Abstract
Recent work has demonstrated that glucocorticoids, nucleoside analogues, and other cancer chemotherapeutics induce apoptosis in chronic lymphocytic leukemia (CLL) cells. In this study, we investigated the involvement of protease activation in these responses using selective peptide inhibitors of the interleukin-1β converting enzyme (ICE)/caspase family and a Ca2+-activated protease we recently implicated in thymocyte apoptosis. Apoptosis was associated with proteolytic cleavage of poly(adenosine diphosphate [ADP]-ribose) polymerase (PARP) and increased caspase protease activity, and cell-permeant caspase antagonists [zVAD(OMe)fmk and Boc-D(OBzl)cmk] blocked apoptosis in response to the glucocorticoid methylprednisolone or the nucleoside analogue fludarabine, indicating that caspase activation was required for these responses. However, a peptide-based inhibitor of the Ca2+-dependent lamin protease (zAPFcmk) also completely suppressed DNA fragmentation and the cleavage of lamin B1 . Strikingly, treatment of cells with zAPFcmk alone led to characteristic PARP cleavage, depletion of the precursor forms of two ICE family proteases (CPP32 and ICH-1), and phosphatidylserine exposure, suggesting that blockade of the lamin protease led to activation of the ICE family. Our results implicate the lamin protease as a target for Ca2+ during chemotherapy-induced apoptosis in CLL lymphocytes, and they identify a novel functional interaction between the protease and members of the ICE family.
CHRONIC LYMPHOCYTIC leukemia (CLL) is the most common hematologic malignancy in the Western world. The disease typically strikes the elderly and, in its early stages, responds well to chemotherapeutics, particularly nucleoside analogues such as fludarabine and 2-chlorodeoxyadenosine. However, these and other regimens ultimately fail, and cells emerge that are resistant to multiple agents. Although the biochemical and molecular mechanisms contributing to drug resistance are still not clear, a possible breakthrough has recently been obtained with the observation that most (if not all) chemotherapeutics relevant to the treatment of CLL induce apoptosis in the cells,1-3 a pathway of cell death characterized by stereotyped morphologic alterations and DNA fragmentation.4 Furthermore, previous work from our laboratory and others has demonstrated that apoptosis in CLL cells involves a Ca2+-dependent pathway,1-3 but the biochemical targets for Ca2+ in CLL lymphocytes and other cell types remain elusive.
Recent work has demonstrated that the activation of a family of cysteine proteases homologous to interleukin-1β converting enzyme (ICE), collectively termed caspases, is a central event in apoptosis.5 For example, selective viral and peptide-based inhibitors of the family delay or prevent numerous examples of apoptosis in mammalian cells,5 and caspase-mediated cleavage of the 116-kD DNA repair enzyme poly(ADP-ribose) polymerase (PARP)6 is now considered an almost universal marker for apoptosis. Importantly, the caspases are not directly Ca2+-dependent, and it is therefore not clear how DNA fragmentation is regulated in systems such as CLL in which Ca2+ is centrally involved. Notably, experiments with recombinant caspases on isolated nuclei have shown that they cannot by themselves promote the nuclear changes of apoptosis, indicating that other biochemical effectors are involved.
In the search for potential Ca2+ targets in apoptosis, we have recently demonstrated that a Ca2+-dependent nuclear protease activity with characteristics distinct from the caspases is required for Ca2+-mediated DNA fragmentation and cell death in thymocytes.7 This protease cleaves the chromatin-associated proteins lamin B1 and histone H1 and is blocked by the serine protease inhibitor tosyl-L-phenylalanine chloromethyl ketone (TPCK) and a related peptide-based inhibitor (Z-alanine-proline-phenylalanine chloromethylketone, Z-APFcmk), but the protease is totally insensitive to other serine protease inhibitors and peptide antagonists of the ICE family (Z-VADfmk, YVADcmk, and DEVD-CHO). Because apoptosis in CLL cells also occurs via a Ca2+-sensitive mechanism, we designed experiments to address the relative contributions of the ICE family and the Ca2+-dependent lamin protease in chemotherapy-induced apoptosis in CLL cells.
MATERIALS AND METHODS
Materials. The esterified peptide caspase inhibitor, Z-VAD(OMe)fmk, and the mouse anti-PARP monoclonal antibody C2-10 were purchased from Enzyme Systems Products, Inc, Dublin, CA. The peptide inhibitor of the Ca2+-dependent lamin protease, Z-APFcmk, was purchased from Bachem Bioscience (King of Prussia, PA). The serine protease inhibitors Nα-p-tosyl-L-lysine chloromethyl ketone (TLCK) and TPCK were purchased from Sigma Chemical Co (St Louis, MO), and calpain inhibitors I and II were from Boehringer Mannheim Corp (Indianapolis, IN). Monoclonal antibodies for human CPP32 and ICH-1 were purchased from Transduction Laboratories, Lexington, KY. A chicken antiserum specific for lamin B1 was generously provided by Dr Scott Kaufmann, Department of Oncology, Mayo Clinic, Rochester, MN. The rabbit antichicken antibody was from Cappel Laboratories (Durham, NC), and horseradish peroxidase-conjugated antimouse and antirabbit antibodies were from Amersham Corp (Arlington Heights, IL).
Patients, cell isolation, and incubation criteria. All patients fulfilled the National Cancer Institute's (NCI) criteria for the diagnosis of CLL. Some of the patients had received prior therapy, although none within the last 6 months before experimentation.
