Abstract
Although highly responsive, advanced stage follicular lymphoma (FL) is not curable with conventional treatment. This relative resistance is thought to be due to the t(14;18) that results in the constitutive overexpression of the death-inhibiting protein bcl-2. However, the observation that FL cells are sensitive to treatment in vivo and prone to apoptosis on in vitro culture questions whether bcl-2 alone is responsible for the pathogenesis and clinical behavior of this disease. Therefore, multiple genes are likely to be involved in both the lymphomagenesis and the clinical course of FL. We examined whether expression of other bcl-2 family genes might also be operative. Here, we show that FL cells display a different pattern of expression of bcl-2 family proteins from normal germinal center (GC) B cells that are thought to be their normal counterpart. FL cells express the death-suppressor proteins bcl-2, bcl-xL, and mcl-1; whereas GC B cells express bcl-xL and mcl-1 but also the proapoptotic proteins bax-α and bad. Although maintaining constitutive levels of bcl-2 and mcl-1, FL cells are not protected from apoptosis when cultured in vitro. Their propensity to undergo apoptosis is temporally associated with downregulation of bcl-xL. More importantly, activation of FL cells via CD40 not only prevents downregulation but increases the level of bcl-xL expression and results in promotion of survival. These results support the hypothesis that the overexpression of bcl-2 is not the only antiapoptotic mechanism responsible for the pathogenesis of FL. Survival of FL cells is determined by a number of death-inhibiting proteins, among which bcl-xL appears to have the most critical role. Moreover, these findings are consistent with the hypothesis that, although FL cells are malignant, they respond to microenvironmental signals such as CD40L that appear to contribute to their survival through the upregulation of death-inhibiting proteins.
THE CLINICAL ENTITY of follicular lymphoma (FL) is an enigma for both the clinician and biologist.1 Within the non-Hodgkin's lymphomas, this malignancy is among the most sensitive to low-dose oral alkylating agents, low-dose external beam radiation, and steroids. However, although the overwhelming majority of patients with advanced stage FL achieve a very good partial remission, many fewer achieve complete remission and few, if any, are cured using conventional aggressive combination chemotherapy.2 3 These clinical observations support the hypothesis that significant biologic heterogeneity exists within each patient's FL and that both chemosensitive and chemoresistant populations might account for these findings.
This clinical enigma has been further complicated by the discovery that the t(14;18), observed in most FLs,4 encodes for thebcl-2 gene,5,6 a major suppressor of apoptosis.7,8 Because B cells from bcl-2transgenic mice do not die in vitro and result in a lymphoproliferative state in vivo,9 it is contended that the constitutive expression of bcl-2 by FL cells is likely to be responsible for both the genesis of the malignancy as well as its relative chemoresistance. However, the uniform expression of bcl-2 by all FL cells contrasts with their exquisite sensitivity to treatment in vivo and their propensity to undergo apoptosis spontaneously in vitro and provides support for the notion that bcl-2 expression cannot by itself explain the pathogenesis and/or the clinical behavior of this malignancy.
Since the cloning of bcl-2 from FL cells, many other genes have been identified that positively and negatively contribute to cell death and these molecules are referred to as the extended bcl-2 family of cell death proteins.10 The best characterized of these include the death suppressors bcl-xL11 and mcl-1,12 the death regulator bad,13 and the death effectors bax-α,14bcl-xS,11 and bak.15-17 Although the molecular mechanism(s) by which these proteins prevent or induce cell death has yet to be elucidated, it has been shown that the survival of a cell is tightly regulated by their expression and association.10 Death is promoted when bax-α is in excess, such that bax-bax homodimers are formed. Both bcl-2 and bcl-xL prevent the accumulation of free excess bax-α by forming bcl-2–bax and bcl-xL–bax heterodimers. Similarly, mcl-1 is thought to function like bcl-2 and bcl-xL18,19 and appears to be relevant for the survival of normal peripheral blood B cells.20 Death can also be promoted by death regulators such as bad that bind to bcl-2 and bcl-xL, thereby displacing bax-α from its heterodimeric form, allowing its homodimerization, and thereby inducing apoptosis.13 The relevance of the above-noted pathways with regard to chemosensitivity and/or resistance of malignant cells is rapidly increasing. Supporting this notion is the observation that bcl-xL expression dramatically reduces sensitivity of tumor cells to chemotherapeutic agents, both in vitro and in vivo.21,22 Similarly, reduced expression of bax-α by solid tumors appears to correlate with chemoresistance and translates into shorter clinical survival.23-25
In the present report, we examined the expression of death suppressor, regulator, and effector proteins in FL cells. Here, we show that FL cells display a unique phenotype of predominantly antiapoptotic protein expression compared with the balance of antiapoptotic and proapoptotic proteins observed in normal germinal center (GC) B cells. Moreover, we show that the apoptosis of FL cells occurs in vitro despite their high constitutive bcl-2 levels and, in contrast, is associated with decreasing expression of bcl-xL. Finally, we show that one mechanism that can prevent the decrease in bcl-xLexpression is signaling via the CD40 molecule, suggesting that this pathway might be relevant to the survival of these cells in vivo. Taken together, these observations suggest that microenvironmental signals can alter the expression of survival proteins, thereby contributing to the clinical and biologic behavior of FL cells.
