Abstract
We have conducted a long-term prospective study of children undergoing bone marrow transplantation (BMT) to assess morbidity and mortality for liver disease. One hundred eleven consecutive children were enrolled between June 1985 and June 1995 and were followed-up for a median of 5.5 years after BMT. Before transplant 48/111 children (43%) had abnormal alanine aminotransferase (ALT), none were HBsAg+ and 4/111 were anti-HCV+. After BMT 4/111 patients (3.6%) died of liver failure. No relationship was found between pretransplant hepatitis B (HBV) or C (HCV) infection or elevated transaminases and development of severe liver damage. Eighty-two out of one hundred and eleven patients (74%) had abnormalities of ALT after BMT, transient (n = 54) or persistent (n = 28). None developed clinical signs or symptoms of end stage liver disease or of cirrhosis during follow-up. No significant difference in prevalence of liver disease, was found between children with normal or abnormal ALT at BMT (relative risk [RR] = 1.04). HCV infection could be implicated in the etiology of chronic liver disease in 14/28 patients; 2 other patients were found infected by HBV alone (1 case) or combined with HCV (1 case). In the remaining 12 the etiology of chronic liver disease could not be defined. Posttransplant hepatitis B occurred in 4/111 children (3.6%), including a recipient from a donor who had been previously vaccinated against HBV, while no patient who had been vaccinated developed hepatitis B. The rate of posttransplant seroconversion to anti-HCV was 15%.
LIVER COMPLICATIONS are thought to be a major clinical problem in patients treated with autologous or allogeneic bone marrow transplantation (BMT). Mortality caused by hepatic failure ranges from 4% to 15% in different series of adult patients and the impact of morbidity is far from negligible, involving more than 80% of cases.1,2 A variety of etiologic factors may contribute to posttransplant liver disease, including drug toxicity, irradiation, viral, septic, and fungal infections, graft versus host disease (GVHD). In most of these conditions, clinical manifestations are rather similar and often do not allow discrimination of casualties in this complex setting.3 Many authors have tried to identify pretransplant risk factors that may be predictive of severe liver complications, such as the veno-occlusive disease of the liver (VOD) by retrospective and prospective studies. An abnormal transaminase value before BMT or the presence of pretransplant hepatitis C virus infection are reported to be independent risk factors for VOD in some published reports,4,5 but other studies do not confirm this association.6-8 Available data refer mainly to adult patients, while very few reports on young patient populations have been published.9 10
We have conducted a prospective study in children undergoing BMT for hematologic malignancies to assess incidence, etiology, severity, and outcome of liver disease. The study design also evaluated the serologic status of BM donors and the relationship between donor/recipient viral markers, including those acquired by previous hepatitis B vaccination and posttransplant serologic expression of hepatitis virus infection.
PATIENTS AND METHODS
Design of the study and patient recruitment. This study was designed as a prospective survey of hepatic complications in a cohort of consecutively enrolled children undergoing allogeneic BMT. The study was initiated in June 1985 and included all patients undergoing allogeneic BMT at the Department of Pediatric Hematology, S. Gerardo Hospital, Monza (University of Milan, Italy) between that date and June 1995. Patients were followed-up at regular intervals to death or to December 1996. During this follow-up, periodical evaluation of liver disease was performed by clinical and biochemical assessments. Markers of hepatitis B and C virus infection were evaluated in serum at the time of periodic check-up or using retrospective analysis of appropriately stored serum samples.