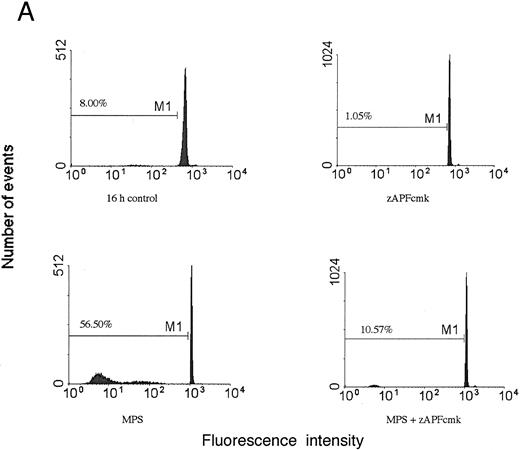
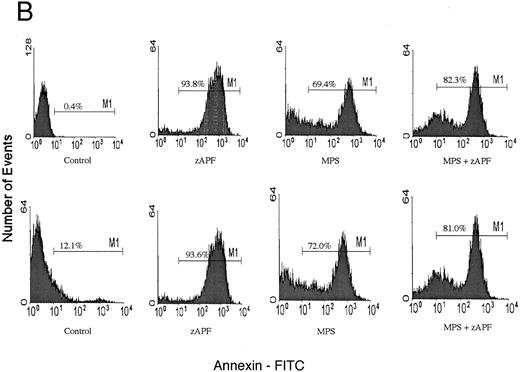
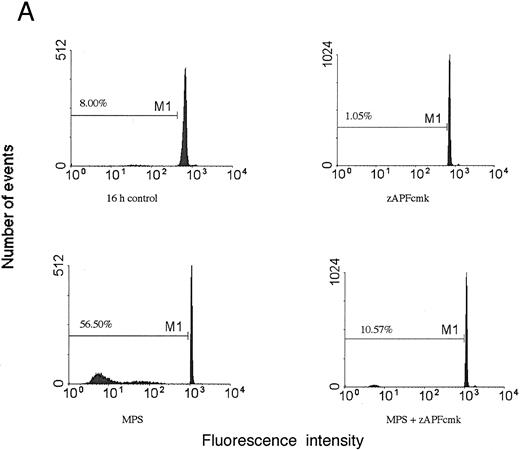
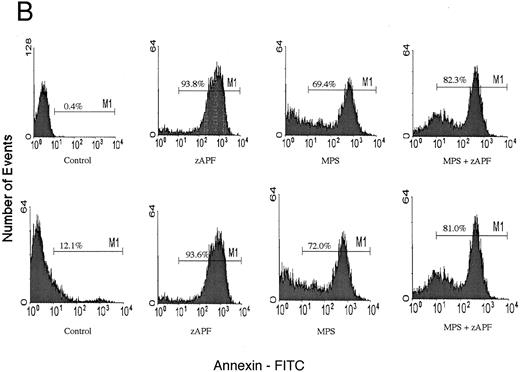
Induction of apoptosis in a representative CLL patient isolate. (A) Cells were incubated in the absence or presence of 10 μmol/L methylprednisolone with or without 25 μmol/L zAPFcmk for 48 hours, and apoptosis was quantified by PI staining and FACS analysis. Representative results are presented from one patient (not included in Table 1). (B) Glucocorticoid treatment induces exposure of phosphatidylserine. Cells were incubated in the absence or presence of 10 μmol/L methylprednisolone with or without 25 μmol/L zAPFcmk, and PS exposure was measured by binding of annexin V as described in Materials and Methods. Representative results from two experiments (patients 24 and 25 from Table 1).
Induction of apoptosis in a representative CLL patient isolate. (A) Cells were incubated in the absence or presence of 10 μmol/L methylprednisolone with or without 25 μmol/L zAPFcmk for 48 hours, and apoptosis was quantified by PI staining and FACS analysis. Representative results are presented from one patient (not included in Table 1). (B) Glucocorticoid treatment induces exposure of phosphatidylserine. Cells were incubated in the absence or presence of 10 μmol/L methylprednisolone with or without 25 μmol/L zAPFcmk, and PS exposure was measured by binding of annexin V as described in Materials and Methods. Representative results from two experiments (patients 24 and 25 from Table 1).
Immunophenotyping by dual-parameter flow cytometry showed coexpression of CD5 with B-cell antigen and isotypic light chain expression. Clinical staging was based on the system described by Rai.8 Freshly isolated peripheral blood was fractionated by Ficoll-Hypaque (Winthrop Pharmaceuticals, New York, NY) sedimentation at 4°C. Nonadherent mononuclear cells were then immediately suspended in complete RPMI-1640 medium supplemented with 10% fetal calf serum, 10 mmol/L HEPES (pH 7.5), and antibiotics, at a cellular concentration of 1 to 2 × 106 cells/mL. Cell viability was assessed by trypan blue exclusion and exceeded 95% following the isolation procedure.
DNA fragmentation analysis. Quantification of apoptosis by propidium iodide (PI) staining and fluorescence-activated cell sorting (FACS) analysis was performed as described previously.9 Following incubation with various agents in vitro, cells were pelleted by centrifugation and resuspended in phosphate-buffered saline containing 50 μg/mL PI, 0.1% Triton X-100, and 0.1% sodium citrate. Samples were stored at 4°C for 16 hours and vortexed prior to FACS analysis (FL-3 channel; Becton Dickinson FACScan, Mountain View, CA).
Annexin V binding. Exposure of surface phosphatidylserine was quantified by surface annexin V staining as described previously.10 Cells were resuspended in binding buffer containing 1 μg/mL fluorescein isothiocyanate (FITC) conjugated annexin V (Nexins Research BV, Hoeven, The Netherlands) and incubated for 30 minutes at 4°C, and cells were analyzed by flow cytometry (FACScan, Becton Dickinson).
ICE activity assay. Protease activity measurements were conducted as described previously.11 Cells were lysed in 1 mL buffer A containing 25 mmol/L HEPES (pH 7.4), 5 mmol/L EDTA, 2 mmol/L dithiothreitol (DTT), and 10 μmol/L digitonin for 15 minutes on ice. The lysates were clarified by centrifugation (12,000g), and supernatants were incubated with 50 μmol/L Asp-Glu-Val-Asp-aminomethyl coumarin DEVD-AMC (Enzyme Systems Products, Inc) at 37°C in the dark. Relative activities were then measured in a spectrofluorimeter (400 nm excitation, 505 nm emission); blanks included supernatants processed as outlined above without dye and supernatants incubated with excess BACMK (25 μmol/L).
