Abstract
A variety of strategies have been attempted in the past to stably transduce natural killer (NK) cells with cytokine or other cellular genes. Here, we demonstrate the successful delivery of the interleukin-2 (IL-2) gene into two human NK cell lines, IL-2–dependent NK-92 and IL-2–independent YT, by retroviral transduction. An MuLV-based retroviral vector expressing human IL-2 andneor markers from a polycistronic message was constructed and transduced into a CRIP packaging cell line. By coincubation of NK cells with monolayers of CRIP cells or by using retrovirus-containing supernatants in a flow-through method, 10% to 20% of NK cells were stably transduced. Upon selection in the presence of increasing G418 concentrations, transduced NK cells were able to proliferate independently of IL-2 for more than 5 months and to secrete up to 5.5 ng/106 cells/24 h of IL-2. IL-2 gene-transduced NK-92 cells had an in vitro cytotoxicity against tumor targets that was significantly higher than that of parental cells and secreted interferon gamma (IFNγ) and tumor necrosis factor alpha (TNFα) in addition to IL-2. Moreover, the in vivo antitumor activity of IL-2 gene-transduced NK-92 cells against established 3-day liver metastases in mice was greater than that of parental nontransduced NK cells. Stable expression of the IL-2 transgene in NK cells improved their therapeutic potential in tumor-bearing hosts. Thus, transduced NK cells secreted sufficient quantities of bioactive IL-2 to proliferate in vitro and mediated the antitumor effects both in vitro and in vivo in the absence of exogenous IL-2. These results suggest that genetic modification of NK cells ex vivo could be useful for clinical cancer therapy in the future.
NATURAL KILLER (NK) cells are immune effector cells that are able to lyse virally infected or transformed targets without previous sensitization. Freshly purified NK cells from human peripheral blood have been recently shown to use both perforin-mediated secretory and nonsecretory apoptotic pathways of killing.1 In addition, NK cells activated by interleukin-2 (IL-2), especially a subset designated as A-NK cells, were found to be able to kill a variety of tumor cell targets in vitro and in vivo.2 Previously, we showed that a single adoptive transfer of human A-NK cells and IL-2 induced regression of established liver metastases in nude mice.3,4 Similarly, transfers of murine A-NK cells into immunocompetent mice bearing pulmonary metastases of B16 melanoma were shown to be therapeutically effective.5 Antitumor activity of A-NK cells in vitro and in vivo was strictly dependent on the presence of exogenous IL-2 at doses sufficient to support functions of these effector cells.3-5 At high concentrations, exogenous IL-2 administered in vivo to tumor-bearing hosts induces toxicity, which has been a limiting factor in the wider utilization of adoptive immunotherapies with IL-2–dependent effector cells for the treatment of animals or patients with cancer.6 To bypass the need for exogenous IL-2, a strategy of genetically modifying the effector cells to express IL-2 can be considered. Such genetically modified effector cells secreting IL-2 are likely to be independent of exogenous IL-2 but retain their functions, particularly in the ability to kill tumor cell targets.
A variety of tumor cell targets, normal tissue cells, or progenitor cells have been successfully transduced with cytokine genes.7,8 However, NK cells appear to be resistant to retroviral transduction, as well as to infection by other viral vectors. For reasons that are not understood, NK cells do not survive the infection and selection process. On the other hand, NK cell lines appear to be more permissive to gene transfer. Two recent reports described instances of stable gene transfer into NK cell lines, one of the CD18 gene into mutant YT-1 cells by electroporation,9and the other, of the chimeric ζ-chain gene into NK 3.3 cells by a retroviral vector.10
In this report, we demonstrate the successful retroviral transduction of the human IL-2 gene into two NK cell lines: IL-2–dependent NK-92 and IL-2–independent YT2C2. The transduced and selected NK cell lines produced and secreted bioactive IL-2 in quantities sufficient to support various functions, including the growth of the NK-92 line. Furthermore, upon adoptive transfer, NK-92 cells transduced with the IL-2 gene (TR-IL-2-NK-92) significantly prolonged the survival of nude mice with established liver metastases. This report describes the process of NK cell transduction, characteristics of the stable NK cell transfectants generated, and their use for immunotherapy in tumor-bearing hosts.
MATERIALS AND METHODS
Cell lines.
The NK-92 cell line was established from a patient with rapidly progressive non-Hodgkin's lymphoma11 and was kindly provided by Dr H.G. Klingemann. This line was maintained in myeloid long term culture medium (MLTC; Terry Fox Laboratory, Vancouver, BC, Canada) containing 12.5% fetal calf serum (FCS), 12.5% horse serum, and 10−4 mol/L 6-mercaptoethanol and supplemented with 10−6 mol/L hydrocortisone (Sigma, St Louis, MO) and 1,200 IU/mL IL-2 (Chiron, Emeryville, CA). The surface marker expression and cytolytic activity of this line against K562 and Daudi are similar to those of A-NK cells.11 The YT2C2 cell line was established from a patient with thymic lymphoma12 and was kindly provided by Dr Kendal Smith. This line was maintained in RPMI 1640 medium (GIBCO, Grand Island, NY) supplemented with 10% (vol/vol) FCS (GIBCO). K562, a chronic myelogenous leukemia cell line, and Daudi, a Burkitt lymphoma cell line, were maintained in RPMI 1640 medium supplemented with 10% FCS. CTLL-2, an IL-2–dependent murine cytotoxic T-cell line, was maintained in RPMI 1640 medium supplemented with 10% FCS and 300 IU/mL IL-2. HR, a human gastric carcinoma cell line, was established in our laboratory from a liver metastasis, as previously described, and was maintained in DMEM (GIBCO) supplemented with 10% FCS.13 These lines were tested monthly for mycoplasma using Gen Probe reagents (Gen Probe Inc, San Diego, CA).
IL-2 expression vector.
The DFG-hIL-2-Neo vector was constructed in our laboratory as previously described.14 Briefly a 490-bp DNA fragment encoding human IL-2 cDNA was obtained from Dr T. Taniguchi (Osaka University, Osaka, Japan) and digested with the restriction enzymes BamHI and NcoI. The neomycin resistance (neor) gene was generated by PCR using a pMC1 Neo Poly A plasmid (Stratagene, La Jolla, CA) as a selectable marker and placed under the regulation of the internal ribosome entry site (IRES) of encephalomyocarditis virus. The DFG-hIL-2-Neo vector contains a full-length human IL-2 cDNA under the regulation of the retroviral LTR.
The DFG-LacZ-Neo vector was similarly constructed and used as a control in some experiments.
IL-2 gene-producing packaging cell line.
The packaging cell line, CRIP, was derived from NIH-3T3 mouse fibroblasts.15 The DFG-IL-2-Neo vector was transduced into this line by a calcium phosphate method.16 Transfectants were selected by culture in the presence of 400 μg/mL G418 (GIBCO), and G418-resistant colonies were tested by enzyme-linked immunosorbent assay (ELISA) for the ability to produce IL-2. The best colony, which produced 103 ng IL-2/mL/5 ×105 cells in 48 hours, was expanded and IL-2 gene-producing cells were used as a source of infectious virus. Supernatants of these cells contained a high titer of the virus (105 to 106 colony-forming units [cfu]/mL). The DFG-LacZ-Neo vector was also transduced into CRIP cells by the same procedure.
IL-2 gene transduction into NK-92 cell lines.
NK-92 cells cultured at a concentration of 4 × 105 to 1 × 106 cells/mL were supplemented with fresh MLTC medium 24 hours prior to transduction by the flow-through method described by Chuck and Palsson.17 NK-92 cells were pelleted and resuspended in an aliquot of the CRIP supernatant (1 × 106 cells/mL plus 8 μg/mL polybrene). Aliquots (2 mL) of this suspension were added to each well of a six-well plate containing Transwell-Col tissue culture membranes (pore size, 0.4 μm; Costar, Cambridge, MA). The supernatant was allowed to filter through by gravity (30 to 40 minutes at 37°C). It was then harvested from the lower chamber and reapplied to the cells remaining on the surface of the membrane. This process was repeated six or seven times. After the last cycle of flow-through, the cells were harvested from the membranes, pooled, and resuspended in the conditioned culture medium (4 × 105 cells/mL) containing 1,200 IU/mL IL-2. The flow-through transduction process was repeated after 2 to 3 days of culture.
YT2C2 cells were transduced by cocultivation with the retrovirus-producing packaging cell line at an E:T cell ratio of 5:1 to 10:1 for 48 hours in the presence of 2 μg/mL polybrene (Sigma). This cocultivation was repeated twice and was followed by selection in the presence of 200 μg/mL G418 for 4 weeks. After selection, the cells were maintained in RPMI 1640 medium containing G418 without exogenous IL-2.
As a control, NK-92 cells and YT2C2 cells were also transduced with the LacZ gene, using the LacZ gene–producing packaging cell line or its supernatant and the procedure already described.
Selection of IL-2/Neo–transduced NK cells.