MATERIALS AND METHODS
Cells.
Lymph nodes were obtained from seven patients undergoing diagnostic biopsies who were diagnosed with follicular, small cleaved cell type lymphoma (working formulation). The malignant B cells were carrying the t(14;18) translocation, as shown by polymerase chain reaction (PCR).26 27 Tonsils were obtained, as discarded tissues, from children undergoing tonsillectomy. All tissue samples from patients were obtained following institutional guidelines at Dana-Farber Cancer Institute (Boston, MA), Brigham and Women's Hospital (Boston, MA), and Children's Hospital (Boston, MA).
Organs were cut with a scalpel blade and incubated two times with Collagenase IV and DNAse I (Sigma, St Louis, MO) for two rounds of 15 minutes at 37°C, 5% CO2. They were then passed through a fine wire mesh to prepare a single-cell suspension. Mononuclear cells were isolated by Ficoll-hypaque gradient (density, 1.077 g/mL; Pharmacia, Uppsala, Sweden). When not used directly for experiments at the time of preparation, they were frozen in liquid nitrogen.
Purification of normal and neoplastic B cells.
Mononuclear cells were first submitted to E rosetting with sheep red blood cells. Residual non-B cells were next removed from the E− population by incubation with a cocktail of anti-CD3, -CD11b, -CD14, -CD56 monoclonal antibodies (MoAbs), as described,26 27 followed by magnetic beads coated with goat antimouse IgG and IgM antibodies (BioMags; Perspective Biosystems, Framingham, MA) and application of a magnetic field. The purity of the isolated B cells was always greater than 97%. Total tonsillar B cells were further FACS-sorted into three different subsets, as described below.
Antibodies.
Surface staining of normal and neoplastic tissue cells was performed using the following antibodies: phycoerythrin (PE)-labeled anti-CD3 and fluorescein isothiocyanate (FITC) anti-CD4; biotin (BIO)-labeled anti-CD8; FITC-labeled anti-CD10 (CALLA); anti-CD14; FITC-, PE-, or BIO-labeled anti-CD19; PE-labeled anti-CD20; and PE-labeled anti-CD56 (Coulter, Miami, FL); FITC-labeled anti-CD24 (Immunotech, Marseille, France); and PE-labeled anti-CD38 (Becton Dickinson and Co, Mountain View, CA). BIO-labeled anti-human IgD was purchased from Southern Biotechnology (SBA, Birmingham, AL). Polyclonal FITC-conjugated rabbit anti-human IgM, anti-IgG, anti-IgA and anti-IgD, anti-human κ light chain, and polyclonal PE-conjugated rabbit anti-λ light chain were purchased from Dako (Carpenteria, CA). Polyclonal BIO-conjugated goat anti-human IgD [F(ab)2] and streptavidin-PE were obtained from SBA. Streptavidin-tricolor was purchased from Caltag Laboratories (South San Francisco, CA).
Cytokines.
Recombinant human interleukin-1β (IL-1β; 5 ng/mL; Endogen, Cambridge, MA), IL-2 (50 U/mL; Genetics Institute, Cambridge, MA), IL-3 (5 ng/mL), IL-6 (10 ng/mL), IL-11 (10 ng/mL; Genetics Institute), IL-4 (2 ng/mL), IL-7 (10 ng/mL; Immunex, Seattle, WA), and IL-10 (10 ng/mL; Genzyme, Cambridge, MA) were supplemented to the medium separately or in combination (IL-2 + IL-10).
Cell surface staining and cell sorting.
Three-color immunofluorescence analysis was used for identification of neoplastic populations in the pathological samples and identification of different B-cell populations within the tonsillar mononuclear cells. Tonsillar B cells were stained with BIO-labeled anti-IgD (followed by streptavidin TRICOLOR), PE-labeled anti-CD38, and FITC-labeled anti-CD19 antibodies. Naive B cells were identified as being CD19+, IgD+, CD38− cells; GC cells being CD19+, IgD−, CD38+; and memory B cells being CD19+, IgD−, CD38−.28 These three different subpopulations were isolated by FACS-sorting. Cell surface immunofluorescence, flow cytometric analysis, and cell sort were performed, as previously described,29 on a Coulter Elite (Coulter Co) at 4°C. Only cells exhibiting low forward angle and low right angle light scattering properties (lymphoid gate) were analyzed and, when needed, sorted.