Immunoblotting. Cells were lysed for 1 hour at 4°C in a lysis buffer containing 150 mmol/L NaCl, 1% Triton X-100, 1 mmol/L phenylmethylsulfonyl fluoride (Sigma Chemical Co), and 25 mmol/L Tris (pH 7.5). Debris was sedimented by centrifugation for 5 minutes at 12,000g and the pellets and/or supernatants were solubilized for 5 minutes at 100°C in Laemmli's sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer containing 100 mmol/L dithiothreitol. Polypeptides were resolved at 100 V on 12% gels and electrophoretically transferred to 0.2 μm nitrocellulose membranes (Schleicher & Schuell Inc, Keene, NH) for 1 hour at 100 V. Membranes were blocked for 1 hour (PARP and ICE family experiments) or overnight (lamin B1 experiments) in a TBS-T buffer (25 mmol/L Tris, pH 8.0, 150 mmol/L NaCl, and 0.05% Tween-20) containing 3% (wt/vol) nonfat dried milk. Blots were then probed overnight with antibodies to PARP or CPP32 or for 1 hour with a chicken antilamin B1 antiserum, and blots were developed using species-specific secondary and/or tertiary antisera. Immunoreactive material was then visualized by enhanced chemiluminescence (ECL; Amersham Corp) according to the manufacturer's instructions.
RESULTS
Previous work has shown that glucocorticoid hormones and nucleoside analogues induce DNA fragmentation characteristic of apoptosis in some (but not all) CLL lymphocytes in vitro and in vivo.3 We treated cells from 25 different CLL patients for 48 hours with 10 μmol/L methylprednisolone (MPS), a concentration that was found to be optimal in dose-response analyses in previous work1 and measured apoptosis by PI staining and FACS analysis (Table 1; representative results from one patient are presented in Fig 1A). Apoptotic cells can be readily distinguished from viable cells with this procedure by their subdiploid DNA content. Variable responses were observed, consistent with our previous findings.3 Surface annexin staining and FACS analysis demonstrated that glucocorticoid treatment induced surface phosphatidylserine exposure on the cells, which is another hallmark biochemical feature of apoptotic cell death (Fig 1B). An optimal concentration12 of the nucleoside analog, fludarabine, also promoted the appearance of cells with a hypodiploid DNA content, demonstrating that the effects were not limited to steroid treatment.
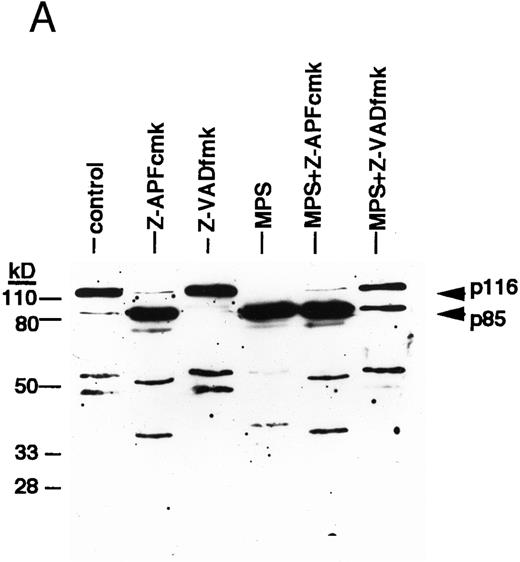
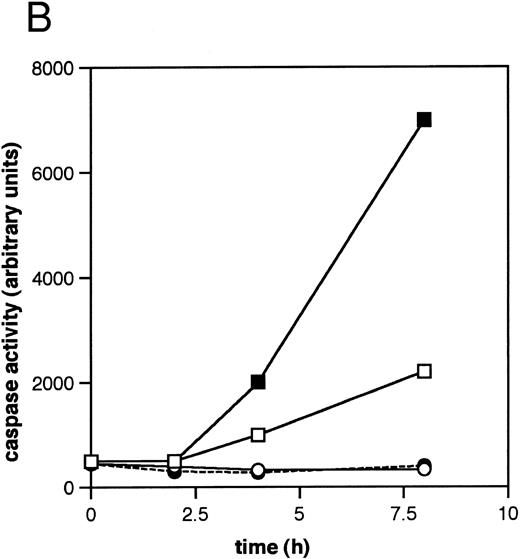
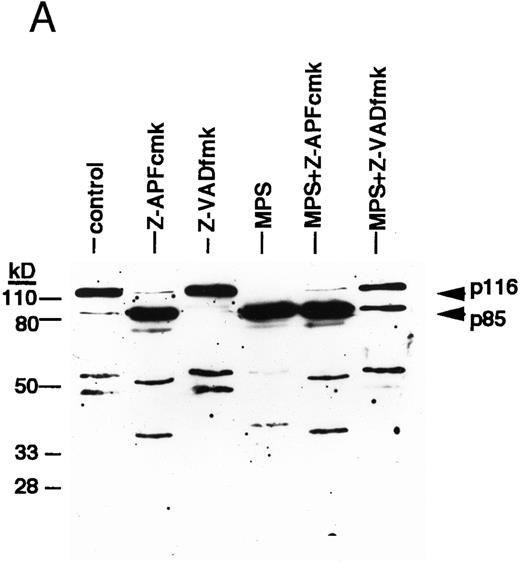
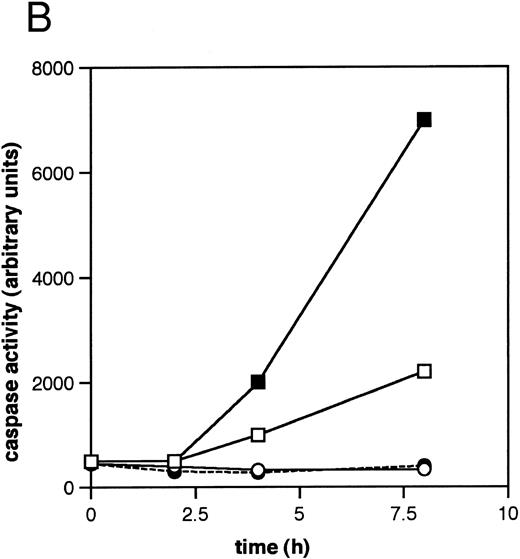
ICE family protease activation in CLL apoptosis. (A) Cleavage of PARP. Cells were incubated for 16 hours in the absence or presence of 10 μmol/L methylprednislone with or without 25 mmol/L zAPFcmk or 25 mmol/L zVADfmk, and PARP integrity was assessed by immunoblotting. Arrows indicate positions of intact PARP (p116) and the 85 kD caspase-generated PARP fragment (p85). Results from one experiment (patient 22) representative of four independent replicates with different patient isolates. (B) Measurement of caspase activity. Cells were incubated for the times indicated in the absence or presence of 10 μmol/L methylprednisolone, and hydrolysis of the ICE protease substrate DEVD-AMC was measured in a spectrofluorimeter as described in Materials and Methods. Results from two representative patient isolates (not included in Table 1) analyzed on the same day. Levels of DNA fragmentation were measured in parallel (16 hours): (□), patient A, control — 2%; (▪), MPS — 30%; (○), patient B, control — 9%; (•), MPS — 16%. Similar results were observed in three other patient isolates.
