25 U/kg twice daily.
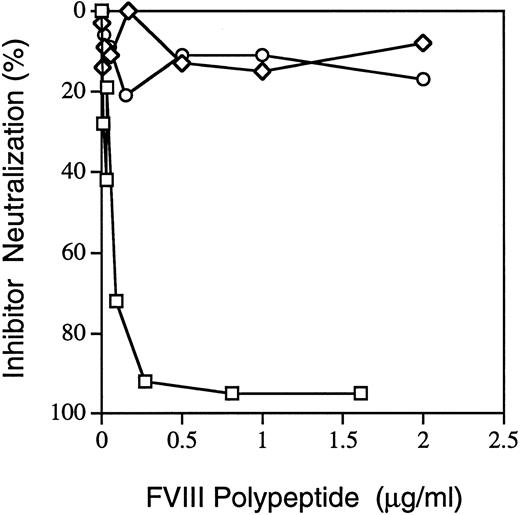
Inhibitor neutralization by factor VIII fragments.When the inhibitor patient's plasma (2-month sample) was preincubated with recombinant factor VIII fragments, A2 (residues 373-740) at 0.8 μg/mL (Fig 2) neutralized 95% of the patient's inhibitor titer against factor VIII in normal plasma. At 0.5 to 2 μg/mL, light chain or C2 only neutralized an average of 15% and 12%, respectively. The initial sample, time 0 in Fig 1, gave very similar results (data not shown). Thus, most of the inhibitory effect is caused by antibodies directed against epitopes within the A2 domain.
Neutralization of factor VIII inhibition by factor VIII peptides. Recombinant A2 (□) or C2 (⋄) or plasma-derived light-chain (○, A3-C1-C2) polypeptides were examined for their ability to neutralize inhibitor patient plasma's factor VIII inhibitor titer. Details are described in Materials and Methods; only the A2 domain neutralized the patient's inhibitor more than 15%. Results are from the sample drawn 2 months after the inhibitor was first detected; similar results were found for the initial sample (not shown). The A2 domain has a molecular weight of 43 kD; light chain is 73 to 80 kD; C2 is 24 kD. Thus, the relative molar amounts of A2 are about half of C2 and twice the light chain at any given concentration.
Neutralization of factor VIII inhibition by factor VIII peptides. Recombinant A2 (□) or C2 (⋄) or plasma-derived light-chain (○, A3-C1-C2) polypeptides were examined for their ability to neutralize inhibitor patient plasma's factor VIII inhibitor titer. Details are described in Materials and Methods; only the A2 domain neutralized the patient's inhibitor more than 15%. Results are from the sample drawn 2 months after the inhibitor was first detected; similar results were found for the initial sample (not shown). The A2 domain has a molecular weight of 43 kD; light chain is 73 to 80 kD; C2 is 24 kD. Thus, the relative molar amounts of A2 are about half of C2 and twice the light chain at any given concentration.
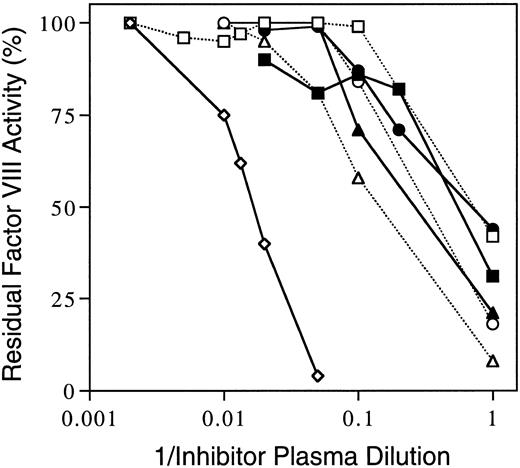
Inhibition of human-porcine hybrid factor VIII proteins.Aliquots of the inhibitor patient's plasmas from the 3- and 7-month inhibitor peaks were incubated with equal volumes of factor VIII–deficient plasma reconstituted with recombinant human B–domainless factor VIII with or without substitutions of sequences derived from the porcine cDNA that code for homologous A2 domain residues. Figure 3 presents inhibition curves for the 3-month sample. The titer against the all human sequence was 90 BU/mL whereas the series of six hybrid proteins ranged from 2 to 8 BU/mL. Thus, the inhibitor was at least an order of magnitude less reactive to the hybrid proteins as opposed to the all human protein construct. A comparable pattern was seen when the 7-month inhibitor plasma dilution curves were determined against the same series of recombinant proteins (not shown).
