Abstract
Epstein-Barr virus (EBV)-specific CD8+ cytotoxic T cells are thought to be critical for the control of EBV, which persists in healthy individuals as a latent infection of B cells. However, recent observations have indicated that CD8+ T-cell responses are not uniformly cytotoxic and that CD8+ T cells may be subdivided into type 1 and type 2 subsets that parallel the classically described Th1 and Th2 subsets of CD4+ T cells. Using two-color flow cytometric analysis of intracellular cytokine expression at the single-cell level, we have identified two distinct but overlapping subsets of EBV-specific CD8+ T cells, the first of which expressed high levels of interferon γ (IFNγ), but little or no interleukin-4 (IL-4), whereas the second subset was IFNγ+/IL-4+ double-positive. A significant proportion of EBV-specific CD8+ T cells also expressed IL-13. Subsequent analysis of a panel of 27 EBV-specific CD8+ T-cell clones showed inverse relationships between EBV-specific cytotoxicity and secretion of IL-4, IL-10, and IFNγ, respectively. IL-10 was not secreted by the 11 most strongly cytotoxic clones, suggesting that IL-10 secretion may provide a functional definition of an EBV-specific type 2 CD8+ T-cell subset with reduced EBV-specific cytotoxicity. Finally, we have demonstrated that EBV-specific CD8+ T cells that express type 2 cytokines possess the ability to activate resting B cells. EBV-specific CD8+ T cells thus have the potential to reactivate latent EBV infection in vivo and may contribute to the development of EBV-associated lymphoproliferative disorders and lymphoma.
EPSTEIN-BARR VIRUS (EBV) is a ubiquitous human gammaherpesvirus that persists throughout life as a latent infection in small resting B cells. Primary infection in early childhood is typically asymptomatic, but infection of young adults may result in infectious mononucleosis, a self-limited lymphoproliferative disorder characterized by fever, pharyngitis, and cervical lymphadenopathy. During latent infection of resting B cells in vivo, viral gene expression is limited to Epstein-Barr nuclear antigen (EBNA) 1 and latent membrane protein (LMP) 2A.1,2 EBV transforms B cells in vitro, permitting the establishment of immortalized lymphoblastoid cell lines (LCL). A broader set of viral latent genes is expressed in LCL, encompassing EBNAs 1 through 6; LMP1, 2A, and 2B; and two highly expressed but nontranslated small RNA species, EBER 1 and 2. The EBNAs 2 through 6 and LMPs 1 and 2 are recognized as targets by virus-specific CD8+ cytotoxic T cells (CTL).3,4EBNA1 is not recognized by CTL by virtue of its possession of a series of gly-ala repeat sequences that inhibit the HLA class I antigen processing pathway.5 Lack of recognition of EBNA1 by CTL may contribute to the ability of EBV to persist in resting B cells.
There is a general consensus that CD8+ CTL play a critical immunosurveillance role in the control of persistent EBV infection. EBV-specific CTL responses are impaired in cyclosporin-immunosuppressed transplant patients,6,7 who are at high risk of developing EBV-associated posttransplant lymphoproliferative disorders (PTLD) and lymphoma.8-11 In addition, patients with established PTLD have been successfully treated by transfer of in vitro-stimulated autologous, EBV-specific CTL,12,13 and EBV-specific CTL have been shown to inhibit development of EBV-associated lymphomas in chimeric SCID/hu mice.14-16
Although the CD8+ cytotoxic response to EBV is undoubtedly important in maintaining an asymptomatic host-virus equilibrium, recent work has shown that the CD8+ response may not be uniformly cytotoxic.17 Furthermore, there is an increasing body of evidence for the existence of type 1 and type 2 subsets of CD8+ T cells similar, but not identical, to the Th1 and Th2 subsets of CD4+ T cells.18 Broadly speaking, type 1 T cells express interleukin-2 (IL-2), interferon γ (IFNγ), and tumor necrosis factor α/β (TNFα/β); mediate delayed-type hypersensitivity; and are cytotoxic; whereas type 2 T cells express IL-4, IL-5, IL-6, IL-10, and IL-13 and provide efficient help for B cell activation, proliferation, and differentiation.18These observations prompted us to investigate whether diversity at the levels of cytokine expression and cytotoxic function was evident in the CD8+ response to EBV. In this report, two-color flow cytometric assays for intracellular cytokine gene expression at the single-cell level show that EBV-specific CD8+ T cells from normal adult donors uniformly express IFNγ, but also demonstrate the presence of IFNγ+/IL-4+ and IFNγ+/IL-13+ subsets. Subsequent clonal analysis showed significant inverse relationships between EBV-specific cytotoxicity and secretion of IL-4, IL-10, and IFNγ, respectively. Finally, we also show that EBV-specific CD8+ T cells that secrete type 2 cytokines are efficient activators of normal resting B cells.
MATERIALS AND METHODS
Human subjects.
Venous blood samples were drawn from three normal individuals, KR (HLA A2, A3, B35, B61), JTC (HLA A1, A28, B8, B27), and MJC (HLA A1, A2, B8, B27, Cw1). Peripheral blood lymphocytes (PBL) were separated by centrifugation over Fico/Lite-LymphoH (Atlanta Biologicals, Norcross, GA), washed, and cryopreserved.