Immunohistochemistry.
Cryostat sections of lymph nodes involved by FL were fixed in acetone for 10 minutes, washed with PBS, and incubated with anti-CD3, -CD20 (Coulter), and -CD40L (Pharmingen, San Diego, CA) MoAbs and the follicular dendritic cells (FDC)-specific MoAb, DRC-1 (Dako) for 30 minutes. Slides were then washed with PBS, incubated with BIO horse antimouse antibody (Vector Laboratories, Burlingame, CA), and then incubated with avidin-biotinylated peroxidase complex (Vector Laboratories) for 40 minutes, followed by reaction with diaminobenzidine/hydrogen peroxide. Sections were subsequently stained with 2% methyl green.
Cell culture.
Purified FL and GC cell populations were cultured in RPMI 1640 (Mediatech, Herndon, VA) supplemented with 10% fetal calf serum (FCS; PAA Laboratories Inc, Newport Beach, CA), 2 mmol/L L-glutamine and 15 μg/mL gentamicin (GIBCO BRL, Gaithersburg, MD), in the presence or in the absence of soluble CD40 ligand (CD40L)30 and cytokines. Soluble CD40 ligand was a generous gift of Dr Peter Lane (Basel, Switzerland).
Western blotting.
At the time of the initiation of culture and after 24 and 48 hours of culture, B cells were harvested (in 2 cases, lymphoma cells were also harvested after 3, 4, 5, and 7 days) and cell lysates were prepared with lysis buffer containing 10 mmol/L Tris-HCl (pH 7.6), 5 mmol/L EDTA, 50 mmol/L NaCl, aprotinin (5 μg/mL), pepstatin (1 μg/mL), soybean trypsin inhibitor (2 μg/mL) 1 mmol/L phenylmethylsulfonyl fluoride, and 1% NP-40 (Sigma). Whole cell lysates (3 × 106 B-cell equivalents per lane or equivalent amount of protein per lane) were analyzed on 12% gels in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Immunoblotting was performed using MoAbs or polyclonal antisera against bcl-2 (1:1,500, mouse monoclonal antibody; DAKO); bcl-x (1:1,000, rabbit polyclonal antiserum; Transduction Laboratories); bad (1:250, mouse MoAb; Transduction Laboratories, Lexington, KY); bax and mcl-1 (1:500, rabbit polyclonal antiserum; Santa Cruz, Santa Cruz, CA). As secondary reagent, horseradish peroxidase–antimouse IgG F(ab′)2 (1:3,000; Amersham, Arlington Heights, IL) or horseradish peroxidase–antirabbit IgG (1:10,000; Biorad, Hercules, CA) were used in the immunoblots. Immunodetection was performed using the Renaissance (TM) enhanced chemiluminescence system (NEN, Boston, MA).
Quantitative assessment of apoptosis.
Quantitative assessment of apoptosis on normal and neoplastic B cells was determined by terminal deoxynucleotidil transferase (TdT)–mediated dUTP-FITC nick end labeling (TUNEL)31 (Boehringer Mannheim GmbH, Mannheim, Germany) (see Fig 3) and with an Annexin V-based apoptosis detection kit (R&D Systems, Flanders, NJ).32
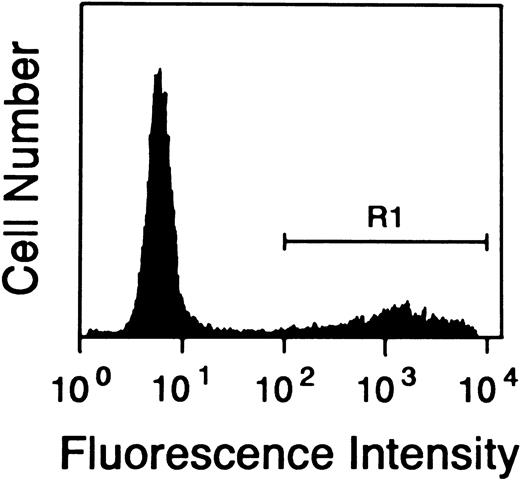
Quantitative assessment of apoptosis in FL cells cultured in media alone. FL cells were cultured as described and the percentage of apoptotic cells was assessed by TUNEL staining in flow cytometry. The figure shows the percentage of cells (R1 = 23.6%) TUNEL positive after 4 days of culture in one representative sample. Similar results were obtained from all seven of the patients studied.
Quantitative assessment of apoptosis in FL cells cultured in media alone. FL cells were cultured as described and the percentage of apoptotic cells was assessed by TUNEL staining in flow cytometry. The figure shows the percentage of cells (R1 = 23.6%) TUNEL positive after 4 days of culture in one representative sample. Similar results were obtained from all seven of the patients studied.
Cell cycle analysis.