ICE family protease activation in CLL apoptosis. (A) Cleavage of PARP. Cells were incubated for 16 hours in the absence or presence of 10 μmol/L methylprednislone with or without 25 mmol/L zAPFcmk or 25 mmol/L zVADfmk, and PARP integrity was assessed by immunoblotting. Arrows indicate positions of intact PARP (p116) and the 85 kD caspase-generated PARP fragment (p85). Results from one experiment (patient 22) representative of four independent replicates with different patient isolates. (B) Measurement of caspase activity. Cells were incubated for the times indicated in the absence or presence of 10 μmol/L methylprednisolone, and hydrolysis of the ICE protease substrate DEVD-AMC was measured in a spectrofluorimeter as described in Materials and Methods. Results from two representative patient isolates (not included in Table 1) analyzed on the same day. Levels of DNA fragmentation were measured in parallel (16 hours): (□), patient A, control — 2%; (▪), MPS — 30%; (○), patient B, control — 9%; (•), MPS — 16%. Similar results were observed in three other patient isolates.
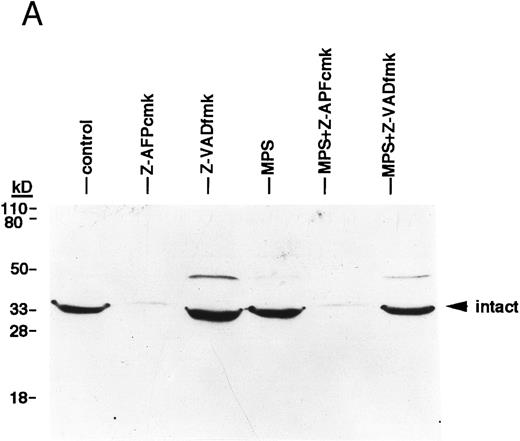
We next investigated the involvement of the caspases in these responses using biochemical assays for their activation. Glucocorticoid-induced cell death was associated with nearly complete cleavage of PARP to an 85-kD fragment characteristic of caspase-mediated proteolysis,6 an event that was inhibited by zVADfmk (Fig 2A). Glucocorticoids also induced time-dependent hydrolysis of a fluorigenic caspase substrate (DEVD-AMC) in apoptosis-sensitive patient isolates, but not in apoptosis-resistant ones (Fig 2B). Furthermore, the cell-permeant caspase antagonist zVAD(OMe)fmk5 inhibited glucocorticoid-induced DNA fragmentation in a majority of patient samples (Fig 3A, Table 1) and prevented DNA fragmentation in some CLL isolates treated with the nucleoside analog, fludarabine (Fig 3A), demonstrating that the effects were not limited to glucocorticoid-induced apoptosis. Together, these results indicate that caspase activation is required for induction of apoptosis in CLL cells.