Selection was accomplished by gradually increasing the concentration of G418 in the medium while decreasing IL-2. Forty-eight hours after transduction, the cells were suspended in medium containing 50 μg/mL G418 and 1,200 IU/mL IL-2 at a concentration of 4 × 105cells/mL and cultured for 14 days. Control cultures established in parallel contained parental (nontransduced) cells plated in the same medium and transduced NK cells cultured in the absence of G418. The cultures were centrifuged on Ficoll-Hypaque gradients to remove dead cells, washed, and replated at 4 × 105 cells/mL in medium containing 0.1 mg/mL G418 and 1,200 IU/mL IL-2. After 1 week of culture, the cells were transferred to the medium containing 300 IU/mL IL-2 and 0.2 mg/mL G418. Following 1 additional week of selection, the cells were cultured in medium containing only IL-2 (300 IU/mL) and then were transferred to IL-2–free medium. During the selection process, cell viability was determined by the trypan blue exclusion method. Parental NK cells are referred to as P-NK-92 or P-YT cells. Transferred and selected NK cells are referred to as TR-IL2-NK-92 or TR-IL2-YT cells throughout.
β-Galactosidase staining.
Forty-eight hours after transduction and 6 weeks after selection in the presence of G418 medium, parental or LacZ gene–transduced NK cells were stained using the fluorescent LacZ substrate, fluorescein di-β-galactopyranoside (FDG), as previously described.4Briefly, 3 × 105 cells were washed twice in PBS and resuspended in a 100-μL aliquot of 1 mmol/L FDG (Sigma) in medium prewarmed to 37°C. The cell suspension was incubated in the 37°C water bath for 1 minute and then placed on ice, and 0.5 mL ice-cold PBS was added to each tube. After 15 minutes on ice, the cells were analyzed by flow cytometry.
Proliferation assay.
To assess proliferation, NK cells were resuspended in RPMI 1640 supplemented with 10% FCS but without IL-2 at a concentration of 1 × 104 cells/200 μL in wells of a U-bottom microliter plate (Costar) and incubated for 4 days. For the final 16 hours of incubation, 1 μCi 3H-thymidine (New England Nuclear, Boston, MA; 147.9 GBq/mmol) was added to each well. Cells were harvested onto glass-fiber filters using a semiautomatic cell harvester (Scatron, Sterling, VA). 3H-thymidine uptake was determined using an LKB Betaplate counter (Pharmacia, Gaithersburg, MD).
Flow cytometry.
Expression of IL-2 and the IL-2R α, β, or γ chains or other surface markers on parental and transduced NK-92 or YT cells was measured by flow cytometry as previously described.18 The following antibodies were used for staining: anti-IL-2 mAb (BioSource International, Camarillo, CA), anti-CD25 mAb for IL-2Rα chain (Becton Dickinson, San Jose, CA), anti-IL-2Rβ chain mAb (Endogen, Boston, MA), and TUGh4 mAb for IL-2Rγ chain (kindly provided by Dr K. Sugamura). Rat IgM kappa (BioSource, International, Camarillo, CA) was used as an isotype control for anti-IL-2 mAb. Rat IgG (TAGO, Burlingame, CA) was used as an isotype control of TUGh4 mAb. Labeled anti-CD3, -CD56, -CD28, and -CD11a mAbs and isotype control antibodies were purchased from Becton Dickinson (San Jose, CA). Anti-CD54 (ICAM-1) was purchased from Immunotech (Marseille, France). For staining, cell suspensions were adjusted to the concentration of 1 × 106 cells/mL in 0.1% (wt/vol) sodium azide-PBS and incubated with pretitered mAb for 30 minutes at 4°C. Cells were then washed with 0.1% sodium azide-PBS, fixed with 1% (wt/vol) paraformaldehyde (PFDH)-PBS, and analyzed in a FACScan flow cytometer (Becton Dickinson). Isotype controls were routinely included. To detect intracellular IL-2 or IL-2R chains, cells were first prefixed with 0.5% (wt/vol) PFDH for 20 minutes at 4°C and then permeabilized with cold acetone for 3 minutes, washed, and stained with Abs to IL-2 or IL-2R chains. Indirect immunostaining was used for detection of the γ-chain protein, in which pretitered TUGh4 mAb (1:100 dilution) was the primary reagent, and FITC-labeled goat anti-rat IgG was used to develop the staining reaction.
ELISA.
Parental or transduced NK cells were washed, resuspended in enriched medium at the concentration of 1 × 106 cell/mL, and incubated for 48 hours. Supernatants were collected, filtered, and tested for the presence of IL-2, TNFα, or IFNγ by ELISA (Endogen, Cambridge, MA). Supernatants of NK-92 cells transiently transduced with the IRAP gene were also tested for IRAP by ELISA (R & D Systems, Minneapolis, MN). The kits were calibrated against World Health Organization International Cytokine Standards, and the assays were performed under quality-control conditions described previously by us.19
Immunohistochemistry for IL-2.
Cell suspensions were washed in PBS and cytocentrifuged onto glass slides using a Shandon centrifuge (Sewickley, PA). The cells were prefixed for 5 minutes in 2% PFDH, permeabilized, and fixed with 0.1% Triton X/PFDH 2% for 2 minutes. Cytospins were stained for the presence of IL-2 using anti-IL-2 polyclonal Ab (Becton Dickinson) at an optimal working dilution of 20 μg/mL, which was determined in preliminary titration experiments with phytohemagglutinin (PHA)-stimulated Jurkat cells. Immunostaining for IRAP was performed using anti-human Ab (Amgen, Boulder, CO) followed by goat anti-mouse Ig. A standard streptavidin-biotin complex immunoperoxidase (sABC-HRP) technique was used for staining, and the color was developed with 3-amino-9-ethyl-carbazole ([AEC] Biomeda, Foster City, CA).20 The slides were counterstained with hematoxylin (Biomeda), mounted in a glycerol-based mounting medium, and evaluated in an Olympus BH-2 light microscope (Olympus, Tokyo, Japan).
In some experiments, Cy3 staining was used. Slides were fixed in 4% PFDH for 10 minutes, permeabilized in 0.1% saponin in PBS for 5 minutes, and incubated with polyclonal anti-IL-2 Ab (Becton Dickinson) at a working dilution of 1:700. Goat anti-rabbit IgG conjugated with Cy3 (Jackson Immuno Research, West Grove, PA) was used as a second antibody (1:1,000). The slides were counterstained with 2 μg/mL Hoechst dye 33342 for 2 minutes and then examined in a fluorescent microscope.
In all experiments, isotype Abs purchased from Dako and Sigma were used as controls. Prior to staining, polyclonal anti-IL-2 Ab was preincubated with an excess of IL-2 (Chiron, Emeryville, CA) to verify its specificity. This absorption was shown to completely eliminate cytokine-specific staining.
IL-2 bioassay with CTLL-2 cells.
Supernatants of NK cells transduced with the IL-2 gene were tested for the presence of bioactive IL-2 in 4-day 3H-thymidine (TdR) incorporation assays using the IL-2–dependent CTLL-2 cell line as described by us previously.14
RT-PCR for IL-2 and IL-2R mRNA.
For amplification of the IL-2 gene, the following oligonucleotide primers were used: IL-2 sense, 5′-GTCACAAACAGTGCACCTAC-3′; and IL-2 antisense, 5′-CCCTGGGTCTTAAGTGAAAG-3′. To analyze expression levels of IL-2 mRNA from parental or transduced NK cell lines, quantitative competitive (QC) RT-PCR established in our laboratory was performed as previously described.21
Cytotoxicity assays.
Untreated NK cells or PFDH-fixed NK cells were used as effectors. PFDH-fixed NK-92 cells were prepared as previously described1 to prevent secretion of cytoplasmic granules and cytokines while preserving cell surface–bound ligands. Briefly, cells were fixed with 1% (wt/vol) PFDH in PBS for 10 minutes at room temperature (RT), washed twice with PBS, and incubated overnight in RPMI 1640 medium at 37°C. Supernatants of NK-92 cells were used in some assays to assess their effects on the viability or growth of tumor cell targets.
51Cr-release cytotoxicity assays were performed as previously described,1 4 using target cells labeled with 100 μCi 51Cr (NEN; 5 μCi/mmol/L) and incubated with effector cells at E:T ratios of 8:1 to 0.5:1 in U-bottom 96-well plates (Costar) for 4 hours. Spontaneous release and maximal release were determined by incubating target cells in medium alone or in 5% Triton X-100, respectively. The assay was always performed in triplicate. The percent specific lysis was calculated according to the formula, percent specific lysis = (mean cpm experimental release − mean cpm spontaneous release/mean cpm maximal release − mean cpm spontaneous release) × 100.
3H-thymidine release assay was a modification of the JAM test described by Matzinger.22 Target cells were labeled with 5 μCi/mL methyl 3H-thymidine (NEN; 147.9 GBq/mmol) and incubated with effector cells at E:T ratios of 16:1 to 2:1 for 1 hour at 37°C. Cells were disrupted by repeated (three times) freezing (at −20°C) and thawing (at RT) and harvested onto fiberglass filters, and their radioactivity was counted in an LKB Betaplate counter (Pharmacia). The percentage of specific3H-thymidine release was determined using the formula, percent cytotoxicity = 100 × (C − E)/C, in which E (experimental) is the cpm of target cells in the presence of effector cells, and C (control) is the cpm of target cells alone.
