Abstract
B-lymphopoiesis decreases with age. We studied how aging affects bone marrow stromal cells, because they provide the growth factors and cell contacts required for B-lymphopoiesis. No differences were noted in the cell-surface phenotype of young and old primary-cultured stromal cells. Fluorescence-activated cell sorter-purified stromal cells from old mice were deficient in the ability to support the proliferation of interleukin-7 (IL-7)–specific B-lymphoid cell lines. The kinetics of this response indicated that IL-7 was not immediately available from stromal cells of either age and was further delayed on aged stromal cells. The levels of IL-7 protein within stromal cells were equivalent between young and old animals, suggesting that the production of IL-7 was not altered by aging. Negligible amounts of IL-7 were found either freely secreted or in the extracellular matrix of cultures of young and old marrow. Contact between the lymphoid cells and the primary stromal cells was required for detectable proliferation, suggesting that cell contact was required for the release of IL-7. We propose that stromal cells regulate B-lymphopoiesis by limiting the amount of IL-7 available to the developing precursors. Therefore, we conclude that the age-related decrease in the function of bone marrow stromal cells is related to the impaired release of IL-7.
BONE MARROW stromal cells have been called “the essential cells of the hematopoietic microenvironment” and the “keys to hematopoietic development.”1,2 Stromal cell lines have been a rich source for the discovery of new growth factors and cytokines.2 It has been assumed that stromal cells regulate hematopoiesis by controlling the availability of these growth factors to the developing precursors. However, the hypothesis that stromal cells regulate hematopoiesis has not been thoroughly tested and the mechanism of this regulation is unknown. Stromal cell lines can be induced to increase the transcription of certain growth factors.2 It is also possible that stromal cells have the capacity to limit the secretion and availability of the protein products. Therefore, the hypothesis that stromal cells regulate hematopoiesis has not been rigorously tested and an understanding of the mechanism(s) of any regulatory process is lacking.
Complicating the study of stromal cells is the fact that the stroma of the marrow consists of many different cell types. A predominant type of stromal cell is the adventitial reticular cell (hereafter the only cell we term stromal cell). This cell is alkaline phosphatase positive and has extensive processes that adhere to many hematopoietic cells.3 One important adhesion molecule on stromal cells is vascular cell adhesion molecule-1 (VCAM-1) (CD106).4,5 Stromal cells are the only cells in the marrow that produce interleukin-7 (IL-7).6 Because both stromal cell contact and IL-7 are essential for B-lymphopoiesis, stromal cells are required for B-lymphopoiesis.7 8 Therefore, the study of the production of IL-7 by stromal cells provides a unique opportunity to determine if stromal cells regulate B-lymphopoiesis by controlling the availability of IL-7 or if stromal cells are just the constitutive source for this growth factor.
Stromal cells in the marrow are rare (∼15,000 cells per femur, or 0.125% of the marrow cellularity).6 In a marrow cell suspension, stromal cells are mostly contained in tight aggregates, in which early stem cells and selected hematopoietic precursors are sequestered.5,6,9 Therefore, it is very difficult to study the function of freshly isolated stromal cells. However, long-term bone marrow culture systems have been developed that mimic certain characteristics of the marrow microenvironment.10-12 The long-term bone marrow culture system for B-lymphocytes (LTBMC-B), as developed by Cheryl Whitlock and Owen Witte,11,12 has a stromal layer consisting of macrophages and stromal cells.13-15 These primary stromal cells are the culture equivalent of the adventitial reticular cells of the marrow in that they express alkaline phosphatase activity, VCAM-1, and α-actin and are the only source of IL-7 within LTBMC-B.15 Because stromal cells can be easily purified from LTBMC-B in sufficient numbers to study their function, we have used the LTBMC-B system as a model to determine if stromal cells regulate B-lymphopoiesis by limiting the availability of IL-7.
One physiologic example of how stromal cells may participate in the regulation of B-lymphopoiesis is aging. It has recently been appreciated that B-lymphopoiesis, like T-cell production in the thymus, decreases with age.16-22 In contrast, myelopoiesis and erythropoiesis appear to remain constant as an animal ages.23,24 Our previous work showed that one component of the mechanism for the age-related decrease in B-lymphopoiesis is a reduction in the ability of stromal cells to support the proliferation of pro-B cells.17 We hypothesized that the production of IL-7 by stromal cells is the underlying mechanism for this functional impairment. One group has already suggested that there is an age-related decrease in the amount of IL-7 in LTBMC-B.25However, those investigators only examined the conditioned media from intact LTBMC-B, which precludes knowing if the decreases found were due to changes in the amount of IL-7 produced per stromal cell, the number of stromal cells present in the cultures, or changes in the uptake of IL-7 by the B-cell precursors present. It is therefore imperative to examine the activities of purified stromal cells, because recently we reported age-related changes in the number of stromal cells per LTBMC-B and in the response of B-cell precursors from LTBMC-B to IL-7.26 Most importantly, the investigators from that previous study never showed that the response by the indicator line to LTBMC-B supernatant was specific to IL-7. Synergistic factors, such as insulin-like growth factor-1 (IGF-1), stem cell factor (SCF), and flt3-ligand (FL), act on B-cell precursors only in combination with IL-7.27-30 Therefore, it is unknown if the activity measured in their assays was that of IL-7 alone or of IL-7 in combination with another factor. These weaknesses prevent accurate conclusions about changes in the production of IL-7 by bone marrow stromal cells with increasing age.
The overall purpose of the current study is to directly test if the production of IL-7 by stromal cells is altered with age. The results suggest that the mechanism for the age-related decline in stromal cell function involves the release of IL-7 rather than its production. IL-7 does not appear to be constitutively secreted by stromal cells from young or old animals. Instead, it appears that the secretion of IL-7 by aged stromal cells is delayed in comparison with young stromal cells, and the availability of IL-7 requires cell-cell contact with B-cell precursors. This finding suggests that stromal cells have the ability to limit the release of IL-7, which we propose is how stromal cells regulate B-lymphopoiesis.
MATERIALS AND METHODS
Animals.
Female BALB/c mice 1 and 24 months of age were obtained from the National Institute on Aging (Bethesda, MD). IL-7 −/− mice and their wild-type, age-matched controls (IL-7 +/+) were obtained from Dr R. Murray (DNAX, Palo Alto, CA). Upon receipt, the animals were housed at the Animal Research Facility at Loyola University (Chicago, IL). The animals were killed between 4 and 10 days after receipt. All animals were visually inspected for splenomegaly, lymph node enlargement, and tumors. Mice presenting these conditions were removed from these studies.
Preparation of Whitlock-type LTBMC-B.
Whitlock-type LTBMC-B11,12 were initiated from pooled femoral bone marrow of at least 7 female mice 1 or 24 months of age and were grown in RPMI 1640 supplemented with 5% fetal bovine serum (selected lot no. 11112473; Hyclone, Logan, UT), L-glutamine, penicillin and streptomycin, and 5 × 10−5 mol/L β-2-mercaptoethanol. By 4 weeks, a complex adherent layer, consisting of stromal cells and macrophages, had formed and was supporting ongoing B-lymphopoiesis. In some cultures, murine recombinant IL-7 (rIL-7; Genzyme, Cambridge, MA) was added at all culture feedings beginning at 2 weeks after initiation at a final concentration of 0.5 ng/mL. Supplementation with IL-7 began 2 weeks after culture initiation to prevent the maintenance and expansion of already committed B-cell progenitors in the marrow preparations. The final concentration of 0.5 ng/mL was chosen because it will stimulate B-cell progenitors but is fourfold lower than the minimum level needed to stimulate macrophage progenitors.31 Lymphoid cells were removed by washing with 0.02% EDTA and enumerated by trypan blue exclusion and phase-microscopy.
Discrimination of stromal cells by flow cytometry.
Stromal cells in LTBMC-B were differentiated as described before15 based on differential forward versus side scatter and the uptake of acetylated low-density lipoprotein (acLDL), labeled with 1,1′-dioctadecyl-1-2.3.3′.3-tetramethyl-indocarocyanine perchlorate (DiI; Biomedical Technologies [Stoughton, MA] or Molecular Probes [Eugene, OR]). LTBMC-Bs were incubated for 3 hours with 5 μg acLDL at 37°C and 7.5% CO2. Lymphoid cells were removed by the addition of 0.02% EDTA. The adherent cells (stromal cells and macrophages) were then harvested by treatment with either 0.25% trypsin-EDTA (Life Technologies, Grand Island, NY; in cell sorting experiments) or a second treatment with 0.02% EDTA (for phenotypic analyses), followed by gently scraping with a silicon rubber policeman. The live stromal cell population was discriminated on a FACStar Plus (Becton Dickinson, San Jose, CA) using 488 nm excitation from an argon laser and detected using a 575-nm band pass filter. All analyses were performed 4 to 7 weeks after culture initiation.