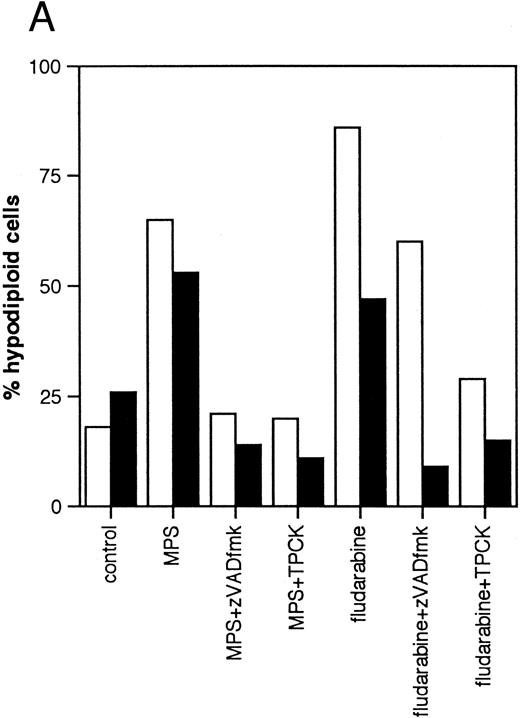
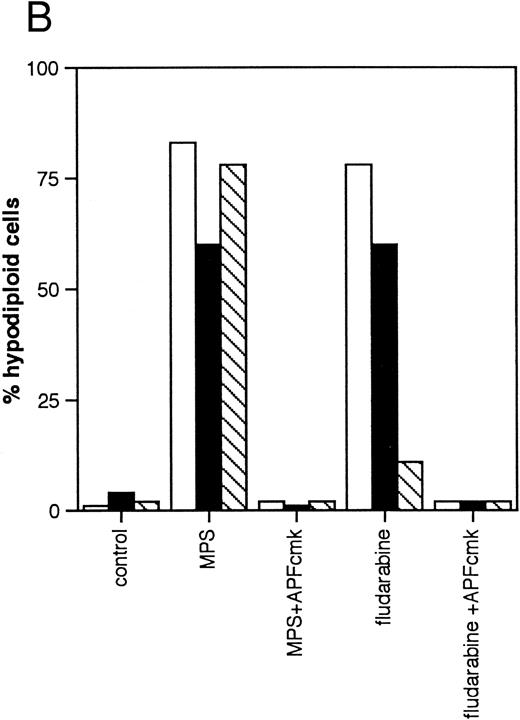
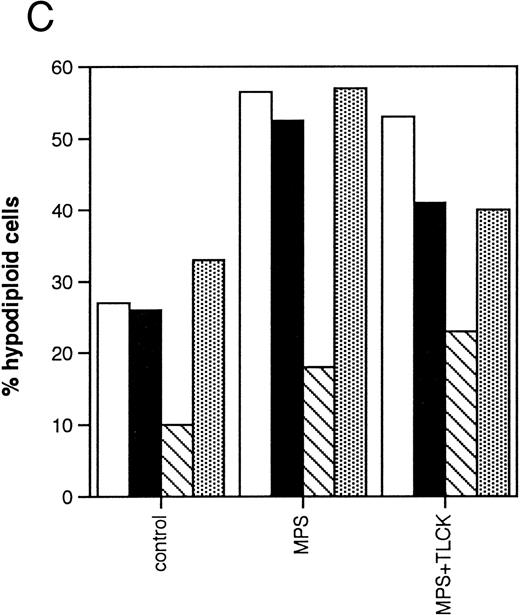
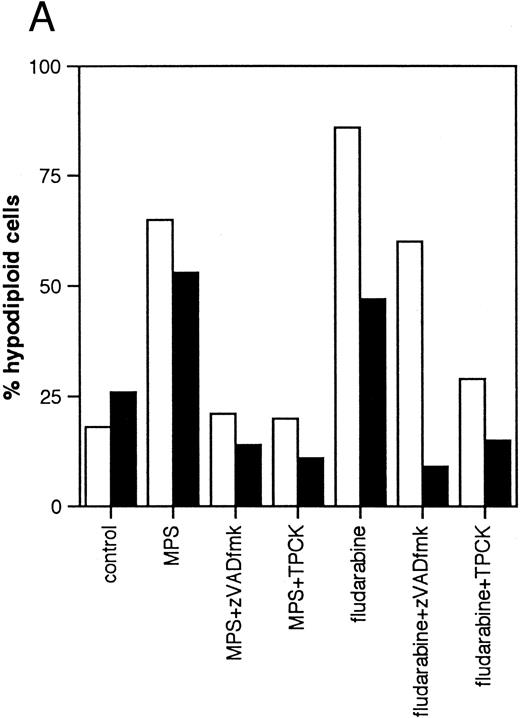
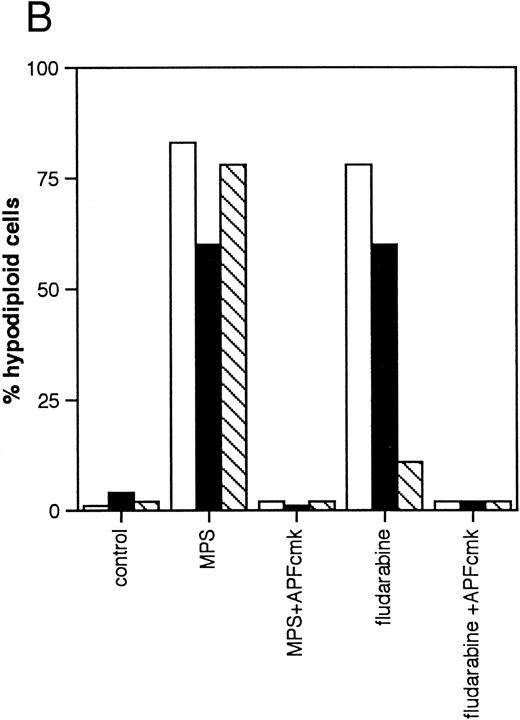
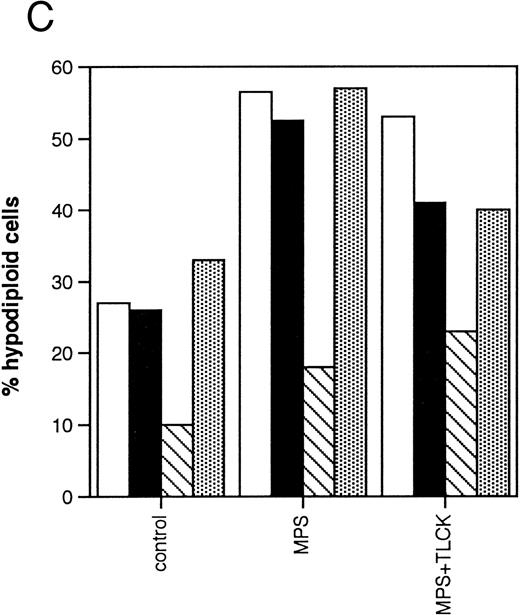
Effects of protease inhibitors on apoptosis. (A) Comparison of the ICE antagonist zVADfmk with the serine protease antagonist TPCK. Cells were incubated for 48 hours in the absence or presence of 10 μmol/L methylprednisolone or 10 μmol/L fludarabine with or without 25 μmol/L zVADfmk or 25 μmol/L TPCK, and the percentage of hypodiploid cells was determined by PI staining and FACS analysis as outlined in Materials and Methods. Results from two representative experiments (patients 6 and 21 in Table 1) (□, experiment 1, ▪, experiment 2). (B) Effects of the peptide antagonist zAPFcmk. Cells were incubated as described above with or without 25 μmol/L zAPFcmk, and the percentage hypodiploid cells was determined by PI staining. Results from three representative experiments (patients 8, 11, and 9 in Table 1) (□, experiment 1; ▪, experiment 2; ▧, experiment 3). (C) Effects of TLCK. Cells were incubated for 48 hours in the absence or presence of 10 mmol/L methylprednisolone with or without 50 mmol/L TLCK, and the percentage of hypodiploid cells was determined by PI staining and FACS analysis. Results from four representative patients (5, 6, 7, and 20 in Table 1) (□, patient 5; ▪, patient 6; (▧), patient 7; (▧), patient 20).
