Abstract
Clonal rearrangements of the Ig heavy chain (IGH ) locus consisting of either intrachromosomal (VDJ ) rearrangements or interchromosomal translocations are a consistent feature of all B-cell malignancies and may be used both diagnostically and to monitor response to therapy. Many of these rearrangements are targeted to the IGHJ segments, but only some can be amplified with regular polymerase chain reaction (PCR) techniques. To permit PCR amplification of potentially all IGHJ rearrangements, we have devised a method incorporating self-ligation of restriction endonuclease-digested DNA fragments with long-distance PCR (long-distance, inverse PCR [LDI-PCR]). We show here, using only 4 nested oligonucleotide primers, the successful amplification and DNA sequencing of all IGHJ rearrangements up to 5.4 kb in length from a panel of 13 cases and cell lines of various types of B-cell malignancy. In all cases, both VDJ and DJ IGH rearrangements and translocation breakpoints were amplified. Six cases exhibited t(14; 18)(q32; q21). All translocation breakpoints were cloned and sequenced. Three cases exhibited a rearrangement to the BCL2 major breakpoint region (MBR). However, 2 other cases exhibited rearrangements between the MBR and the minor cluster region (mcr). These 2 cases broke within 44 bp of each other, confirming the presence of an additional 3′ BCL2 breakpoint cluster region. The final case fell immediately 3′ of the 3′ UTR of the BCL2 gene adjacent to an Alu repeat. No other BCL2 breakpoints within this region have been reported. Four cases exhibited t(11; 14)(q13; q32). All 3 cases with translocations targeted to the IGHJ segments were successfully amplified and sequenced, including 1 case in which the BCL1 translocation could not be detected by DNA blot using the currently available probes. All three translocation breakpoints fell outside the BCL1 major translocation cluster between 20 and 40 kb telomeric and showed no clustering. Two of the three fell within or adjacent to Alu repeat regions. LDI-PCR is a simple and robust technique that allows PCR amplification of nearly all IGHJ rearrangements.
THE IGH LOCUS LIES immediately adjacent to the telomere of the long arm of chromosome 14 and comprises 51 functional VH segments (and at least 30 pseudogenes), some 30 to 50 DH segments, 6 JH segments, and 9 CH segments.1 In normal B-cell development, these gene segments undergo regulated recombination to produce a functional VDJ gene.2 B cells that fail to undergo this process correctly lack surface Ig and die by programmed cell death within the bone marrow.3,4 Because VDJ rearrangement occurs very early in B-cell differentiation, all malignancies of the B-cell lineage exhibit clonal rearrangement and/or deletion of the IGH locus.5-7 Clonal IGH rearrangements can be detected either by DNA blot using a JH probe or by polymerase chain reaction (PCR) using a consensus JH primer in combination with primers designed within conserved regions of the VH gene.8-10 PCR-based techniques can be used both diagnostically and to monitor the response to therapy with unprecedented sensitivity.11-13 However, a potential disadvantage of using consensus IGH primers is that only a fraction (about 50% to 70% in most studies) of the rearranged IGH alleles are amplified and some 20% of cases of B-cell precursor acute lymphoblastic leukemia (BCP-ALL) remain PCR-negative using these techniques.14-16 In the context of BCP-ALL, the most common cause of PCR failure is due to DJ recombination and, because of the complex nature of the DHJH recombinations, only a few DHJH recombinations can be amplified by PCR.16 Prospective identification of cases with biallelic DHJH recombination may be biologically important, because this subgroup of BCP-ALL may represent transformation of the earliest B-cell precursor before full activation of the VDJ recombinase.