Stimulator/target cells.
LCL were prepared by infection of PBL from normal individuals with the B95-8 strain of EBV in the presence of cyclosporin A (1.0 μg/mL). All LCL were subsequently maintained in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 3 mmol/L glutamine, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 5 × 10−5 mol/L 2-mercaptoethanol (RPMI/10). Activated normal B cells were prepared essentially as described by Banchereau et al.19 Briefly, PBL were depleted of T cells by panning for 2 hours over OKT3-coated (50 μg/mL in 2 mL phosphate-buffered saline [PBS], o/n) 6-well Costar plates (Costar Corp, Cambridge, MA). Nonadherent cells were subsequently cultured in RPMI 1640 supplemented with 10% human AB serum (Advanced Biotechnologies Inc, Columbia, MD), 3 mmol/L glutamine, 100 IU/mL penicillin, 100 μg/mL streptomycin, and 5 × 10−5 mol/L 2-mercaptoethanol (RPMI/10Hu) in 24-well Costar plates (2 × 105/well) with 2 × 104/well irradiated (7,500 rad) mouse L cells transfected with human Cdw32 (FcγRII; kindly provided by Kevin Moore, DNAX, Palo Alto, CA), 100 ng/mL anti-CD40 (monoclonal antibody [MoAb] 89; Immunotech Inc, Westbrook, ME), and 50 U/mL recombinant human IL-4 (PharMingen, San Diego, CA). B cells were fed every 3 to 4 days with fresh RPMI/10Hu plus 50 U/mL IL-4. B cells were infected o/n with rVV at a multiplicity of injection of 10. The rVV expressing EBV latent genes LMP2A and EBNAs 2 through 6 were kindly provided by Mike Kurilla (University of Virginia Health Sciences Center, Charlottesville, VA) and rVV-TK− was provided by Mike Mackett (University of Manchester, Manchester, UK).
T-cell lines and clones.
EBV-specific human T-cell lines were initiated by culturing PBL (2 × 106/mL) with autologous irradiated (7,500 rad) B95-8 transformed LCL (5 × 104/mL) in RPMI/10Hu. After 9 to 11 days, T cells (2 × 105/mL) were restimulated with irradiated LCL (2 × 105/mL). Recombinant human IL-2 (50 to 100 U/mL; provided by the Biological Response Modifiers Program, National Cancer Institute, Bethesda, MD) was added to the cultures at this time or 3 to 4 days after restimulation. T-cell lines were subsequently maintained by restimulation with irradiated LCL every 14 days, with interim half-changes of fresh medium plus IL-2 every 3 to 4 days.
T-cell clones were derived by limiting dilution from established T-cell lines. Briefly, T cells were cultured in 96-well plates (Falcon; Becton Dickinson, Lincoln Park, NJ) at 100, 30, 10, 3, and 1 cell(s)/well with autologous irradiated LCL (5 × 104/well) in 150 μL/well RPMI/10Hu plus 100 U/mL IL-2. After 7 days, cultures received an additional 100 μL/well RPMI/10Hu plus 100 U/mL IL-2. Positive wells at limiting dilution (12 wells or fewer positive cultures per 96-well plate) were restimulated and expanded into 24-well plates (Costar) and subsequently maintained in 24- or 12-well plates by periodic restimulation/feeding as described above. Phenotype analysis was by flow cytometry using a FACScan (Becton Dickinson, San Jose, CA) with MoAbs specific for CD3 (OKT3), CD8 (OKT8), and CD4 (PE-Leu3a; Becton Dickinson). Anti-CD3 and anti-CD8 were unconjugated MoAbs, the binding of which was detected with fluorescein isothiocyanate (FITC) goat antimouse Ig (Sigma, St Louis, MO).
Cytotoxic T-cell assays.
Cytotoxicity was tested in a standard chromium release assay. Targets, which included autologous and HLA class I-mismatched LCL, NK-sensitive K562 cells, and recombinant vaccinia virus (rVV)-infected activated normal B cells, were labeled with Na2[51Cr]O4 for 1 hour and washed three times before incubation in 96-well round-bottom plates (1 × 104/well; Falcon, Becton Dickinson) with effector T cells at the indicated E:T ratios for 4 to 5 hours. Released 51Cr in the supernatants was measured with a Cobra Auto-Gamma counter (Packard, Meriden, CT).
Lymphoproliferation assays.
T cells (1 × 104/well) were incubated with irradiated autologous or HLA class I-mismatched LCL (5 × 104/well) in 96-well flat-bottom plates in 200 μL/well RPMI/10Hu plus 20 U/mL IL-2. After 4 days, 3H-TdR was added (1 μCi/well), and 6 hours later the plates were harvested with a Filtermate 196 cell harvester (Packard) and 3H-TdR incorporation was measured with a Matrix 96 Direct Beta Counter (Packard).
Flow cytometric analysis of intracellular cytokines.
