Abstract
Shear-induced platelet aggregation (SIPA) involves von Willebrand Factor (vWF) binding to platelet glycoprotein (GP)Ib at high shear stress, followed by the activation of αIIbβ3. The purpose of this study was to determine the vWF sequences involved in SIPA by using monoclonal antibodies (MoAbs) to vWF known to interfere with its binding to GPIb and to αIIbβ3. Washed platelets were exposed to shear rates between 100 and 4,000 seconds−1 in a rotational viscometer. SIPA was quantitated by flow cytometry as the disappearance of single platelets (DSP) in the sheared sample in the presence of vWF, relative to a control in the absence of shear and vWF. At a shear rate of 4,000 seconds−1, DSP was increased from 5.9% ± 3.5% in the absence of vWF to 32.7% ± 6.3% in the presence of vWF. This increase in SIPA was not associated with an elevation of P-selectin expression. vWF-dependent SIPA was completely abolished by MoAb 6D1 to GPIb and partially inhibited by MoAb 10E5 to αIIbβ3. Three MoAbs to vWF were compared for their effect on SIPA at 4,000 seconds−1 in the presence of vWF: MoAb 328, known to block vWF binding to GPIb in the presence of ristocetin, MoAb 724 blocking vWF binding to GPIb in the presence of botrocetin, and MoAb 9, an inhibitor of vWF binding to αIIbβ3. Similar to the effect of MoAb 6D1, MoAb 328 completely inhibited the effect of vWF, whereas MoAb 9 had a partial inhibitory effect, as MoAb 10E5 did. In contrast, MoAb 724, as well as its F(ab′)2 fragments, promoted shear-dependent platelet aggregation (165% of the DSP value obtained in the absence of MoAb 724), indicating that MoAb 724 was responsible for an enhanced aggregation, which was independent of binding to the platelet Fcγ receptor. In addition, the enhancement of aggregation induced by MoAb 724 was abrogated by MoAb 6D1 or 10E5 to the level of SIPA obtained in the presence of vWF incubated with a control MoAb to vWF. Finally, the activating effect of MoAb 724 was also found under static conditions at ristocetin concentrations too low to induce platelet aggregation. Our results suggested that on binding to a botrocetin-binding site on vWF, MoAb 724 mimics the effect of botrocetin by inducing an active conformation of vWF that is more sensitive to shear stress or to low ristocetin concentration.
ELEVATED LEVELS of shear stress are found associated with arteriotic stenosis, which may be secondary to atherosclerosis or vascular spasm. Under these conditions, platelet adhesion and aggregation can occur and induce thrombotic occlusion. In this process, von Willebrand factor (vWF) is the main protein required for the interaction with platelet receptors glycoprotein (GP) Ib and GPIIb-IIIa, the αIIbβ3integrin.1 Under static conditions, binding to GPIb has been described in the presence of nonphysiological agents, such as the antibiotic ristocetin or the snake venom protein botrocetin, and involves distinct sequences localized within the first type A repeat (A1 domain) extending from amino acids (aa) 497 to 716 and containing a disulfide bond between Cys 509 and Cys 695.2-4 However, in the absence of these agents, fluid-phase vWF is unable to bind to GPIb, presumably because of the presence of inhibitory sequences that cooperate to maintain an inactive conformation of vWF.5 In physiological conditions, binding of vWF to the endothelial extracellular matrix occurs through its A1 domain and allows subsequent interaction with platelet GPIb under high shear rate conditions.6-8 However, plastic-immobilized vWF has also been reported to bind to GPIb under static conditions.9Thus, the exact mechanism by which vWF binds to GPIb in vivo remains to be determined.
An approach to determine the effect of high shear rates on fluid-phase vWF interaction with platelets is the so-called shear-induced platelet aggregation (SIPA), originally reported by Moake et al.10This process differs from platelet aggregation observed under static or low shear rate conditions, which involves binding of the Arg-Gly-Asp (RGD) sequence of fibrinogen to adenine diphosphate (ADP)- or thrombin-activated αIIbβ3. In contrast, two essential features are observed in SIPA: platelet activation by exogenous agents is not a prerequisite for αIIbβ3 activation, and the main effector is not fibrinogen, but vWF. Based on studies performed with viscometers, in which uniform shear fields can be applied in the absence of exogenous modulators, the following working model has been proposed: in high shear rate conditions, fluid-phase vWF becomes able to bind to GPIb. The αIIbβ3 integrin becomes activated through intracellular signals generated by the vWF-GPIb complex formation. Activated αIIbβ3 binds to the RGD sequence of vWF thereby stabilizing platelet aggregation.11 By using antibodies against GPIb or αIIbβ3 as well as platelet-rich plasma from patients with von Willebrand disease (vWD), Glanzmann's thrombasthenia, or Bernard-Soulier syndrome, the effect of vWF as the main effector of platelet aggregation in high shear rate conditions has been confirmed.12 13
The aim of our study is to investigate the involvement of different sequences of vWF on platelet aggregation at high shear rates generated by means of a rotational viscometer. To this end, we used monoclonal antibodies (MoAbs) to vWF, which inhibit its binding to GPIb or to αIIbβ3. MoAb 328 has been previously reported as an inhibitor of ristocetin-induced platelet aggregation and vWF binding to platelet GPIb.14,15 In addition, at a wall shear rate of 1,600 seconds−1, this MoAb completely blocks platelet adhesion to immobilized vWF in a perfusion chamber.6 MoAb 724 inhibits vWF binding to platelet GPIb in the presence of botrocetin and binds to normal vWF with a higher affinity than to vWF from type 2B vWD, which has an increased reactivity for GPIb.16 MoAb 9 inhibits vWF binding to activated platelet αIIbβ3 in static conditions.17 We show that in high shear rate conditions and in the presence of vWF, MoAbs 328 and 9 are able to block platelet aggregation, whereas MoAb 724 enhances platelet aggregation, via a Fcγ-receptor independent mechanism. To our knowledge, this is the first description of an antibody against the A1 domain of vWF that increases platelet aggregation.