In the mature B-cell malignancies, and notably in the B-cell non-Hodgkin lymphomas (B-NHL), the most common cause of PCR failure is the occurrence of chromosomal translocations in which segments of other chromosomes become juxtaposed with the IGH locus, normally via either the Joining (J) or the Switch (S) segments.17,18 Translocations targeted to the IGH locus occur in greater than 70% of all B-NHL; moreover, specific translocations are largely associated with specific histological subgroups of disease.19 In some cases, clustering of the breakpoints within both the incoming oncogene and the IGH locus has allowed the development of PCR assays.20,21 More recently, long-distance PCR has extended the repertoire of PCR-detectable translocation breakpoints.22 However, in other cases, involving notably the MYC and BCL1 loci, the breakpoints can be dispersed over several hundred kilobases, beyond the present capacity of PCR technology.23 24 The breakpoints in partner chromosomes involved in IGH translocations are rarely within coding regions of putative oncogenes, and therefore the major consequence of the translocation is deregulated expression rather than a fusion protein. Thus, these translocations do not lend themselves to reverse transcriptase mediated methods of cloning such as the rapid amplification of cDNA ends (RACE).
To address these problems, we have sought to develop a PCR-based assay that would allow rapid amplification of all recombination events within the IGH locus, at both J and S segments. Inverse PCR, a method involving the amplification of restriction endonuclease-digested DNA ligated at low concentrations to form monomeric circles, represents a simple strategy for amplifying DNA sequences flanked on one side by a region of known sequence.25 By combining this with techniques for long-distance PCR and using oligonucleotide primer pairs complementary to regions within IGH, we have developed a method for the rapid molecular cloning of IGH rearrangements that we have termed long-distance inverse PCR (LDI-PCR). We report here the development and preliminary use of LDI-PCR and show the successful amplification of nearly all JH rearrangements using only 4 nested oligonucleotide primers.
MATERIALS AND METHODS
Cell lines and samples.Details of the samples and cell lines studied in this report are shown in Table 1. The translocation breakpoints in many of the cell lines have been characterized previously by DNA blot and/or bacteriophage cloning and sequencing. References to the derivation of the cell lines used in this study are either given in the text or are available on request.
Southern blot analysis.DNA blot was performed as previously described.7 Briefly, high molecular weight DNA was prepared by digestion with restriction endonucleases for 3 to 4 hours at 37°C. Restriction digests included Bgl II, HindIII, Pst I, Sac I, and Xba I (GIBCO-BRL, Gaithersburg, MD), because these have restriction sites within the IGH enhancer/Sμ region.26 DNA fragments were electrophoresed in 0.8% agarose and transferred to positively charged nylon membranes (Hybond N+; Amersham, Amersham, UK) by capillary transfer. Hybridization to a radiolabeled IGHJ probe (clone C76R51A) and, in selected cases, to BCL1 and BCL2 probes was performed as described previously.27 28 Filters were autoradiographed at −80°C with intensifying screens for up to 96 hours.
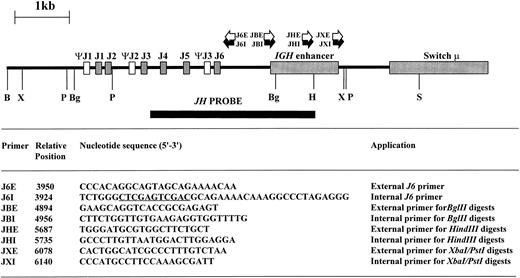
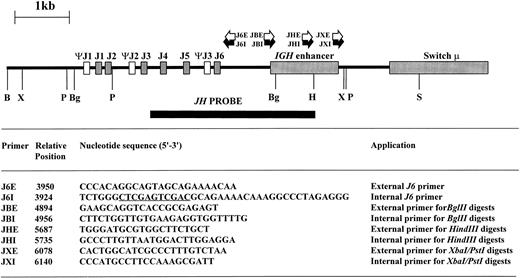
Inverse PCR.High molecular weight DNA was digested with restriction enzymes chosen from the results of DNA blot to yield small DNA IGHJ fragments; the enzyme chosen varied from sample to sample as shown in Table 1. Phenol:chloroform extraction was performed to remove residual enzymatic activity, and 0.4 μg of digested DNA was then ligated at 15°C overnight in a total volume of 500 μL with 5 U of T4 DNA ligase (Promega, Madison, WI). The ligated DNA was then purified using the Wizard DNA clean-up system (Promega) and eluted in a final volume of 40 μL. Primers were designed within the JH and IGH enhancer regions based on published sequences26 29-32 so that the amplification products would include short stretches from these regions in addition to the rearranged or translocated regions 5′ of JH. Additionally, restriction sites to permit forced cloning into plasmid vectors were introduced (Fig 1). The sequence, position, and orientation of the primers are shown in Fig 1.