Inhibition of human/porcine hybrid factor VIII proteins by inhibitory patient's plasma. Inhibition of factor VIII clotting activities of recombinant human factor VIII des-Ser741-Arg1648 (⋄)15 was compared to inhibition of six hybrid proteins with substitutions of porcine sequence in the A2 domain using dilutions of inhibitor patient plasma from the 3-month (peak titer) sample. The homologous substitutions were of human residues 387-740 (▪), 387-604 (□), 387-508 (•), 484-508 (○), 489-508 (▴), and 484-488 (▵) as described by Healey et al.16
Inhibition of human/porcine hybrid factor VIII proteins by inhibitory patient's plasma. Inhibition of factor VIII clotting activities of recombinant human factor VIII des-Ser741-Arg1648 (⋄)15 was compared to inhibition of six hybrid proteins with substitutions of porcine sequence in the A2 domain using dilutions of inhibitor patient plasma from the 3-month (peak titer) sample. The homologous substitutions were of human residues 387-740 (▪), 387-604 (□), 387-508 (•), 484-508 (○), 489-508 (▴), and 484-488 (▵) as described by Healey et al.16
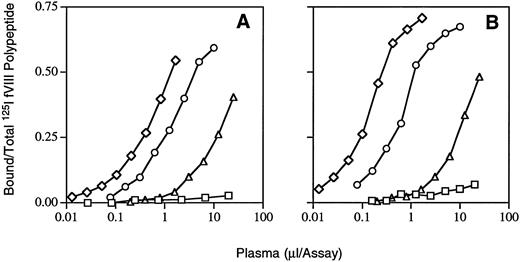
Immunoprecipitation with purified factor VIII fragments.Dose-response curves for binding of inhibitor plasma antibodies to 125I-labeled peptide fragments are shown in Fig 4. From the linear portion of the curve, immunoprecipitation units (IPU) per milliliter were calculated as described in Materials and Methods for the initial and 2-month plasma inhibitor samples. Average anti-A1 binding was less than 10 IPU for both, anti-A2 was 938 and 2,748, anti–light chain was 308 and 934, and anti-C2 28 and 30, respectively. Thus, the antibody binding titers for both anti-A2 and anti–light chain increased threefold during a period that the inhibitor titer (Fig 1) also rose. Similarities in the slopes of the binding curves of the two samples (Fig 4) suggest that the increased antibody binding titers were more likely caused by higher concentrations of the same or similar antibodies as opposed to changes in affinity.
Immunoprecipitation of inhibitor patient antibodies bound to factor VIII peptides. Increasing volumes of inhibitor patient plasma were added to and incubated with 0.75 nmol/L 125I-factor VIII fragments in solution phase and labeled fragments bound to antibodies were precipitated by binding to protein G-Sepharose beads (see Materials and Methods). (A) and (B) are results from the inhibitor patient anti–factor VIII plasmas from initial (0) and 2-month samples, respectively, after the inhibitor was first detected (see Fig 1). Polypeptides were plasma-derived A1 (□), A2 (⋄) and light chain (○), and recombinant C2 (▵).
Immunoprecipitation of inhibitor patient antibodies bound to factor VIII peptides. Increasing volumes of inhibitor patient plasma were added to and incubated with 0.75 nmol/L 125I-factor VIII fragments in solution phase and labeled fragments bound to antibodies were precipitated by binding to protein G-Sepharose beads (see Materials and Methods). (A) and (B) are results from the inhibitor patient anti–factor VIII plasmas from initial (0) and 2-month samples, respectively, after the inhibitor was first detected (see Fig 1). Polypeptides were plasma-derived A1 (□), A2 (⋄) and light chain (○), and recombinant C2 (▵).
Mutation detection and screening.Sequencing amplified fragments from the inhibitor patient's factor VIII gene, including exons 8 through the 5′ sequence of exon 14, showed a single C to T transition in the first base of codon 593 in exon 12 (numbering according to Gitschier et al18 ) predicting an Arg (CGC ) to Cys (TGC) substitution. To confirm this mutation on separate amplified products, amplification mismatch was used to introduce a new BstUI restriction site when the normal codon 593 sequence is present. The patient's fragment failed to cleave, as previously reported for one other patient followed locally.17 In screening DNAs from 70 other unrelated mild hemophilia A patient families, 4 others lacked the mismatch amplified BstUI site in exon 12. A C to T transition predicting Cys593 was confirmed by DNA sequencing in each.