MATERIALS AND METHODS
Preparation of washed platelets.
Blood was obtained from healthy individuals who had not ingested any medication for 2 weeks before donation. The blood was drawn into 15% (vol/vol) acid citrate dextrose (ACD), pH 5.8. Platelet rich plasma (PRP) was obtained by centrifugation at 100g for 20 minutes at 37°C. To the PRP, ACD (1 mL for 40 mL) and apyrase (2 U/mL; Sigma, St Louis, MO) were added and platelets were isolated by centrifugation (500g for 15 minutes at 37°C). Platelets were washed twice with HEPES buffer, pH 6.7 (10 mmol/L HEPES; N-[2-hydroxyethyl]piperazine-N'-[ethanesulfonic acid], 0.136 mol/L NaCl, 2.7 mmol/L KCl, and 2 mmol/L MgCl2) and 0.35% bovine serum albumin (BSA) in the presence of apyrase (2 U/mL) and ACD (1 mL for 40 mL). Finally, the platelets were resuspended in HEPES buffer pH 7.5, containing 1 mmol/L CaCl2, and BSA 0.15%, and they were used after 1 hour incubation at 37°C. Platelets were counted with an electronic particle counter (Model Z1, Coulter Electronics, Margency, France), and the concentration was adjusted such that the final concentration was 1.5 × 108 platelets/mL.
Purifications and radiolabeling of vWF.
Human vWF was purified from outdated high purity vWF concentrates (a gift from Dr C. Mazurier, LFB, Lille, France) as described.6 In some experiments, vWF was labeled with Na125I (Amersham, Les Ulis, France) and Iodogen (Pierce Chemical Co, Rockford, IL) as described.6 Specific radioactivity varied from 1 to 4 μCi/μg of protein. Labeled protein was used within a week. Fibrinogen was purchased from Biogenic (Maurin, France). Botrocetin was purified from Bothrops Jararaca (Sigma) as described.14
Antibodies.
Different MoAbs to vWF have been previously reported: MoAb 9 inhibits vWF binding to platelet αIIbβ3 and its epitope has been localized between aa 1704 and 1746 by screening recombinant cDNA fragments of vWF expressed in Escherichia coli17; MoAb 328 blocks vWF binding to GPIb in the presence of ristocetin, but not botrocetin.14,15 MoAb 724 blocks binding of vWF to botrocetin and binding to GPIb in the presence of botrocetin but not ristocetin.16 Comparative immunoblotting analysis indicated that both MoAbs 328 and 724 recognize the T116 dimeric tryptic fragment (aa 449-728), but that only MoAb 328 reacts with the III-T2 fragment containing aa 273 to 511 and 674 to 728 and lacking the central part (aa 512-673) of the A1 domain. Comparison of functional studies and immunostaining analysis allowed mapping the 724 epitope to the central part of the A1 loop (aa 565-587) and the 328 epitope to the distal part of the loop in either one or both segments proximal to the disulfide loop (aa 480-511 and/or 674-718).15,16 In some experiments, MoAb 418 to vWF, in which the epitope is located between aa 2 and 53, was used as a control.17 We also used MoAbs 6D1 and 10E5 (a kind gift from Dr B.S. Coller, SUNY, Stony Brook, NY), which are directed against vWF binding sites on platelet GPIb-IX and αIIbβ3, respectively.18 MoAb to P-selectin (S12) was obtained from Dr R.P. McEver (Department of Medicine, University of Oklahoma, Oklahoma City, OK). MoAb PAC-1 to activated αIIbβ3 was provided by Dr Shattil (Scripps Research Institute, La Jolla, CA).19Isotypic controls were from Immunotech (Marseille, France).
Preparation of F(ab′)2 fragments of MoAb 724.
F(ab′)2 fragments of MoAb 724 were obtained after digestion with pepsin (Sigma) at 37°C for 1 hour by using a pepsin/protein ratio of 1/50 (wt/wt). F(ab′)2fragments were further purified by chromatography on protein-A Sepharose (Pharmacia, Uppsala, Sweden). Purity of the fragments was assessed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis under reducing and nonreducing conditions. F(ab′)2fragments of MoAb 724 were used at 10- or 20-μg/mL concentrations.
Shear experiments.
The rotating device is a Couette type viscometer used after some modifications.20 The main part of the viscometer consists of two coaxial cylinders: an outer cylinder rotating at various angular speed (Ω) and an inner cylinder of a smaller diameter, which is kept static. The shear rate (γ) applied to the samples in the cylinder gap (e) can be calculated as γ = Ω R/e, where R is the radius of the outer cylinder (10 mm) and e is set at 0.5 mm. The equipment can generate steady state flow conditions by achieving shear rates varying between 50 and 4,000 seconds−1, within 5 seconds. The rotating device is kept at a constant temperature of 20°C by a water-bath thermostating unit (Bioblock, Illkirch, France). Washed platelet suspensions (1.5 × 108/mL) were exposed for 5 minutes to a continuous shear rate in the absence or presence of purified vWF (10 μg/mL) and different MoAbs to vWF or platelet receptors (20 μg/mL) in a final volume of 240 μL. Preincubation with antibodies was performed for 5 minutes at 20°C except for the antiplatelet MoAbs, which were preincubated with platelets at 37°C. After exposure to shear, samples were fixed with 1% paraformaldehyde by addition of a 10-fold concentrated solution and mixed for 30 seconds. A control platelet sample was obtained by incubating the platelet suspension in the absence of vWF in the cylinder gap for 5 minutes without exposure to shear, followed by fixation in the same way.