MTT assay was also performed as previously described.23Briefly, adherent target cells were plated at the density of 5,000/well in 96-well flat-bottom plates (Costar) and cultured for 24 hours at 37°C in 5% CO2 in air to prepare the monolayers for assay. Effector cells were added to the monolayer cultures at E:T ratios of 16:1 to 2:1, and following centrifugation, the plates were incubated for 24 hours. Effector cells and detached target cells were then removed by washing. Next, a 200-μL aliquot MTT (Sigma) solution (0.5 mg/mL in DMEM supplemented with 10% FCS) was added to each well and the plates were incubated for additional 4 hours at 37°C. MTT solution was then removed, and a 150-μL aliquot of dimethyl sulfoxide (Sigma) was added to each well to solubilize the formazan crystals that formed in viable target cells. The plates were rotated on a plate shaker for 10 minutes at RT. Absorbance was read immediately at a wavelength of 540 nm on a scanning multiwell spectrophotometer. The percentage of cell death was calculated using the formula, percent cell death = 100 × (C − E)/(C − B), where C is the optical density (OD) reading in wells containing target cells alone (control), B is the OD reading of wells containing medium (background), and E is the OD reading of adherent targets remaining in the wells after incubation with effector cells (experimental).
Adoptive transfer of NK cells.
P-NK-92 or TR-IL-2-NK-92 cells or human A-NK cells generated as previously described1 were used for immunotherapy of established 3-day liver metastases in BALB/c nude mice.4Liver metastases were induced by a single intrasplenic injection of human HR cells (5 × 106/0.2 mL) into nude mice immunosuppressed by treatment with cyclophosphamide (200 mg/kg; Sigma) and anti-asialo GMI (AS-GMI) Ab (0.2 mg/mouse; Waco, Dallas, TX). Three days after intrasplenic injection of HR cells, 5 × 106effector cells/mouse (P-NK-92 and exogenous IL-2, TR-IL-2-NK-92 alone, or A-NK cells and exogenous IL-2) were delivered intrasplenically to mice in groups of 10 animals per treatment. As a negative control, HBSS (GIBCO) was injected. Within 10 minutes of these injections, the spleens were removed. Exogenous IL-2 (60,000 IU in 0.5 mL HBSS) was delivered IP to mice treated with P-NK-92 and A-NK cells twice daily for 5 days. During this period of IL-2 therapy, all mice were treated with anti-AS-GMI Ab by IP injection twice to eliminate endogenous NK activity. Mice were evaluated for 60 days to assess survival.
Statistical analysis.
Statistical analysis of the results was performed using the Mann-Whitney U test. Differences were considered significant for P values less than .05.
RESULTS
Transient transduction of NK cells.
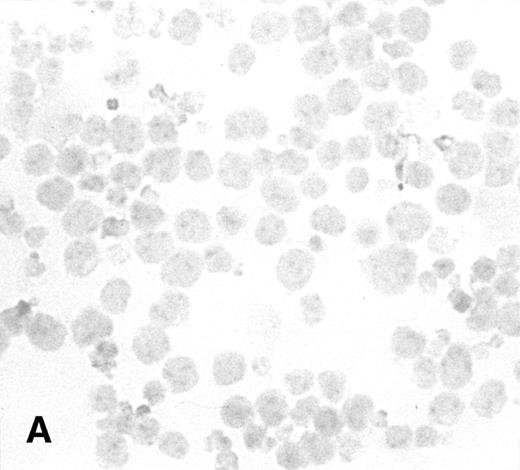
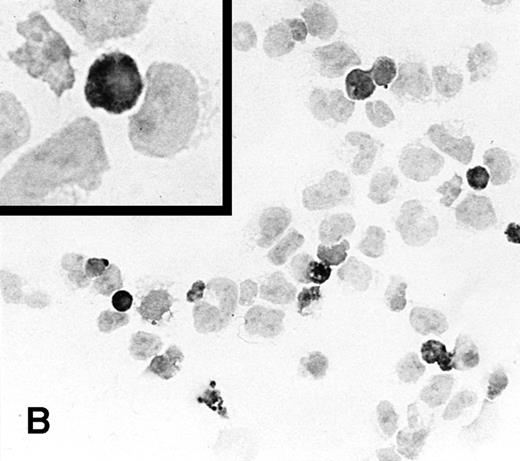
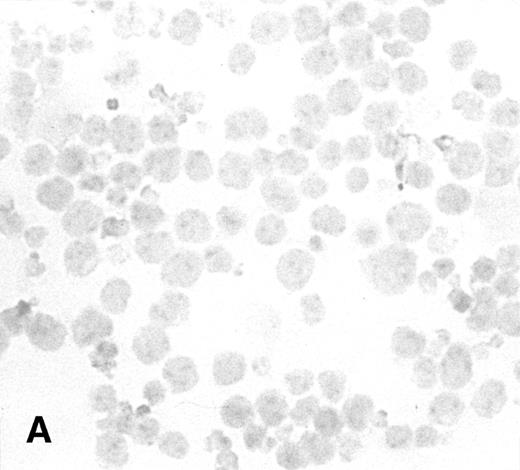
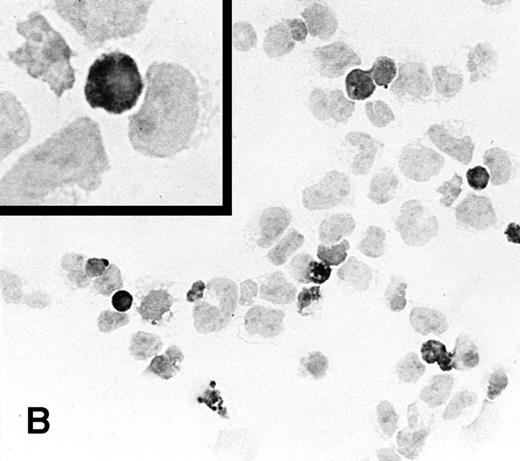
To determine the ability of an MuLV-based retroviral vector to stably infect NK cells, expression of IL-2 and Neo markers was evaluated. The efficiency of a flow-through method was determined by immunostaining for expression of the transgene. As a control, MFG-IRAP–infected NK-92 cells were used. Figure 1 shows staining for IL-2 of cultured P-NK-92 cells, which were negative for intracytoplasmic IL-2, and of transiently transduced NK-92 cells, which contained 10% to 20% stained cells. The percentage range of positive cells was determined by counting a total of 200 cells on each immunostained cytosmear prepared from three different cultures of transiently transduced NK-92 cells. These cultures were not tested by ELISA for the level of IL-2 in the supernatants because of the presence of exogenous IL-2. Instead, a culture of NK-92 cells transduced with the IRAP gene and containing 2% to 3% IRAP-positive cells by immunostaining (not shown) was tested in ELISA for the level of IRAP in the supernatant. At 72 hours posttransduction, the supernatant contained 8.4 ng IRAP/106 cells/48 h. P-NK-92 cells did not produce IRAP.
Immunostaining for IL-2 in transduced but nonselected NK-92 cells. (A) P-NK-92 cells incubated for 48 hours in the absence of exogenous IL-2 prior to staining. (B) Transduced nonselected (48 hours posttransduction) IL-2/Neo/NK-92 cells incubated for 48 hours in the absence of exogenous IL-2 prior to staining. Insert shows an IL-2–expressing NK cell (original magnification ×1,000). The staining reaction was developed with AEC, and the cells were counterstained with hematoxylin. For A and B, original magnification is ×500.
Immunostaining for IL-2 in transduced but nonselected NK-92 cells. (A) P-NK-92 cells incubated for 48 hours in the absence of exogenous IL-2 prior to staining. (B) Transduced nonselected (48 hours posttransduction) IL-2/Neo/NK-92 cells incubated for 48 hours in the absence of exogenous IL-2 prior to staining. Insert shows an IL-2–expressing NK cell (original magnification ×1,000). The staining reaction was developed with AEC, and the cells were counterstained with hematoxylin. For A and B, original magnification is ×500.
The transduced YT cells were assessed for the ability to secrete IL-2 by ELISA performed 48 hours after transduction. The supernatants consistently contained low levels of IL-2 (7 to 10 pg/106cells/48 h), while those of parental YT cells contained none.
Selection of transduced NK cells.
The process of selection was modified to include a series of stepwise increases in the concentration of G418 separated by periods of culture in the absence of G418 to give the surviving cells an opportunity to recover. Throughout selection, the growth medium was supplemented with exogenous IL-2 (1,200 IU/mL) to avoid massive cell death due to IL-2 withdrawal. The selection process was usually accomplished in 4 to 5 weeks, at which time the surviving cells were transferred to the IL-2–free medium and thereafter cultured without exogenous IL-2.