Patient characteristics. One hundred and eleven consecutive children were included during the 10-year study period. They received allogeneic BMT for the following hematological disorders: acute lymphoblastic leukemia (ALL, n = 68), acute myeloid leukemia (AML, n = 22), juvenile chronic myeloid leukemia (JCML, n = 2), chronic myeloid leukemia (CML, n = 7), severe aplastic anemia (SAA, n = 4), refractory anemia with blast excess (RAEB, n = 7), Histiocytosis X (n = 1). Mean age at the time of transplant was 9 years and 5 months (range: 11 months to 18 years). There were 73 boys and 38 girls. The allogeneic BM was obtained from siblings in 99 cases, from twins in 2 cases, from parents in 3 cases and from unrelated donors in 7 cases. Table 1 illustrates the clinical characteristics of these patients. Twenty-two children underwent BMT in first complete remission (CR), 55 in second CR, and 6 in third or further CR. Median duration of first CR, in cases transplanted in CR other than first, was 30 months from diagnosis (range 1 to 61 months). The median timing from second CR to BMT was 4.8 months, ranging from 1 to 14 months. Seven patients were treated in chronic phase of CML while 16 cases were transplanted in advanced disease (relapse, resistent disease or blastic phase of RAEB or JCML). Transplant protocols, including conditioning regimen, GVHD prophylaxis and antiviral prophylaxis, varied according to the year of transplant, disease status and type of BMT donor (related or unrelated). The majority of patients were conditioned with cyclophosphamide (CY), 120 mg/kg body weight given as two daily doses of 60 mg/kg plus hyperfractionated total body irradiation (fTBI) 12 Gy given as 200 cGy twice daily for 3 days, alone (16 patients), or in combination with high-dose cytarabine (HDARAC), 3 g/m2 for 2 days (14 patients), high-dose vincristine (VCR) 4 mg/m2 in 4 days (46 patients), etoposide (VP16) 60 mg/kg in 1 day (15 patients). Eleven patients were conditioned with busulphan (BU) 16 mg/kg given as 4 daily doses, plus CY 120 mg/kg given as two daily doses of 60 mg/kg. Melphalan 140 mg/m2 was added to Cy and BU in 5 more patients. Children with SAA were conditioned with CY 200 mg/kg given in divided doses on 4 days (4 cases). GVHD prophylaxis consisted of cyclosporine (CSA) (in 101 patients) or CSA plus methotrexate (in 7 patients receiving BM from matched unrelated donors and in 1 child with SAA) as currently used.11 No GVHD prophylaxis was given to the two children transplanted from twin donors. No antiviral prophylaxis was adopted in children transplanted before 1988 (21 cases). From 1989 on, patients undergoing BMT from family donors were treated with acyclovir 250 mg/m2 intravenously (IV) every 8 hours (for donor and patient negative for serum cytomegalovirus antibodies) or 500 mg/m2 IV every 8 hours (for cases with donor and/or recipient positive for anti-CMV antibodies) from the day before marrow infusion up to day + 30. In those cases transplanted from an unrelated donor, patients instead received foscarnet 30 mg/kg every 12 hours. No patient received prophylaxis for VOD or for fungal infections. Growth factors were not given on a regular basis, but only if clinically indicated.
All BM donors were tested for serum HBsAg, anti-HBs, anti-HBc and since June 1991, for serum anti-HCV. Thirty-one BMT donors and 31 BMT recipients, including 25 donor/recipient pairs, had been previously vaccinated (7 months to 5 years before inclusion in this study) against hepatitis B, according to Italian Public Health Policy.
Assessment of liver disease. In all 111 children, alanine aminotransferase (ALT), total and fractionated bilirubin, albumin, and prothrombin time were evaluated before the conditioning regimen, at 2 to 3 day intervals during the first month after transplant, weekly for the following 2 months, and every 2 weeks for a further 9 months. Patients were then evaluated at least every 3 months until the second year after BMT and every 12 months thereafter up to the last observation. Tests were performed at closer intervals when clinically indicated. At the same time intervals all patients underwent clinical evaluation for the presence of symptoms or signs of liver disease. Clinical criteria adopted for the diagnosis of liver failure, VOD, and GVHD have been previously published.6
Hepatitis B and C serum markers. HBV markers, including HBsAg, HBeAg, anti-HBs, anti-HBc, and anti-HBe were serially tested in serum by commercially available radioimmunoassay (Abbott Laboratories, North Chicago, IL).
Antibodies to HCV (anti-HCV) were detected by second generation Ortho-enzyme-linked immunosorbent assay (ELISA) tests (Ortho Diagnostic Systems, Raritan, NJ). ELISA positive sera were tested by RIBA-2 (Chiron Corp, Emeryville, CA) to confirm specificity and to define the pattern of antibody response against different viral agents. HCV-RNA was tested in serum by nested polymerase chain reaction (PCR) according to a previously described method12: two sets of primers, specific for the 5′ untranslated region (5′UTR) were used (external primers: 5′-GCCATGGCGTTAGT-ATGAGT-3′ (sense) and 3′-TCCAGAGCATCTGGCACGTA-5′ (antisense); internal primers: 3′-TATCCCACGAACGCTCACGG-5′ (antisense) and 5′GTGCAGCCTCCAGGACCCC-3′ (sense). Amplification products were visualized by ethidium bromide staining. Two normal sera and water were used as negative controls in each experiment and a known positive reference sample was also included. When HCV-RNA was amplified, the HCV genotype was then defined by spot hybridization with genotype specific oligonucleotide probes, as previously described.13
Blood components administered to the patients were obtained from HBsAg-negative volunteer donors. Anti-HCV was also tested from June 1991.