Effects of protease inhibitors on apoptosis. (A) Comparison of the ICE antagonist zVADfmk with the serine protease antagonist TPCK. Cells were incubated for 48 hours in the absence or presence of 10 μmol/L methylprednisolone or 10 μmol/L fludarabine with or without 25 μmol/L zVADfmk or 25 μmol/L TPCK, and the percentage of hypodiploid cells was determined by PI staining and FACS analysis as outlined in Materials and Methods. Results from two representative experiments (patients 6 and 21 in Table 1) (□, experiment 1, ▪, experiment 2). (B) Effects of the peptide antagonist zAPFcmk. Cells were incubated as described above with or without 25 μmol/L zAPFcmk, and the percentage hypodiploid cells was determined by PI staining. Results from three representative experiments (patients 8, 11, and 9 in Table 1) (□, experiment 1; ▪, experiment 2; ▧, experiment 3). (C) Effects of TLCK. Cells were incubated for 48 hours in the absence or presence of 10 mmol/L methylprednisolone with or without 50 mmol/L TLCK, and the percentage of hypodiploid cells was determined by PI staining and FACS analysis. Results from four representative patients (5, 6, 7, and 20 in Table 1) (□, patient 5; ▪, patient 6; (▧), patient 7; (▧), patient 20).
We then investigated the involvement of the nuclear scaffold protease in these responses. A peptide inhibitor of the nuclear Ca2+-dependent lamin protease (zAPFcmk)7 completely blocked DNA fragmentation (Table 1; Fig 3B) and the cleavage of lamin B1 (Fig 4) in all patient isolates tested. Qualitatively similar results were obtained with the serine protease inhibitor TPCK (Fig 3A; Table 1). In contrast, equivalent concentrations of the serine protease antagonist TLCK had little effect on DNA fragmentation (Fig 3C).
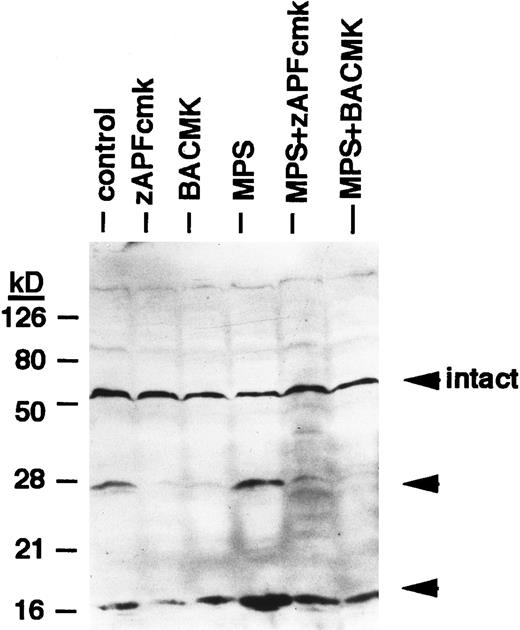
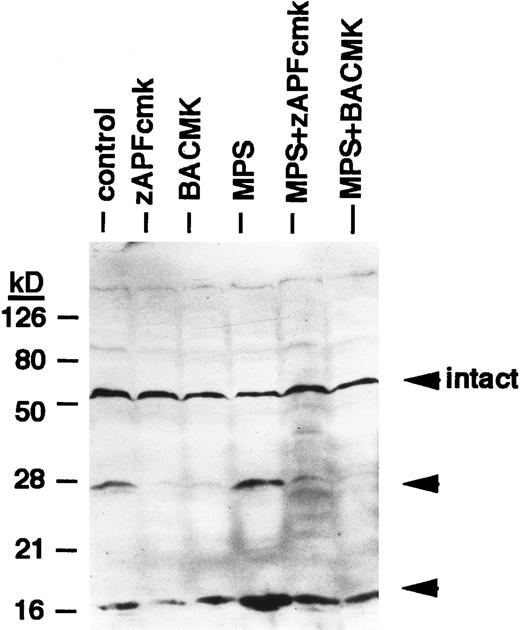
Effects of protease inhibitors on lamin B1 cleavage. Cells were incubated for 6 hours in the absence or presence of 10 μmol/L methylprednisolone with or without 10 μmol/L zAPFcmk or 25 μmol/L BACMK, and lamin B1 was detected by immunoblotting. DNA fragmentation levels in these samples were quantified in parallel (by the diphenylamine assay) and are as follows: control, 8%; zAPFcmk, 3%; BACMK, 3%; MPS, 33%; MPS + zAPFcmk, 10%; MPS + BACMK, 5%. Arrows indicate positions of intact lamin B1 and two characteristic lamin fragments. Results of one experiment typical of three independent replicates.
Effects of protease inhibitors on lamin B1 cleavage. Cells were incubated for 6 hours in the absence or presence of 10 μmol/L methylprednisolone with or without 10 μmol/L zAPFcmk or 25 μmol/L BACMK, and lamin B1 was detected by immunoblotting. DNA fragmentation levels in these samples were quantified in parallel (by the diphenylamine assay) and are as follows: control, 8%; zAPFcmk, 3%; BACMK, 3%; MPS, 33%; MPS + zAPFcmk, 10%; MPS + BACMK, 5%. Arrows indicate positions of intact lamin B1 and two characteristic lamin fragments. Results of one experiment typical of three independent replicates.