YT cells, which are IL-2–independent, were selected as already described, but the medium used for selection was not supplemented with IL-2.
Growth of NK cell lines transduced with the IL-2 gene.
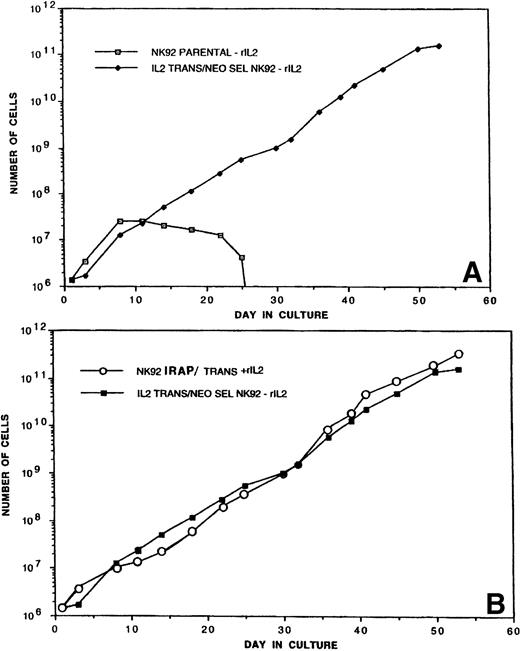
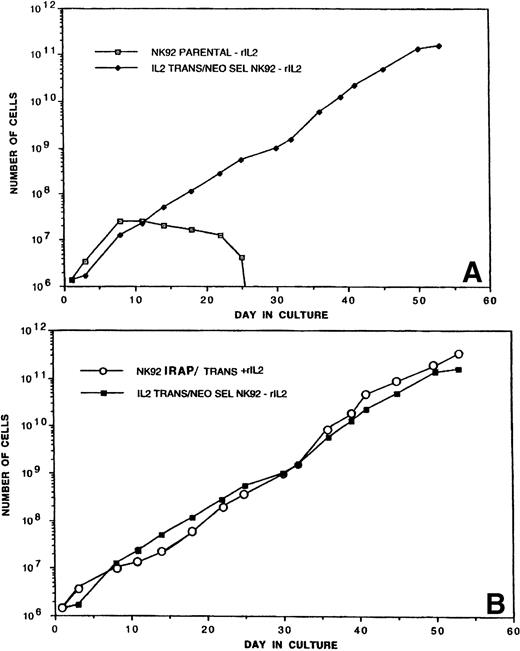
TR-IL-2-NK-92 cells were initially cultured in the presence or absence of exogenous IL-2, and their proliferation was compared with that of parental cells. Figure 2A shows that P-NK-92 cells did not continue proliferating in the absence of exogenous IL-2. In contrast, TR-IL-2-NK-92 cells proliferated exceedingly well, continuing to double in number every 24 hours. These cells have been maintained in a continuous culture for over 6 months.
(A) Proliferation of P-NK-92 and selected TR-IL-2-NK-92 in cultures not supplemented with exogenous IL-2. For culture of P-NK-92 cells, exogenous IL-2 was removed just before day 0. One of 2 experiments performed is shown. (B) Proliferation of TR-IL-2-NK-92 cells and NK-92 cells transduced with the retroviral vector containing the IRAP gene as control. The latter were IL-2–dependent and were cultured in the presence of exogenous IL-2. Note that the growth rates of transduced experimental and control NK-92 cells were similar.
(A) Proliferation of P-NK-92 and selected TR-IL-2-NK-92 in cultures not supplemented with exogenous IL-2. For culture of P-NK-92 cells, exogenous IL-2 was removed just before day 0. One of 2 experiments performed is shown. (B) Proliferation of TR-IL-2-NK-92 cells and NK-92 cells transduced with the retroviral vector containing the IRAP gene as control. The latter were IL-2–dependent and were cultured in the presence of exogenous IL-2. Note that the growth rates of transduced experimental and control NK-92 cells were similar.
It was possible that transfer of a retroviral vector into NK-92 cells enhanced their growth irrespective of the IL-2 gene. To test this possibility, we also transduced NK-92 cells with the retroviral IRAP gene and cultured them in the presence of exogenous IL-2 (1,200 IU/mL). Proliferation of TR-IRAP-NK-92 + IL-2 was similar to that of P-NK-92 + IL-2 and of TR-IL-2-NK-92 cultured in the absence of IL-2 (Fig 2B). In contrast, control TR-IRAP-NK-92 cells cultured in the absence of IL-2 did not proliferate beyond day 25 (data not shown). These control experiments demonstrated that transfer of a retroviral vector into NK-92 cells was not itself responsible for spontaneous proliferation of NK cells.
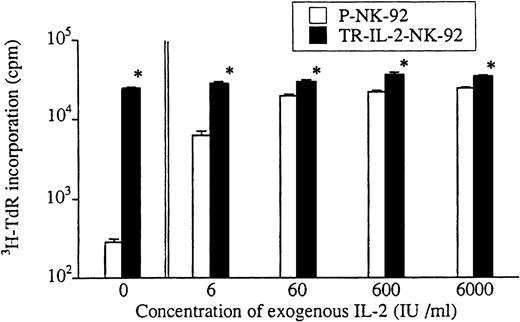
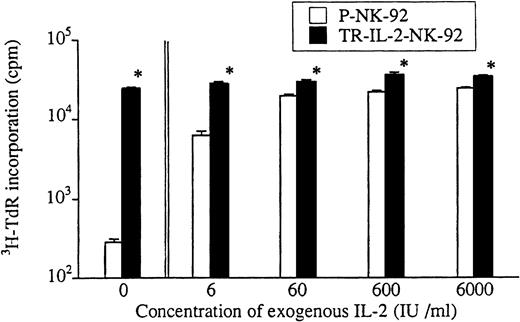
Growth of transduced and parental NK-92 cells was also compared in short-term 3H-TdR incorporation assays (Fig3). In the absence of exogenous IL-2 or in the presence of low IL-2 concentrations, TR-IL-2-NK-92 cells proliferated significantly more rapidly than parental cells. Growth of P-NK-92 cells was strictly IL-2–dependent, and at the concentration of 60 IU/mL, it reached a plateau. Addition of exogenous IL-2 at concentrations greater than 60 IU/mL had little effect on growth of the parental cell line. On the other hand, growth of TR-IL-2-NK-92 cells was independent of the presence of exogenous IL-2.
Proliferation of parental or selected and transduced NK cell lines in short-term cultures. Cells were adjusted to the concentration of 1 × 104 cells/200 μL in wells of a U-bottom microtiter plate and incubated ± IL-2 at various concentrations for 4 days. 3H-TdR incorporation was measured during the last 16 hours of culture. *P < .05 for differences in growth between parental and transduced NK cells.
Proliferation of parental or selected and transduced NK cell lines in short-term cultures. Cells were adjusted to the concentration of 1 × 104 cells/200 μL in wells of a U-bottom microtiter plate and incubated ± IL-2 at various concentrations for 4 days. 3H-TdR incorporation was measured during the last 16 hours of culture. *P < .05 for differences in growth between parental and transduced NK cells.
Only two cycles of coincubation with CRIP cells were necessary for transduction of the IL-2 gene into YT cells, which then became resistant to G418. Irrespective of the presence or absence of exogenous IL-2, transduced and selected YT cells grew significantly more rapidly than parental cells (175% ± 2% v 100% with parental cells in the absence of IL-2). Since P-YT cells are IL-2–independent and addition of exogenous IL-2 at levels of 6 to 6,000 IU/mL had no effect on their rate of growth, this rapid growth of TR-IL-2-YT cells may be attributable to activation of the autocrine IL-2 pathway in the transfectants. Control YT cells transduced with the LacZ gene did not proliferate better than P-YT cells (data not shown).
Expression of IL-2 protein and secretion of IL-2 by transduced NK cells.
To determine the ability of TR-IL-2-NK-92 or TR-IL-2-YT cell lines to secrete IL-2, supernatants of transduced and parental cell lines were tested by ELISA. IL-2 was secreted by transduced NK-92 cells consistently in the range of several ng/106 cells/24 h (Table 1). Fluctuations in the level of secreted IL-2 over time may be attributed to its utilization by IL-2–dependent NK-92 cells. TR-IL-2-YT cells secreted considerably lower quantities of IL-2 than TR-IL-2-NK-92 cells. Both transduced NK-92 and YT cells have continued to secrete IL-2 for up to 3 months in culture (Table1). In contrast, the parental cell lines produced no detectable IL-2.
The bioactivity of IL-2 secreted by TR-IL-2-NK-92 cells was next assessed in a CTLL bioassay. A standard curve based on the rate of CTLL-2 proliferation in the presence of various concentrations of exogenous IL-2 was prepared to calibrate the level of IL-2 in supernatants of NK-92 cells (data not shown). TR-IL-2-NK-92 cells secreted bioactive IL-2 in quantities comparable to those measured in immunoassays (850 pg/mL). In contrast, P-NK-92 cells contained minimal levels of bioactive IL-2 (16 pg/mL), probably representing residual exogenous IL-2 (data not shown).