Quantitation of SIPA.
An aliquot (10 μL) of the sheared or control sample was diluted in 1 mL of fluorescence-activated cell sorting (FACS)-flow buffer (Becton Dickinson, Le Pont-de Claix, France). SIPA was measured in a FACScan flow cytometer (Becton Dickinson) by modification of a method reported for whole blood.21 Data acquisition was performed by counting the particle number during a constant time (30 seconds) to measure identical volumes in different samples. Washed platelets were analyzed by forward light scatter and side light scatter without prior labeling. The population of single platelets was defined by gating the control platelet sample (no vWF and no shear). SIPA in the sheared samples was compared by counting the gated population of single platelets and results were expressed as the percentage of disappearance of single platelets (DSP): DSP = [(n0 − n) /n0] × 100, where n0 represents the single platelet population of the nonsheared control sample and n represents the one of the sheared sample.
Fluorescence analysis of platelet samples.
Platelets (20 μL of the fixed samples) were incubated for 30 minutes at 4°C with an appropriate dilution of the primary antibody (control IgM or PAC1 at 20 μg/mL, control IgG or S12 at 10 μg/mL). After washing in 80 mmol/L Na2HPO4, 20 mmol/L KH2PO4 buffer, pH 7.4 phosphate-buffered saline (PBS), antibody binding was assessed by flow cytometry by incubating the platelets for 30 minutes at 4°C in the dark with a 100-fold dilution of fluorescein isothiocyanate (FITC)-conjugated goat F(ab′)2 fragments directed against mouse IgG or IgM (Caltag Laboratories, South San Francisco, CA). Acquisition of a constant number of particles (usually 10,000) was used for the samples, and fluorescence was detected by using the 525-nm band pass filter of the flow cytometer.
Shear-independent platelet aggregation.
All shear-independent aggregation experiments were performed with fresh platelets. Washed platelets (108/mL) were incubated in the presence of fibrinogen (200 μg/mL) and 1 μmol/L ADP (Stago, Asnières, France) or 2 μg/mL collagen (Nycomed, Munich, Germany), and aggregation was measured in a dual channel aggregometer (Chrono-Log Corp, Coultronics, France). Ristocetin-induced aggregation was performed in the presence of vWF (10 μg/mL) and varying concentrations (0.5-1 mg/mL) of ristocetin (abp, New York, NY). In some cases, F(ab′)2 fragments of MoAb 724 (10 μg/mL) were preincubated with vWF before aggregation studies. PRP aggregation studies were also performed by using 0.7 mg/mL of ristocetin and varying concentrations of MoAb 724 (0.8 to 10 μg/mL).
Binding of 125I-vWF to platelets.
Platelets were isolated from PRP and fixed with paraformaldehyde (2%) in 0.15 mol/L NaCl, 25 mmol/L Tris-HCl buffer, pH 7.4, containing 0.1% BSA.14125I-vWF was preincubated with varying concentrations of MoAb 418 or MoAb 724 (0 to 20 μg/mL) during 30 minutes at 20°C. The final mixture contained 108platelets/mL, 125I-vWF (0.5 μg/mL), MoAb to vWF, and ristocetin (0.6 mg/mL). After 1 hour incubation at 20°C, duplicate aliquots (100 μL) were layered onto 200 μL of 25% sucrose in microfuge tubes and centrifuged for 3 minutes at 10,000g. The tube tip containing bound ligand was separated from supernatant. Bound and free radioactivities were counted in a γ counter (LKB Instruments SA, Bromma, Sweden). The percentage of total bound radioactivity was calculated as bound/(free + bound) radioactivity. Specific binding was obtained by subtracting nonspecific binding in the absence of ristocetin from total binding.
Statistical analysis.
Means ± standard error of the means (SEM) were calculated from three experiments performed in duplicate. Statistical significance of differences between means was evaluated by using Student'st-test for paired samples.
RESULTS
Effect of shear rate and vWF on platelet aggregation.
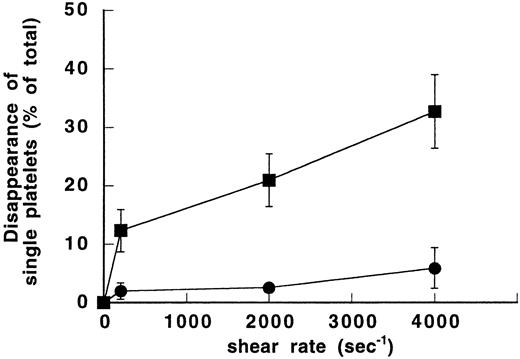
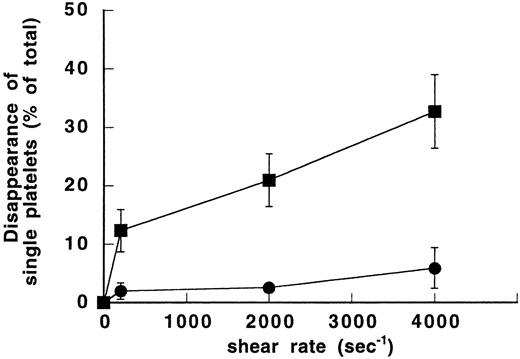
To verify the dependency of SIPA on vWF, different shear rates (0, 200, 2,000, and 4,000 seconds−1) were applied to two series of samples, either in the absence or presence of purified vWF. When DSP in the single platelet region was plotted as a function of shear rate, clear differences were observed between the vWF-free and the vWF-containing samples (Fig 1). In the absence of vWF, the increase of DSP from the unsheared sample (0%) to the sheared sample at 4,000 seconds−1 (5.9% ± 3.5%) was not statistically significant. In contrast, in the vWF-containing samples, SIPA increased significantly with shear as shown by DSP of 20.9% ± 4.5% at 2,000 seconds−1and 32.7% ± 6.3% at 4,000 seconds−1 (P< .01 and P < .005, respectively, relative to the unsheared sample). At these shear rates, DSP values were increased significantly in the presence of vWF (P < .01). In addition, in the presence of vWF, the dot plots showed aggregated platelets at shear rates of 2,000 and 4,000 seconds−1. Therefore, our data indicate that vWF is required for shear-dependent aggregation of washed platelets at a shear rate above 2,000 seconds−1.