Phenotypic examination of stromal cells.
After acLDL treatment and harvesting as described above, aliquots of the adherent cells from LTBMC-B were incubated on ice with the following antibodies: MK/2 (monoclonal anti–VCAM-1, rat IgG1; ATCC, Rockville, MD), polyclonal rabbit anti-SCF (Genzyme), or monoclonal hamster anti-murine stromal cell antibodies. The hamster anti-stromal cell monoclonal antibodies were derived from a fusion of splenocytes from Armenian hamsters, which had been immunized with stromal cells, and mouse myeloma P3X63Ag8.653. The antibody-bound adherent cells were then incubated with the appropriate biotinylated secondary antibody, donkey anti-rat IgG, donkey anti-rabbit IgG, or goat anti-hamster IgG [all F(ab′)2; purchased from Jackson ImmunoResearch, West Grove, PA], followed by streptavidin-allophycocyanin (APC; Becton Dickinson or Pharmingen [San Diego, CA]). The APC was excited at 647 nm from a krypton laser and emission was detected through a 670-nm band pass filter. All data were gated on the live stromal cell population.
Proliferation of IL-7–dependent pre-B–cell lines.
Four independently derived pre-B–cell lines (cμ+sμ−; BC76, BC77, BC715, and 2E8; all gifts of P.W. Kincade, Oklahoma Medical Research Foundation, Oklahoma City) were used throughout these experiments. These cells were derived from LTBMC-B initiated with marrow from BALB/c mice. For the characterization of growth factor responsiveness of these cells, 5 × 104 cells were cultured for 72 hours in 100 μL final volume of media with the following recombinant cytokines: IL-7, SCF, FL (all from Genzyme), or IGF-1 (Biosource International, Camarillo, CA). Where noted, conditioned media from stromal cells (as described below) were titrated into the usual media. One microcurie of 3H-thymidine (20 Ci/mmol; NEN Research, Boston, MA) was added to each well 4 hours before harvesting onto glass fiber filter strips (Cambridge Technology, Watertown, MA). The amount of 3H-thymidine incorporated was determined with a 1900CA Tri-Carb liquid scintillation analyzer (Packard, Meriden, CT).
Stromal cell-mediated proliferation of IL-7–dependent pre-B–cell lines.
Ten thousand primary stromal cells were directly fluorescence-activated cell sorter (FACS)-sorted from LTBMC-B into 96-well flat-bottom plates. Three days after the stromal cells were sorted, all of the media was removed (stromal cell conditioned media [SCCM]) and stored at −20°C. IL-7–dependent pre-B cells (1 × 104; as described above) were added to the stromal cells in 100 μL of media and cocultured with the stromal cells for 4 days. For experiments testing the necessity of cell-cell contact for stromal cell activity, 24-well plates were used with either 25,000 or 50,000 stromal cells per well. An equal number of IL-7–dependent pre-B cells were added, either in direct contact with the stromal cells or separated by 0.45-μm pore size membrane (Fisher, Pittsburgh, PA). The stromal cell lines RS2 and RS4 were derived in our laboratory by FACS sorting stromal cells from LTBMC-B at limiting dilution. In experiments using these stromal cell lines, the stromal cells were plated 24 hours before the addition of the IL-7–dependent pre-B cells. In some experiments, either murine rIL-7 (Genzyme) or a neutralizing mouse anti-human IL-7 monoclonal antibody (M25; cross-reactive against mouse IL-7; Genzyme) was added to the coculture at the time of culture initiation. The cells were harvested by treatment with trypsin and the lymphoid cells were identified and enumerated by trypan blue exclusion and phase microscopy. For cell cycle analysis, the cells were fixed with 200 μL phosphate-buffered saline (PBS), 200 μL heat-inactivated fetal bovine serum, and 1.2 mL 70% cold ethanol for 1 to 4 days at 4°C. The cells were then washed twice and stained with 0.5 mg/mL propidium iodide with 0.1 nmol/L EDTA and 0.05 ng/mL RNAse A. Cells were stained in the dark at room temperature for 1 to 3 hours before FACS analysis.
Measurement of secreted mouse IL-7 by enzyme-linked immunosorbent assay (ELISA).
We developed an ELISA for mouse IL-7 using commercially available antibodies. Immulon 4 microtiter plates (Dynatech Laboratories, Chantilly, VA) were coated overnight at 4°C with 5 μg/mL monoclonal mouse anti-human/mouse IL-7 antibody (M25; Genzyme) in 50 μL per well. The plates were washed with PBS using a microtiter plate washer (Elcatech, Winston-Salem, NC) and patted on paper towels to remove excess liquid. Nonspecific binding was diminished by blocking each well with 200 μL of 20% (vol/vol) fetal calf serum in PBS for 2 hours at 37°C. The plates were then washed as described previously. For the standard curve, twofold serial dilutions were made with murine rIL-7 (Genzyme) diluted in culture media for final IL-7 concentrations ranging from 25 to 0.049 ng/mL. Forty microliters of each concentration was added per well. Culture medium alone served as a negative control. For each sample to be tested, 40 μL of supernatant or extract was placed into each of three wells. Plates were incubated at 37°C for 2 hours and then washed with PBS containing 0.05% (vol/vol) Tween 20. Fifty microliters of goat anti-murine IL-7 antibody (lot no. BH03; R&D Systems, Minneapolis, MN) at a final concentration of 5 μg/mL was added to each well and incubated at 37°C for 45 minutes. The plate was then washed with PBS containing 0.05% Tween 20 and patted dry. This pairing and order of anti–IL-7 antibodies gave the best results of six combinations tried (data not shown). The goat anti-murine IL-7 antibody was detected with 50 μL of 2 μg/mL of mouse anti-goat IgG conjugated with biotin (Jackson Immunoresearch). The plate was incubated at 37°C for 45 minutes and washed with PBS containing 0.05% Tween 20 and patted dry. Fifty microliters of a 1:1,000 dilution of streptavidin-alkaline phosphatase (Southern Biotechnology, Birmingham, AL) was added to each well. The plate was then incubated at 37°C for 30 minutes. The plate was washed with PBS containing 0.05% Tween 20 and patted dry, followed by a wash with PBS alone and patted dry. P-nitrophenyl phosphate (Sigma, St Louis, MO) was mixed at 1 mg/mL with 10 mmol/L diethanolamine, 0.5 mmol/L MgCl2, pH 9.5, and the solution was used to show the streptavidin-alkaline phosphatase. In some experiments, the sensitivity was further enhanced with GIBCO/BRL ELISA Amplification System as substrate (Life Technologies). The optical density of each well was read at 405 nm with a Vmax Kinetic Microplate Reader (Molecular Devices, Menlo Park, CA), and the data were analyzed on Softmax version 2.3 (Molecular Devices) for Macintosh. The instrument was set up for automatic plate shaking. Standard curves were read on a four-parameter curve. Experimental samples were plotted on the resulting standard curve and were considered to be quantitative if found on the linear portion of the curve. Those on the tail of the curve were determined to be qualitatively positive for IL-7. Those below the tail of the curve were determined to be negative for IL-7.
Extraction of IL-7 from the extracellular matrix of LTBMC-B.
The extraction of IL-7 from the matrix of LTBMC-B was based on the method described by Clarke et al.32 LTBMC-B were grown in 100-mm2 tissue culture dishes. All supernatant was aspirated from the cultures and replaced with 2 mL of sterile 0.6 mol/L NaCl in Dulbecco's PBS without calcium and magnesium. The cultures were sealed with Parafilm (American National Can, Neeneh, WI) and incubated on ice for 2 hours with gentle rocking. The salt solution (extract) was removed under sterile conditions and 1 mL was placed into two siliconized 1.7-mL microcentrifuge tubes. The extract was centrifuged at 13,000 rpm for 5 minutes. The supernatant was transferred to a clean siliconized microcentrifuge tube and stored at −20°C until ready to be tested in an IL-7 ELISA.
IL-7–dependent proliferation of freshly isolated pro-B cells.