Determination of the percentage of FL cells at each stage of the cell cycle was performed by assessment of DNA content after staining with ethidium bromide, according to an established protocol.33
RESULTS
Distinct patterns of Bcl-2 related proteins are expressed in functionally distinct B-cell populations.
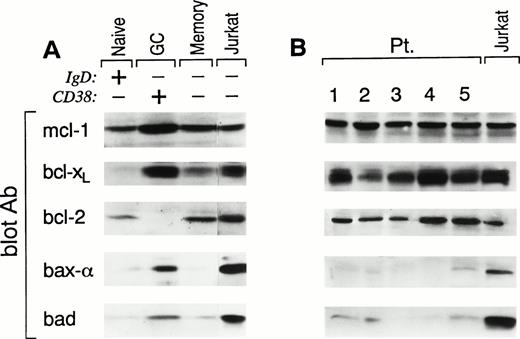
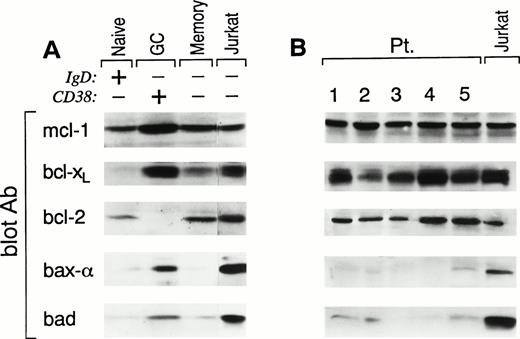
After an encounter with antigen, recirculating naive B cells migrate into secondary lymphoid organs where they downregulate their Ig receptors and differentiate into GC B cells.34 Within the GC, antigen-specific B cells rapidly mutate their Ig variable region genes. After positive selection by interaction with antigen presented by follicular dendritic cells, antigen-specific B cells clonally expand and then either differentiate into antibody-secreting plasma cells or long-lived memory B cells.34 The above-noted differentiative sequence results in functionally distinct populations of B cells that display unique cell surface phenotypic patterns,28 including (1) naive B, sIgD+CD38−; (2) GC B, IgD−CD38+; and (3) memory B, sIgD−CD38−. Purified B cells from three human tonsils were prepared and then analyzed by three-color FACS analysis (CD19, sIgD, and CD38) for B-cell populations. Of the total CD19+ cells, 45% to 55% displayed a naive, 30% to 45% displayed a GC, and 10% to 15% displayed a memory phenotype. Each of the above populations were sorted with a resulting purity of greater than 97%. Cell lysates from each population were prepared and analyzed by Western blot (3 × 106 cell equivalents or equivalent amount of protein per lane) for the expression of death suppressor (bcl-2, bcl-xL, and mcl-1), death regulator (bad), and death inducer (bax-α) proteins. Cell lysates from Jurkat cells, grown in log phase, were used as positive controls. As shown in Fig 1A, naive B cells expressed levels of mcl-1 and bcl-2 comparable to that seen in Jurkat, but bcl-xL was below the level of detection. Compared with Jurkat, bax-α was virtually undetectable and bad expression was approximately 10-fold less. In contrast, GC B cells express high levels of bcl-xL, mcl-1, and bax-α and detectable levels of bad but, as previously observed, do not have detectable bcl-2 expression. Finally, memory B cells, like naive B cells, express both bcl-2 and mcl-1 but, in addition, they also express bcl-xL, although at lower levels than GC B cells. Bax-α is undetectable and bad is comparable to the low levels seen in naive B cells. These results show that functionally distinct B-cell populations show discrete patterns of bcl-2 family protein expression and are associated with the biologic proclivity of these cells to survive and/or die in vivo.
Pattern of expression of bcl-2 family members in human tonsillar B-cell populations (A) and FL cells (B). Samples from (A) FACS-sorted CD38−IgD+ naive B cells, CD38+IgD− GC B cells, and CD38−IgD− memory B cells and (B) samples from highly purified FL cells of five representative patients were lysed and analyzed by SDS-PAGE and Western blotting using: anti–mcl-1 polyclonal antiserum (first panel); anti–bcl-x polyclonal antiserum (second panel); anti–bcl-2 MoAb (third panel); anti–bax polyclonal antiserum (fourth panel); or anti-bad MoAb (fifth panel). Expression of the different proteins was quantitated in each lane using a Scanner phosphoimager (Alpha Innotech Corp, San Leonardo, CA). Jurkat cell line (last lane in A and B), grown in log phase, was used as control. A total of 3 × 106 cell equivalents were used per test. Similar results were obtained when experiments were performed on tonsils or FL cells using an equivalent amount of protein per lane.