Statistical analysis. Descriptive statistics, such as location and dispersion measures, were calculated, when appropriate. The distributions of baseline characteristics in groups defined according to ALT levels were compared by means of Fisher exact test for association and Student's t-test. The relative risk (RR) of liver disease after BMT in patients with or without ALT elevation before BMT was estimated as an odds ratio.
RESULTS
Clinical outcomes. The median follow-up time after BMT for the 111 patients was 5.5 years, with a minimum potential follow-up of 1 year for each patient. Fifty-eight children died 2 to 1,645 days after they had undergone BMT (median time to death: 98 days) due to the following: leukemia relapse (n = 37), infection (n = 8), multiorgan failure (MOF ) (n = 4), graft failure (n = 3), adult respiratory distress syndrome (ARDS) (n = 3), pulmunary hemorrage (n = 2), GVHD (n = 1), car crash (n = 1). Fifty-three patients were still alive at December 1996, with a follow-up ranging from 485 to 4,001 days after transplant.
Evidence of liver disease before BMT. Sixty-three out of 111 children (57%) had normal ALT (≤42 IU/L) before BMT while 48 cases (43%) had abnormal values before conditioning regimen (mean ALT = 145.7 IU/L, SD 127.5). As shown in Table 2, the two groups were comparable regarding hematological diagnosis, disease status, age at transplant, and gender. None of the patients was HBsAg positive, while anti-HBc was detectable in 15/63 children with normal liver enzymes, two of whom also showed anti-HCV positivity, and in 8/48 patients with elevated ALT values. Besides the two anti-HBc and anti-HCV positive cases, two other patients with normal transaminases were found to be anti-HCV positive.
Liver disease after BMT. The frequency and type of liver disease occurring after transplant in the 111 children is described in Table 3. Overall, 86 patients (77%) had liver complications while in 25 (23%) clinical and biochemical parameters remained normal for the entire follow-up period. VOD leading to MOF occurred in 4 children (3.6%). Hematological diagnosis in these cases was ALL in second CR in two, RAEB in leukemic transformation in one, and AML in relapse in one. The conditioning regimen included CY and fTBI for all patients; in three of them a third drug was added (HDARAC, VCR, and VP16). Two children had normal and two had abnormal ALT levels at BMT. Therefore, these patients did not have peculiar characteristics distinguishable from the others that did not develop VOD.
ALT elevation was observed in 82 cases (74%): it was transient (<6 months) in 54 patients (49%) while 28 (25%) showed persistent or recurrent transaminases abnormalities. In the majority of cases liver disease was asymptomatic and was identified biochemically, while symptomatic acute hepatitis was observed in only seven children (6%). These patients underwent BMT with normal ALT and anti-HCV negative serology. Antibodies to HBV were detectable in five of them. During the acute hepatitic episode after BMT, no changes in HBV and HCV serology were observed in five patients, while of the two children who had negative serology for both HBV and HCV before transplant, one seroconverted to anti-HCV and the other to HBsAg positivity.
No patient developed clinical evidence of cirrhosis or chronic liver failure. Hepatomegaly and significant reduction of liver function tests were observed only in the four children who died of MOF. Median peak value of ALT was 267 IU/L, range 64 to 2,362 IU/L (normal value [nv] ≤42 IU/L); median bilirubin peak was 2.8 mg/dL, range 1.2 to 22.7 mg/dL (nv ≤ 1). Median timing of first ALT and bilirubin elevation occurred at day +15 and +10, respectively (day 0 = day of BM infusion).