This protocol is adapted from that described by Openshaw et al.20 EBV-specific CD8+ T cells were rested for 14 days after antigen stimulation before activation by phorbol myristate acetate (PMA) and ionomycin. Briefly, CD8+ T cells (7.5 × 105/mL) were incubated at 37°C for 6 hours in RPMI/10Hu plus 50 ng/mL PMA, 500 ng/mL ionomycin, and 3 μmol/L monensin (in some experiments, monensin treatment was replaced by addition of 10 μg/mL Brefeldin A for the final 3 hours of incubation). Control, nonactivated cultures were incubated in the presence of monensin or Brefeldin A only. The cells were harvested, washed, and fixed with 2% paraformaldehyde in PBS for 20 minutes at room temperature, after which they were washed and stored overnight in PBS at 4°C. For intracellular staining, the cells were washed and permeabilized by incubation in PBS plus 1% bovine serum albumin (BSA) and 0.5% saponin (S-7900; Sigma) for 10 minutes at room temperature. In early experiments, activated and control cells were stained with FITC-anti-IFNγ and phycoerythrin (PE)-anti–IL-4 or PE-anti–IL-13 (all from PharMingen). However, later experiments used FITC-anti-IFNγ, PE-anti–IL-4, and isotype-matched controls (FITC-anti-Igγ2a and PE-anti-Igγ1) from Becton Dickinson. To confirm activation, T cells were also stained with PE-anti-CD69 (Becton Dickinson). After staining, cells were washed twice with PBS plus 1% BSA and 0.5% saponin, washed once with PBS plus 0.5% BSA, and fixed a second time with 2% paraformaldehyde in PBS. Analysis was conducted with a FACScan, using LYSIS II software and WinMDI 2.5 software (kindly made available by Joe Trotter, The Scripps Research Institute, La Jolla, CA).
Assays for cytokine secretion.
Supernatants were harvested from T cells incubated for 48 hours in microwells (1 × 105 T cells/well in 100 μL RPMI/10Hu) precoated with OKT3 (50 μg/mL, o/n). Supernatants were microfuged for 5 minutes at 12,000g to remove cell debris and stored at −20°C. Secretion of IFNγ, IL-4, and IL-10 by CD8+ T cells was measured by enzyme-linked immunosorbent assay (ELISA), using commercial assay kits (Biotrak; Amersham Corp, Arlington Heights, IL). Cytokine levels were quantified against standard curves, in accordance with the manufacturer's instructions. The limits of sensitivity of these assays were less than 2 pg/mL for IL-4 and IFNγ and less than 3 pg/mL for IL-10.
Ribonuclease (Rnase) protection assay.
Cytokine mRNA expression by EBV-transformed LCL was assessed by multiprobe Rnase protection assays, essentially as described.21 Probe sets were from PharMingen.
Measurement of B-cell activation.
Purified resting B cells were isolated from PBL by positive selection with CD19-Dynabeads (Dynal A.S., Oslo, Norway). Briefly, 7 to 9 × 107 PBL were incubated with 4 × 107CD19-Dynabeads in 4 mL PBS/2% FCS at 4°C for 60 minutes. Dynabead-rosetted B cells were magnetically separated, washed five times with PBS/2% FCS, and subsequently isolated by incubation with 200 μL PBS/2% FCS plus 60 μL CD19-Detachabead reagent (Dynal A.S.) for 90 minutes at room temperature. Yields were generally 4 to 6 × 106 B cells of 95% to 98% purity by flow cytometric analysis with FITC-anti-CD3/PE-anti-CD19 (Becton Dickinson). Residual T-cell contamination was less than 1%. CD8+T-cell lines or clones (5 × 104/well) were irradiated (3,000 rad) and cocultured with B cells (5 × 104/well) in 96-well flat-bottom plates (Falcon) precoated with OKT3 (50 μg/mL in PBS, o/n). B-cell activation and proliferation were measured by 3H-TdR incorporation after 4 days (as described above).
RESULTS
Characterization of EBV-specific polyclonal T-cell lines.
EBV-specific polyclonal T-cell lines were established from three normal donors by stimulation of PBL with autologous LCL. The cell lines possessed variable proportions of CD8+ T cells, from 47% (JTC) to greater than 98% (MJC), and all exhibited strong HLA-restricted cytotoxic function against autologous LCL (from 34.3% specific lysis at an E:T ratio of 10:1 for JTC T cells to 53.9% lysis at the same E:T ratio for MJC T cells), with minimal cytotoxicity against HLA class I-mismatched LCL and NK-sensitive K562 cells. The MJC T-cell line was further characterized as being HLA A2.1-restricted, with specificity for EBV latent gene products LMP2A (36.7% lysis at an E:T ratio of 10:1), EBNA3 (29.7% lysis), and EBNA6 (26.5% lysis) in cytotoxicity assays against rVV-infected autologous anti-CD40–activated normal B cells. Cytotoxic specificity for EBNA2, EBNA4, and EBNA5 was not detected; specificity for EBNA1 and LMP1 was not tested. Of particular significance, activation of the MJC and KR CD8+ T cells with anti-CD3 (OKT3) or autologous LCL in each case resulted in secretion of both IFNγ and IL-4 detectable by ELISA (not shown).