Pattern of expression of bcl-2 family members in human tonsillar B-cell populations (A) and FL cells (B). Samples from (A) FACS-sorted CD38−IgD+ naive B cells, CD38+IgD− GC B cells, and CD38−IgD− memory B cells and (B) samples from highly purified FL cells of five representative patients were lysed and analyzed by SDS-PAGE and Western blotting using: anti–mcl-1 polyclonal antiserum (first panel); anti–bcl-x polyclonal antiserum (second panel); anti–bcl-2 MoAb (third panel); anti–bax polyclonal antiserum (fourth panel); or anti-bad MoAb (fifth panel). Expression of the different proteins was quantitated in each lane using a Scanner phosphoimager (Alpha Innotech Corp, San Leonardo, CA). Jurkat cell line (last lane in A and B), grown in log phase, was used as control. A total of 3 × 106 cell equivalents were used per test. Similar results were obtained when experiments were performed on tonsils or FL cells using an equivalent amount of protein per lane.
Unbalanced expression of bcl-2 family proteins in FL.
Because FL has been considered to be the neoplastic counterpart of GC B cells,35 we examined the expression of the identical bcl-2 family proteins in seven patients with FL. For these experiments, lymph nodes that were highly infiltrated by FL cells (>65%), as assessed by immunohistochemistry, were selected and B cells were isolated (CD19 > 97%). Phenotypic analysis showed that B cells from each of these nodes were homogeneously expressing CD19, CD10, and CD38 and expressing monoclonal light chain. Moreover, all patients in this study expressed the t(14;18), as shown by an amplifiable PCR product for the bcl-2/IgH rearrangement. Using the identical approach used to analyze normal B-cell populations, FL B cells were lysed and analyzed for bcl-2 family protein expression by Western blotting (Fig 1B). As expected, all FLs studied expressed homogeneous, high levels of bcl-2 protein. In addition, both bcl-xL and mcl-1 were also expressed in FL cells at levels comparable to those observed in normal GC B cells. In contrast to GC B cells, both bax-α and bad are either undetectable or at very low levels.
In vitro apoptosis of FL cells is temporally associated with downregulation of bcl-xL, which is prevented by CD40 activation.
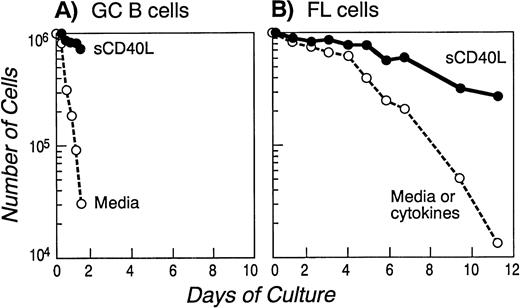
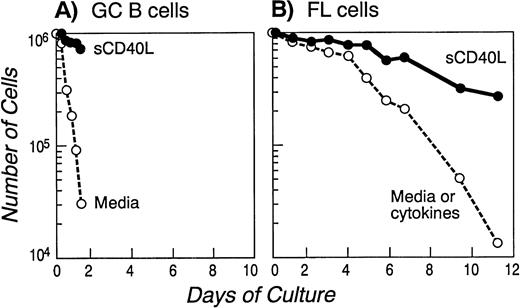
Consistent with previous reports,36 GC B cells cultured in media alone underwent apoptosis within 18 hours (>90% specific death; Fig 2A). In contrast, in vitro cultured FL cells have been reported to survive for 24 to 48 hours and then die by apoptosis over the next 5 to 7 days.37 We confirmed these results in samples from all seven patients and a representative cell survival curve is depicted in Fig 2B. Spontaneous FL cell death in vitro was mediated by apoptosis, as determined by DNA nick end labeling (TUNEL assay), to detect single-strand DNA fragmentation (Fig 3). Apoptosis was also confirmed in all the samples with the Annexin V method (data not shown). We attempted to determine whether the inability of FL cells to survive in vitro was due to the absence of a known cytokine; therefore, we repeated these experiments in the presence of functional concentrations of cytokines including IL-1β, IL-2, IL-3, IL-4, IL-6, IL-7, IL-10, and IL-11 and the combination of IL-2 and IL-10 (known to deliver a differentiative signal to GC B cells).38 As shown in Fig2B, none of the conditions described above significantly altered the FL cell survival curve.
Onset of apoptosis in GC (A) and FL cells (B) cultured in media alone or in the presence of cytokines: prolongation of survival in the presence of soluble CD40L. FACS-sorted GC B cells (A) or purified FL cells of a representative patient (B) were cultured in media alone or in the presence of several cytokines (see Materials and Methods) or soluble CD40L for the indicated time and apoptosis was assessed quantitatively by Trypan Blue exclusion at the indicated time points. Similar results were obtained with GC cells isolated from two other tonsils and with FL cells from six additional patients.