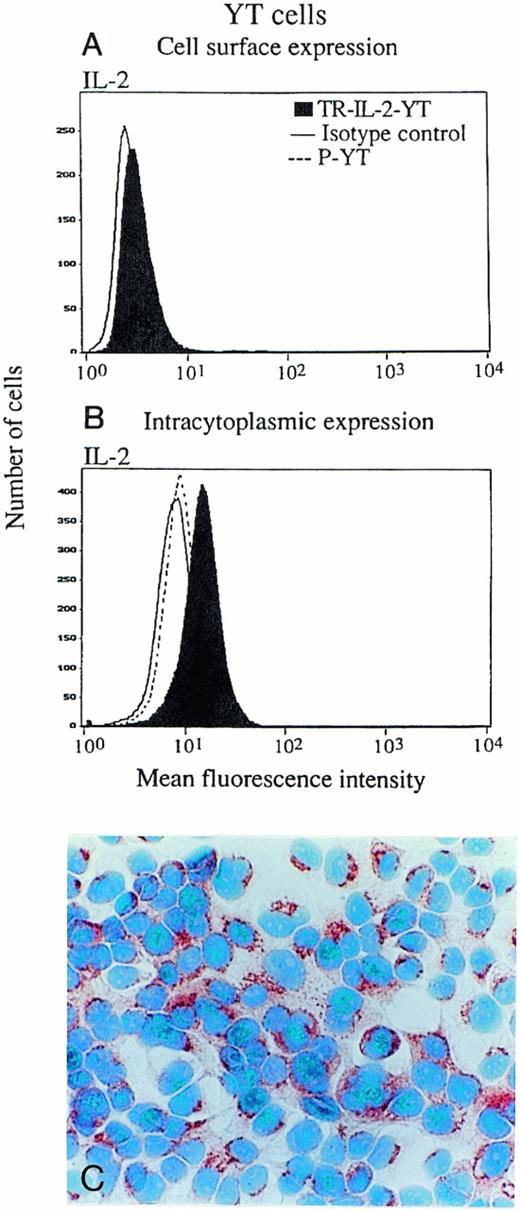
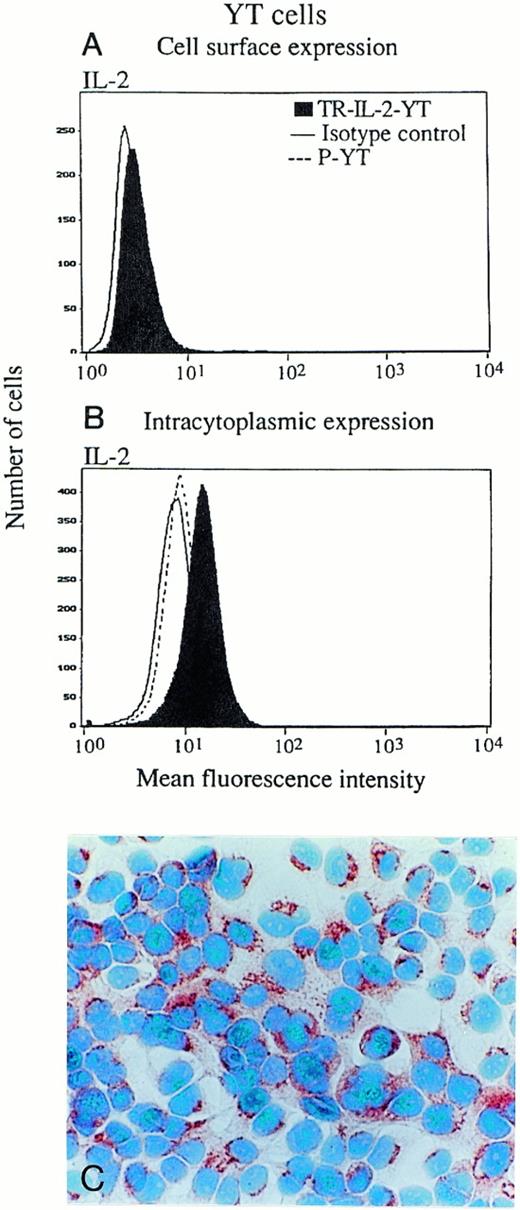
In addition, the proportion of cells in culture expressing surface and/or intracellular IL-2 was determined by both immunoperoxidase staining and flow cytometry (Fig4). Both NK-92 and YT cells expressed a minimal amount of surface-associated IL-2 detectable by flow cytometry (data for YT cells are shown in Fig 4A), and there were no differences between transduced cells and parental cells. Intracellular IL-2 protein was detectable in all transduced NK-92 (Fig 4C) and all YT cells (Fig4B), as indicated by the shift of the IL-2 peak to the right. P-NK-92 cells also contained low levels of intracellular IL-2 (NK-92 cells are IL-2–dependent), but YT cells did not. To more precisely determine the cellular localization of IL-2, immunoperoxidase or Cy5 staining was performed in the presence of brefeldin, and all TR-IL-2-NK-92 cells were found to express IL-2 protein in the Golgi zone, an indication that the protein was synthesized in the transduced cells (data not shown). In contrast, weak and diffuse expression of IL-2 protein was observed in the cytoplasm of the parental cells (not shown), perhaps due to uptake of exogenous IL-2 from the medium.
IL-2 expression in parental or IL-2 gene–transduced and selected NK cell lines. (A) and (B) Nonpermeabilized or permeabilized cells, respectively, were stained with the FITC-labeled antibody for IL-2 and examined for surface or intracytoplasmic expression of IL-2 by flow cytometry. One representative experiment of 3 performed is shown for YT cells. Similar data were obtained in 3 experiments performed on NK-92 cells. (C) Immunostaining for IL-2 in stably transduced and selected NK-92 cells (original magnification ×1,000).
IL-2 expression in parental or IL-2 gene–transduced and selected NK cell lines. (A) and (B) Nonpermeabilized or permeabilized cells, respectively, were stained with the FITC-labeled antibody for IL-2 and examined for surface or intracytoplasmic expression of IL-2 by flow cytometry. One representative experiment of 3 performed is shown for YT cells. Similar data were obtained in 3 experiments performed on NK-92 cells. (C) Immunostaining for IL-2 in stably transduced and selected NK-92 cells (original magnification ×1,000).
RT-PCR for IL-2 and IL-2R mRNA.
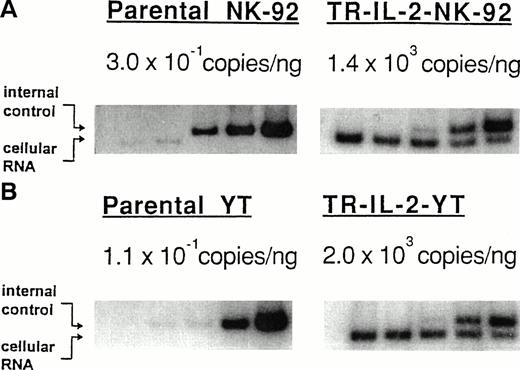
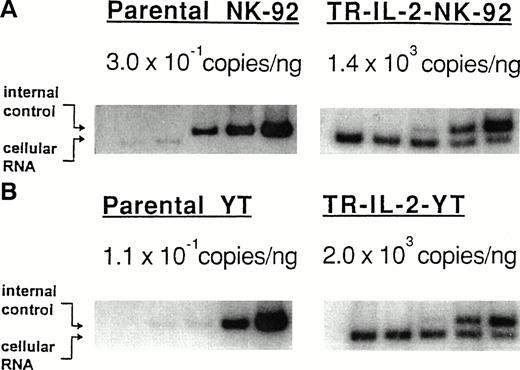
Both transduced and selected NK-92 and YT cells expressed a high number of IL-2 mRNA copies as determined by QC-RT-PCR (Fig5; TR-IL-2-NK-92 cells, 1,400 copies/ng total RNA; TR-IL-2-YT cells, 2,000 copies/ng). In contrast, parental cells expressed a marginally small number of IL-2 mRNA copies (P-NK-92 cells, 0.304 copies/ng total RNA; P-YT cells, 0.117 copies/ng).
Expression of IL-2 mRNA in the parental or IL-2 gene–transduced and selected NK cell lines by QC-RT-PCR. Total RNA extracted from NK cell lines (100 ng from parental cells, 1 ng from IL-2 gene–transduced cells) was reverse-transcribed and amplified using primers specific for IL-2. Southern hybridization was performed with radiolabeled cDNA for IL-2 to confirm the identify of the PCR product. The ratio of cpm in the internal control to cpm in cellular RNA was plotted to calculate the number of copies of IL-2 mRNA/ng total cellular RNA (not shown). (A) NK-92 cells, (B) YT cells.