B220+CD43+IgM− pro-B cells were isolated from fresh marrow of young mice by flow cytometry as described previously.17 The following antibodies were used: rat-antimouse CD45R/B220 (RA3-6B2) conjugated with phycoerythrin, rat-antimouse CD43 (S7) conjugated with biotin (both from Pharmingen), and fluorescein-conjugated affinity-purified donkey antimouse IgM (μ-chain specific; Jackson ImmunoResearch, West Grove, PA). The anti-CD43 was shown with streptavidin-APC that was purchased from Becton Dickinson. Ten thousand sorted pro-B cells (<2% contaminating sIgM+) were cultured in the presence of IL-7 and stromal cell conditioned media in 100 μL total volume at 37°C and 7.5% CO2 for 4 days. The lymphoid cells were harvested and enumerated using trypan blue exclusion and phase microscopy.
Immunofluorescent staining for cytoplasmic IL-7 analyzed using flow cytometry.
The procedure for cytoplasmic FACS staining for IL-7 was based on that of Prussin and Metcalf.33 AcLDL labeling and harvesting of adherent cells with trypsin were performed as above, except that 10 μg/mL Brefeldin A (Sigma) was included during the labeling protocol and the labeling procedure was extended to 4 hours. The adherent cells were fixed with 4% cold paraformaldehyde for 10 minutes. All subsequent washes contained 0.1% saponin (Sigma) and 0.01 mol/L HEPES (Life Technologies). The nonspecific uptake of IgG staining by the cells was blocked with 10% donkey serum and 100 μg/mL mouse Ig (Jackson Immunoresearch) for 10 minutes at room temperature. Cells were incubated with either 1 μg rabbit anti-human IL-7 antibody (affinity-purified, cross-reactive with mouse IL-7; Biosource) or purified rabbit IgG (Jackson Immunoresearch). The rabbit anti–IL-7 antibody and rabbit IgG were centrifuged at 100,000g for 1 hour to remove antibody aggregates. After incubation for 30 minutes, 5 μg/mL of biotin-conjugated donkey antirabbit Ig [F(ab′)2; Jackson Immunoresearch] was then added to the cells for 30 minutes. Streptavidin-APC (Becton Dickinson or Pharmingen) was used to show the biotinylated antibodies and analyzed on a FACStar Plus. The flow cytometry data were collected from the stromal cell-gated population. No specific staining for IL-7 was found without permeabilization of the stromal cells (data not shown). The levels of staining for cytoplasmic IL-7 were reduced by preincubating the rabbit anti-human/mouse IL-7 antibodies with a nonsaturating amount of murine IL-7 for 1 hour (data not shown).
Measurement of macrophage colony-stimulating factor (M-CSF) activity in stromal cell conditioned media (SCCM).
SCCM was collected as described above and used in a soft agar colony assay for macrophages (colony-forming units-macrophage [CFU-M]). Freshly harvested bone marrow cells from young (1 to 3 months of age) mice were placed in 0.3% soft agar with 20% SCCM as previously described.15 Colonies (>20 cells) with macrophage morphology were scored with an inverted microscope after 7 days of culture at 37°C. Recombinant M-CSF (Genzyme) was used as a positive control.
RESULTS
Previously, we found an age-related decline in the ability of bone marrow stromal cells to support pro-B–cell proliferation.17 This alteration in stromal cell function consistently occurred only between 12 and 24 months of age. Therefore, the present study compares stromal cells from young (1 month old, at the peak of B-lymphopoiesis) and old (24 months old) BALB/c mice. Our work has documented the relative homogeneity of the primary-cultured stromal cells in cell surface phenotype and production of cytokines.15 Because of the variation among stromal cell lines in both phenotype and function (Deryugina and Müller-Sieburg2 and personal unpublished observations), only freshly isolated stromal cells from primary Whitlock-type LTBMC were used throughout this study.
Bone marrow stromal cells do not change in composition or expression of cell-surface molecules with increasing age.
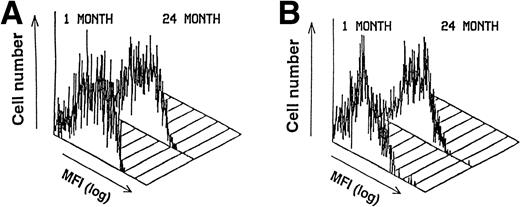
The cell surface phenotype of the stromal cells was examined to determine whether (1) the expression of any specific molecules was altered or (2) the composition of the stromal cell population was changed with increasing age. An adhesion molecule expressed on stromal cells in vitro and in vivo that is essential for the adhesion of B-cell progenitors to stromal cells is VCAM-1.4,5 The expression pattern and intensity of VCAM-1 on the stromal cells did not change with age (Fig 1A). SCF delivers a synergistic growth signal to B-cell progenitors in the presence of IL-7 and can participate in the adhesion between B-cell progenitors and stromal cells.30 One isoform of SCF is also expressed on the surface of the stromal cells.34 35 Examination of membrane-bound SCF by flow cytometry showed that the relative levels of SCF also did not change with age (Fig 1B). We also tested a panel of 11 new monoclonal antibodies directed against antigens on stromal cells that are currently being characterized. Each of these antibodies binds 75% to 100% of the stromal cells from LTBMC-B. The expression and distribution of each of the stromal cell antigens detected by these monoclonal antibodies were unaffected by the aging process (data not shown). Therefore, neither the composition of the stromal cells nor their cell-surface phenotype is greatly affected by aging.
The cell surface phenotype of primary-cultured stromal cells does not change with age. FACS histograms of the distribution of (A) VCAM-1 and (B) SCF on primary cultured stromal cells. Gates were set to include only live stromal cells based on FSC, SSC, and acLDL uptake. Eleven other less well-characterized monoclonal antibodies against stromal cells were also tested and no differences in either surface intensity or cellular distribution were found with increasing age (data not shown).
The cell surface phenotype of primary-cultured stromal cells does not change with age. FACS histograms of the distribution of (A) VCAM-1 and (B) SCF on primary cultured stromal cells. Gates were set to include only live stromal cells based on FSC, SSC, and acLDL uptake. Eleven other less well-characterized monoclonal antibodies against stromal cells were also tested and no differences in either surface intensity or cellular distribution were found with increasing age (data not shown).
Age-related changes in the ability of stromal cells to support the proliferation of IL-7–specific cell lines.
IL-7 is the primary lymphopoietic growth factor necessary for early B-cell proliferation,8 and it is produced solely by the stromal cells in Whitlock-type bone marrow cultures and in the bone marrow.6,15 Furthermore, the neutralization or absence of IL-7 activity results in a phenotype remarkably similar to that seen in the bone marrow of aged mice.7 8 Therefore, we wanted to determine if IL-7 production by stromal cells was changed with increasing age. As described below, very little or no IL-7 was found in stromal cell conditioned media. Therefore, we determined the amount of IL-7 produced by stromal cells by measuring the proliferation of IL-7–dependent, B-lineage indicator cell lines when directly in contact with stromal cells.
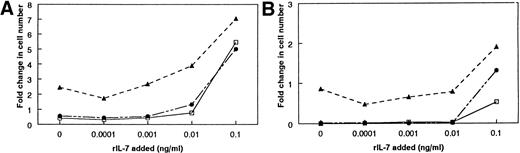
To accurately measure cytokines with a bioassay, the responder cell line cannot proliferate to any other factor present in the experimental system. Four independently derived pre-B–cell lines were initially used to minimize the chances that another stromal-derived growth factor(s) was participating with IL-7 in driving proliferation. Stromal cells from LTBMC-B produce SCF, IGF-1, and FL, which synergize with IL-7 to increase the proliferation of certain B-cell progenitors. We determined that the IL-7–dependent cell lines were unresponsive to SCF, IGF-1, and FL, either alone or in combination with IL-7 (Table1). Furthermore, none of the cell lines appeared to respond to any other soluble factors secreted by stromal cells (see below). The proliferation of the responder cell lines on stromal cells was completely inhibited by 0.1 μg/mL of neutralizing antibody against IL-7, demonstrating the IL-7 dependency of the cells (data not shown). However, it was still possible that the IL-7–dependent cell lines could respond to another growth factor secreted by stromal cells or present on the surface of stromal cells. Such a factor might elicit a proliferative response by itself or synergize with IL-7 to increase proliferation. Therefore, we tested these possibilities with stromal cells from IL-7 −/− mice (IL-7 −/− stromal cells). None of the IL-7–dependent cell lines proliferated in response to the IL-7 −/− stromal cells, confirming the requirement for IL-7 and showing that no other stromal cell-derived factor can induce an independent response (Fig2). Three of the cell lines (BC715, BC77, and 2E8) showed no evidence for proliferation to a stromal cell-derived synergistic factor when cultured in the presence of a suboptimal concentration of rIL-7 (Fig 2A). Therefore, the growth of these cells on stromal cells is a measure of IL-7 alone. On the other hand, culture of the BC76 cell line with IL-7 −/− stromal cells and exogenous rIL-7 did increase the amount of proliferation (Fig 2B), suggesting that BC76 is responsive to an unknown stromal-derived factor that synergizes with IL-7. Therefore, proliferation of BC76 on stromal cells measures a combined activity of the stromal cells and is not an accurate measure of the amount of IL-7 produced by the stromal cells.