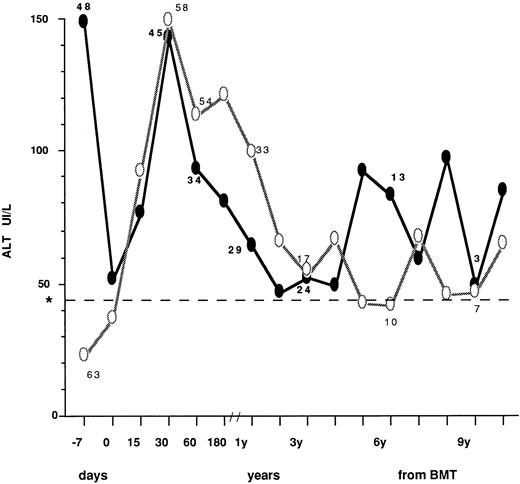
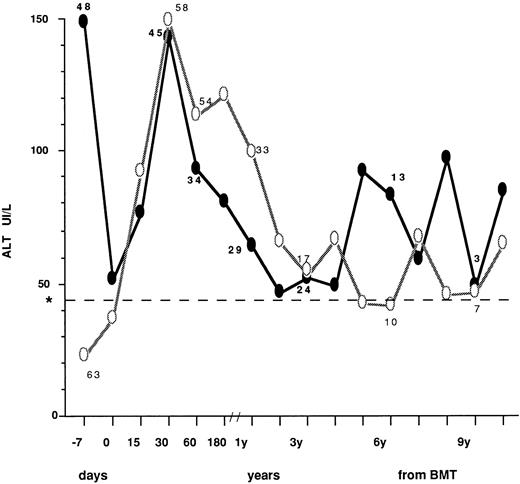
The ALT profiles after BMT in patients who had normal (63 cases) or abnormal (48 cases) values before transplant are described in Fig 1, where mean values at different time intervals are described. In patients with high pre-BMT levels, a sharp reduction was observed at day 0, most likely as a consequence of the conditioning regimen. After BMT, transaminase peak values were observed within 30 days in all patients. Thereafter, ALT profile showed a fluctuating pattern that was quite similar in the two groups. Table 4 describes the type of posttransplant liver disease in children undergoing BMT with normal versus abnormal transaminase levels, also in relation to pre BMT viral markers. Overall, during follow-up, 47 patients (78%) with normal ALT before BMT developed enzyme abnormalities after BMT that were transient (<6 months) in 32 (asymptomatic in 25 and clinically overt in seven) and more persistent in 15. Conversely, 11/48 children with abnormal ALT before BMT (23%) showed a persistent ALT normalization during follow-up after transplant. VOD leading to MOF was equally distributed in patients with normal or elevated ALT, while less severe VOD was not observed in the whole population. No significant difference in prevalence of liver disease, diagnosed as ALT elevation, was found between children presenting with normal and abnormal liver enzyme levels at BMT (RR = 1.04, P > .05). Of the 86 patients serologically negative for HBsAg and anti-HCV, 19 (22%) had no liver disease, 2 (2.3%) died with MOF and 65 (75.6%) developed liver disease that progressed to chronicity in 22. Six out of 21 patients positive for anti-HBc before transplant did not develop liver complications, 13 had transient or persistent ALT elevation, and two died of MOF. Of the four children positive for anti-HCV at transplant, two were also anti-HBc+. All had liver disease during follow-up (Table 4).
ALT profiles after BMT in children who had normal (○) or abnormal (•) values before transplant. The number of cases observed in the two groups is indicated at the following intervals: −7.0, 30, and 60 days; 1, 3, 6, and 9 years from BMT. Day 0 = day of marrow infusion. *Dotted line shows the ALT upper normal value (=42 IU/L).
ALT profiles after BMT in children who had normal (○) or abnormal (•) values before transplant. The number of cases observed in the two groups is indicated at the following intervals: −7.0, 30, and 60 days; 1, 3, 6, and 9 years from BMT. Day 0 = day of marrow infusion. *Dotted line shows the ALT upper normal value (=42 IU/L).
HBV and HCV serum markers during posttransplant follow-up. Fifty-seven children (51%) were negative for HBV antibodies at transplant. Anti-HBs alone was present in 33 cases (30%), anti-HBc in one (0.9%), and both in 20 (18%). After BMT only two patients (2%) remained negative for all HBV markers while anti-HBs alone was detected in four (4%) and 105 children (95%) became positive for anti-HBs and anti-HBc. HBV antibodies were detected transiently in 59/109 (54%) (possibly as a result of passive immunization) and persistently up to the end of follow-up, in 50 cases (46%). Four children (3.6%) became HBsAg positive: two developed acute hepatitis that resolved completely, one had chronic hepatitis, and the remaining one who was anti-HBs and anti-HBc positive before transplant, had received the marrow from an HLA identical sister vaccinated for HBV, and had normal transaminase values long after seroconversion. This patient became a chronic carrier and developed chronic hepatitis when he became also anti-HCV positive (Fig 2). Table 5 illustrates the effects of previous HBV vaccination of the BMT donor or recipient or both. The probability of developing HBsAg positivity after BMT appeared to be influenced by previous HB vaccination in recipients rather than donors although this difference did not reach statistical significance.