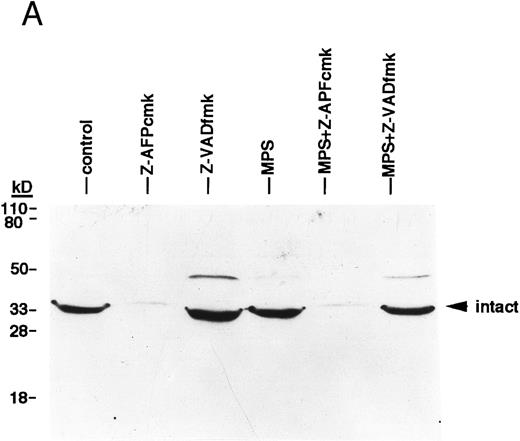
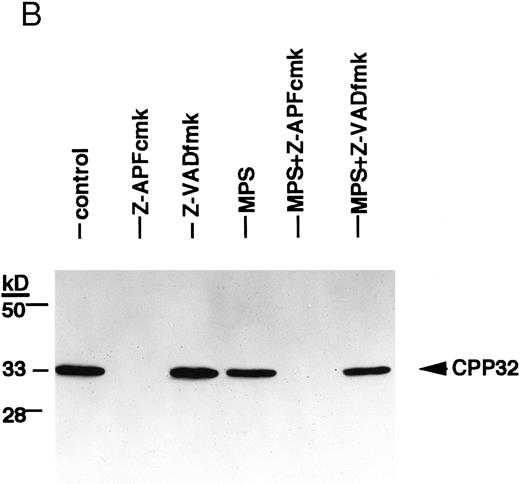
Finally, we conducted experiments to determine whether activation of the lamin protease is regulated by the caspases in CLL cells. The caspase inhibitor BACMK (Fig 4) and zVADfmk (not shown) prevented lamin cleavage, suggesting that the caspases act upstream of the lamin protease. On the other hand, not only did zAPFcmk fail to prevent glucocorticoid-induced PARP cleavage, treatment with the inhibitor alone resulted in nearly complete cleavage of PARP (Fig 2A), proteolytic processing of ICH-1/caspase 2 (Fig 5A) and CPP32/caspase 3 (Fig 5B), and extensive surface phosphatidylserine exposure (Fig 1B). Therefore, although zAPFcmk completely suppresses lamin cleavage and DNA fragmentation, it promotes activation of at least two caspases, proteolytic degradation of one of their key substrates, and a specific surface change associated with apoptosis. Together, these results strongly suggest that the Ca2+-dependent lamin protease acts downstream of the caspases in CLL lymphocytes.
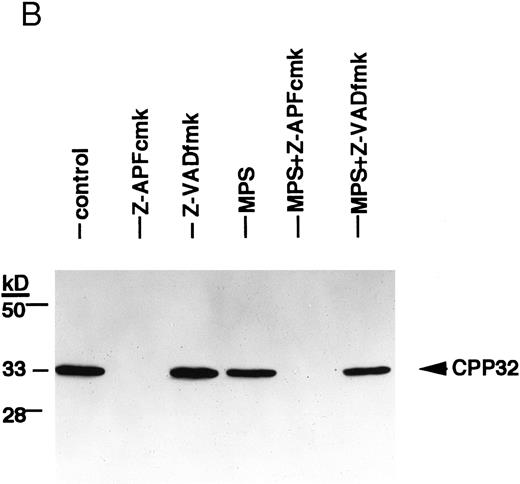
Effects of the Ca2+ protease inhibitor on processing of two different members of the ICE family. (A) Effects on CPP32. Cells were incubated for 16 hours in the absence or presence of 10 μmol/L methylprednisolone with or without 10 μmol/L zAPFcmk or 25 μmol/L zVADfmk, and levels of the precursor form of CPP32 were analyzed by immunoblotting. Results of one experiment (patient 22, Table 1) that were typical of three independent replicates. (B) Effects on ICH-1L . Cells were incubated as described above and levels of the precursor form of ICH-1L were determined by immunoblotting. Results of one experiment (patient 23, Table 1) that were typical of three independent replicates.
Effects of the Ca2+ protease inhibitor on processing of two different members of the ICE family. (A) Effects on CPP32. Cells were incubated for 16 hours in the absence or presence of 10 μmol/L methylprednisolone with or without 10 μmol/L zAPFcmk or 25 μmol/L zVADfmk, and levels of the precursor form of CPP32 were analyzed by immunoblotting. Results of one experiment (patient 22, Table 1) that were typical of three independent replicates. (B) Effects on ICH-1L . Cells were incubated as described above and levels of the precursor form of ICH-1L were determined by immunoblotting. Results of one experiment (patient 23, Table 1) that were typical of three independent replicates.
DISCUSSION
The observation that cancer chemotherapeutics induce apoptosis in CLL cells and other tumor cell types13 provides a mechanistic framework for their actions and suggests the existence of novel mechanisms for drug resistance involving specific suppression of physiologic cell death. Detailed information about the biochemical and molecular mechanisms involved in apoptosis is now emerging, with the implication of the caspases in the effector phase of the process being one of the most notable.5 With this in mind, we investigated the potential involvement of the caspases in chemotherapy-induced apoptosis in CLL lymphocytes. The results show that specific cleavage of the caspase substrate, PARP, is a feature of glucocorticoid-induced cell death. Furthermore, direct measurement of caspase activity using a fluorigenic substrate demonstrated a correlation between the effects of glucocorticoid on caspase activation and DNA fragmentation. Time course analysis indicates that caspase activation slightly precedes DNA fragmentation (J. Chandra, unpublished observations), consistent with a role for caspase activation at an early stage of the response. Finally, cellpermeant peptide caspase inhibitors blocked endogenous endonuclease activation in most patient samples. Together, our results show for the first time that activation of one or more members of the ICE family participate in glucocorticoid-induced apoptosis in CLL cells.