Determination of the responsiveness of four IL-7–dependent pre-B cell lines to other stromal cell-derived factors. The response of the cells to IL-7 +/+ and −/− stromal cells was compared in the presence of suboptimal concentrations of rIL-7. Ten thousand pre-B cells were cocultured with 10,000 FACS-purified stromal cells for 4 days. The pre-B cells were then harvested and enumerated. (A) The response of BC715. (B) The response of BC76. The response of BC715 represents one of three cell lines that did not respond to any other growth factor besides IL-7. This was concluded because the presence of the IL-7 −/− SC does not appear to affect the amount of proliferation. Similar results were obtained with the pre-B–cell lines BC77 and 2E8. The response of BC76 represents a cell line that responded to another growth factor in combination with IL-7 because, in the presence of rIL-7, the amount of proliferation is greater on the IL-7 −/− SC than without any stromal cells. (□) No SC; (▴) IL-7 +/+ SC; (•) IL-7 −/− SC.
Determination of the responsiveness of four IL-7–dependent pre-B cell lines to other stromal cell-derived factors. The response of the cells to IL-7 +/+ and −/− stromal cells was compared in the presence of suboptimal concentrations of rIL-7. Ten thousand pre-B cells were cocultured with 10,000 FACS-purified stromal cells for 4 days. The pre-B cells were then harvested and enumerated. (A) The response of BC715. (B) The response of BC76. The response of BC715 represents one of three cell lines that did not respond to any other growth factor besides IL-7. This was concluded because the presence of the IL-7 −/− SC does not appear to affect the amount of proliferation. Similar results were obtained with the pre-B–cell lines BC77 and 2E8. The response of BC76 represents a cell line that responded to another growth factor in combination with IL-7 because, in the presence of rIL-7, the amount of proliferation is greater on the IL-7 −/− SC than without any stromal cells. (□) No SC; (▴) IL-7 +/+ SC; (•) IL-7 −/− SC.
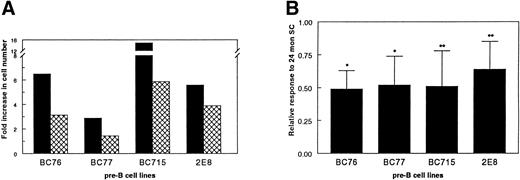
The IL-7–specific cell lines were used as a relative measure of the amount of IL-7 produced from stromal cells obtained from young and old mice. The growth of each of the indicator cell lines was significantly less on the stromal cells from old mice when compared with the stromal cells from young mice (Fig 3). The ability of the stromal cells from 24 month-old-mice to support IL-7–dependent proliferation averaged only 52% of that of the stromal cells from 1-month-old mice. These data indicate that the ability of stromal cells to produce and/or secrete IL-7 is diminished with aging.
Impaired ability of stromal cells from aged mice to support the proliferation of IL-7–dependent pre-B–cell lines. The IL-7–responsive pre-B–cell lines were used to compare the ability of stromal cells to support IL-7–mediated proliferation. Ten thousand pre-B cells were cocultured with 10,000 FACS-purified stromal cells (SC) from 1-month-old mice (▪) or 24-month-old mice () for 4 days. The pre-B cells were then harvested and enumerated. (A) The results from one of four or five experiments with each cell line. (B) The relative ability of aged SC to support IL-7–mediated proliferation as compared with young SC. The amount of proliferation supported by the young SC for each experiment was set as 1.00. The results shown are the average ± SD from four or five experiments with each pre-B–cell line. *P < .02; **P < .05.
Impaired ability of stromal cells from aged mice to support the proliferation of IL-7–dependent pre-B–cell lines. The IL-7–responsive pre-B–cell lines were used to compare the ability of stromal cells to support IL-7–mediated proliferation. Ten thousand pre-B cells were cocultured with 10,000 FACS-purified stromal cells (SC) from 1-month-old mice (▪) or 24-month-old mice () for 4 days. The pre-B cells were then harvested and enumerated. (A) The results from one of four or five experiments with each cell line. (B) The relative ability of aged SC to support IL-7–mediated proliferation as compared with young SC. The amount of proliferation supported by the young SC for each experiment was set as 1.00. The results shown are the average ± SD from four or five experiments with each pre-B–cell line. *P < .02; **P < .05.
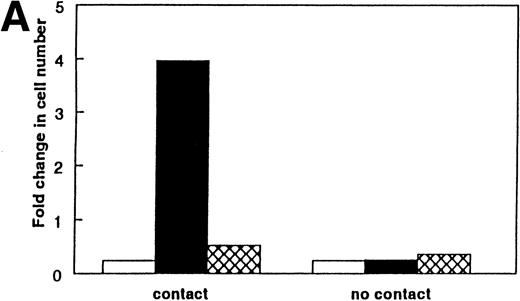
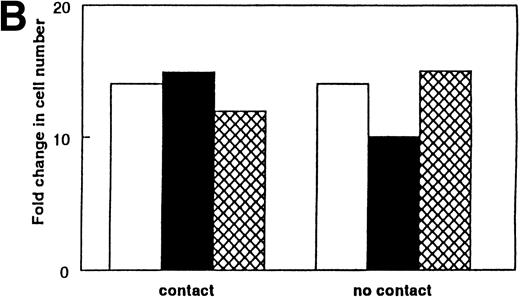
If available IL-7 is deficient in aged marrow, then the replacement of IL-7 in aged marrow should boost the numbers of lymphoid cells. This was tested in two ways. First, the addition of 0.025 to 0.1 ng/mL of rIL-7 to purified stromal cells from aged marrow resulted in levels of proliferation of IL-7–dependent cells approximately equal to that on stromal cells from young mice (data not shown). As a separate experimental system, we supplemented LTBMC-B with rIL-7. Whitlock-type LTBMC-B of aged (24 months old) marrow produced threefold fewer B-lymphoid cells per culture than cultures initiated from young (1 month old) marrow.26 The numbers of lymphoid cells within the supplemented cultures of aged marrow increased 17-fold to greater levels than that found in the unsupplemented LTBMC-B of young marrow (Fig 4). Identical supplementation of LTBMC-B of young marrow induced only a threefold increase (Fig 4). In vivo, the primary stage of B-cell deficiency in the aged marrow is the pre-B–cell stage.17 Supplementing cultures of aged marrow with IL-7 increased the numbers of cμ+sμ− pre-B cells 32-fold, whereas the pre-B cells in supplemented cultures of young marrow increased only 2-fold. Therefore, we conclude that, in LTBMC-B from aged mice, one reason for the reduced yield of B-cell progenitors produced by these cultures is insufficient available IL-7. We also predict that a diminished availability of IL-7 is one reason for the reduced number of pre-B cells in aged mice.17
Supplementation of LTBMC-B of marrow from young and old mice with IL-7 increases the number of B-lineage cells produced per culture. The addition of rIL-7 at 0.5 ng/mL for 2 weeks increased the number of B-lineage cells produced in LTBMC-Bs of young and aged marrow. The total number of lymphoid cells per culture was determined using trypan blue exclusion and phase microscopy. The results were calculated from 2 to 5 pooled cultures. (▪) No added IL-7; () added IL-7.
Supplementation of LTBMC-B of marrow from young and old mice with IL-7 increases the number of B-lineage cells produced per culture. The addition of rIL-7 at 0.5 ng/mL for 2 weeks increased the number of B-lineage cells produced in LTBMC-Bs of young and aged marrow. The total number of lymphoid cells per culture was determined using trypan blue exclusion and phase microscopy. The results were calculated from 2 to 5 pooled cultures. (▪) No added IL-7; () added IL-7.
Evaluation of IL-7 and soluble inhibitory factors secreted by stromal cells.