Serologic and biochemical profiles in a leukemic child who became an HBsAg, HBeAg carrier 7 months after BMT from a sister vaccinated for HBV.
Serologic and biochemical profiles in a leukemic child who became an HBsAg, HBeAg carrier 7 months after BMT from a sister vaccinated for HBV.
Data on anti-HCV, tested with second generation ELISA, with RIBA confirmatory test, included all patients; for those cases treated before 1991, stored frozen serial serum samples were investigated. Detection of HCV-RNA by PCR was performed more recently, and only in anti-HCV positive cases with available serum samples obtained before and after BMT. Before transplant, four children were anti-HCV positive (Table 4): one of them became negative following an acute hepatitic episode observed after cyclosporine withdrawal; the other three remained anti-HCV positive during follow-up. One of them had an acute hepatitis, followed by normal ALT values, while the other two showed a chronic hepatitis. Seroconversion to anti-HCV was documented in 16/107 cases (15%): three of them (19%) seroreverted to anti-HCV negativity. One did so after an acute hepatitis following withdrawal of immunosuppression, while in the other two serorevertion had no evident impact on their chronic, asymptomatic liver disease. The other 13 patients with persistent anti-HCV positivity had an acute (n = 3) or chronic (n = 10) hepatitis during follow-up. Two patients with chronic hepatitis had a transient biochemical worsening during the tapering of immunosuppression.
HCV-RNA could be investigated in 12 anti-HCV positive children. Ten of them resulted HCV-RNA positive (one before and after BMT and nine during follow-up), and these viraemic children developed acute (n = 1) or chronic (n = 9) liver disease after BMT. No patient had liver failure or a decompensated liver disease. HCV genotype was HCV one in eight, HCV two in one and could not be determined with our method in the last one.
Patients with chronic liver disease. Twenty-eight out of 53 (53%) patients alive at December 1996 showed an asymptomatic chronic liver disease, with recurrent or persistent ALT elevation lasting for more than 6 months. Fourteen out of 28 such cases were anti-HCV positive and HCV-RNA was investigated and detected in nine of them. One child was HBsAg and anti-HCV positive (Fig 2), one became HBsAg positive after BMT. Overall the cause of chronic liver disease could be attributed to HCV and/or HBV infection in 16/28 children (57%). In the remaining 12 patients, the etiopathogenesis of liver damage was still less obvious. The 12 children became anti-HBc and anti-HBs positive after BMT (eight transiently and four persistently) but the clinical significance of this finding remains obscure, as no time correlation between the appearence of HBV antibodies and the outcome of liver disease could be found.
DISCUSSION
Although there have been a number of reports, mainly retrospective, on the incidence and type of liver disease occurring after BMT in adult patients,4,5,6,12,14-16 few studies have been addressed to young patient populations and have often focused on particular aspects.7,9,10,17 18 Therefore, our study is the first survey on a large cohort of children followed prospectively after BMT, to assess morbidity and mortality for liver disease. All patients transplanted in our Unit during the study period were included, with no selection regarding pretransplant liver status or hematologic diagnosis.
The incidence of severe hepatic complications in our series was low (3.6%), and was independent of pretransplant transaminase levels and of infection with the hepatitis B or C virus. Predisposal towards severe posttransplant liver disease resulting from pretransplant hepatitis infection could not be fully evaluated as only a few infected patients underwent BMT. On the other hand, the influence of pretransplant ALT could be adequately assessed as about half of the cases presented with abnormal enzyme levels at BMT. The lack of any correlation between pretransplant liver disfunction and occurrence of VOD contrasts with other studies showing that ALT abnormalities had a significant impact on the occurrence of VOD,4,14,19 with even the severity related to enzyme levels.4-14 It has to be noted that other pretransplant factors such as fungal infection, Karnofsky performance score, the use of busulfan in conditioning regimen in adults and children, gender, age, etc, have been shown to be either important or insignificant as risk factors in different series.4,15-17,20 This disparity on the incidence, risk factors, and fatality rates from Center to Center is likely to be related to the inherent toxicity of different regimens, patient selection and even VOD definition.21 However, our data indicate that in pediatric patients, transplant outcome is not influenced by preconditioning enzyme levels, and therefore there is no reason to postpone or negate BMT on the basis of ALT elevation. Interestingly, we observed the same pattern of liver diseases and viral markers in a small series of 12 consecutive children treated with autologous BMT at our institution during the same time period. Indeed, no child developed VOD or any other severe posttransplant liver complications, although five out of 12 had elevated ALT values before BMT and one was anti-HCV positive. Seroconversion to anti-HCV positivity occurred in four cases (two with normal and two with abnormal ALT pre-BMT). As regards to HBV infection, no patient developed HBsAg positivity during the observation period, while HBV antibodies were found in 8/12 children before and 10/12 after BMT. Five patients had no liver disease during follow-up (one anti-HCV positive) while 7 developed a hepatitis that was acute in 3 and chronic in 4 of them (unpublished).