Onset of apoptosis in GC (A) and FL cells (B) cultured in media alone or in the presence of cytokines: prolongation of survival in the presence of soluble CD40L. FACS-sorted GC B cells (A) or purified FL cells of a representative patient (B) were cultured in media alone or in the presence of several cytokines (see Materials and Methods) or soluble CD40L for the indicated time and apoptosis was assessed quantitatively by Trypan Blue exclusion at the indicated time points. Similar results were obtained with GC cells isolated from two other tonsils and with FL cells from six additional patients.
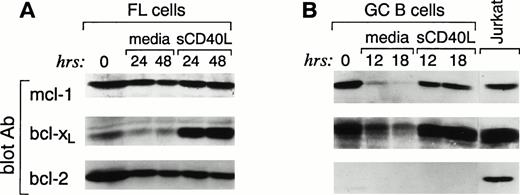
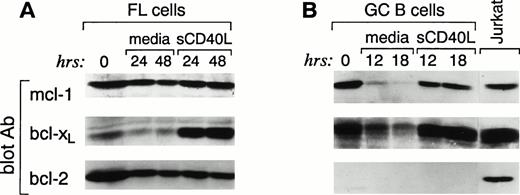
Because FL cells express high constitutive levels of death suppressors bcl-2, bcl-xL, and mcl-1 but no death effectors bax-α or bad, it appears paradoxical that these cells undergo apoptosis in vitro. Therefore, we postulated that proclivity to apoptosis might be associated with a change in the expression of bcl-2 family proteins during in vitro culture of FL cells. FL cells were cultured for 24 and 48 hours, and expression of the same bcl-2 family members was examined. FL cells cultured in media showed stable high expression of bcl-2 over the 48-hour culture period (Fig 4A). Likewise, the mcl-1 protein was not altered over the same time interval. In contrast, bcl-xL showed a rapid decline with threefold decrease from basal level within 24 hours. Neither bax-α nor bad was induced over this time interval (data not shown). Identical results were observed when, in three cases, the cells were also analyzed after culture in the presence of the above-mentioned cytokines (data not shown). These results suggest that the rapid decrease in bcl-xL is associated with the in vitro death of FL cells. Moreover, they suggest that the high constitutive expression of bcl-2 alone is insufficient to prevent apoptosis in vitro.
Changes in bcl-xL expression in FL cells (A) and GC (B) upon culture in media alone or in the presence of sCD40L; bcl-2 levels are not affected. Highly purified FL cells (A) and FACS-sorted CD38+ GC B cells (B) were cultured in media alone or in the presence of soluble CD40L. At the indicated time intervals, cells were isolated, cell lysates were prepared, and 3 × 106 cells were analyzed by SDS-PAGE and Western blotting using anti–mcl-1 polyclonal antiserum (first panel), anti–bcl-x polyclonal antiserum (second panel), and anti–bcl-2 MoAb (third panel). Expression of the different proteins was quantitated in each lane using a Scanner phosphoimager (Alpha Innotech Corp).
Changes in bcl-xL expression in FL cells (A) and GC (B) upon culture in media alone or in the presence of sCD40L; bcl-2 levels are not affected. Highly purified FL cells (A) and FACS-sorted CD38+ GC B cells (B) were cultured in media alone or in the presence of soluble CD40L. At the indicated time intervals, cells were isolated, cell lysates were prepared, and 3 × 106 cells were analyzed by SDS-PAGE and Western blotting using anti–mcl-1 polyclonal antiserum (first panel), anti–bcl-x polyclonal antiserum (second panel), and anti–bcl-2 MoAb (third panel). Expression of the different proteins was quantitated in each lane using a Scanner phosphoimager (Alpha Innotech Corp).
Previous studies have suggested that culture of both normal and neoplastic B cells with CD40L improves survival in vitro. We therefore sought to determine whether CD40 signaling would alter the expression of bcl-2 family proteins in GC and FL cells and whether such an effect would be temporally associated with protection from apoptosis. Purified GC B and FL cells were isolated as described above and cultured in the presence of media or soluble CD40L. As shown in Fig 2A and B, this resulted in significantly improved survival. After 12 to 18 hours of culture of GC cells in media alone, Western blot analysis showed a dramatic downregulation of mcl-1 and bcl-xL protein expression (Fig 4B). However, at this time point, virtually all cells were dead (Fig 2A). In contrast, CD40 signaling dramatically suppressed apoptosis and, at 18 hours, greater than 80% of the cells were alive (Fig 2A). Western blot analysis of CD40-activated GC B cells showed no change in the expression of bcl-2, which remained below the level of detection (Fig 4B). Mcl-1 expression did not significantly change at both 12 and 18 hours. In contrast, consistent with previous reports,39 CD40-mediated activation maintained and upregulated the expression of bcl-xL (Fig 4B).