IL-7 is found only in a secreted form.36 Therefore, we next attempted to compare the levels of IL-7 that were secreted by the stromal cells from young and old mice. To detect secreted IL-7, we developed a sensitive ELISA for mouse IL-7. Detectable limits were 0.1 ng/mL of IL-7, and quantitation was derived from the linear portion of the standard curve, which fell at concentrations greater than 0.782 ng/mL (Table 2). IL-7 was detected in the media in 21 of 39 individual LTBMC-B cultures. However, in only 6 of these cultures were the levels great enough to be quantifiable (Table2). The paucity of IL-7 in the LTBMC-B did not appear to be solely due to its rapid uptake by the lymphoid progenitors present in the LTBMC-B, because IL-7 was detected in the conditioned media from FACS-purified stromal cells only occasionally (2 of 5 experiments) and was below quantitative limits (Table 2).
It was recently reported that IL-7 can bind to sulfated glycoaminoglycans of the extracellular matrix.32 The binding of IL-7 to heparin or heparin sulfate can be dissociated by treatment with 0.6 mol/L NaCl.32 In control experiments, we found that treatment with 0.6 mol/L NaCl efficiently eluted at least 30 ng of rIL-7 that was bound to 0.25 mg/mL of heparin (data not shown). We then used this elution procedure to determine if IL-7 was bound in the matrix of intact LTBMC-B. Even after concentration of the eluate, IL-7 was usually undetectable or present only in the qualitative range (<0.782 ng/mL; Table 2). To investigate the possibility that IL-7 was rapidly degraded in the stromal cell cultures, 10 ng/mL of rIL-7 was added to FACS-purified stromal cells for up to 3 hours. The concentration of recoverable IL-7 was reduced by 19% in the first 15 minutes, but did not significantly change thereafter. This suggests that the lack of IL-7 in the supernatant is not due to rapid degradation, binding to the matrix, or uptake by stromal cells.
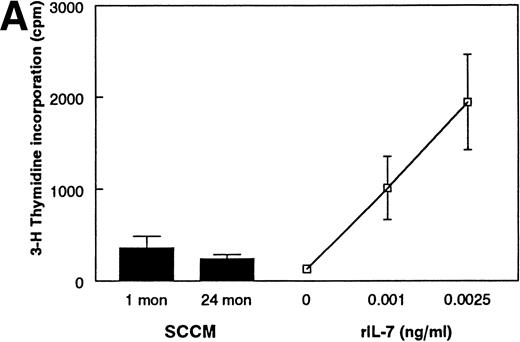
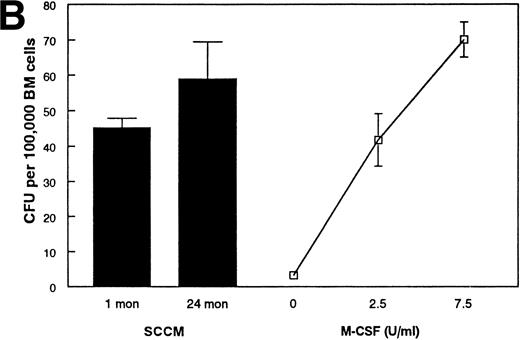
As an alternative method, we developed a bioassay using the IL-7–dependent cell lines to compare the amount of IL-7 in the conditioned media from purified stromal cells. Although this approach is extremely sensitive (routinely detectable at ≤1 pg/mL and quantitative at ≥10 pg/mL IL-7), IL-7 activity in the conditioned media from FACS-purified stromal cells from 1- or 24-month-old mice was below the level of detectability (Fig 5A). Furthermore, it appears that the absence of IL-7 activity in the SCCM is not solely due to the general degradation of proteins in the SCCM, because M-CSF activity is detected in SCCM from both age groups (Fig5B). These results suggest that quantitative amounts of IL-7 are not freely secreted by primary cultured stromal cells.
Examination of SCCM. (A) Measurement of IL-7 secreted by stromal cells from young and old mice. A bioassay for IL-7 was used to measure the amount of IL-7 secreted by FACS-purified stromal cells in 72 hours. SCCM was used at a final concentration of 10%. The results shown are the average ± SD of 3 to 4 replicate wells using the pre-B–cell line BC715; similar results were found using two other pre-B–cell lines, BC76 and 2E8. Similar results were also obtained when 20% and 30% SCCM were used and with SCCM from 4 separate collections (data not shown). The responses to 0.001 and 0.0025 ng/mL rIL-7 are shown for comparison. (B) Production of secreted M-CSF activity by stromal cells. The amount of M-CSF activity present in SCCM from FACS-purified stromal cells was determined by a CFU-M assay. The number of macrophage colonies having greater than 20 cells was determined using a dissecting microscope after culture in semisoft agar for 7 days. The data are reported as the average ± SD of 3 to 4 plates per group. The results shown are from one representative experiment of three performed with different collections of SCCM after 72 hours. The results shown used SCCM at 20%; no age-related differences were observed when the SCCM was used at 10% (data not shown). The responses to 2.5 and 7.5 U/mL rM-CSF are shown for comparison.
Examination of SCCM. (A) Measurement of IL-7 secreted by stromal cells from young and old mice. A bioassay for IL-7 was used to measure the amount of IL-7 secreted by FACS-purified stromal cells in 72 hours. SCCM was used at a final concentration of 10%. The results shown are the average ± SD of 3 to 4 replicate wells using the pre-B–cell line BC715; similar results were found using two other pre-B–cell lines, BC76 and 2E8. Similar results were also obtained when 20% and 30% SCCM were used and with SCCM from 4 separate collections (data not shown). The responses to 0.001 and 0.0025 ng/mL rIL-7 are shown for comparison. (B) Production of secreted M-CSF activity by stromal cells. The amount of M-CSF activity present in SCCM from FACS-purified stromal cells was determined by a CFU-M assay. The number of macrophage colonies having greater than 20 cells was determined using a dissecting microscope after culture in semisoft agar for 7 days. The data are reported as the average ± SD of 3 to 4 plates per group. The results shown are from one representative experiment of three performed with different collections of SCCM after 72 hours. The results shown used SCCM at 20%; no age-related differences were observed when the SCCM was used at 10% (data not shown). The responses to 2.5 and 7.5 U/mL rM-CSF are shown for comparison.
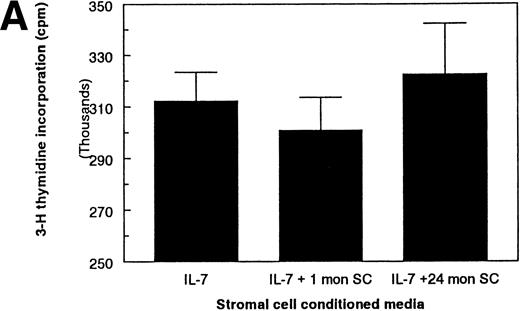
B-lymphopoiesis is regulated by both positive and negative regulators (reviewed in Kincade37). Therefore, the reduced amount of proliferation on the stromal cells from aged mice could also be due to an increase in the concentration of these inhibitors. This possibility was tested by examining the activity of the stromal cell conditioned media on the proliferation of the IL-7–dependent pre-B–cell lines in the presence of rIL-7. The addition of stromal cell conditioned media (up to 30%) did not affect the proliferation of the IL-7–dependent cells in the presence of IL-7 (Fig 6A). Because the IL-7–dependent cell lines used here do not respond to other known positive growth factors, it could be argued that the absence of detectable inhibitory factors was due solely to the use of cell lines that are no longer sensitive to these factors. Therefore, to ensure that the detection of an inhibitory factor was not missed, FACS-purified pro-B cells (B220+CD43+IgM−) were used in place of the IL-7–dependent cells. Stromal cell conditioned media from either age group also did not inhibit the proliferation of freshly isolated pro-B cells in the presence of IL-7 (Fig 6B). Unlike the IL-7–dependent pre-B–cell lines, the freshly isolated pro-B cells respond to the synergistic factors produced by stromal cells. Therefore, the increase in activity of the stromal cell conditioned media over IL-7 alone is probably due to the presence of other synergistic growth factors produced by the stromal cells. The absence of inhibitory activity of the stromal cell conditioned media was found regardless of the concentration of IL-7 used (range, 0.05 to 5.0 ng/mL; data not shown). Furthermore, the activity of exogenously added rIL-7 in cocultures of stromal cells and the IL-7–dependent pre-B cells was not inhibited by the presence of stromal cells from aged mice (data not shown), suggesting that neither soluble nor cell-membrane bound inhibitory factors were involved. Therefore, the reduced ability of stromal cells from aged mice to support the proliferation of IL-7–dependent B-cell precursors is not due to an increased production of inhibitory factors.