Our study enabled us to evaluate medium to long-term hepatic problems after BMT. Although severe events were rare, confirming earlier observations made in retrospective studies and more heterogeneous patient series1,6 in 78% of the cases an increase in transaminase levels was observed. Liver enzyme elevation was persistant in 28/82 cases. This liver disease was often asymptomatic and could only be detected biochemically. The etiology of such a condition could not always be defined and only in a subgroup did it appear to be related to HBV or HCV infection, while in the majority of cases other causes may have contributed. The observation that the highest ALT peak levels occurred within the first month posttransplant (Fig 1) would suggest that etiologic factors such as drug toxicity, acute GVHD, infection from bacterial, fungal, or nonhepatotropic viruses, total parenteral nutrition, could be involved.3
As regards to chronic liver disease, no signs of severe sequelae such as cirrhosis or hepatic decompensation have been observed so far, suggesting a rather benign and slow evolution. However, it has to be stressed that this favorable outcome may be overestimated, as it is well known that progressive liver disease has even been documented in patients with normal ALT22,23 and it can be fully evaluated only by means of liver biopsy. Continued follow-up of our cohort of patients will clarify whether these conclusions remain valid.
Chronic hepatitis was associated with anti-HCV positivity in 14/28 patients, confirming that HCV remains epidemiologically important even after the introduction of blood and marrow donor screening. In fact the rate of seroconversion has decreased when compared to the results obtained in a previous study24 (from 29% tested with first generation methods to 15% by means of the more specific and sensitive second generation ELISA) but the risk of becoming infected after BMT is still present.
In two additional children, chronic hepatitis could be related to either HBV infection alone (one patient) or in combination with HCV infection (one patient). In the remaining 12 cases, the etiology of chronic liver disease was less defined. These children became positive for HBV antibodies, but there was a lack of time correlation between seroconversion and hepatic events. As previously observed in this patient population, other causes such as GVHD, drug related hepatitis, or serologically atypical infection with HBV or HCV may contribute to chronic liver disease in this setting.12,25 26
The finding that four children became HBsAg positive after transplant, confirms that these patients are extremely prone towards becoming infected with blood-born viruses. This susceptibility is present even for HBV, even though blood and marrow donors have been screened before donation since the early 70's. In this respect, the results on the effect of previous vaccination against HBV of patients and/or BMT donors were not conclusive, although there was a trend in favor of recipient prophylaxis being more protective. These data, unlike previous reports on the efficacy of donor vaccination,27 together with the intriguing observation that HBV infection occurred also in one child who received BM from a vaccinated donor, would suggest that complex and poorly known immunologic events are involved in the immune response after BMT.
In conclusion, our results indicated that ALT abnormalities before BMT did not have a significant impact on the occurrence of severe VOD, which in turn was a rare event (less than 4%) in this unselected, prospectively followed patient population. On the other hand, less severe liver disease was very frequent (78%) and while clinically asymptomatic, progressed to chronicity in a relevant proportion of children. Chronic liver disease was related to HCV and HBV infection in more than 50% of patients, while its etiology in the remaining children is less definite.
Despite the screening of blood and marrow donors for HBV and more recently for HCV markers, the rate of posttransplant infection with those viruses indicates that viral hepatitis still remains an important clinical problem in this setting, although the prognosis of chronic HCV and HBV infection appears more benign in children when compared to adult patients.
ACKNOWLEDGMENT
The authors thank J. Upton for her secretarial and linguistic assistance.
Supported by “Progetto Epatiti Virali, Istituto Superiore Di Sanita” and “Comitato Ml Verga Per Lo Studio E La Cura Delle Leucemie Del Bambino.”
Address reprint requests to Anna Locasciulli, MD, Ematologia Pediatrica Nuovo Ospedale “S. Gerardo,” Via Donizetti, 106, 20052 Monza (MI) Italy.