Intracellular cytokine expression by EBV-specific polyclonal CD8+ T cells.
Whereas ELISAs showed that EBV-specific CD8+ T cells secreted IFNγ and IL-4, they could not determine whether IFNγ and IL-4 were coexpressed by CD8+ T cells or whether cytokine expression segregated into discrete IFNγ+/IL-4− and IFNγ−/IL-4+ CD8+ subsets. Recently developed flow cytometric techniques for detection of intracellular cytokine expression at the single-cell level enabled us to address this question.
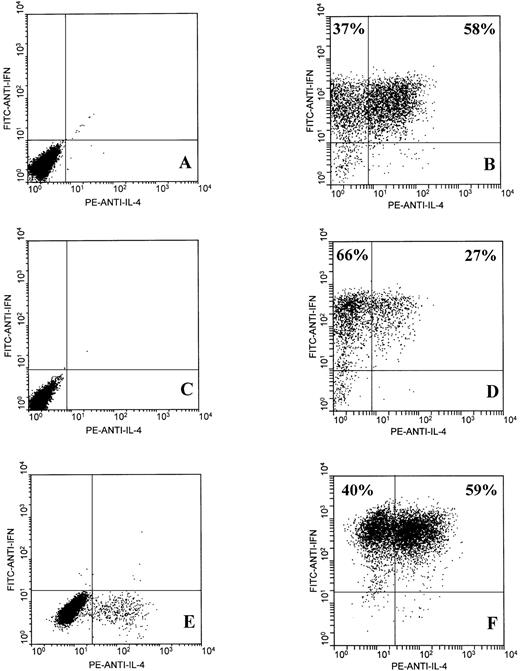
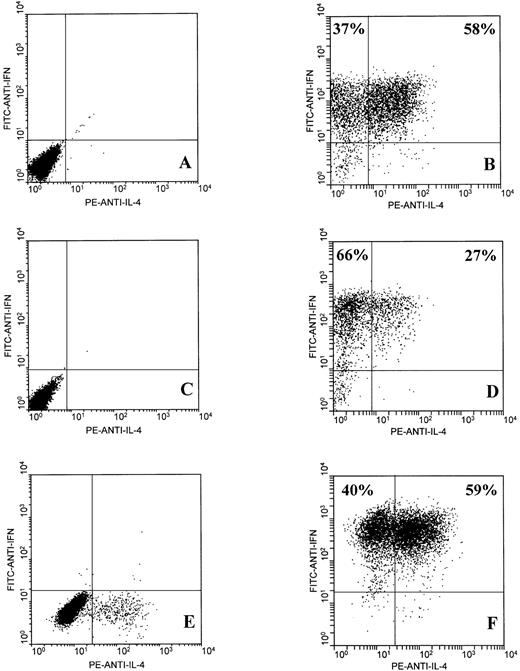
CD8+ T cells from the JTC and KR lines were purified by magnetic bead separation, yielding CD8+ T-cell preparations that were greater than 98% pure. The MJC T-cell line was greater than 98% CD8+ and was not subject to further enrichment. The KR and MJC CD8+ T-cell lines had been subjected to 8 antigen-driven restimulation cycles of 14 days, and the JTC CD8+ T cells had been through 5 restimulation cycles at the time of these assays. Subsequent clonal analysis (see below) confirmed that a large majority of the T cells within the lines were antigen-specific at this point. Two-color flow cytometric analysis of intracellular IFNγ and IL-4 expression by EBV-specific activated CD8+ T cells showed the presence of two distinct but overlapping subsets: one subset that secreted IFNγ but little or no IL-4 and a second subset that secreted both IFNγ and IL-4 (Fig 1B, D, and F). Furthermore, IFNγ+/IL-4+ double-positive cells constituted the major subset of CD8+ T cells from donors JTC (58%) and MJC (59%). The finding that EBV-specific CD8+ T cells from all three normal donors include significant populations of IL-4–secreting cells strongly suggests that this phenotype is not exceptional. Nonactivated (ie, resting) CD8+ T cells from all three donors failed to stain for IFNγ or IL-4, except for a small proportion (∼5%) of MJC CD8+ T cells, which expressed IL-4 and may represent residual activated cells from earlier antigen stimulation (Fig 1A, C, and E). Similarly, FITC-anti-IgG2a and PE-anti-IgG1 isotype controls did not stain either activated or nonactivated CD8+ T cells (not shown).
Two-color flow cytometric analysis of intracellular IFNγ and IL-4 expression by EBV-specific CD8+ T cells. T cells were unstimulated (A, C, and E) or stimulated for 6 hours with PMA and ionomycin (B, D, and F), as described in Materials and Methods. T-cell lines from three healthy adult donors were tested: JTC (A and B), KR (C and D), and MJC (E and F).
Two-color flow cytometric analysis of intracellular IFNγ and IL-4 expression by EBV-specific CD8+ T cells. T cells were unstimulated (A, C, and E) or stimulated for 6 hours with PMA and ionomycin (B, D, and F), as described in Materials and Methods. T-cell lines from three healthy adult donors were tested: JTC (A and B), KR (C and D), and MJC (E and F).