Absence of soluble inhibitory activities present in SCCM. SCCM from FACS-purified stromal cells does not inhibit the proliferation of (A) IL-7–dependent BC715 cells or (B) freshly isolated pro-B cells in the presence of 1.0 ng/mL rIL-7. In (B), FACS-purified pro-B cells (B220+CD43+IgM−) from young mice were used to maximize the detection of any factors present in the SCCM. The additional activity of the SCCM with IL-7 on freshly isolated pro-B cells is probably due to the presence of synergistic factors that are not active on the IL-7–dependent pre-B–cell lines. No inhibitory activities were found in SCCM with 0.1 to 5.0 ng/mL of IL-7, at 20% and 30% final volumes of SCCM, and on the other IL-7–dependent pre-B–cell lines (data not shown). At least 3 separate collections of SCCM have been tested with the freshly isolated pro-B cells and with each cell line.
Absence of soluble inhibitory activities present in SCCM. SCCM from FACS-purified stromal cells does not inhibit the proliferation of (A) IL-7–dependent BC715 cells or (B) freshly isolated pro-B cells in the presence of 1.0 ng/mL rIL-7. In (B), FACS-purified pro-B cells (B220+CD43+IgM−) from young mice were used to maximize the detection of any factors present in the SCCM. The additional activity of the SCCM with IL-7 on freshly isolated pro-B cells is probably due to the presence of synergistic factors that are not active on the IL-7–dependent pre-B–cell lines. No inhibitory activities were found in SCCM with 0.1 to 5.0 ng/mL of IL-7, at 20% and 30% final volumes of SCCM, and on the other IL-7–dependent pre-B–cell lines (data not shown). At least 3 separate collections of SCCM have been tested with the freshly isolated pro-B cells and with each cell line.
Comparison of the amount of IL-7 within stromal cells.
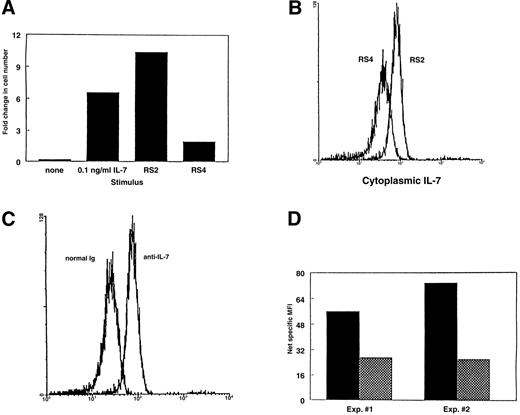
Because the amount of IL-7 secreted by stromal cells appears to decrease with aging, we asked if this was due to decreased production of IL-7 by the stromal cells. To examine the production of IL-7, we determined the cytoplasmic levels of IL-7 protein within the stromal cells. Previously, we used immunocytochemistry to show the presence of IL-7 within both primary-cultured and freshly isolated stromal cells.6 15 Flow cytometric analysis of cytoplasmic IL-7 using the same anti–IL-7 antibodies offered a semiquantitative approach for the comparison of IL-7 levels within stromal cells from young and old mice. Justification of this methodology is shown in Fig7. Two stromal cell lines were derived from LTBMC-B in our laboratory by limiting-dilution FACS sorting. The stromal cell line RS2 supported a greater amount of proliferation of the IL-7–dependent cell lines than the RS4 stromal cell line (Fig 7A). When analyzed for content of cytoplasmic IL-7 by flow cytometry, RS2 cells showed a higher specific staining with anti–IL-7 antibody (Fig7B). The net mean fluorescent intensity (MFI) specific for IL-7 was calculated by subtracting the MFI obtained with staining with normal Ig from the MFI obtained with staining with anti–IL-7 antibody (representative histograms from staining RS2 cells are shown in Fig7C). The net specific MFI for IL-7 showed that the relative content of IL-7 protein in the RS2 cells is 2 to 3 times higher than in the RS4 cells (Fig 7D). Therefore, cytoplasmic flow cytometry provides an accurate relative comparison of the levels of IL-7 protein within stromal cells and correlates with the ability to support the proliferation of IL-7–dependent pre-B cells in coculture.
Justification of cytoplasmic FACS as a method to compare the amount of IL-7 within the cytoplasm of stromal cells. (A) Comparison of the ability of 2 stromal cell clones to support IL-7–mediated proliferation of BC715 pre-B cells. (B) Histograms showing the levels of cytoplasmic IL-7 staining of the same 2 stromal cell clones as determined by flow cytometry. (C) Histograms comparing the levels of cytoplasmic staining of RS2 cells using normal Ig and anti–IL-7. (D) Net specific IL-7 staining for the same 2 stromal cell clones calculated as the difference in MFIs between staining with anti–IL-7 and control Ig antibodies. (▪), RS2; (▩), RS4.
Justification of cytoplasmic FACS as a method to compare the amount of IL-7 within the cytoplasm of stromal cells. (A) Comparison of the ability of 2 stromal cell clones to support IL-7–mediated proliferation of BC715 pre-B cells. (B) Histograms showing the levels of cytoplasmic IL-7 staining of the same 2 stromal cell clones as determined by flow cytometry. (C) Histograms comparing the levels of cytoplasmic staining of RS2 cells using normal Ig and anti–IL-7. (D) Net specific IL-7 staining for the same 2 stromal cell clones calculated as the difference in MFIs between staining with anti–IL-7 and control Ig antibodies. (▪), RS2; (▩), RS4.
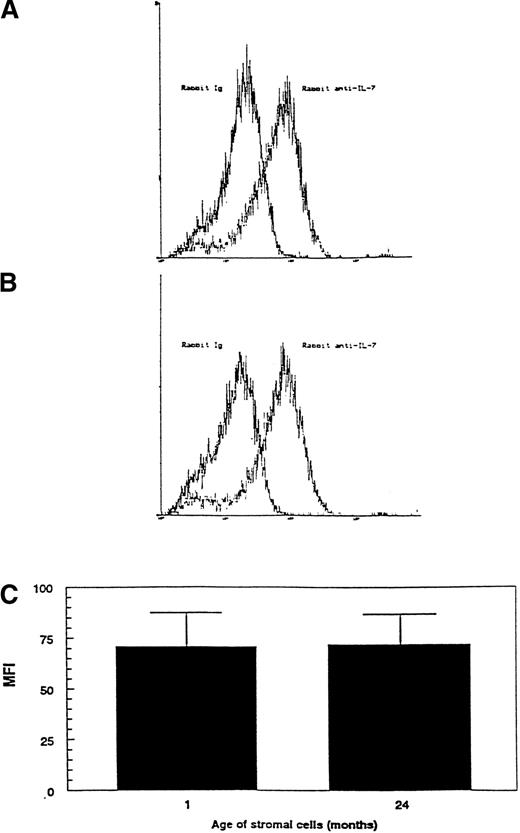
FACS analysis was used to compare the intracytoplasmic levels of IL-7 protein within stromal cells from primary cultures of marrow from 1- and 24-month-old mice. Representative histograms comparing the cytoplasmic staining of stromal cells from young and old mice with normal Ig and anti–IL-7 antibody are shown in Fig8A and B. This confirms our previous work using immunocytochemistry that the primary stromal cells are relatively homogenous in IL-7 expression at early times of culture.15There was no significant difference between stromal cells from young and old marrow in the net specific MFI for cytoplasmic IL-7 (Fig 8C). Therefore, although stromal cells from aged mice are impaired in their ability to support the proliferation of IL-7–dependent cell lines, the level of IL-7 protein within the stromal cells does not appear to change with age.
Comparison of the relative amount of IL-7 within the cytoplasm of stromal cells from young and old mice. The relative amount of IL-7 within stromal cells was determined by cytoplasmic flow cytometry as described in Fig 7 and in Materials and Methods. The histograms compare the levels of cytoplasmic staining for normal Ig and anti–IL-7 antibody in stromal cells from (A) 1-month-old mice and (B) 24-month-old mice. (C) Net specific IL-7 staining was calculated as the difference in MFIs between staining with anti–IL-7 and control Ig antibodies. The average ± SD was calculated from four separate experiments using stromal cells from two independent culture initiations is shown. In each experiment, two to four pooled LTBMC-Bs were used as the source of stromal cells.