Comparisons with other patients with factor VIII-Cys593.Table 1 summarizes factor VIII results and presents haplotypes among all six families (A through F ) with Cys593 that have been identified in the Seattle series. Affected members all had clinically mild hemophilia A with low-normal factor VIII antigen levels. In family D, de novo occurrence was documented and the others are associated with at least three distinct haplotypes indicating recurrent mutation events; patients E and F share a common haplotype but have different ethnic backgrounds. None of the 13 affected members from the five other families has shown any evidence of an inhibitor, either clinically or in factor VIII clotting activity assays, although none has received as intense or as prolonged a course of factor VIII concentrate therapy as did inhibitor patient F.
One possibility for the reduced level of factor VIII clotting activity in patients with the mutant Cys593 factor VIII protein is that the unpaired sulfhydryl group forms a disulfide bond with that of another polypeptide. To examine this possibility, 5 mmol/L cysteamine (a mild reducing reagent) was incubated for 10 minutes with normal and patients B2 or B3 (Table 1) plasmas and the factor VIII clotting activity determined. Clotting activities of each sample (including normal and both patient plasmas) were 10% to 20% lower than those before incubation with cysteamine, excluding an enhancing effect by reducing weak disulfide bonds.
Dilutions of inhibitor patient F's plasmas were incubated with plasma from one noninhibitor patient (B3, Table 1) with a Cys593 mutation. The inhibitor titer was compared to that obtained with a fourfold diluted normal plasma sample with a similar factor VIII clotting activity. The inhibitor plasma sample from patient F from 1 month after detection gave titers of 70 and 95 BU/mL when incubated with normal and patient B3 plasmas, respectively; the 6-month sample showed 60 BU/mL compared with 90 BU/mL, respectively. These results indicate that the inhibitor titer and/or avidity of the inhibitor patient's neutralizing antibody was similarly reactive with native, Arg593, as with mutant, Cys593, proteins.
DISCUSSION
One month after 5 weeks of daily therapy with factor VIII concentrates, spontaneous bleeding and a high-titer (peak of 128 BU/mL) inhibitor developed in a patient with previously mild hemophilia A (patient F, Table 1). Until then, factor VIII concentrates had effectively supported traumatic and surgical hemostasis, indicating tolerance for both transfused and his own hemophilic factor VIII proteins. Over the next 18 months, he infused factor VIII daily and the inhibitor titer increased and decreased twice, eventually becoming quite low (<1 BU/mL).
From inhibitor neutralization assays with factor VIII polypeptides, the patient's predominant inhibitor (95%) was restricted to an epitope or epitopes within the A2 domain. There was no detectable inhibition of porcine factor VIII by patient F's plasma. Hybrid porcine–human factor VIII proteins in which A2 domain sequences including residues 484 through 508 were porcine were examined. As in a severe hemophilia A inhibitor patient and three others with autoantibodies to factor VIII,16 substitution of porcine sequences within this 25-residue segment in human factor VIII was sufficient to reduce the patient F inhibitor titer over 10-fold. Thus, over 90% of the inhibitor is restricted to this sequence in the A2 domain. In contrast, typical inhibitors in severely affected hemophilia A patients usually have epitope specificity within the C2 domain with or without similar A2 domain specificity.6
Patient F's DNA encoding the A2 domain and flanking regions were then sequenced and a single C to T transition was identified. This predicts an Arg593 to Cys mutation, one previously described in five other families with mild hemophilia A,2 one of which was from Seattle.17 Using amplification mismatch and BstUI digestion,17 four additional families with mild hemophilia A were identified, and the same mutation was confirmed by sequencing. Arg593 to Cys is a recurrent CpG dinucleotide transition2; haplotype differences among the six families in Table 1 rule against a founder effect. In contrast to patient F, none of 13 other patients with Cys593 (Table 1) has developed a clinically significant inhibitor. In seven previous reports of patients with nonsevere hemophilia A and inhibitors where a mutation was described (Table 2) one had a Cys593 mutation,19 although characteristics of his immune response were not presented.