Restriction map of germline IGHJ region showing restriction sites used in digests together with site and orientation of oligonucleotide primers. Sequence information used to design the oligonucleotide primers was derived from published sequences.26 29-32 The position of the 5′ end of the oligonucleotide primers is indicated with respect to the 5′ BamHI site. Xho I and Sal I sites within J6I are underlined. B, BamHI; Bg, Bgl II; H, HindIII; P, Pst I; S, Sac I; X, Xba I.
Restriction map of germline IGHJ region showing restriction sites used in digests together with site and orientation of oligonucleotide primers. Sequence information used to design the oligonucleotide primers was derived from published sequences.26 29-32 The position of the 5′ end of the oligonucleotide primers is indicated with respect to the 5′ BamHI site. Xho I and Sal I sites within J6I are underlined. B, BamHI; Bg, Bgl II; H, HindIII; P, Pst I; S, Sac I; X, Xba I.
The first PCR reaction was of 30 cycles (94°C for 1 minute followed by 30 cycles of 94°C for 1 minute and 68°C for 6 minutes followed by a terminal extension at 68°C for 7 minutes). The reaction mixture (50 μL total volume) contained 1 μL (approximately 10 ng) of the ligated DNA, 15 μL of 3.3× GeneAmp XL Buffer II (Perkin-Elmer, Foster City, CA), 200 μmol/L dNTPs, each primer at 0.2 μmol/L, 1 U rTth polymerase (Perkin-Elmer), and 1.0 to 1.2 mmol/L Mg(OAc)2 . A hot start was achieved by the addition of 5 μL of a 10× Mg(OAc)2 solution after heating the reaction at 85°C for 2 minutes. One microliter of the PCR reaction was then reamplified for 30 cycles using identical conditions with nested primers. Amplification was conducted on a programmable heating block (Biometra TRIO Thermoblock; Biometra, Göttingen, Germany).
After PCR amplification, the products were purified to remove excess salts and then digested with restriction enzymes and run on 0.8% agarose. Bands corresponding to the size expected from Southern hybridization were excised from the gel, purified with Qiaex II (Qiagen, Hilden, Germany), and ligated into pBluescript KS+ (Stratagene, La Jolla, CA). Aliquots of the ligation reaction were transformed into electrocompetent XL-1 blue cells using an EasyjecT Plus electroporator (Flowgen, Lichfield, UK) set at 2 kV, 25 μF, and 481 Ω. Bacteria were plated on X-Gal/IPTG/Ampicillin plates, and white colonies were picked and were grown overnight at 37°C. Plasmid DNA was extracted using the Qiaprep Spin Plasmid system (Qiagen), digested, and run on 0.8% agarose. Plasmids containing the correct size insert were sequenced using an ABI PRISM automated sequencer and AmpliTaq DNA polymerase, FS (Perkin-Elmer). Sequences were compared with the Genbank and EMBL databases using BLAST and FASTA programs via computing facilities made available by the Human Genome Mapping Project Resource Centre (Hinxton Hall, Cambridge, UK). DH segments were identified according to the criteria described.33
RESULTS
Details of the cases and cell lines studied are shown in Table 1. These were selected because previous studies had shown that many of the translocation breakpoints would not be amenable to amplification by regular PCR techniques (Dyer et al,27 Kiem et al,34 Jadayel et al,35 and M.J.S.D., unpublished observations).
In this preliminary study, all cases were analyzed by DNA blot, first to determine the sizes and number of the IGHJ rearrangements with various restriction enzymes that cut within the IGHJ-Cμ intron and second to determine the approximate location of breakpoints within the BCL1 and BCL2 loci. However, subsequent studies have shown that this step is not necessary, because LDI-PCR will detect nearly all IGHJ rearrangements within any given sample (T.G.W. and M.J.S.D., unpublished observations).