Comparison of the relative amount of IL-7 within the cytoplasm of stromal cells from young and old mice. The relative amount of IL-7 within stromal cells was determined by cytoplasmic flow cytometry as described in Fig 7 and in Materials and Methods. The histograms compare the levels of cytoplasmic staining for normal Ig and anti–IL-7 antibody in stromal cells from (A) 1-month-old mice and (B) 24-month-old mice. (C) Net specific IL-7 staining was calculated as the difference in MFIs between staining with anti–IL-7 and control Ig antibodies. The average ± SD was calculated from four separate experiments using stromal cells from two independent culture initiations is shown. In each experiment, two to four pooled LTBMC-Bs were used as the source of stromal cells.
Induction of the response to IL-7 is delayed on stromal cells from aged mice.
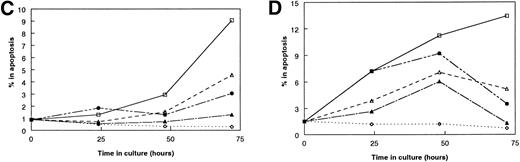
We hypothesized that the mechanism for the age-related dysfunction of stromal cell from aged mice is the impaired secretion of IL-7. If this hypothesis is correct, one may predict that the detection of IL-7 secreted by aged stromal cells would be delayed when compared with young stromal cells. To test this prediction, we determined when we could first detect IL-7 activity after culturing on stromal cells. Changes in the percentage of the IL-7–dependent cell lines in the S/G2/M stages of the cell cycle were used as a measure of IL-7 activity to allow for more accurate assessment at early times of culture when only small changes in cell numbers have occurred. The detection of IL-7 activity from stromal cells was defined as when the percentage of cells in S/G2/M was greater than the percentage of cells in cell cycle that were cultured without stromal cells and without IL-7 (0 IL-7). With stromal cells from young mice, IL-7 activity was routinely first detected within the first 24 to 48 hours. In contrast, the onset of detectable IL-7 activity was not observed until about 24 hours later when cultured with stromal cells from old mice (Fig9). The delay in the increase in cells actively cycling on aged stromal cells was not due to the death of the responder cells, because few cells in the cocultures were undergoing apoptosis through the first 72 hours of culture (Fig 9). Instead, it appeared that the IL-7–specific cells were accumulating in the G0/G1 stages of the cell cycle (data not shown). These results suggest that the age-related deficiency in stromal cell function is due to the delayed secretion of IL-7.
Kinetics of the responsiveness of IL-7–specific cell lines to stromal cells. (A and B) The kinetics of the secretion of IL-7 by FACS-purified stromal cells was determined by measuring changes in the percentage of responding IL-7–specific cells in S/G2/M stages of the cell cycle. (C and D) Age-related differences in the kinetics of the responses to stromal cells are not due to the death of the responding cells. (□) 0 ng/mL IL-7; (▵) 0.001 ng/mL IL-7; (◊) 1.0 ng/mL IL-7; (▴) 1-month-old SC; (•) 24-month-old SC. (A) and (C) show representative results with the IL-7–specific cell line BC77; (B) and (D) show representative results with BC715. Each cell line was tested in two or three separate experiments. In all panels, the responses of the IL-7–specific cells to 0.001 and 1.0 ng/mL of rIL-7 are shown solely for comparison.
Kinetics of the responsiveness of IL-7–specific cell lines to stromal cells. (A and B) The kinetics of the secretion of IL-7 by FACS-purified stromal cells was determined by measuring changes in the percentage of responding IL-7–specific cells in S/G2/M stages of the cell cycle. (C and D) Age-related differences in the kinetics of the responses to stromal cells are not due to the death of the responding cells. (□) 0 ng/mL IL-7; (▵) 0.001 ng/mL IL-7; (◊) 1.0 ng/mL IL-7; (▴) 1-month-old SC; (•) 24-month-old SC. (A) and (C) show representative results with the IL-7–specific cell line BC77; (B) and (D) show representative results with BC715. Each cell line was tested in two or three separate experiments. In all panels, the responses of the IL-7–specific cells to 0.001 and 1.0 ng/mL of rIL-7 are shown solely for comparison.
IL-7 activity produced from stromal cells requires direct cell contact with B-cell precursors.
We next examined if direct cell-cell contact between the stromal cells and the IL-7–specific B-cell precursors is required for the proliferation of the IL-7–dependent cell lines. Direct cell-cell contact between the stromal cells and the B-cell precursors was prevented using 0.45-μm membrane inserts. We found that minimal proliferation of the IL-7–dependent cell lines occurred without direct cell-cell contact between the pre-B cells and the stromal cells (Fig 10A). Normal levels of proliferation occurred when rIL-7 was exogenously added to the wells (Fig 10B). Furthermore, proliferation of the IL-7–dependent cell lines occurred in the presence of rIL-7 regardless of whether the IL-7 was added to the stromal cell side or the B-cell precursor side of the transwell chamber, indicating that IL-7 could pass through the transwell membrane if it was freely secreted by the stromal cells (data not shown). Therefore, direct cell-cell contact between B-cell precursors and stromal cells is required for stromal-derived IL-7 to induce proliferation.
Requirement for direct cell-cell contact for the secretion of IL-7 from stromal cells. Cell contact between the IL-7–dependent pre-B cells and the stromal cells was prevented by use of a 0.45-μm membrane (no contact). (A) IL-7–dependent proliferation did not occur without direct cell contact with the stromal cells. (B) Proliferation of IL-7–dependent pre-B cells to rIL-7 was not affected by the presence or absence of cell contact with stromal cells. The results shown in (B) used rIL-7 at a concentration of 0.25 ng/mL; similar results were found with IL-7 at 1.0 ng/mL (data not shown). The results shown in (A) and (B) used the pre-B–cell line BC77; similar results were found with BC715. (□) No SC; (▪) 1-month-old SC; () 24-month-old SC.
Requirement for direct cell-cell contact for the secretion of IL-7 from stromal cells. Cell contact between the IL-7–dependent pre-B cells and the stromal cells was prevented by use of a 0.45-μm membrane (no contact). (A) IL-7–dependent proliferation did not occur without direct cell contact with the stromal cells. (B) Proliferation of IL-7–dependent pre-B cells to rIL-7 was not affected by the presence or absence of cell contact with stromal cells. The results shown in (B) used rIL-7 at a concentration of 0.25 ng/mL; similar results were found with IL-7 at 1.0 ng/mL (data not shown). The results shown in (A) and (B) used the pre-B–cell line BC77; similar results were found with BC715. (□) No SC; (▪) 1-month-old SC; () 24-month-old SC.
DISCUSSION
Although most processes of bone marrow hematopoiesis are unaffected by aging, it has recently become appreciated that there is a significant age-related decrease in B-lymphopoiesis.16-22 In our previous work, we found functional alterations in both the stromal cells and in the B-cell precursors.17 Because the activity of IL-7 in the marrow is directed mainly on lymphoid development, as is the age-related decrease in lympho-hematopoiesis, we hypothesized that the production of IL-7 by stromal cells would be the underlying deficiency of the stromal cells from aged mice. One previous study suggested that a decrease in the relative quantity of IL-7 protein in LTBMC-B occurs with increasing age.25 However, it is unclear if the activity measured in that study was IL-7 alone or IL-7 in combination with another factor. Our use of four independently derived IL-7–dependent cell lines, three of which appear to be uniquely IL-7–specific, clearly proves that the stromal cells from aged mice are impaired in their ability to support IL-7–mediated proliferation. However, because a decrease in the amount of IL-7 protein within stromal cells does not occur with increasing age and because the response of the IL-7–specific cells to stromal cells from old mice is delayed when compared with stromal cells from young mice, the functional alteration of the stromal cells appears to be at the level of IL-7 secretion. Because of the modest level of IL-7 in stromal cell conditioned media or within the extracellular matrix and the requirement of the stromal cells for cell contact with the IL-7–dependent cells for the release of IL-7 activity, we propose that the regulation of B-lymphopoiesis by stromal cells occurs by modulating the availability of IL-7 at the level of protein secretion, not production.