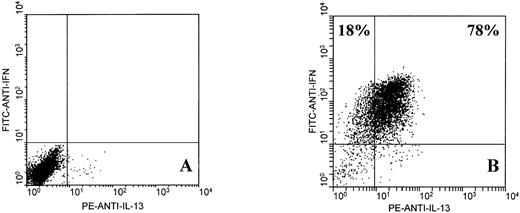
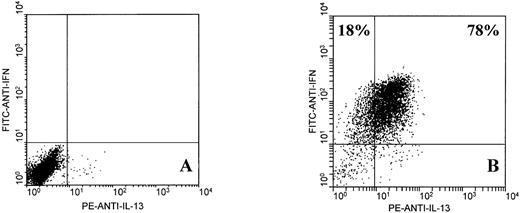
Further analysis showed that EBV-specific CD8+ T cells from normal donors expressed IL-13, a type 2 cytokine that shares many of the functional characteristics of IL-4.22 Two-color analysis of IFNγ and IL-13 expression by activated JTC CD8+ T cells (78% of which expressed intracellular IL-13) is shown in Fig 2. Single-color analysis showed that IL-13 was also expressed by 41% of MJC CD8+ T cells and 79% of KR CD8+ T cells (not shown). In all cases, nonactivated CD8+ T cells did not express IL-13.
Two-color flow cytometric analysis of intracellular IFNγ and IL-13 expression by EBV-specific CD8+ T cells from donor JTC. T cells were unstimulated (A) or stimulated for 6 hours with PMA and ionomycin (B), as described in Materials and Methods.
Two-color flow cytometric analysis of intracellular IFNγ and IL-13 expression by EBV-specific CD8+ T cells from donor JTC. T cells were unstimulated (A) or stimulated for 6 hours with PMA and ionomycin (B), as described in Materials and Methods.
Correlation of CD8+ T-cell phenotype with patterns of cytokine secretion by antigen-presenting autologous LCL.
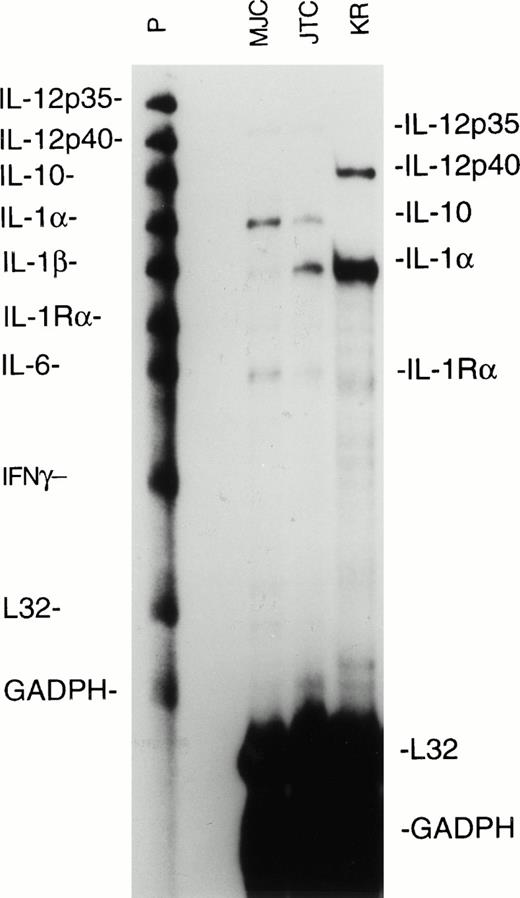
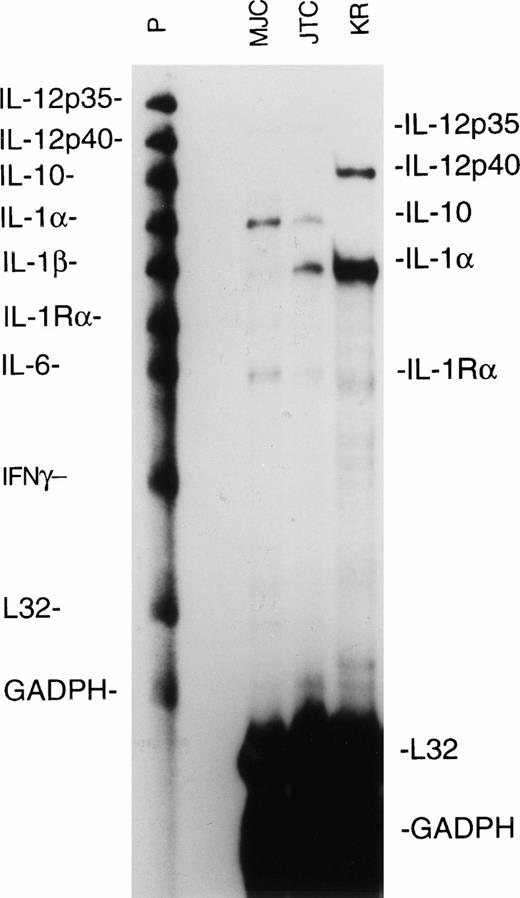
Cytokines secreted by the antigen-presenting cells, in this case EBV-transformed LCL, can exert a strong influence on the differentiation of the responding T cells. Notably, IL-12 is a powerful promoter of type 1 T-cell responses, whereas IL-4 and IL-10 will inhibit type 1 responses and favor development of type 2 responses. Using a multiprobe RNase protection assay, we measured cytokine mRNA levels expressed by the MJC, KR, and JTC LCL used to stimulate the EBV-specific CD8+ T-cell lines. We found that the MJC and JTC LCL expressed the p35 subunit of IL-12 at barely detectable levels and that the KR LCL expressed the p40 subunit of IL-12, but none of the LCL expressed both the p35 and p40 subunits required for functional IL-12 (Fig 3). The MJC and JTC LCL expressed IL-10 mRNA, whereas IL-10 expression by KR LCL was almost undetectable (Fig 3). Expression of IL-10, combined with the failure of the LCL to express IL-12, may contribute to a permissive environment for the development of CD8+ T-cell responses with type 2 characteristics. We did not test the LCL for expression of IL-4, a type 2-promoting cytokine, because IL-4 is not generally expressed by B cells, and we have consistently failed to detect IL-4 mRNA expression in a wide variety of LCL.23