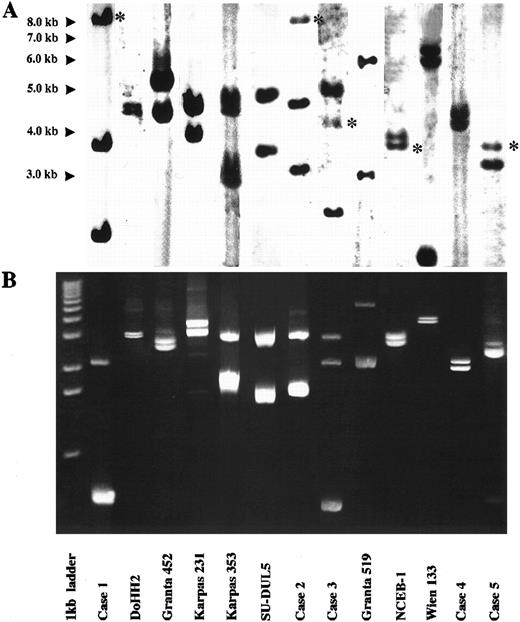
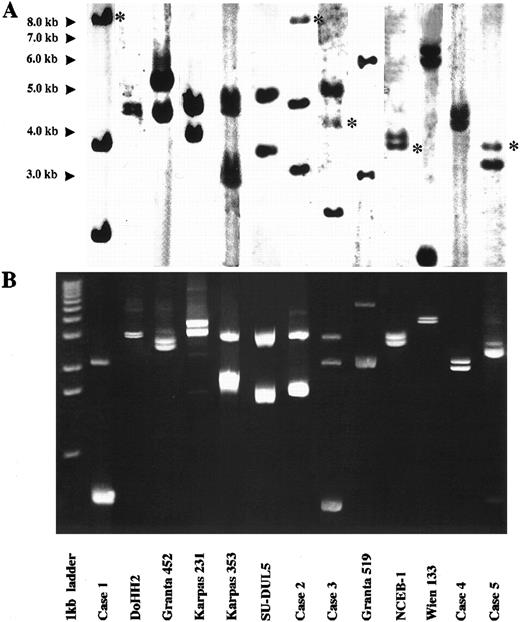
LDI-PCR was performed on the 13 cases. The restriction enzyme used in each digestion/ligation reaction is shown in Table 1. Twenty-five of the 26 rearranged IGHJ alleles and three germline IGHJ alleles seen on DNA blot were successfully amplified. Moreover, the sizes of the PCR products corresponded precisely to those anticipated from the DNA blot data, indicating that no major artifacts had occurred during LDI-PCR amplification (Fig 2). Subsequent sequencing data were compared with database sequences and those obtained from phage cloning and also showed no major artifacts (see below). Both alleles were amplified in the one PCR reaction even though they were sometimes of widely differing sizes. There was no preferential amplification of the shorter allele to the exclusion of the larger. Large amounts of PCR products of up to 5.4 kb were routinely obtained. Only one rearranged IGHJ allele failed to amplify and this was in the cell line Wien 133, which exhibited a complex, three-way translocation t(8; 14; 12)(q24.1; q32.3; q24.13).36 In this cell line there was an additional breakpoint between the JH5 and JH6 segments that precluded amplification with the primers used in this series of experiments.
Results of Southern blot with JH probe and LDI-PCR in cases studied. For each case, LDI-PCR products correspond in size to rearranged bands on Southern blot once unamplified distance between primers is allowed for. (A) Southern blot analysis of 10 μg of genomic DNA. Asterisks refer to germline bands. (B) Five microliters of LDI-PCR product was run on 0.8% agarose stained with ethidium bromide. A 1-kb ladder was used as a molecular weight marker.
Results of Southern blot with JH probe and LDI-PCR in cases studied. For each case, LDI-PCR products correspond in size to rearranged bands on Southern blot once unamplified distance between primers is allowed for. (A) Southern blot analysis of 10 μg of genomic DNA. Asterisks refer to germline bands. (B) Five microliters of LDI-PCR product was run on 0.8% agarose stained with ethidium bromide. A 1-kb ladder was used as a molecular weight marker.