In our previous work on CLL cells, we showed that glucocorticoid-induced apoptosis is associated with an early, sustained increase in the cytosolic Ca2+ concentration, and Ca2+-buffering agents blocked DNA fragmentation and delayed cell death.1 We proposed that one target for Ca2+ in these cells is the endogenous nuclear endonuclease responsible for chromatin cleavage, as incubation of isolated nuclei from untreated, drug-sensitive CLL cells in the presence of Ca2+ resulted in oligonucleosomal DNA fragmentation characteristic of apoptosis in whole cells.1,3 Indeed, the level of endonuclease activity detected by this approach predicts the level of DNA fragmentation observed in whole cells exposed to glucocorticoid,3 indicating that it is tightly coupled to the response, and the endonuclease remains an attractive candidate target for Ca2+ in the cells. However, the results of the present study indicate that the nuclear lamin protease is another important target for Ca2+ in CLL cells. Activation of this protease is also required for endonuclease activation in whole thymocytes and isolated thymocyte nuclei,7 and it is therefore possible that the link between drug sensitivity and endonuclease activity identified in our previous work is related to levels of this protease. Other recent work has demonstrated that the cytosolic caspases MCH-214,15 and CPP3216 can also degrade the lamins in other model systems. However, we can exclude a requirement for caspase-3 activation in our system, because activation of the protease in response to treatment with zAPFcmk alone did not result in either lamin cleavage or DNA fragmentation in CLL cells. Although this has not yet been cloned, preliminary efforts in this laboratory and another suggest that the nuclear scaffold protease is structurally related to the proteosome,17 a multisubunit protease complex18 that has recently been implicated in apoptosis.19-21 Precisely how this protease regulates endonuclease activation will be the focus of future investigation.
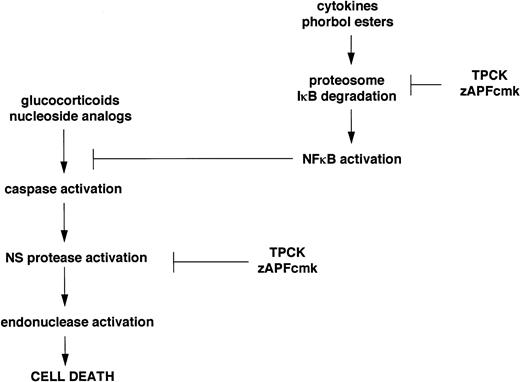
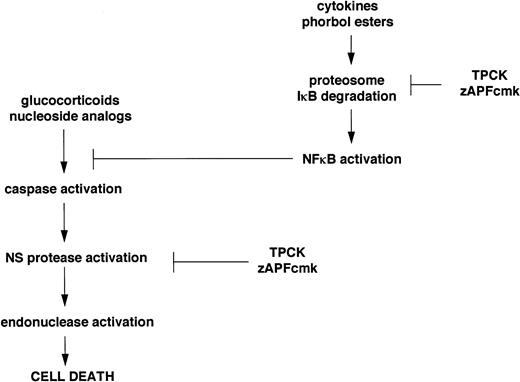
Inhibitors of the Ca2+-dependent lamin protease promote PARP cleavage, processing of two distinct members of the ICE family, and surface exposure of phosphatidylserine, while they completely block drug-induced lamin B1 cleavage and endonuclease activation. Although the biochemical mechanisms underlying these seemingly contradictory observations are still under investigation, our ongoing work suggests a possible explanation, outlined schematically in Fig 6. Previous work has shown that one of the inhibitors of the nuclear scaffold (NS) protease,7 TPCK, blocks the activity of the transcription factor, NFκB, by preventing proteolytic degradation of its inhibitor, IκB.22 Other recent work has shown that NFκB inhibits apoptosis,23-27 and protease inhibitors that block IκB degradation can directly induce apoptosis in certain model cell lines.25 The biochemical properties of TPCK and zAPFcmk are similar (they both possess a phenylalanine residue at the critical P1 site that binds the protease active site), and in electrophoretic mobility shift (EMSA) assays, we have found that the levels of active NFκB, which are very high in most of the isolates we have tested, are rapidly lowered by both TPCK and zAPFcmk (J. Chandra, unpublished observations). Therefore, if NFκB participates in the suppression of apoptosis in CLL, its inhibition by zAPFcmk could account for the caspase activation and phosphatidylserine exposure observed in CLL cells treated with the inhibitor. However, TPCK and zAPFcmk also completely inhibit Ca2+-mediated lamin B1 cleavage and endonuclease activation in isolated nuclei, so that although upstream events of apoptosis still occur, the process is arrested upstream of the nuclear events in cells treated with these inhibitors. Importantly, our results predict that agents that inhibit the NFκB protease without blocking the NS protease would be extremely effective inducers of apoptosis in CLL cells. Our ongoing work suggests that proteosome inhibitors (calpain inhibitor I, MG-132, and lactacystin) may fall into this category: they induce apoptosis in all of the patient isolates we have tested so far, including cells that are substantially resistant to glucocorticoids or nucleosides. Hopefully, by elucidating the biochemical mechanisms involved, we can use this information to identify better treatments for CLL and other hematopoietic malignancies.
Schematic representation of the dual effects of TPCK and zAPFcmk on the apoptotic pathway in CLL lymphocytes.
Schematic representation of the dual effects of TPCK and zAPFcmk on the apoptotic pathway in CLL lymphocytes.
Supported by a grant from the Leukemia Research Foundation, Inc and the American Cancer Society (RPG-9716901-CCOD). K.O.K. is the recipient of a Deutsche Krebshilte (Mildred-Scheel-Stiftung) Scholarship.
Address reprint requests to David J. McConkey, PhD, Department of Cell Biology, Box 173, The University of Texas M.D. Anderson Cancer Center, 1515 Holcombe Blvd, Houston, TX 77030.