Ribonuclease protection assay for cytokine mRNA synthesis by EBV-transformed MJC, JTC, and KR LCL. RNA was extracted from LCL during log-phase growth, and aliquots from 106cell-equivalents were analyzed with the hCK-2 probe set (PharMingen). Lanes containing untreated probes (P) and mRNA-protected probes (MJC, JTC, and KR) are shown. Ribosomal L32 and GADPH are cellular housekeeping mRNAs. The autoradiogram was from 24 hours of exposure.
Ribonuclease protection assay for cytokine mRNA synthesis by EBV-transformed MJC, JTC, and KR LCL. RNA was extracted from LCL during log-phase growth, and aliquots from 106cell-equivalents were analyzed with the hCK-2 probe set (PharMingen). Lanes containing untreated probes (P) and mRNA-protected probes (MJC, JTC, and KR) are shown. Ribosomal L32 and GADPH are cellular housekeeping mRNAs. The autoradiogram was from 24 hours of exposure.
Clonal analysis of cytotoxic function and cytokine secretion.
Functional analysis of 27 EBV-specific CD8+ T-cell clones isolated by limiting dilution from the MJC CD8+ T-cell line showed extensive diversity with regard to patterns of cytokine secretion and cytotoxic function (summarized in Table 1). All the clones secreted IFNγ at variable but high levels (26 of 27 clones >400 pg/mL by ELISA). In contrast, levels of IL-4 secretion varied over a wide range (4 pg/mL to >400 pg/mL by ELISA), although all clones were IL-4+. This finding was initially surprising, because analysis of intracellular cytokine expression by the parent MJC CD8+line had indicated the existence of an IL-4− subset (Fig 1F). Although it is possible that our cloning conditions favor the selection of IL-4–secreting T cells, the apparent discrepancy may simply be a reflection of different levels of sensitivity between ELISA and flow cytometric analysis of cytokine expression. Only 10 of 25 clones secreted IL-10 at a level detectable by ELISA. A minority of clones exhibited little or no cytotoxicity against autologous LCL, but showed HLA-restricted specificity for EBV in proliferation assays against autologous and HLA class I-mismatched LCL (not shown).
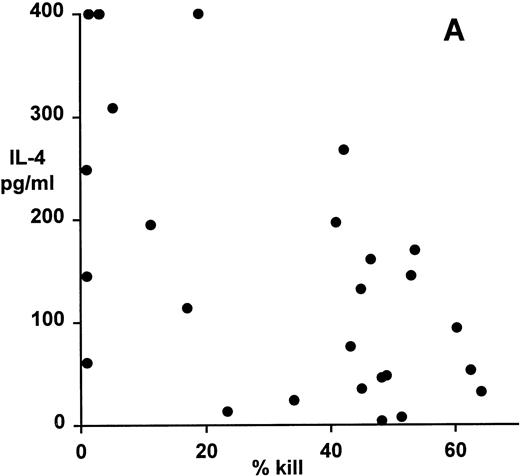