All PCR products including both VDJ and DJ rearrangements and chromosomal translocations were cloned and sequenced.
IGH rearrangements.All cases showed clonal IGHJ rearrangements by DNA blot. In 10 cases, these were biallelic. However, the cell line NCEB-1, which exhibited t(11; 14) (q13; q32), showed only one IGHJ rearrangement with the other allele retaining germline configuration in all digests. Additionally, case no. 5, which exhibited t(10; 14)(q22; q32), showed only one IGHJ rearrangement in several digests, indicating that one IGHJ allele had been deleted. Subsequent studies indicated that the translocations in both NCEB-1 and case no. 5 had occurred to downstream switch regions and were therefore not amenable to LDI-PCR amplification using the set of primers described here (data not shown). The Wien 133 cell line exhibited 3 IGHJ rearrangements.36
VDJ and/or DJ rearrangements were amplified in all cases. Sequence data for selected cases are shown in Table 2. VDJ rearrangements consisted of germline VH genes as well as VH genes that had undergone somatic mutation. Case no. 4, a B-NHL in leukemic phase that exhibited a t(1; 14)(p22; q32) cytogenetically, was found to have a productive VDJ rearrangement on one IGHJ allele and a nonproductive DJ rearrangement on the other. The latter consisted of a D4-23/JH5 rearrangement with germline DH sequences 5′ of this (Table 2). This case, like NCEB-1 and case no. 5, presumably had undergone translocation to one of the downstream IGH switch regions.
Unusually, NCEB1 retained a germline IGHJ allele. This was confirmed by sequencing of the amplified allele.
BCL2 translocations.Most BCL2 translocations in follicular and diffuse NHL cluster to two defined regions of the 3′ region of the BCL2 gene termed the major breakpoint region (MBR) and the minor cluster region (mcr).37 The distribution differs in other B-cell malignancies such as B-cell chronic lymphocytic leukemia (B-CLL), in which rearrangements to the 5′ region are common.27
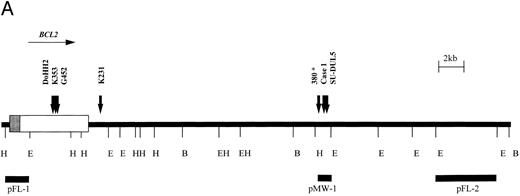
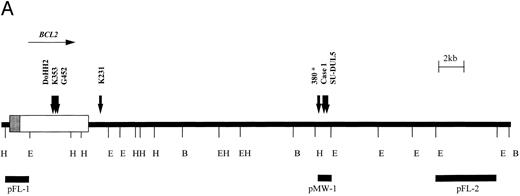
Six cases with translocation t(14; 18)(q32; q21) were studied using the BCL2 probes shown in Fig 3. Cell lines DoHH2, Granta 452, Karpas 231, and Karpas 353 exhibited rearrangement of the BCL2 MBR probe, pFL-1. Case no. 1 was a case of B-CLL with t(14; 18)(q32; q21) that showed a 3′ BCL2 breakpoint between the MBR and mcr as detected by probe pMW-1. Cell line SU-DUL5, derived from a case of transformed high-grade NHL, exhibited a complex three-way translocation involving MYC,BCL2, and IGHJ (Kiem et al34 and M.J.S.D., unpublished observations), but also showed rearrangement with this probe. The position of the BCL2 probes relative to one another and the position of the BCL2 breakpoints as determined by LDI-PCR and sequencing are shown in Fig 3A.
t(14; 18) translocations. (A) Restriction map of the 3′ region of the BCL2 gene showing position of breakpoints cloned in this study. Note the clustering of breakpoints in the region of the pMW-1 probe and the variant breakpoint in Karpas 231. *Leukemia 380, breakpoint not characterized in this study but cloned previously.39 (B) Sequence of pMW region and breakpoint sequences in case no. 1, SU-DUL5, and 380. JH sequences are underlined and n regions are in lowercase. JH segment involved in translocation is shown. B, BamHI; E, EcoRI; H, HindIII.