Effect of vWF on shear-induced aggregation of washed platelets. Different shear rates were applied for 5 minutes at 20°C to washed platelet suspensions (1.5 × 108/mL) either in the absence of vWF (•) or in the presence of 10 μg/mL purified vWF (▪). After exposure to shear, samples were fixed with 1% paraformaldehyde. Forward and side light scatter dotplots were obtained by counting a constant volume in a flow cytometer. The single platelets region was determined in the buffer-containing unsheared sample and used as the reference value for calculation of disappearance of single platelets (DSP). Means ± SEM from three experiments performed in duplicate were expressed as a function of shear rate. In the absence of vWF, DSP of sheared platelets was hardly modified compared with the unsheared sample. In the vWF-containing samples, percent of DSP increased significantly with shear.
Effect of vWF on shear-induced aggregation of washed platelets. Different shear rates were applied for 5 minutes at 20°C to washed platelet suspensions (1.5 × 108/mL) either in the absence of vWF (•) or in the presence of 10 μg/mL purified vWF (▪). After exposure to shear, samples were fixed with 1% paraformaldehyde. Forward and side light scatter dotplots were obtained by counting a constant volume in a flow cytometer. The single platelets region was determined in the buffer-containing unsheared sample and used as the reference value for calculation of disappearance of single platelets (DSP). Means ± SEM from three experiments performed in duplicate were expressed as a function of shear rate. In the absence of vWF, DSP of sheared platelets was hardly modified compared with the unsheared sample. In the vWF-containing samples, percent of DSP increased significantly with shear.
Effect of shear rate and vWF on PAC1 and S12 epitope expression.
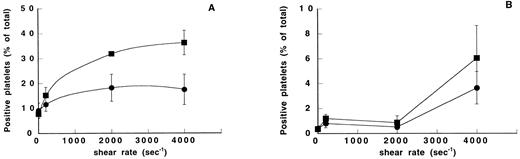
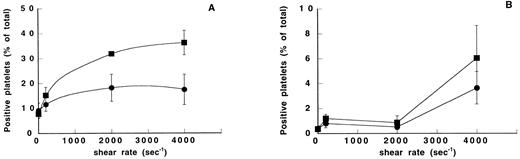
Washed platelets were assessed for their ability to express activation epitopes for activated αIIbβ3 (PAC-1) and P-selectin (S12) in response to exogenous agonists. We found that collagen-induced aggregation resulted in 60% positive platelets both for activated αIIbβ3 and for P-selectin, whereas ADP-induced aggregation resulted in 25% positive platelets for activated αIIbβ3 and 5% for P-selectin (data not shown). These results were compared with the expression of the PAC1 and S12 epitopes in platelet suspensions exposed to different shear rates in the absence or presence of vWF (Fig 2). We found a shear-dependent increased expression of activated αIIbβ3(Fig 2A). At 4,000 seconds−1, PAC-1 expression was almost twofold higher in the vWF-containing samples (36.3% ± 4.9%) than in vWF-free samples (17.5% ± 6.1%, P < .001). However, in samples devoid of vWF, we found a slightly higher PAC-1 expression at 4,000 seconds−1 than in unsheared samples (9.2% ± 3%). In contrast, up to 2,000 seconds−1 no detectable expression of P-selectin was seen (<1%) and there was no significant difference between the control samples and the vWF-containing samples (Fig 2B). At the highest shear rate of 4,000 seconds−1, the samples exhibited a slightly increased level of P-selectin expression (3.6% ± 1.3% and 6% ± 2.6% in the absence and presence of vWF, respectively) comparable with that found in ADP-activated platelets. This result indicates that a significant contribution of α-granule–secreted proteins, such as fibrinogen, may be ruled out in these shear rate conditions.
Effect of shear rate and vWF on PAC-1 and S12 epitope expression. Washed platelets exposed to different shear rates were incubated with PAC-1 directed to activated αIIbβ3 or S12 to P-selectin. The percentage of positive platelets was assessed by flow cytometry by incubating the platelets with an FITC-conjugated secondary antibody. Means ± SEM were calculated from three experiments performed in duplicate. Platelets were exposed to different shear rates in the absence (•) or in the presence of 10 μg/mL vWF (▪). (A) PAC-1 expression. (B) S12 expression. Note differences in the scale of the y-axis.
Effect of shear rate and vWF on PAC-1 and S12 epitope expression. Washed platelets exposed to different shear rates were incubated with PAC-1 directed to activated αIIbβ3 or S12 to P-selectin. The percentage of positive platelets was assessed by flow cytometry by incubating the platelets with an FITC-conjugated secondary antibody. Means ± SEM were calculated from three experiments performed in duplicate. Platelets were exposed to different shear rates in the absence (•) or in the presence of 10 μg/mL vWF (▪). (A) PAC-1 expression. (B) S12 expression. Note differences in the scale of the y-axis.
Effect on SIPA of MoAbs to vWF interfering with platelet-vWF interactions.