We next examined whether CD40 signaling altered the expression of bcl-2 family proteins in FL cells as it did in GC cells. After CD40 activation, although survival was prolonged (Fig 2B), no increase in cycling cells was observed and less than 2% of either freshly isolated or CD40-activated FL cells were in S and G2/M stages of the cell cycle (data not shown). Western blot analysis showed no significant change in mcl-1 and bcl-2 at 24 and 48 hours of culture (Fig 4A). In contrast, the baseline bcl-xL expression was significantly upregulated at 24 and 48 hours (Fig 4A). However, in two cases, when the analysis was extended up to 7 days, bcl-xL protein began to decline after 4 days and restimulation at that time did not prolong the survival of FL cells and it did not upregulate bcl-xL protein expression (data not shown).
These results show that, although neoplastic, FL cells are still capable of responding to a physiologic CD40-mediated signal. The above results suggest that FL B cells might also receive such signals in vivo resulting in upregulation of bcl-xL.
CD40 ligand-positive T cells infiltrate neoplastic follicles in patients with FL.
CD40 ligand-positive T cells have been reported to be present in significant numbers in the follicles of FL.40 We confirmed the findings in lymph node sections stained with lineage-restricted MoAbs and MoAbs to CD40L, from six of the seven FL patients studied. Neoplastic follicles showed infiltrating T cells that were immunoreactive for CD40L (data not shown).
DISCUSSION
In the present report, we show that highly enriched FL cells display a unique pattern of expression of bcl-2 family proteins compared with the major functional populations of human B cells. Unlike GC B cells, which are thought to be the normal cellular counterparts of FL cells,35 FL cells express only death-suppressor proteins (bcl-2, bcl-xL, and mcl-1), whereas normal GC B cells also express bax-α and bad. Although all FL cells studied express high, constitutive levels of bcl-2, this did not protect them from undergoing apoptosis when cultured in vitro. In contrast, rapid downregulation of bcl-xL was associated with the propensity of FL cells to undergo apoptosis in vitro. Activation of FL cells via CD40, but not via any cytokine receptor tested, resulted in both maintenance and upregulation of bcl-xL and promotion of survival. These findings are consistent with the hypothesis that, although FL cells are malignant, they respond to microenvironmental signals that appear to contribute to their in vivo survival through the selective induction of death protective proteins, predominantly of bcl-xL.
Because FL and GC B cells share a common follicular organization (including presence of FDCs) as well as a unique cell surface phenotype characterized by the expression of CD19, CD20, CD10, and CD38, FL cells have long been considered to be the neoplastic counterpart of GC B cells.35 Similarly, both FL and GC B cells undergo somatic hypermutation and clonal expansion.34,41 In contrast to these similarities, FL and GC B cells behave differently in vivo and in vitro. In vivo, FL cells are largely G0 B cells with little, if any, propensity to die, whereas most GC B cells are actively cycling and are prone to apoptotic death. In vitro, both populations undergo apoptosis, with FL cells dying more slowly than GC B cells. Our results provide insight into the molecular events responsible for these differences. Although GC B cells do not express bcl-2, they do express the death suppressor bcl-xL and the death inducers bax-α and bad. The balance of these death-regulatory proteins appear to be responsible, at least in part, for the propensity of GC B cells to either undergo apoptosis or alternatively to differentiate and survive as memory B cells, depending on the signals they receive from their microenvironment. In contrast, highly enriched FL cells express only death-suppressor proteins (bcl-2, bcl-xL, and mcl-1) and no significant levels of either bax-α or bad. Therefore, FL cells appear to have an even more efficient mechanism with which to counter physiological death signal(s) because they express both potent death suppressors without any death effectors. Consistent with this hypothesis is the observation that, in bcl-x/bcl-2 transgenic mice, mature B cells are more effectively protected from death signals than they are in transgenic mice expressing either death suppressor alone.42 The capacity of bcl-2+bcl-xL+bax− FL cells to undergo apoptosis in vitro questions the current model in which bax-α is required to mediate death and bcl-2/bcl-xLprotect from apoptosis by heterodimerization with bax-α.14,43 In support of this observation are studies inbax-deficient and bcl-2–deficient mice showing that bcl-2 may not always act through interaction with bax-α, because the affected tissues ofbax−bax− mice are not identical to the affected tissues ofbcl-2−bcl-2−.44Moreover, heterodimerization with bax-α is also not always required for bcl-xL to exert its death-repressing activity.45,46 Other death-promoting proteins that have been recently cloned, such as bak15-17 and bik,47 48 can interact with bcl-2 and bcl-xLand therefore might be critical for the survival-promoting activity of these proteins.