t(14; 18) translocations. (A) Restriction map of the 3′ region of the BCL2 gene showing position of breakpoints cloned in this study. Note the clustering of breakpoints in the region of the pMW-1 probe and the variant breakpoint in Karpas 231. *Leukemia 380, breakpoint not characterized in this study but cloned previously.39 (B) Sequence of pMW region and breakpoint sequences in case no. 1, SU-DUL5, and 380. JH sequences are underlined and n regions are in lowercase. JH segment involved in translocation is shown. B, BamHI; E, EcoRI; H, HindIII.
All six BCL2 translocation breakpoints were cloned and sequenced. On the translocated allele, there was loss of homology with IGH sequences upstream of JH6. In DoHH2, Granta 452, and Karpas 353, upstream sequences showed identity with the BCL2 MBR, with the BCL2 breakpoints occurring at nucleotides 3231, 2999, and 3053, respectively, of the cDNA sequence.38
The sequences juxtaposed to JH6 in case no. 1 and in the cell line SU-DUL5 showed homology with a 108-bp breakpoint sequence previously cloned from a cell line (380) derived from a patient with B-cell ALL with t(14; 18) (q32; q21) in which the breakpoint fell between the MBR and the mcr.39 Further sequencing around the breakpoints showed that all three clustered within a 300-bp region, with two falling within 44 bp of each other (Fig 3B).
In the cell line Karpas 231 (amplified from a Bgl II digestion), the sequence of 70 bp immediately 5′ of JH6 showed no homology to any other sequence on the database but thereafter contained Alu repetitive elements. However, sequence from the other end of the 2.9-kb clone showed identity with BCL2 cDNA from the Bgl II site at nucleotide 4686 onwards, showing that the breakpoint in this cell line fell some 4.5 kb downstream of the MBR 70 bp 3′ of an Alu region.
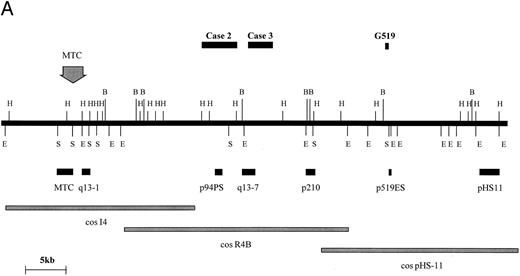
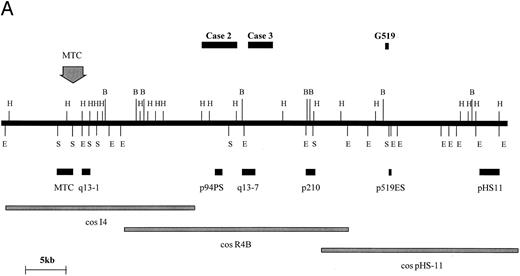
BCL1 translocations.Four cases with t(11; 14) (q13; q32) were studied consisting of two cell lines (Granta 519 and NCEB-1) and two fresh cases of mantle-cell lymphoma in leukemic phase (Table 1, cases no. 2 and 3). In 1 of these (case no. 3), the t(11; 14)(q13; q32) was not clearly identified cytogenetically. Rearrangements of the BCL1 locus in these 4 cases were sought by DNA blot with a panel of probes spanning the BCL1 locus as previously described (Fig 4A and Jadayel et al28 ). NCEB-1 showed rearrangement with a BCL1 major translocation cluster (MTC) probe, case no. 2 with probe p94PS, and case no. 3 with probe q13-7. However, no rearrangement of any of the available BCL1 probes was detected in the cell line Granta 519. Fluorescent in situ hybridization (FISH) of this cell line was therefore performed with the BCL1 cosmids I4, R4B, and pHS-11; the breakpoint fell between cosmids I4 and pHS-11, although none of the probes detected a split signal (data not shown).