The influence of different MoAbs to vWF on SIPA was compared in sheared samples (4,000 seconds−1) in the presence of vWF. In a particular set of experiments, relative to the DSP obtained in the absence of antibody (24.6% ± 3.2%) a complete inhibition of SIPA was seen in the presence of MoAb 328, which is known to block the vWF-GPIb interaction (DSP of 4% ± 2.1%). Furthermore, MoAb 328 completely suppressed the formation of large as well as small aggregates and restored the single platelet population count to the value observed in the control vWF-free sheared sample. In contrast, MoAb 9 that blocks the vWF-αIIbβ3interaction, was able to inhibit SIPA by 45% (11.5% ± 5.2%).
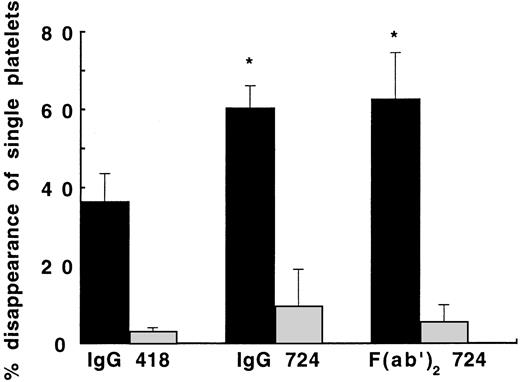
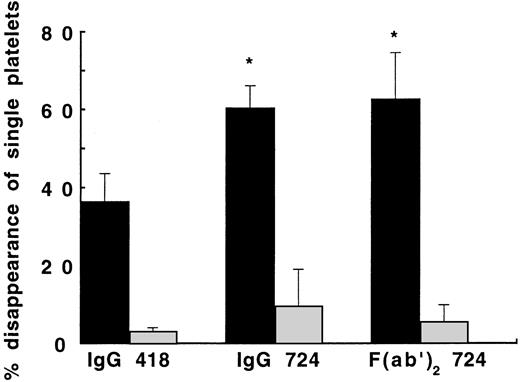
Surprisingly, we found a completely different effect of MoAb 724 to vWF, which blocks vWF binding to GPIb in the presence of botrocetin, as shown by the strongly enhanced SIPA at a shear rate of 4,000 seconds−1 (Fig 3). This effect was clearly mediated by vWF because it was not observed when platelets were exposed to shear in the absence of vWF. In contrast, no effect was observed in samples incubated with MoAb 724 versus control MoAb 418, in the absence of shear, whether vWF was present or absent (data not shown). To rule out an interaction with the Fcγ-receptor, we also studied 724 F(ab′)2 fragments for their ability to modify SIPA at 4,000 seconds−1. Both MoAb 724 and its F(ab′)2 fragments were able to significantly enhance SIPA because DSP reached 165% of the value obtained in the absence of MoAb 724 (P < .05), indicating that the effect was Fcγ-receptor independent (Fig 3). In keeping with an enhancing effect by MoAb 724 of vWF-dependent platelet activation at 4,000 seconds−1, we found a 150% increase of P-selectin expression relative to control MoAb. Similar results were obtained for PAC-1 expression. Interestingly, similar to the effect of MoAb 328 and 9, we found that the effect of anti-platelet MoAbs 6D1 (anti-GPIb) and 10E5 (anti-αIIbβ3) resulted in a complete or partial inhibition, respectively. In addition, MoAb 6D1 on the one hand and MoAb 10E5 on the other hand were equally effective in inhibiting SIPA in the presence of vWF and either the control MoAb 418 or MoAb 724 (Table 1). Furthermore, at a physiological temperature of 37°C a similar increase of DSP was observed on addition of MoAb 724 compared with 20°C.
Effect of MoAb 724 to vWF on SIPA. Washed platelet suspensions (1.5 × 108/mL) and either buffer (▧) or purified vWF (10 μg/mL, ▪) were exposed to a shear rate of 4,000 seconds−1 for 5 minutes at 20°C in the presence of IgG of MoAb 418 as control, IgG of MoAb 724, or F(ab′)2 fragments of MoAb 724 (20 μg/mL). DSP was calculated as outlined in the legend to Fig 1. Means ± SEM were calculated from three experiments performed in duplicate. In the presence of MoAb 724 or its F(ab′)2 fragments, a significant enhancement of SIPA was observed in the vWF-containing samples, whereas this effect was not seen in the vWF-free samples. *P < .05, for the effect of MoAb 724 (IgG or F(ab′)2 fragments) versus MoAb 418 in the vWF-containing samples.
Effect of MoAb 724 to vWF on SIPA. Washed platelet suspensions (1.5 × 108/mL) and either buffer (▧) or purified vWF (10 μg/mL, ▪) were exposed to a shear rate of 4,000 seconds−1 for 5 minutes at 20°C in the presence of IgG of MoAb 418 as control, IgG of MoAb 724, or F(ab′)2 fragments of MoAb 724 (20 μg/mL). DSP was calculated as outlined in the legend to Fig 1. Means ± SEM were calculated from three experiments performed in duplicate. In the presence of MoAb 724 or its F(ab′)2 fragments, a significant enhancement of SIPA was observed in the vWF-containing samples, whereas this effect was not seen in the vWF-free samples. *P < .05, for the effect of MoAb 724 (IgG or F(ab′)2 fragments) versus MoAb 418 in the vWF-containing samples.
Effect of MoAb 724 on shear-independent platelet aggregation.