Without question, bcl-2 plays an important role in normal and neoplastic B-lymphocyte survival in vivo. With increasing age,bcl-2–deficient mice display profound B and T lymphopenia.44 Conversely, increased B-cell survival in vivo and in vitro is observed in bcl-2-Ig–transgenic mice.9 In these mice, mature B cells accumulate with age, which is thought to be due to expansion of the memory B-cell pool rather than altered B-cell selection within the GC.49Although these mice uniformly develop B-cell hyperplasia, none develops low-grade FL and only some progress to high-grade lymphoma, after acquisition of a c-myc rearrangement.50 These results are consistent with the hypothesis that a bcl-2 translocation alone does not appear to be sufficient to induce FL or any other neoplasm. In agreement with this is the observation that clones harboring a PCR-amplifiable bcl-2 translocation can be detected in the secondary lymphoid organs51 and peripheral blood of normal individuals.52 Interestingly, the frequency of these translocations appears to increase significantly with age.53
Increasing evidence supports the conclusion that bcl-2 is not sufficient to regulate apoptosis in all cell types or in response to all stimuli. Expression of bcl-2 does not protect the phenotypically immature B-cell lymphoma WEHI-231 from anti-μ–induced cell death.54 Similarly, anti-μ–induced apoptosis of a Burkitt cell line is not accompanied by modulation of bcl-2 expression.55 Likewise, our results show that, despite constitutive high levels of bcl-2, FL cells undergo apoptosis in vitro. Moreover, no modifications in bcl-2 protein level can be detected accompanying the onset of apoptosis, whereas a rapid downregulation of bcl-xL is observed. This result parallels the previous observations in T cells in which a dramatic decrease in bcl-xL, but not bcl-2 protein levels, precedes apoptosis induced by IL-2 withdrawal.46
CD40/CD40L interaction is thought to play a key role for proliferation, isotype switching, memory formation, and rescue from apoptosis in both normal and malignant B cells.36,55-58 In all of the studies described above, a dissociation exists between the rescue from apoptosis achieved by CD40 stimulation and induction of bcl-2. In fact, bcl-2 is induced relatively late during CD40-mediated GC B cells rescue.36 Similarly, bcl-2 is not induced in Burkitt's lymphoma or WEHI 231 cell lines rescued by CD40 activation after anti-μ–induced apoptosis.36,55 In contrast, it has been recently suggested that upregulation of bcl-xL could be a key event in CD40-mediated survival in both normal tonsillar B cells39 and the immature B-cell lymphoma WEHI-231 cells.59
To date, the outcome of CD40 ligation of FL cells has been controversial. Intermediate and high-grade lymphoma B-cell lines appear to be inhibited in their growth by either anti-CD40 antibody or soluble CD40 ligand,60 whereas FL cells appear to be stimulated to grow.37 We show here that activation of FL cells by the soluble form of CD40L, but not via any cytokine receptor thus far tested, results in upregulation of bcl-xL and protection of apoptosis in vitro. In this respect, FL B cells appear to behave as normal GC B cells. Therefore, FL cells are capable of receiving microenvironmental signals that might contribute to their survival. The biologic relevance of these findings is supported by the observation that CD40L-expressing CD3+/CD4+ T cells are admixed with FL cells within the neoplastic follicles,40 as we also confirmed in infiltrated lymph nodes from all patients tested. We have also confirmed these findings by flow cytometric analysis, in which approximately 70% to 80% of the T cells were CD4+and 10% of these cells coexpress CD40L on their cell surface (data not shown). Therefore, CD40L-positive T cells might provide signals via CD40 recapitulating in vivo our in vitro findings. However, CD40L on T cells may not provide the only viability signal to FL cells. The physical interaction between FDC and GC B lymphocytes, as well as FDC and FL cells, has been shown to rescue the latter from apoptotic cell death.61 62 However, the pattern of CD40L expression in the GCs is clearly distinct from that of FDC. Therefore, the molecular interactions that regulate the antiapoptotic effect of FDCs are unknown.
Our results suggest that microenvironmental signals are at least in part responsible for the modulation of FL survival in vivo. Blockade of such signals may facilitate the entry of FL cells into the death pathway(s). Precise definition of such signals and pathways might potentially provide novel approaches to alter the sensitivity of FL to therapy as well as to develop novel treatment modalities for this disease.
ACKNOWLEDGMENT
We are grateful for the expert technical assistance of J. Borus for the PCRs. We also acknowledge the help of Michelle Yoon. We also acknowledge Dr F. Caligaris-Cappio for critical reading of the manuscript. We are thankful to Toby S. Meyerson, Esq of Paul, Weiss, Rifkind, Wharton & Garrison, and Jerry I. Speyer, President Tishman Speyer Properties Inc for their support to P.G.
Supported by National Institutes of Health Grants No. P01-CA66996 and CA55207 (A.S.F). P.G. is supported by a fellowship from the American-Italian Cancer Foundation; by Toby S. Meyerson, Esq of Paul, Weiss, Rifkind, Wharton & Garrison; and by Jerry I. Speyer, President, Tishman Speyer Properties, Inc.
Address correspondence to Paolo Ghia, MD, PhD, Dana-Farber Cancer Institute, D738, 44 Binney St, Boston, MA 02115.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.