BCL1 breakpoints. (A) Partial restriction map of the BCL1 locus illustrating cosmid and probe locations and breakpoints cloned in this study. Breakpoint of Granta 519 marked with its derived probe (p519ES, a 500-bp EcoRI/Sac I fragment). Note that this breakpoint falls within a window not covered by other probes. Map taken from Williams et al40 and Raynaud et al.41 (B) Sequences of breakpoints cloned in cases with t(11; 14); JH sequences are underlined and JH segment involved in breakpoint is shown. Bold type refers to Alu repeats. B, BamHI; E, EcoRI; H, HindIII; S, Sac I.
BCL1 breakpoints. (A) Partial restriction map of the BCL1 locus illustrating cosmid and probe locations and breakpoints cloned in this study. Breakpoint of Granta 519 marked with its derived probe (p519ES, a 500-bp EcoRI/Sac I fragment). Note that this breakpoint falls within a window not covered by other probes. Map taken from Williams et al40 and Raynaud et al.41 (B) Sequences of breakpoints cloned in cases with t(11; 14); JH sequences are underlined and JH segment involved in breakpoint is shown. Bold type refers to Alu repeats. B, BamHI; E, EcoRI; H, HindIII; S, Sac I.
BCL1 translocation breakpoints from cases no. 2 and 3 as well as from the Granta 519 cell line were cloned and sequenced (Fig 4B). In case no. 2, the translocated allele showed loss of homology with IGHJ immediately upstream of JH5. The breakpoint in this case occurred 170 bp downstream of an Alu repetitive region. Because DNA blot showed comigration of JH and the BCL1 (p94PS) probes, no further analysis was conducted. In case no. 3, sequences beyond JH6 showed homology with Alu repetitive elements. Given that this case showed rearrangement with the BCL1 q13-7 probe, the breakpoint in this could be mapped to approximately 20 kb telomeric of the MTC. In the cell line Granta 519, sequences beyond JH4 showed no homology with any sequence on the databases. Because both DNA blot and FISH had failed to localize the breakpoint within the BCL1 region, a 0.5-kb EcoRI-Sac I fragment single-copy probe was prepared from the 5.4-kb cloned PCR product and was hybridized with cosmids I4, R4B, and pHS11, which span a 100-kb region 3′ of the BCL1 MTC. There was a strongly positive hybridization with pHS11, showing that the breakpoint fell within this cosmid. Analysis of the restriction map for this region and hybridization with DNA digested with a number of different restriction enzymes indicated that the breakpoint was close to the 5′ end of the pHS11 cosmid. Maps of the BCL1 locus showed that this region was flanked by restriction enzyme sites within which none of the currently available BCL1 probes fell, hence the failure to detect rearrangement with any of these probes.
BCL7A translocation.Because all the IGHJ rearrangements in the Wien 133 cell line with a complex t(8; 14; 12) (q24.1; q32.3; q24.1) had been previously cloned and sequenced from bacteriophage clones,36 LDI-PCR was also performed to assess whether this technique introduced mutations at a high frequency. Comparison of the sequences of both the productive VDJ allele and the allele involved directly with BCL7A showed no differences.
DISCUSSION
We have sought to develop a method for the rapid PCR amplification and cloning of all rearrangements and translocations involving the IGH locus. Preliminary results reported here for the IGHJ segments indicate that LDI-PCR is a simple and robust technique allowing amplification of all but one IGHJ allele in 13 cases of B-cell malignancy. Amplification of sequences up to 5.4 kb in length was readily observed. Whether longer PCR products can be routinely obtained with this method is as yet unknown. In this series, restriction enzymes were chosen to produce relatively small DNA fragments. Importantly, both alleles were amplified in the one PCR reaction even when the two alleles differed widely in length (eg, Granta 519). Preferential amplification of the shorter allele to the exclusion of the longer was not observed. Also, comparison with sequences that had been obtained previously by conventional bacteriophage cloning methods in the cell line Wien 133 indicated that LDI-PCR did not introduce artifacts.