Expression of IL-2 mRNA in the parental or IL-2 gene–transduced and selected NK cell lines by QC-RT-PCR. Total RNA extracted from NK cell lines (100 ng from parental cells, 1 ng from IL-2 gene–transduced cells) was reverse-transcribed and amplified using primers specific for IL-2. Southern hybridization was performed with radiolabeled cDNA for IL-2 to confirm the identify of the PCR product. The ratio of cpm in the internal control to cpm in cellular RNA was plotted to calculate the number of copies of IL-2 mRNA/ng total cellular RNA (not shown). (A) NK-92 cells, (B) YT cells.
Expression of IL-2Rs and other markers in parental and transduced NK cell lines.
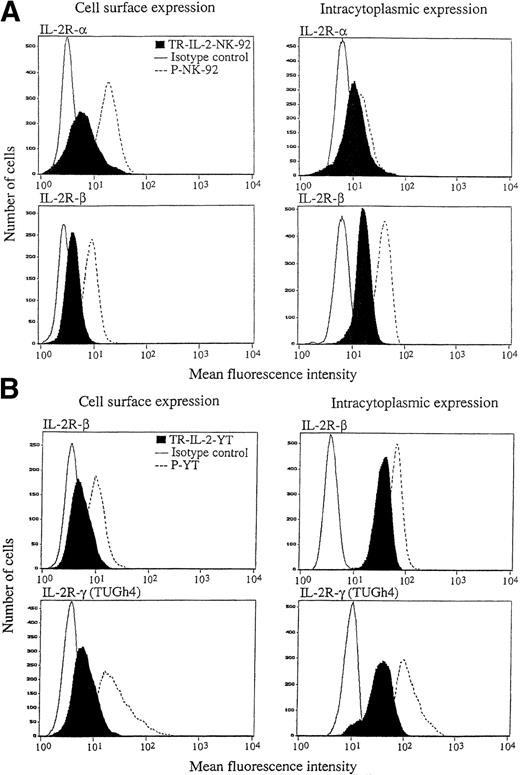
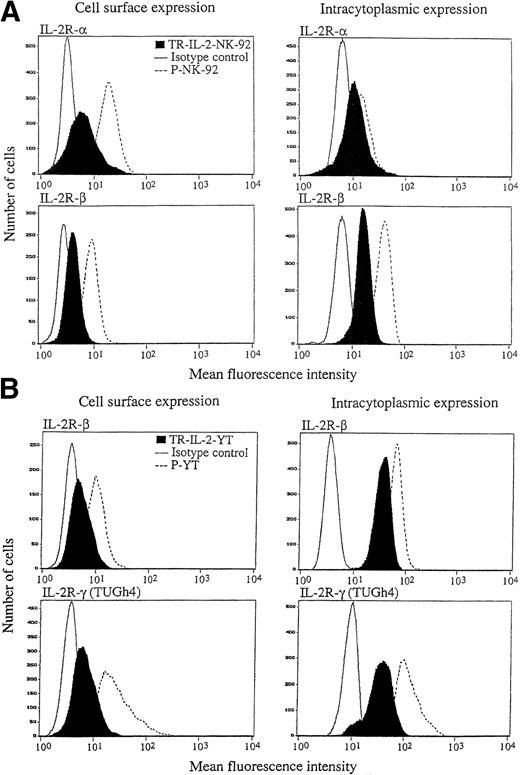
The ability to produce and secrete IL-2 could lead to changes in IL-2R expression on transduced NK cells. Therefore, surface expression of IL-2R α, β, and γ was measured by flow cytometry in the parental and IL-2 gene–transduced and selected NK cell lines. With P-NK-92 cells, flow cytometry was performed after 48 hours' incubation in the absence of exogenous IL-2. The flow cytometric data shown in Fig6 indicate that TR-IL-2-NK-92 cells have lower surface expression of IL-2R α and β than P-NK-92 cells. Expression of the γ chain was also decreased in transduced NK-92 cells relative to P-NK-92 cells (data not shown). Similarly, TR-IL-2-YT cells have lower cell surface expression of IL-2R β and γ chains than P-YT cells. YT cells do not express IL-2R α. These patterns of surface expression of IL-2Rs are expected, because transduced NK cells produce IL-2 and thus IL-2Rs are likely to be occupied by the endogenously produced ligand and internalized or shed from the cell surface.
IL-2R expression on the parental and IL-2 gene–transduced NK cell lines as determined by flow cytometry. Nonpermeabilized or permeabilized cells were stained with the appropriate antibodies for IL-2 α, β, and γ chains and examined by flow cytometry. One representative experiment of 3 performed is shown. (A) NK-92 cells, (B) YT cells.
IL-2R expression on the parental and IL-2 gene–transduced NK cell lines as determined by flow cytometry. Nonpermeabilized or permeabilized cells were stained with the appropriate antibodies for IL-2 α, β, and γ chains and examined by flow cytometry. One representative experiment of 3 performed is shown. (A) NK-92 cells, (B) YT cells.
Intracytoplasmic expression of IL-2R in the IL-2 gene–transduced and selected NK cell lines, as detected by flow cytometry, was also lower than in parental cells (Fig 6). This observation is consistent with a rapid turnover of IL-2R chains in transduced NK cells, which secrete IL-2.
No changes in expression of other surface markers commonly seen on lymphocytes were observed on parental or transduced NK cell lines by flow cytometry. Thus, all transduced and selected NK-92 cells were CD3−CD56+CD11a+CD54+CD45+, as were YT cells, except for CD56 expression. In addition to determining the proportion of cells positive for the various markers, we also looked for possible quantitative differences in their level of expression. However, no changes in the MFI of these markers were seen on transduced cells, with the exception of CD11a (LFA-1), which showed mildly increased MFI in transduced NK-92 but not YT cells (data not shown).
Production of TNFα and IFNγ by transduced NK cell lines.
It was also of interest to examine the production of TNFα and IFNγ, since these cytokines are known to be produced by human NK cells.24 ELISA was used to determine the levels of these two cytokines in supernatants of TR-IL-2-NK cells. P-NK-92 cells produced little or no TNFα and considerable levels of IFNγ in the presence of exogenous IL-2 (Table 2). In contrast, TR-IL-2-NK-92 cells spontaneously produced IFNγ, but at levels substantially lower than in P-NK + IL-2. TR-IL-2-YT cells secreted no TNFα and less IFNγ than P-YT cells.
Cytolytic functions of transduced NK cells.
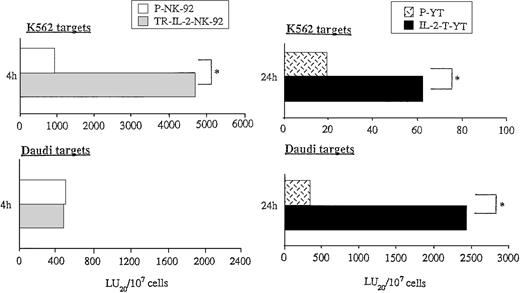
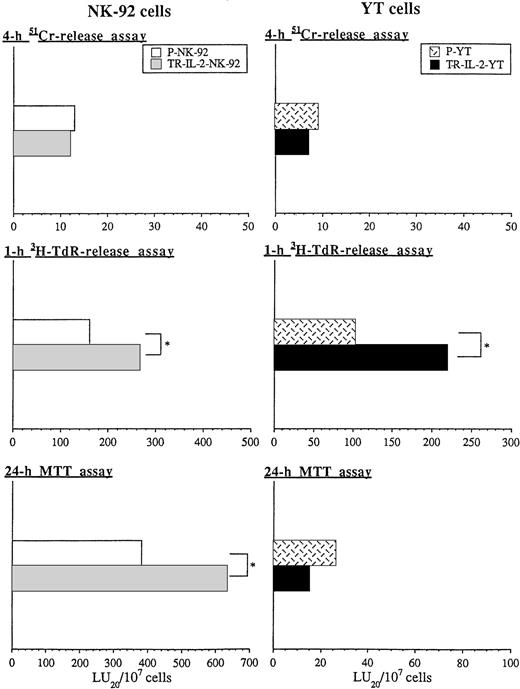
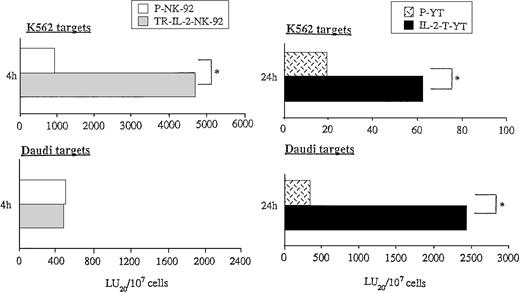
Because exogenous IL-2 is known to upregulate the cytotoxicity of NK cells, we wished to study the effect of endogenous secreted IL-2 on antitumor functions of transduced NK cells. Cytolytic activities of parental or transduced NK cells were measured in short- or long-term51Cr-release assays against NK cell–sensitive targets (K562) and NK cell–resistant targets (Daudi) (Fig7). While K562 targets were lysed by P-NK-92 cells in 4-hour 51Cr-release assays, the cytolytic activity of TR-IL-2-NK-92 cells against K562 was significantly higher than that of parental cells (Fig 7A). In contrast, lysis of Daudi targets by these TR-IL-2-NK-92 cells was comparable to that of parental cells (Fig 7A). Neither P-YT nor TR-IL-2-YT cells lysed K562 or Daudi targets in 4-hour 51Cr-release assays (data not shown). However, both types of cells lysed these targets in 24-hour51Cr-release assays, and TR-IL-2-YT cells had significantly higher cytotoxicity than P-YT cells (Fig 7B).