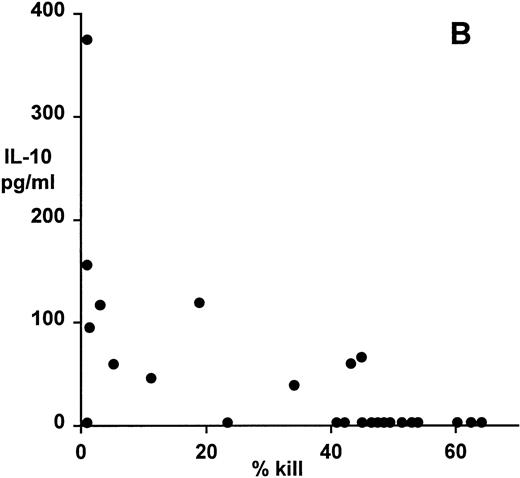
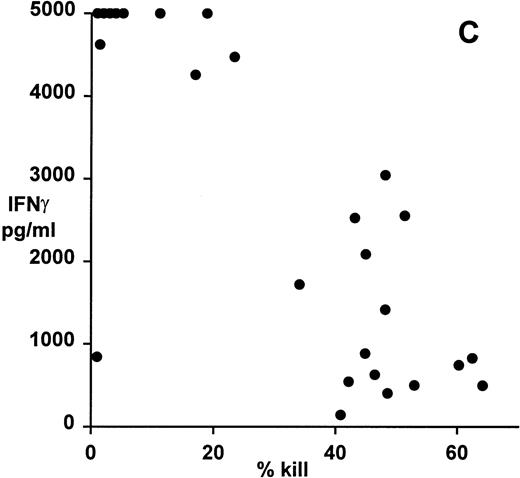
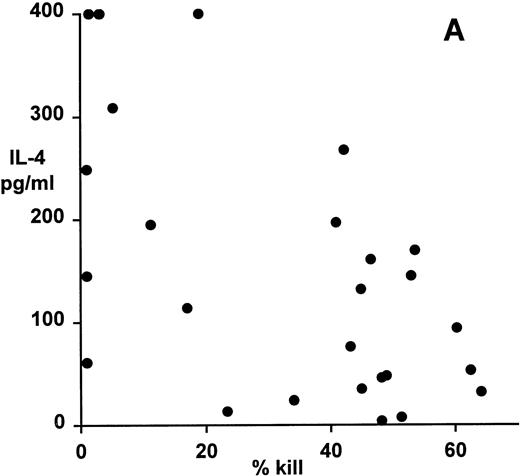
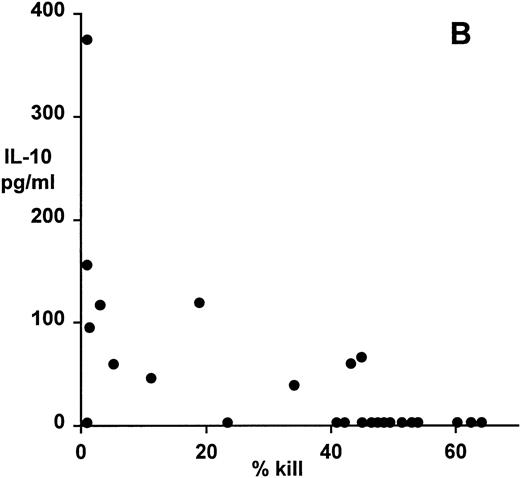
Correlation of clonal cytokine production with cytotoxic function showed a significant inverse relationship between IL-4 production and cytotoxicity (r = −.59, P = .001, from correlation analysis based on simple linear regression; Fig 4A). However, several highly cytotoxic clones also secreted relatively high levels of IL-4 (eg, clones 3F11 and 3D11; see Table 1). There was also a significant inverse relationship between IL-10 production and cytotoxicity (r = −.63, P = .0008; Fig 4B); indeed, none of the top 11 most strongly cytotoxic clones expressed detectable levels of IL-10. Finally, correlation of IFNγ secretion with cytotoxicity again showed a statistically highly significant inverse relationship (r = −.79, P = .0001; Fig 4C). In this case, the distribution suggested two distinct subsets, the first of which produced high levels of IFNγ but was relatively noncytotoxic, whereas the second subset was highly cytotoxic but produced lower levels of IFNγ.
Patterns of EBV-specific cytotoxicity and cytokine secretion by 27 EBV-specific CD8+ T-cell clones from donor MJC. (A) Correlation of cytotoxicity and IL-4 secretion (r = −.59, P < .001). (B) Correlation of cytotoxicity and IL-10 secretion (r = −.63, P < .0008). (C) Correlation of cytotoxicity and IFNγ secretion (r= −.79, P < .0001). Cytotoxicity was measured in a 5-hour51Cr-release assay at an effector:target ratio of 4:1. Cytokine secretion was measured by ELISA of 48-hour supernatants from anti-CD3–activated T-cell cultures, as described in Materials and Methods.
Patterns of EBV-specific cytotoxicity and cytokine secretion by 27 EBV-specific CD8+ T-cell clones from donor MJC. (A) Correlation of cytotoxicity and IL-4 secretion (r = −.59, P < .001). (B) Correlation of cytotoxicity and IL-10 secretion (r = −.63, P < .0008). (C) Correlation of cytotoxicity and IFNγ secretion (r= −.79, P < .0001). Cytotoxicity was measured in a 5-hour51Cr-release assay at an effector:target ratio of 4:1. Cytokine secretion was measured by ELISA of 48-hour supernatants from anti-CD3–activated T-cell cultures, as described in Materials and Methods.
Can EBV-specific CD8+ T cells activate resting B cells?