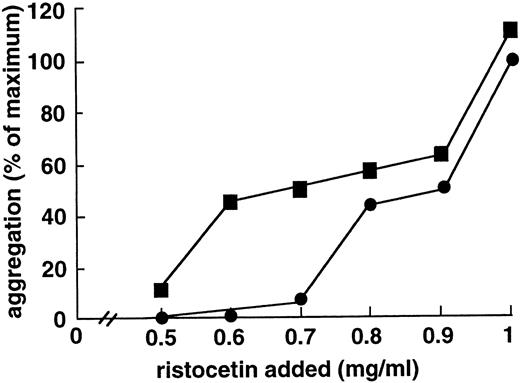
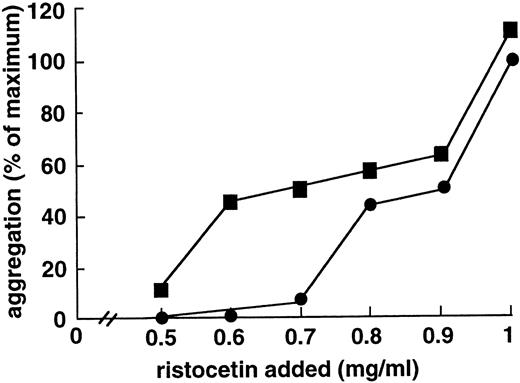
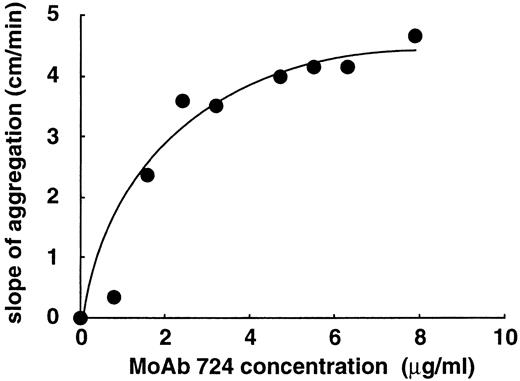
To determine whether the enhancing effect of MoAb 724 was specific to shear, we studied its influence on shear-independent platelet aggregation. To this end, we incubated washed platelets and vWF with ristocetin at concentrations too low to induce a significant aggregation. Interestingly, in this range (0.5 to 0.7 mg/mL), aggregation was significantly increased on addition of F(ab′)2 of MoAb 724 (Fig4). At higher ristocetin concentrations, aggregation values were only slightly higher in the presence of MoAb 724 than in control samples, indicating that the effect of MoAb 724 was bypassed by these relatively high ristocetin concentrations. In addition, we found a similar effect in PRP, because at a low ristocetin concentration of 0.7 mg/mL, MoAb 724 was able to enhance platelet aggregation in a dose-dependent manner (Fig 5). Incubation of washed platelets or PRP with MoAb 724 in the absence of ristocetin did not result in a spontaneous aggregation. This suggests that, by binding to vWF, MoAb 724 enhances the effect of low concentrations of ristocetin and induces platelet activation and aggregation.
Effect of F(ab′)2 fragments of MoAb 724 on shear-independent platelet aggregation induced by different ristocetin concentrations. Washed platelets (108/mL) and purified vWF (10 μg/mL) were incubated with different ristocetin concentrations either in the absence (•) or in the presence of F(ab′)2 fragments (10 μg/mL) of MoAb 724 (▪). Slopes of aggregation were measured and results were expressed relative to the value obtained with 1 mg/mL ristocetin concentration in the absence of MoAb 724, which was arbitrarily set as the maximal aggregation. At low ristocetin concentrations (0.5 to 0.8 mg/mL), F(ab′)2 fragments of 724 increased the aggregation.
Effect of F(ab′)2 fragments of MoAb 724 on shear-independent platelet aggregation induced by different ristocetin concentrations. Washed platelets (108/mL) and purified vWF (10 μg/mL) were incubated with different ristocetin concentrations either in the absence (•) or in the presence of F(ab′)2 fragments (10 μg/mL) of MoAb 724 (▪). Slopes of aggregation were measured and results were expressed relative to the value obtained with 1 mg/mL ristocetin concentration in the absence of MoAb 724, which was arbitrarily set as the maximal aggregation. At low ristocetin concentrations (0.5 to 0.8 mg/mL), F(ab′)2 fragments of 724 increased the aggregation.
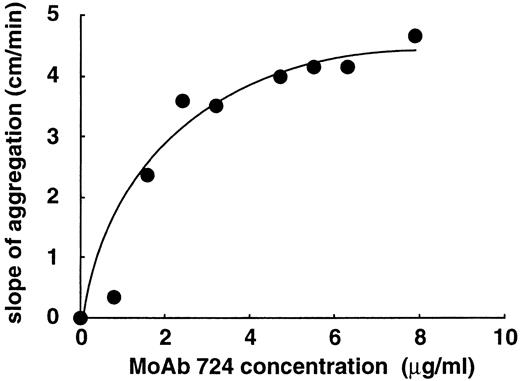
Effect of varying concentrations of MoAb 724 on shear-independent platelet aggregation. PRP was incubated with 0.7 mg/mL of ristocetin, in the presence of MoAb 724. Results were expressed as slopes of aggregation. MoAb 724 was able to increase platelet aggregation in a dose-dependent manner.
Effect of varying concentrations of MoAb 724 on shear-independent platelet aggregation. PRP was incubated with 0.7 mg/mL of ristocetin, in the presence of MoAb 724. Results were expressed as slopes of aggregation. MoAb 724 was able to increase platelet aggregation in a dose-dependent manner.
Effect of MoAb 724 on 125I-vWF binding to platelets.