Cytotoxicity of parental or IL-2 gene–transduced NK cells against NK-sensitive K562 targets or NK-resistant Daudi targets. Cytotoxicity was tested in the absence of exogenous IL-2 in 4- or 24-hour 51Cr-release assays. One representative experiment of 3 performed is shown. *P < .05. (A) NK-92 cells, (B) YT cells.
Cytotoxicity of parental or IL-2 gene–transduced NK cells against NK-sensitive K562 targets or NK-resistant Daudi targets. Cytotoxicity was tested in the absence of exogenous IL-2 in 4- or 24-hour 51Cr-release assays. One representative experiment of 3 performed is shown. *P < .05. (A) NK-92 cells, (B) YT cells.
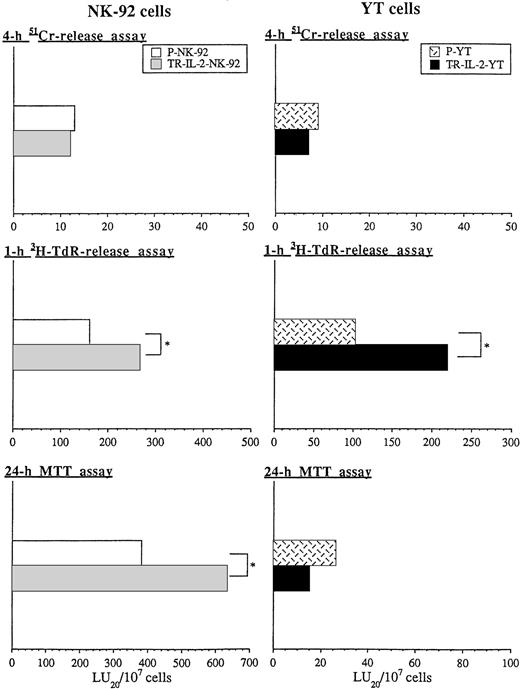
Figure 8 shows the cytotoxicity of parental or transduced NK cell lines against HR human gastric carcinoma targets, which are considered “NK cell–resistant.” HR targets were found to be only slightly sensitive to perforin-mediated killing in 4-hour51Cr-release assays by both transduced or parental NK-92 targets or YT cells (Fig 8). However, these targets were sensitive to nonsecretory apoptotic killing (3H-TdR-release assays) by both NK-92 and YT cells. Furthermore, HR targets were killed significantly better by transduced versus parental NK cells in 1-hour 3H-TdR-release assays. TR-IL-2-NK-92 cells were also significantly more growth-inhibitory to HR targets than parental cells in 24-hour MTT assays. In contrast, TR-IL-2-YT were not, perhaps because they secreted considerably less IL-2 than transduced NK-92 cells.
Cytotoxicity of parental or IL-2 gene–transduced NK cells against human gastric carcinoma targets in 4-hour51Cr-release, 1-hour 3H-TdR-release, and 24-hour MTT assays was tested. One representative experiment of 3 performed is shown. *P < .05. (A) NK-92 cells, (B) YT cells.
Cytotoxicity of parental or IL-2 gene–transduced NK cells against human gastric carcinoma targets in 4-hour51Cr-release, 1-hour 3H-TdR-release, and 24-hour MTT assays was tested. One representative experiment of 3 performed is shown. *P < .05. (A) NK-92 cells, (B) YT cells.
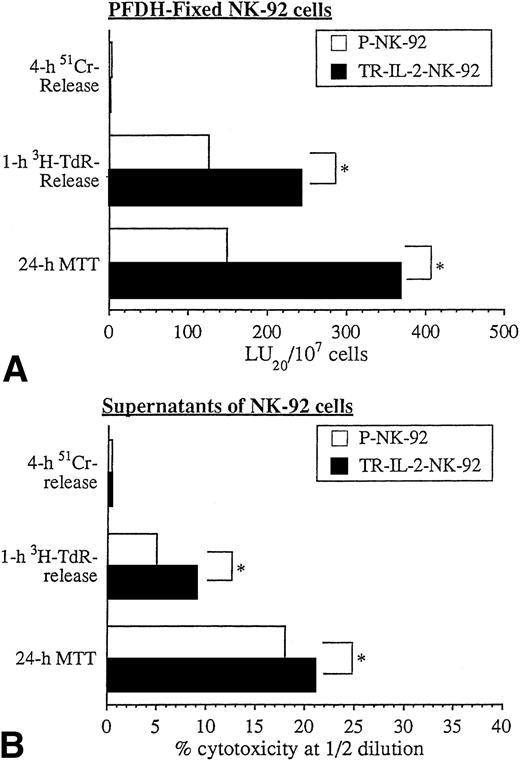
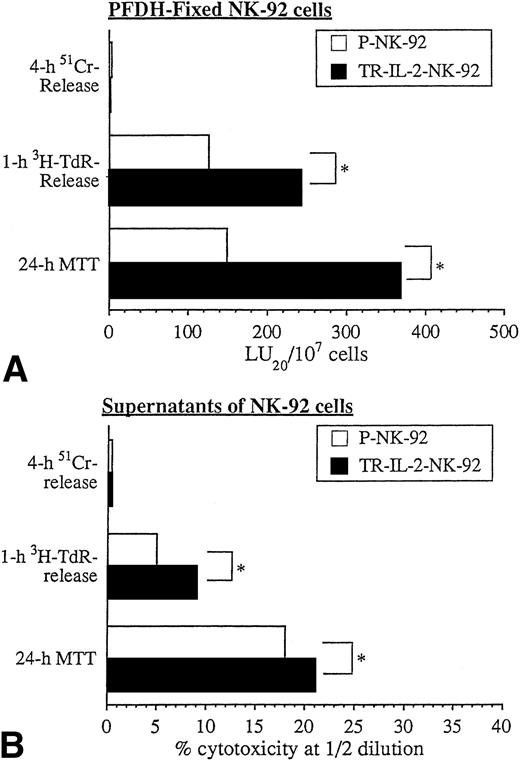
We have shown previously that PFDH-fixed NK cells are unable to secrete granules and mediate cytolysis via the classic perforin-mediated pathway.1 On the other hand, PFDH-fixed NK cells can induce apoptosis and cytostasis in tumor cell targets.1 In agreement with these prior data, PFDH-fixed NK-92 cells did not lyse HR targets in 4-hour 51Cr-release assays. However, they did mediate HR cell apoptosis in 1-hour 3H-TdR-release assays and caused growth arrest of HR targets in 24-hour MTT assays. TR-IL-2-NK-92 cells were significantly more effective than parental cells in these in vitro assays (Fig 9A).
Cytotoxicity of PFDH-fixed parental or IL-2 gene–transduced NK cells or their supernatants against HR targets was tested in various cytotoxicity assays. One representative experiment of 3 performed is shown. *P < .05.
Cytotoxicity of PFDH-fixed parental or IL-2 gene–transduced NK cells or their supernatants against HR targets was tested in various cytotoxicity assays. One representative experiment of 3 performed is shown. *P < .05.
Supernatants of NK-92 cells, which contain spontaneously released cytokines, had little effect on HR targets in 51Cr-release assays (Fig 9B). However, HR targets were readily killed by supernatants in 3H-TdR-release and 24-hour MTT assays, and supernatants produced by TR-IL-2-NK-92 were significantly more effective than those of P-NK-92 cells. Taken together, these data indicate that NK cells transduced with the IL-2 gene and secreting IL-2 mediate cytotoxicity and/or growth inhibition of solid tumor targets in vitro significantly better than nontransduced NK-92 cells.
In vivo antitumor effects of TR-IL-2-NK-92 cells.