Our results, along with the published work of a number of other investigators, have led us to propose a model whereby IL-7 is stored inside stromal cells and secreted only in a tightly regulated and directed manner into discrete microenvironments. IL-7 is absolutely required for marrow B-lymphopoiesis,7,8 and stromal cells are the only cells in the marrow and in the LTBMC-B that produce IL-7.6,15 Although we can detect IL-7 protein within stromal cells6,15 (Fig 8), we cannot detect substantial amounts of secreted IL-7. We find that the anti–IL-7 monoclonal antibody M25 stains the stromal cells in a very punctate pattern, as if the IL-7 is within vesicles (personal observations). Using electron microscopy, Jacobson et al38 found that CD29 (β1 integrin), as identified by the antibody KMI6, is localized on the stromal cells only to places of lymphoid contact in vivo. Therefore, the concept of lymphopoietic-specific stromal niches has been shown. We predict that a B-cell precursor in one of these specialized stromal niches signals the stromal cell to locally release IL-7 directly to that B-cell precursor. This precursor then leaves the niche after receiving the IL-7 to undergo cell division. This explains why the majority of cells responsive to IL-7 alone (CFU–IL-7) are not in close contact with stromal cells in native marrow.5 The capacity of B-cell progenitors to induce signals within stromal cells has been shown by Jarvis and LeBien,39 and Sudo et al40 reported the activation of a stromal cell line by contact with a pre-B–cell line. Recently, Tang et al41suggested that IL-7 secretion by human stromal cells can be negatively regulated, supporting the hypothesis that the secretion of IL-7 is not a constitutive process. All of these findings can be used in support for a model of directed secretion of IL-7 by bone marrow stromal cells.
Special note needs to be made of the cell-cell contact specificity in our system. The proliferation of the IL-7–specific cell lines in the presence of rIL-7 is not affected by the presence of stromal cells. This finding suggests that the direct cell-cell contact between the B-cell progenitors and the stromal cells influences stromal cell function. Because the release of IL-7 from aged stromal cells is impaired even though the amount of IL-7 protein within stromal cells does not change with age and because we cannot detect constitutive secretion of IL-7 in SCCM, we believe that cell-cell contact is required to induce the secretion of IL-7 from the stromal cells. However, we cannot exclude the possibility that the amount of IL-7 secreted is so low that stromal cells must localize secreted IL-7 to reach a biologically active threshold, thereby preventing IL-7 from crossing the transwell membrane and preventing our detection of IL-7 in SCCM. Further experiments are needed to distinguish between these possibilities. Furthermore, in seemingly direct contrast with our results, Hardy et al42 showed contact-independent production of IL-7 activity by stromal cells. The important difference is that they used a stromal cell line while we used primary-cultured stromal cells. Using our bioassay, we readily detect IL-7 in the SCCM from our stromal cell lines (data not shown), suggesting that these stromal cell lines also secrete IL-7 in a contact-independent manner. This difference stresses the importance of using nontransformed cells to study the natural regulatory processes of a specific cell type. Furthermore, it may also suggest that the regulation of IL-7 secretion is a delicate process that is easily disrupted during a cell's immortalization.
A recent study by Selleri et al43 showed the potent effects of small amounts of locally secreted cytokines produced by stromal cells. In their study, stromal cells were stably transfected to produce interferon-γ (IFN-γ). The transfected stromal cells had a 100-fold greater IFN-γ activity than what would be predicted by the amount of IFN-γ that was detected in the stromal cell conditioned media. Thus, negligible amounts of IL-7 might be detected in the culture supernatant, whereas the amount of IL-7 may be relatively high within the lymphopoietic niches provided by the stromal cells. Furthermore, because a majority of stromal cells in vivo produce IL-7,6the lymphopoietic stromal niche may also be a means of limiting B-lymphopoiesis, which would allow the other hematopoietic lineages to develop in the same tissue.
Why does such regulation of B-lymphopoiesis by the stromal cells need to occur? One advantage of stromal cell regulation is its ability to explain a mechanism for positive selection in B-lymphopoiesis. The pre-B–cell receptor is composed of a functional heavy chain with the surrogate light chain (VpreB and λ5) that is required for B-lymphopoiesis.44 One popular hypothesis is that the pre-B–cell receptor has a ligand on stromal cells.45 This predicted ligand-receptor pair may play a role in inducing the secretion of IL-7. This could explain a mechanism for positive selection by precluding the IL-7–mediated expansion of those B-cell precursors that are unable to express a functional heavy chain and therefore cannot express the pre-B–cell receptor. The regulation of the availability of IL-7 also minimizes any inhibitory effects that IL-7 possesses. Grawunder et al46 reported that the differentiation of pre-B-I cells into sIgM+ cells in vitro does not occur when stromal cells and a relatively high level of IL-7 (500 to 5,000 U/mL) are present in cultures. In their system, differentiation can proceed when the exogenous IL-7 is removed.46 However, other laboratories, including our own, observe the differentiation of sIgM− pro-B cells (equivalent to pre-B-I) into sIgM+ cells when stromal cells are used as the only source of IL-7.17,42 Therefore, by limiting the availability of IL-7, stromal cells may limit the amount of proliferation that a precursor undergoes, allowing each cell the chance to further differentiate into an sIgM+ B cell. The diminished availability of IL-7 when associated with stromal cells from aged mice may explain why the percentage of sIgM+ recovered after coculture with stromal cells from aged marrow was greater than that recovered after using stromal cells from young marrow.17
Where do we now stand in understanding the reasons for the age-related decrease in B-lymphopoiesis? It appears that B-lymphopoiesis is the only bone marrow hematopoietic process greatly disrupted by aging.23,24 Therefore, the mechanism(s) for these changes will probably involve only proteins and processes that are involved primarily in lymphopoiesis. It is clear that the ability of stromal cells to support IL-7–dependent proliferation of B-lineage precursor cells is compromised by age. It is also important to note that, in the bone marrow, the B-lineage phenotype of aged mice17 is remarkably similar to mice that either do not express IL-78or are defective in a component of the IL-7 receptor complex or its signaling cascade.7,47,48 These similar phenotypes support the hypothesis that the primary lesions in the bone marrow of aged mice involve IL-7. We predict either that the stromal cells from aged mice are unable to correctly process the signals from the developing B-lineage precursors or that the stromal cells from aged mice are unable to efficiently secrete the IL-7. However, this conclusion is only one of the many possible age-related alterations that occur within stromal cells. Further work is needed to determine if the production/secretion of other stromal-derived lymphopoietic cytokines is also affected by aging. Clinically, because stromal cells remain after marrow ablative treatments,49 50 age-related changes in stromal cells may need to be considered for optimizing strategies for bone marrow transplantation.
In contrast to the significant decreases in the production of IL-7 activity from the stromal cells, it is also apparent that not every activity of the stromal cells is negatively affected by aging. Even though there are fewer stromal cells per LTBMC-B of aged marrow, the number of macrophages per culture does not decrease,26suggesting that the ability of stromal cells to support macrophage development and survival is preserved. In support of this preserved stromal cell activity, we find equal or greater levels of M-CSF activity in SCCM from FACS-purified aged stromal cells (Fig 6). Therefore, the impaired function of stromal cells with increasing age appears to be limited to lymphopoiesis.
In this study, we have shown that the ability of bone marrow stromal cells to support IL-7–mediated proliferation decreases with age. We believe this is one factor in the age-related decrease in pre-B–cell numbers in vivo.17 From our data and based on many reports from the literature, we propose a model of directed IL-7 secretion that, if correct, would be the first example of how stromal cells actually regulate B-lymphopoiesis, as opposed to only providing the necessary cell-cell contacts and growth factors to the developing precursors. This could then help explain how normal marrow homeostatic output is maintained and why, under certain experimental conditions, the recovery rates of the different cell lineages vary.51
ACKNOWLEDGMENT
The authors are grateful to Dr Richard Murray (DNAX) for the kind gift of the IL-7 knockout and wild-type mice. We thank Drs Paul W. Kincade, Deborah Lill-Elghanian, Virginia M. Sanders, Glennda Smithson, and Thomas Ellis for helpful suggestions and discussions, reagents (V.M.S.), and cell lines (P.W.K.). Christopher Sperl and Bansri Dhutia provided excellent technical assistance in some studies. We also thank Dr Ellis and Patricia Simms of the Loyola University FACS Core Facility for assistance with the flow cytometric analysis and cell sorting.
Supported by National Institutes of Health Grants No. DK48663 and AG14218 and grants from the Potts Foundation. R.P.S. is a recipient of a fellowship from the American Foundation for Aging Research. Mice were supplied by the NIA through a dissertation award to R.P.S.
Address reprint requests to Pamela L. Witte, PhD, Department of Cell Biology, Neurobiology, and Anatomy, Loyola University Chicago, 2160 S First Ave, Maywood, IL 60153.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.