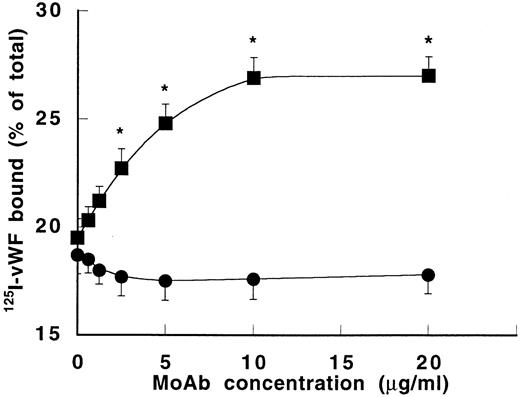
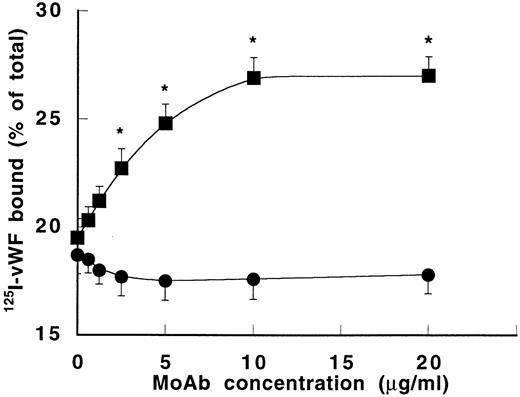
To determine whether MoAb 724 binding to vWF allows increased binding to GPIb, the effect of MoAb 724 on 125I-vWF binding to fixed platelets was studied in the presence of a low ristocetin concentration (0.6 mg/mL). Interestingly, binding of vWF was enhanced in a dose-dependent manner by MoAb 724, whereas it was unchanged in the presence of the control MoAb 418 (Fig 6). In addition, this MoAb 724-dependent increase was significantly higher at the lowest ristocetin concentrations (data not shown), thus comparable with the effect of MoAb 724 on platelet aggregation. Furthermore, we found that F(ab′)2 fragments of MoAb 724 dose-dependently inhibited botrocetin-induced binding of125I-vWF to GPIb with an IC50 of 4 μg/mL (data not shown), confirming previously reported data on the effect of MoAb 724 IgG.16 Finally, because MoAb 724 has been reported as an inhibitor of botrocetin binding to vWF, conversely it was of interest to investigate the ability of botrocetin to interact with MoAb 724 binding to vWF. At the highest concentration added (50 μg/mL), botrocetin was able to completely inhibit the binding of MoAb 724 with immobilized vWF, whereas it had no effect on the binding of a control MoAb.
Effect of MoAb 724 on 125I-vWF binding to platelets. Binding of 125I-vWF (0.5 μg/mL) to fixed platelets (108/mL) was performed in the presence of ristocetin (0.6 mg/mL) and varying concentrations of either MoAb 418 (•) or MoAb 724 (▪). Results were expressed as specific binding to platelets after subtraction of nonspecific binding in the absence of ristocetin. Means ± SEM were calculated from three experiments in duplicate. In the presence of MoAb 724, 125I-vWF binding to platelets was significantly enhanced compared with MoAb 418 (*P< .01).
Effect of MoAb 724 on 125I-vWF binding to platelets. Binding of 125I-vWF (0.5 μg/mL) to fixed platelets (108/mL) was performed in the presence of ristocetin (0.6 mg/mL) and varying concentrations of either MoAb 418 (•) or MoAb 724 (▪). Results were expressed as specific binding to platelets after subtraction of nonspecific binding in the absence of ristocetin. Means ± SEM were calculated from three experiments in duplicate. In the presence of MoAb 724, 125I-vWF binding to platelets was significantly enhanced compared with MoAb 418 (*P< .01).
DISCUSSION
Using a coaxial viscometer to apply shear rates ranging from 200 to 4,000 seconds−1, we report on the activating effect of a MoAb directed against the A1 domain of vWF on SIPA. We thereby provide new information on the molecular mechanism of high shear-dependent vWF interaction with platelets.
It has been reported that SIPA does not require exogenous agents such as ristocetin or botrocetin. To clarify the vWF sequences involved in this interaction, we have chosen the straightforward approach of a purified system, consisting of washed fresh platelet suspensions incubated with purified plasmatic vWF and exposed to varying shear rates. We confirm that SIPA is dependent on vWF at 2,000 and 4,000 seconds−1, whereas at lower shear rates no significant aggregation is observed in the presence or absence of vWF. Thus, our model grossly reproduces the situation observed in patients with afibrinogenemia and indicates that, whereas fibrinogen is the main effector in low shear conditions, vWF is primarily involved as a mediator of platelet aggregation in high shear conditions.12 A complete inhibition was obtained by incubating platelets with MoAb 6D1 or MoAb 328, previously reported to completely block vWF binding to GPIb in static and high shear conditions.18,22 We found that they both completely block SIPA, thereby confirming in a purified system the involvement of vWF in SIPA.13 Interestingly, we found that MoAb 10E5, an anti-αIIbβ3 MoAb that completely blocks vWF binding to activated platelets in static conditions, inhibits SIPA by only about 50%, as reported by another group in different shear conditions.22 Moreover, MoAb 9 to vWF, which completely inhibits vWF binding to activated αIIbβ3 in static conditions, was also found to have a similar partial inhibitory effect on SIPA, indicating a different involvement of each vWF receptor in SIPA. Interestingly, similar results were recently reported on the partial inhibition of SIPA by MoAb GUR76-23 to vWF, recognizing a different epitope from MoAb 9.23
Because normal washed platelets were used, we cannot exclude a role of adhesive α-granule proteins, in particular a role of endogenous vWF or fibrinogen released from platelets. However, this is unlikely because we showed that P-selectin, another α-granule protein, is hardly expressed on the platelet surface even after exposure to the highest shear rate tested. This low expression (6% in the presence of vWF exposed at 4,000 seconds−1 for 5 minutes) can be compared with the value of 10.5% obtained in whole blood samples exposed to 10,000 seconds−1 during 30 seconds.21 In addition, we find a fourfold higher PAC-1 expression in sheared vWF-containing samples compared with nonsheared vWF-free samples, indicating a significant increase of activated αIIbβ3 expressed on the surface after exposure to shear. This value compares very well with the value reported under slightly different conditions of exposure to shear (10,000 seconds−1, 30 seconds).21 This confirms the prevailing model that the vWF-GPIb interaction acts as a platelet activator under high shear conditions and, as recently shown, that activated αIIbβ3, even occupied by ligands, is not sufficient to mediate platelet aggregation under high shear stress conditions.24
Interestingly, SIPA provides a different approach from binding studies in static conditions to analyze the effect of MoAbs to vWF. We have compared two MoAbs, MoAb 724 and 328, that block binding of vWF to GPIb in the presence of botrocetin and ristocetin, respectively.14,16 MoAb 328 is a potent inhibitor of SIPA in high shear conditions. In contrast, MoAb 724 increases SIPA up to 165% of the value obtained in the absence of MoAb 724. This enhancing effect is completely abrogated when platelets are incubated with MoAbs 6D1 or 10E5, so that the remaining SIPA is similar to the value obtained in the absence of MoAb 724. In addition, the effect of MoAb 724 requires an interaction with vWF, because no increase is obtained in the platelet samples devoid of vWF. Similar results with F(ab′)2 fragments were obtained, which rules out an effect mediated by the platelet Fcγ-receptor. Because MoAb 724 binds to normal vWF with a higher affinity than to vWF from type 2B vWD, which has an increased reactivity for GPIb,16 altogether our data suggest that the vWF-MoAb 724 complex acts through the same pathway but with a higher affinity than vWF alone does.