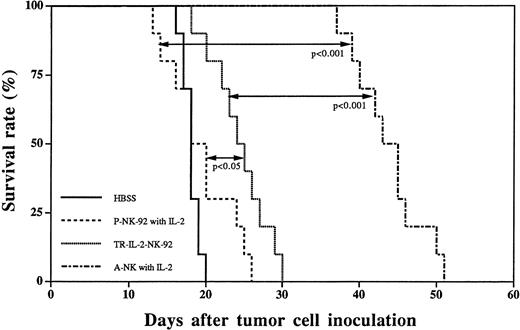
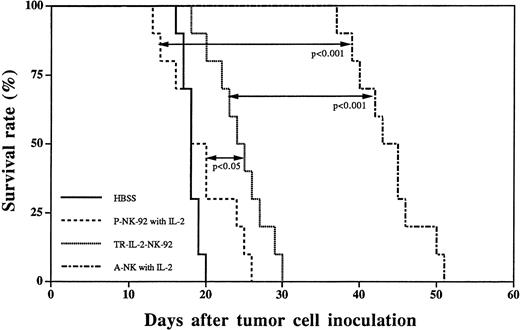
A subset of IL-2–activated human NK cells, A-NK cells, have been previously shown by us to rapidly eliminate established liver metastases of HR, a human gastric carcinoma, and to prolong survival of nude mice treated by adoptive transfer of A-NK cells and IL-2.4 24 In view of our results indicating that TR-IL-2-NK-92 cells have significantly enhanced in vitro antitumor activity against HR targets, we next performed a series of in vivo adoptive immunotherapy experiments in the HR model of liver metastasis, comparing TR-IL-2-NK-92 and P-NK-92 cells. Single adoptive transfer of TR-IL-2-NK-92 cells resulted in significantly prolonged survival of nude mice with 3-day established liver metastases as compared with 3-day therapy with P-NK-92 cells administered together with IP IL-2 (Fig 10). Nevertheless, the therapeutic effect of TR-IL-2-NK-92 cells was not as impressive as with A-NK cells transferred together with exogenous IL-2. P-NK-92 cells plus IL-2 had no therapeutic effect against established liver metastases as compared with tumor-bearing control mice not treated with AIT (Fig10).
Survival curves of nude mice with 3-day established liver metastases following immunotherapy with HBSS control, P-NK-92 cells plus exogenous IL-2, TR-IL-2-NK-92 cells, or A-NK cells plus IL-2. Statistical differences in survival between groups of 10 animals each are indicated.
Survival curves of nude mice with 3-day established liver metastases following immunotherapy with HBSS control, P-NK-92 cells plus exogenous IL-2, TR-IL-2-NK-92 cells, or A-NK cells plus IL-2. Statistical differences in survival between groups of 10 animals each are indicated.
To explain the relatively weak but significant survival advantage seen in mice treated with TR-IL-2-NK-92 cells, we determined the level of IL-2 in serum and in homogenized liver tissues of animals treated as a control with P-NK-92 (no IL-2) or with TR-IL-2-NK-92 cells. No IL-2, IFNγ, TNFα, or IL-12 was detectable in the sera of these animals 24 hours after injection of effector cells (Table3). Only liver homogenates of mice treated with transduced cells contained a detectable but low level of IL-2, which probably explains why the survival benefits of AIT were not as pronounced as those with A-NK cells supplemented with a relatively high dose of exogenous IL-2. On the other hand, levels of TNFα and IFNγ were higher in liver homogenates of mice treated with TR-IL-2-NK-92 versus P-NK-92 cells. In this experiment, IL-12 levels in liver homogenates were also assayed to show that secretion of IL-2 by TR-IL-2-NK-92 cells did not induce IL-12 production in the liver.
To ascertain that mice do not develop a lymphoproliferative disease following adoptive transfer of TR-IL-2-NK-92 cells, we injected two normal animals with these cells and observed them for a period of 5 months. No evidence of sickness, splenomegaly, or wasting has been observed in these animals so far.
DISCUSSION
NK cells are thought to play an important role in protection against various infectious agents and to mediate immune surveillance against metastases.25-28 Antitumor functions of NK cells in vitro and in vivo are known to be dependent on the presence of IL-2.3-5 In fact, human NK cells, which constitutively express IL-2Rβ, are the first lymphoid cells to respond to exogenous IL-2 by upregulation of IL-2Rα chain expression29,30 and by adherence to solid substrates.6 The dependence of NK cells on exogenous IL-2 necessitates that this cytokine be present in concentrations sufficient to support NK activities, when the cells are adoptively transferred for therapy of established tumor or tumor metastases in experimental animals4,24 or in patients with advanced tumors.31 However, toxicities associated with high-dose IL-2 therapy have limited its use alone or together with effector cells in patients with cancer.32 Therefore, we wished to test the hypothesis that human NK cells transduced with the IL-2 gene and producing IL-2 might be able to sustain and mediate antitumor activities without the addition of exogenous IL-2.33
To test this hypothesis, we initiated attempts to introduce the IL-2 gene into human NK cells. However, these effector cells proved to be resistant to gene transfer under a variety of experimental conditions tried. Techniques that have been successful with tumor or tissue cells14,34-36 failed to work with NK cells, as reported by us and others.33 Transfection with viral supernatants has been the most widely used strategy for genetic modification of a variety of cell types.14,36,37 Since NK cells have been previously reported to be susceptible to viral infections,38,39 we decided to deliver the IL-2 gene by transduction of NK cell lines with a retroviral vector. The NK-92 cell line established from a patient with lymphoma11 is IL-2–dependent and cytotoxic against K562 and Daudi targets. The YT cell line, on the other hand, is IL-2–independent and unable to lyse NK-sensitive targets in 4-hour 51Cr-release assays. The use of these NK cell lines for gene transfer gave us an opportunity to establish and test a model system for transduction and selection of fresh NK cells. By cocultivation of NK cells with the CRIP cell line, producing high titers of the viral particles (105 to 106 cfu/mL) containing IL-2 and NeoR genes, we readily obtained transfectants of YT cells. In contrast, cocultures of NK-92 cells with CRIP resulted in a complete destruction of CRIP cell monolayers mediated by cytolytic NK-92 cells. This observation indicated that NK-92 cells were capable of killing virally infected targets rapidly, and that retroviral-mediated transduction of the IL-2 gene into these effectors might be much more difficult than such transduction into YT cells that are unable to kill virally infected targets.
Based on this initial experience, a transduction method facilitating a high rate of gene transfer (ie, rapid binding and internalization of the virus) was sought. A recently described technique of flow-through gene transfer17 appeared to offer this advantage, and we applied it to transduction of NK-92 cells with the viral supernatants produced by CRIP cells. Transduction of 10% to 20% NK-92 cells, which was achieved in this system, allowed for subsequent selection in G418-containing medium, leading to the establishment of stably transduced NK-92 cells expressing the IL-2 gene.
The TR-IL-2-NK-92 cells we have consistently been able to generate are resistant to G418, proliferate well in the absence of exogenous IL-2, express abundant mRNA for IL-2 and IL-2 protein localized to the Golgi zone, and secrete biologically active IL-2 into culture supernatants. In addition, these TR-IL-2-NK-92 cells have an increased ability to kill tumor cell targets in vitro compared with P-NK-92 cells. Furthermore, TR-IL-2-NK-92 cells produce supernatants with antitumor activity. These supernatants were shown to contain IFNγ in addition to secreted IL-2, but little measurable TNFα. The near absence of TNFα in death-inducing supernatants of transduced cells indicates that other members of the TNF family of proteins, such as FasL, might be responsible for tumor cell apoptosis.40 41IL-2–activated NK cells and the transduced NK cell lines express FasL and their supernatants contain sFasL, while HR targets express Fas (N.L. Vujanovic and T.L. Whiteside, unpublished data, October 1997). Thus, sFasL could be responsible for apoptosis of HR cells observed in vitro. The most important observation in these in vitro studies is that TR-IL-2-NK-92 cells kill NK-resistant solid tumor cell targets more efficiently than parental cells. This could translate into a more efficient antitumor effect in vivo, because the transduced cells are likely to survive and function in the absence of exogenous IL-2, while the parental cells depend on the presence of exogenous IL-2. Furthermore, the presence of considerable levels of TNFα in liver homogenates indicates that in vivo TNFα might contribute to elimination of established liver metastases.
In vivo experiments with TR-IL-2-NK-92 cells showed that adoptive transfer of these effector cells in the absence of exogenous IL-2 was therapeutic in an experimental metastasis model. Survival of nude mice with 3-day established liver metastases from HR, a human gastric carcinoma metastatic to liver, was significantly prolonged after a single transfer of 106 TR-IL-2-NK-92 cells. This in vivo effect was not as dramatic as that repeatedly observed by us with A-NK cells plus IL-2.4 25 It is possible that in comparison to A-NK cells, TR-IL-2-NK-92 cells were less able to localize to metastases in vivo. Future AIT studies with labeled effector cells should be able to determine differences, if any, in their localization in the liver. Nevertheless, our data confirm the hypothesis that NK cells engineered to produce IL-2 acquire and maintain significantly greater antitumor functions in vitro and in vivo than parental NK cells in the presence of exogenous IL-2. For example, liver homogenates in mice treated with the transduced NK cells contained higher levels of TNFα and IFNγ than those in control mice (Table 3), and these cytokines might play an important role in tumor cell elimination in vivo.
The reported initial success of stable transduction of NK-92 cells with the IL-2 gene is encouraging and provides a model for further studies aiming at genetic modifications of A-NK cells. These studies are in progress in our laboratory.
Supported in part by the National Institutes of Health Grant No. R01-CA63513 (T.L.W.), the Markey Foundation, the Pathology Educational and Research Foundation, and Deutche Forschungsgemeinschaft Grant Re No. 1155/1-1 (T.E.R.).
Address reprint requests to Theresa L. Whiteside, PhD, University of Pittsburgh Cancer Institute, W1041 Biomedical Science Tower, 211 Lothrop St, Pittsburgh, PA 15213-2582.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.