Intrachromosomal IGH rearrangements and interchromosomal translocations were cloned and sequenced. Both VDJ and DJ rearrangements were amplified. The latter are difficult or impossible to amplify using conventional PCR methods due to the number and complexity of DH rearrangements. Nevertheless, in the context of BCP-ALL, it may be important to detect cases with biallelic DJ rearrangement because these cases may represent the transformation of the earliest B-cell precursors that have failed to activate the full recombinase machinery.16
Regarding chromosomal translocations, BCL1, BCL2, and BCL7A breakpoints were readily amplified and sequenced. Six BCL2 translocations were cloned by LDI-PCR. As anticipated from the DNA blot results, three breakpoints fell within the MBR situated within the 3′ untranslated region of the BCL2 gene. However, 2 further cases (case no. 1 and SU-DUL5) were shown to cluster within 44 bp of one another in a region lying between the MBR and the mcr. This region has been shown to contain the BCL2 breakpoint in 2 other cases of CLL with t(14; 18)(q32; q21).27 Also, further sequencing showed that the BCL2 breakpoint in the B-cell ALL cell line 38039 fell 240 bp centromeric. Together, these data indicate the presence of a third translocation cluster within the 3′ region of the BCL2 gene. Finally, one other 3′ BCL2 breakpoint in the cell line Karpas 231 was mapped 4.5 kb 3′ of the BCL2 MBR, 70 bp 3′ of an Alu repeat. This breakpoint fell outside of any recognized clusters. To our knowledge, no other BCL2 breakpoints have been mapped to this region.
In contrast to the defined clusters of BCL2 translocations, BCL1 translocations are dispersed over a region of at least 350 kb with only about 25% of cases clustering to the MTC.24,40,41 Thus, most BCL1 translocations cannot be amplified with regular PCR techniques. All three BCL1 translocations involving IGHJ were cloned by LDI-PCR. Mapping of these relative to other BCL1 probes showed dispersed breakpoints between 25 and 40 kb telomeric of the BCL1 MTC. Two of the three BCL1 translocations fell in or adjacent to Alu sequences. The breakpoint in the Granta 519 cell line could not be detected by Southern blot using the currently available panel of BCL1 probes and the p519ES probe derived from LDI-PCR may prove useful in defining other breakpoints between probes p210 and pHS11. The BCL1 translocation in the cell line NCEB-1 did not involve the IGHJ segments and it is likely that this translocation was targeted to a centromeric IG switch region, as has been reported in two multiple myeloma cell lines.42 Further development of the LDI-PCR method is being performed to allow the amplification of these translocations.
LDI-PCR allows the rapid cloning of all IGHJ translocations in B-cell malignancies. Analysis of such breakpoints by bacteriophage cloning continues to allow the isolation of new genes and the recognition of new pathogenic mechanisms.36 43-45 The benefits of LDI-PCR are first the speed and ease with which this cloning can now be performed and second that cloning can be performed when only small amounts of material are available. We have successfully amplified IGHJ rearrangements without prior DNA blot using only 10 ng of DNA. Therefore, this technique will be useful when material is limited.
ACKNOWLEDGMENT
The authors thank Dr Pieter de Schouwer (Department of Academic Haematology and Cytogenetics, ICR, Sutton, UK) for his help in designing primers; Rifat Hamoudi, Rachel Hunter, Samantha Dibley, Rachel Jackson, and Bina Desai (Gene Cloning Facility, ICR) for their help with automated DNA sequencing; and Prof A. Hagemeijer (Rotterdam, The Netherlands) and Dr D.G. Oscier (Royal Bournemouth Hospital, Bournemouth, UK) for providing samples used in this study. We also thank the Human Genome Mapping Project Resource Centre (Cambridge, UK) for providing computing facilities.
Supported in part by grants from Royal Marsden Hospital Trust Funds and the Kay Kendall Leukaemia Trust.
Address reprint requests to M.J.S. Dyer, MD, DPhil, Academic Department of Haematology and Cytogenetics, Institute of Cancer Research-Royal Marsden Hospital, Haddow Laboratories, 15 Cotswold Rd, Sutton, Surrey, SM2 5NG UK.