Finally, this report shows that the enhancement of platelet aggregation is not restricted to a shear-dependent effect, because MoAb 724 can enhance platelet aggregation in the presence of ristocetin at low concentrations, unable to induce a detectable increase in turbidity in a classical aggregometer. In addition, we find a significant increase of vWF binding to fixed platelets by MoAb 724 in the presence of low ristocetin concentrations. In contrast, MoAb 724 has no enhancing effect on platelet aggregation in the presence of botrocetin (data not shown). This is in agreement with the fact that MoAb 724 competes with botrocetin for binding to the same site(s) on vWF as shown by the strong inhibitory effect of MoAb 724 on botrocetin-induced vWF binding to GPIb.16 Conversely, highly purified botrocetin was able to completely inhibit the binding of 125I-MoAb 724 to vWF, strongly suggesting that this MoAb shares a common site with botrocetin. It should be mentioned that in using high concentrations of MoAb 724 (50 to 100 μg/mL), an inhibitory effect of botrocetin-induced platelet aggregation has been reported.16 This effect does not contradict the present findings, because these high IgG concentrations are likely to act through a steric hindrance mechanism. According to our hypothesis, the enhancing effect of MoAb 724 on SIPA is secondary to its binding to a regulatory site of vWF, which would be responsible for an increased affinity for platelet GPIb. Once in this “active” conformation, vWF may be more sensitive to high shear conditions explaining the increased SIPA compared with the control. Thus, MoAb 724 may be considered as a modulatory antibody, similar to the effect obtained when botrocetin binds to vWF. This is confirmed by the additive effects of high shear rates and low botrocetin concentrations resulting in extensive aggregate formation (data not shown). This does not exclude a direct effect of shear on the conformation of vWF suggested by others,25 indirectly established in comparing the properties of shear- versus ristocetin-induced vWF binding to platelets.26 Very few studies have attempted to compare the effect of anti-vWF MoAbs in shear-dependent and shear-independent assays. Our data with MoAb 724 may indirectly indicate that the binding is not affected by shearing conditions. Recently, studies performed by atomic force microscopy suggested that elevated shear stress can directly induce conformational change of vWF, although evidence is still missing for the involvement of the A1 domain.27 It would be interesting to use MoAb 724 as a tool to determine with certainty whether the vWF conformation can be affected by shear. However, there is some debate on a role of high shear rates on the structure and function of GPIb so that the active conformation of vWF may be more easily recognized by GPIb, which is itself modified by shear.28
Finally the significance of a platelet-activating antibody directed against an adhesive protein can be addressed. Another antibody directed to vWF has been previously described by Tornai et al,29which could increase vWF binding to both its platelet receptors. This result is different from ours, because we did not find any enhancing effect of MoAb 724 on vWF binding to activated αIIbβ3. Interestingly, the epitope of that MoAb has been localized in the amino terminal part of vWF, within the factor VIII-binding region, thus suggesting different regulatory elements distributed along the vWF subunit. However, our results are in favor of a direct conformation-dependent change of the A1 domain, which contains the GPIb-binding sequence, because the MoAb 724 epitope is located inside the A1 domain.
In conclusion, we have confirmed that SIPA requires vWF and its two platelet receptors, GPIb and activated αIIbβ3. The following working model for the interaction between vWF and GPIb can be proposed based on opposite effects of two MoAbs to the A1 domain of vWF on SIPA. MoAb 328, which inhibits vWF binding to GPIb in the presence of ristocetin, recognizes a site on vWF involved in the shear-dependent interaction with GPIb. In contrast, MoAb 724 recognizes a modulatory site that is common to a botrocetin binding site. High shear rates, as well as high ristocetin concentrations, may bypass the effect of the binding of MoAb 724 to this regulatory site. However, MoAb 724 may unmask the effect of this modulation site when added in combination with lower ristocetin concentrations or with shear rates that induce submaximal aggregation.
ACKNOWLEDGMENT
Drs Coller, McEver, and Shattil are thanked for providing antibodies. We thank Paulette Legendre and Stephan Vauterin for expert technical assistance.
Supported by grants of EC Biomed, PL 93 1685 and the Belgian Royal Academy for Medicine to H.D. while on a postdoctoral leave in INSERM U143 and an INSERM fellowship to N.A.
Address reprint requests to Dominique Baruch, MD, PhD, INSERM U143, 84 rue du General Leclerc, 94276 Bicêtre Cedex, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.