The lack of detectable clotting activity (initially and in subsequent 24-hour postinfusion samples, Table 1) and the suboptimal responses to either infused factor VIII concentrates or DDAVP indicate that patient F's inhibitor crossreacts with both exogenous Arg593 and endogenous Cys593 factor VIII proteins. Furthermore, his plasma inhibitor inhibited factor VIII clotting activity from another patient with Cys593 comparably to inhibition of a similar concentration of normal plasma factor VIII. This shows that his mutation is distinct from a major inhibitor epitope. Molecular modeling was used to explore the approximate distance between residue 593 and the residue 484-508 epitope, as found in some inhibitors to factor VIII in severe (Crm negative) hemophilia A or in autoantibody responses.16
Molecular modeling of factor VIII protein structure is based on homology between the amino acid sequences of the A domains of human factor VIII and human ceruloplasmin, suggesting a common ancestor.25 Furthermore, the location of disulfide bonds and free sulfhydryl groups in factor VIII26 is identical to their occurrence in ceruloplasmin. The coordinates for the three-dimensional structure of ceruloplasmin27 provide an approximation of the structure of factor VIII's A domains. In addition, crystallographic structures of more distantly related proteins, ascorbate oxidase and nitrate reductase, allow identification of conserved structural regions in this protein family, namely the pairs of β-barrel cylindrical structures within each A domain.28 These conserved regions are probably similar in factor VIII. Within the A domains, ceruloplasmin is more homologous with factor VIII than the oxidase or reductase proteins allowing more accurate modeling of factor VIII than previously attempted.29
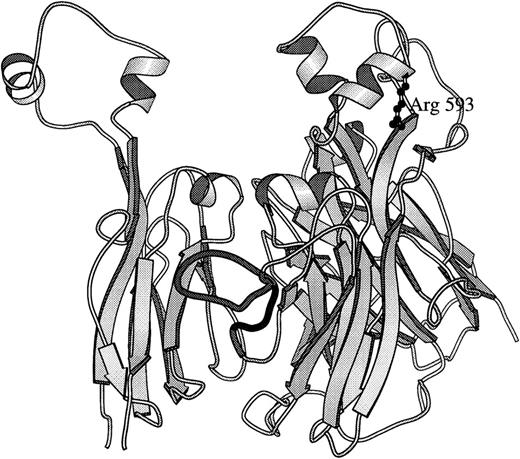
The structure of ceruloplasmin27 was modified to create a simple model to localize the ceruloplasmin homologue to Arg593 and to residues 484-508 (including a major inhibitor epitope sequence) in factor VIII using program “O.”30 As viewed in Fig 5, Arg593 is located within a loop structure at the top of the second A2 domain β-barrel. This loop is highly conserved within the three A domains of ceruloplasmin and factor VIII, with the equivalent one in A2 being the same length. This supports an assumption that the position of Arg593 and folding of this loop is similar to that in ceruloplasmin. There is less homology within the side loop containing the inhibitor epitope, with ceruloplasmin having a longer loop and one that may be partially cleaved in the crystals, limiting its availability for comparison. However, in the A1 domain the comparable ceruloplasmin side loop structure is 21 amino acids long compared to 20 each in the A1 and A2 domains of factor VIII (residues 107-116 and 482-501, respectively). Therefore, the ceruloplasmin A1 domain side loop was substituted in the model for that protein's A2 domain side loop to approximate the folding in factor VIII (Fig 5). Folding of the side loop is constrained by the β-barrel structures and from the model, the side chain of Arg593 is at least 28Å removed. This distance is greater than that observed for an epitope within a specific immunoglobulin-antigen complex.32
Molecular model based on ceruloplasmin structure. A secondary structure drawing, using MOLSCRIPT,31 of relative positions of homologous factor VIII residues in the human ceruloplasmin structure27 is presented. The two β-barrel structures that compose the A2 domain (residues 380 through 711 in factor VIII) are viewed in a vertical presentation (perpendicular to the main axes of the barrel structures) on the right. Arg593 is drawn in ball and stick form extending down from a helical portion of the top loop of the second β-barrel structure of the A2 domain. The second β-barrel of the A1 domain (residues 183-329) is on the left to show the packing of the A2 domain's epitope side loop (residues 484-508)16 that is predominantly between the A1 and A2 domains as shown in the darkly shaded ribbon (residues 482-501). In ceruloplasmin, corresponding side loops from A1 and A3 domains pack between the β-barrels and may stabilize A domain interactions. Broad arrows indicate the orientation of β-pleated sheets; spiral ribbons, α-helices; narrow ribbon cords, turns and coil structures.
Molecular model based on ceruloplasmin structure. A secondary structure drawing, using MOLSCRIPT,31 of relative positions of homologous factor VIII residues in the human ceruloplasmin structure27 is presented. The two β-barrel structures that compose the A2 domain (residues 380 through 711 in factor VIII) are viewed in a vertical presentation (perpendicular to the main axes of the barrel structures) on the right. Arg593 is drawn in ball and stick form extending down from a helical portion of the top loop of the second β-barrel structure of the A2 domain. The second β-barrel of the A1 domain (residues 183-329) is on the left to show the packing of the A2 domain's epitope side loop (residues 484-508)16 that is predominantly between the A1 and A2 domains as shown in the darkly shaded ribbon (residues 482-501). In ceruloplasmin, corresponding side loops from A1 and A3 domains pack between the β-barrels and may stabilize A domain interactions. Broad arrows indicate the orientation of β-pleated sheets; spiral ribbons, α-helices; narrow ribbon cords, turns and coil structures.
Because the top loops of each of the pairs of β-barrels have close interactions, it is possible that Cys593 perturbs this interaction, allowing the epitope side loop to be more exposed. It is also possible that Arg593 in factor VIII is actually closer to the 482-501 loop in factor VIII than the modeled loop structures indicate. Alternatively, the substitution of Cys for Arg may allow a direct side-chain interaction with a disulfide-bonded peptide. However, in contrast to plasma from a patient with Arg1689 to Cys mutation,33 cysteamine failed to increase activity, excluding a peptide bound by a weak disulfide bond. Another possibility is that a T-cell epitope was involved. However, none of these explanations account for his developing binding antibodies to light-chain epitopes.
Binding antibodies directed against the A2 domain and at least two light-chain epitopes are frequently found in alloimmunized severe hemophilia A patients and in some patients with acquired hemophilia due to autoantibodies.6 In immunoprecipitation assays, patient F's plasma bound not only to A2 but significantly to factor VIII light chain and recombinant C2 domain (Fig 4). Higher titer binding to the light chain than C2 suggested that antibodies for A3 and/or C1 were also present. This possibility was examined with direct binding to recombinant A3-C1 in a semi-quantitative immunoprecipitation assay34; A3-C1 binding was present (unpublished observations, November 1996). Thus, multiple epitopes in at least three distinct domains were involved in this mild hemophilia A patient's immune response. These data are consistent with a previous study demonstrating both inhibitory and noninhibitory alloantibodies in severe hemophilia patients.35 Quantitatively, the neutralization assay is less sensitive than immunoprecipitation and only 4 of 34 alloimmune inhibitors in severe patients had the major inhibitory component to an A2 domain (as opposed to C2, occasionally with A3-C1 domain) epitope or epitopes.36
Patient F had had only occasional bleeding episodes after trauma, and prior treatment with factor VIII had not been associated with a clinically significant inhibitor. The inhibitor occurred after major trauma and 5 weeks of high doses of infused factor VIII. The demonstration of multiple epitope involvement in his immune response suggests breakdown of immune tolerance to his endogenous, mutant factor VIII and transfused (both recombinant and plasma-derived) factor VIII proteins with the normal amino acid sequence. However, studies in mice suggest that T cells are made tolerant only to those self peptides that are displayed in sufficient amounts by antigen-presenting cells and for which a T-cell receptor has high affinity. Conversely, potentially self-reactive T cells specific for “cryptic” determinants that do not meet these qualifications would escape tolerance.37 Another consideration is that trauma and/or inflammation heightened his immune responsiveness. Interleukin-1 (IL-1), a major mediator of the inflammatory response to trauma, induces other cytokines, interferons, and chemokines that can activate both T and B lymphocytes.38,39 Interferon-γ and tumor necrosis factor-α upregulate major histocompatibility complex (MHC) II, adhesion and costimulatory molecules40 as well as cellular proteases involved in antigen processing.41 Combined with the antigen load, the display of cryptic determinants to T cells42 may have been enhanced, representing a plausible explanation for his response to multiple epitopes. The presence of cryptic epitopes in factor VIII might account for trace levels of autoantibodies found in normal individuals.43 The similarity of the antibody responses in this mild hemophilia patient with those in severely deficient patients, including patients with inversions where at least partial transcription and perhaps translation occur, demonstrates that prior tolerance is not sufficient to prevent high-titer inhibitors to factor VIII.
NOTES ADDED IN PROOF
Since submission of this study, a molecular model of factor VIII A domains based on ceruloplasmin structure has appeared.44 In comparing the structure in Fig 5 with the factor VIII model's coordinates (provided by Dr Peter Lindley, Daresbury Laboratory, Warrington, UK), the 593 residue remains in a top loop in the same relationship with the side chain loop that contains the inhibitor epitope.
ACKNOWLEDGMENT
Ann Weinmann is thanked for technical assistance, Elinor Adman (University of Washington, Seattle) and Gary Brayer (University of British Columbia, Vancouver, Canada) for use of modeling facilities and graphics programs, and Peter Lindley (Warrington, UK) for providing coordinates for the crystallographic structure of human ceruloplasmin.
Supported in part by National Institutes of Health Grants No. HL-42615 (P.L.) and HL-55273 (D.S.), and by Baxter and Centeon (A.R.T.) for a pilot study involving mild congenital bleeding disorders.
Portions of these data were previously presented ( Blood 88:657a, 1996).
Address reprint requests to Arthur R. Thompson, MD, PhD, Puget Sound Blood Center, 921 Terry Ave, Seattle, WA 98104-1256.