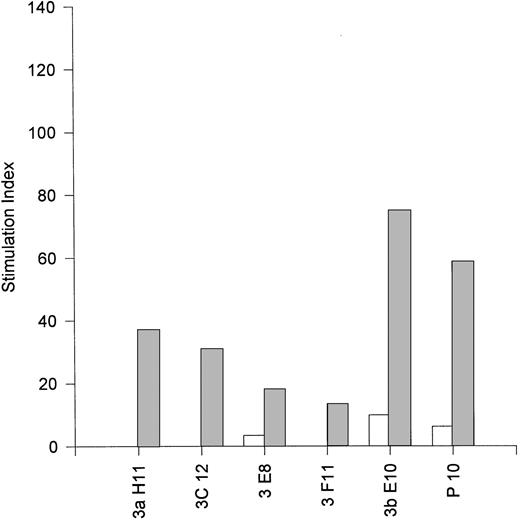
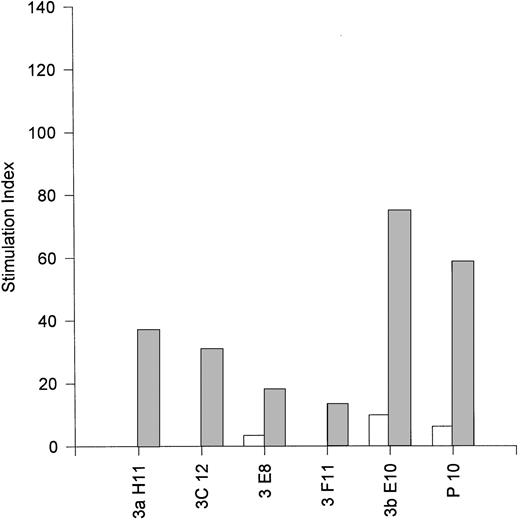
The observation that EBV-specific CD8+ T cells can express IL-4, IL-10, and IL-13, all of which are regarded as type 2 cytokines and are involved in B-cell activation and proliferation, suggested that they may be capable of providing help for B-cell activation. Accordingly, we conducted coculture assays of purified resting B cells with irradiated EBV-specific CD8+ T cells in the presence or absence of plate-bound anti-CD3 MoAb (OKT3). The results presented in Fig 5 show that the MJC CD8+T-cell line and five fully characterized clones derived therefrom (see Table 1) were all able to activate resting B cells in 4-day microwell proliferation assays. The most potent helper CD8+ clone was 3bE10 (stimulation index of 75), which was noncytotoxic and secreted IL-10 in addition to IL-4 (see Table 1). However, we did not observe a correlation between the magnitude of B-cell activation and the level of IL-4 expression by the MJC CD8+ clones; it is possible that the level of IL-13 expression (which was not assessed) may also make a significant contribution to the level of B-cell activation. Four EBV-specific CD8+ T-cell clones from donor JTC were also tested for their ability to activate resting B cells. These clones exhibited uniformly low cytotoxic function against autologous JTC LCL (mean of 6.5% specific lysis at an E:T ratio of 4:1), but mounted strong HLA-restricted lymphoproliferative responses (not shown). All four clones, when stimulated with plate-bound anti-CD3 MoAb, were efficient activators of resting B cells, furnishing B-cell stimulation indices greater than 100 (not shown). The cytokine profiles for the JTC CD8+ clones were not determined.
Activation of resting B cells by an EBV-specific CD8+ T-cell line from donor MJC (P10) and five EBV-specific CD8+ T-cell clones (3aH11, 3bE10, 3C12, 3E8, and 3F11) derived from the MJC CD8+ T-cell line. Small resting B cells were purified from peripheral blood by positive selection with anti-CD19–coupled magnetic beads, as described in Materials and Methods, and cultured for 4 days with γ-irradiated T cells in the presence (▨) or absence (□) of plate-bound anti-CD3. Stimulation indices were calculated as the ratio of 3H-TdR uptake by B cells cultured with T cells divided by 3H-TdR uptake by B cells cultured without T cells. Background counts (typically <100 cpm) by control wells of irradiated T cells were subtracted before calculation of stimulation indices for B-cell proliferation.
Activation of resting B cells by an EBV-specific CD8+ T-cell line from donor MJC (P10) and five EBV-specific CD8+ T-cell clones (3aH11, 3bE10, 3C12, 3E8, and 3F11) derived from the MJC CD8+ T-cell line. Small resting B cells were purified from peripheral blood by positive selection with anti-CD19–coupled magnetic beads, as described in Materials and Methods, and cultured for 4 days with γ-irradiated T cells in the presence (▨) or absence (□) of plate-bound anti-CD3. Stimulation indices were calculated as the ratio of 3H-TdR uptake by B cells cultured with T cells divided by 3H-TdR uptake by B cells cultured without T cells. Background counts (typically <100 cpm) by control wells of irradiated T cells were subtracted before calculation of stimulation indices for B-cell proliferation.
For both the MJC and the JTC CD8+ T-cell clones, B-cell proliferation in the absence of plate-bound anti-CD3 was minimal, indicating that the response was strongly dependent on concomitant activation of the T cells. Control wells of irradiated T cells only, in the presence or absence of anti-CD3, showed no evidence of3H-TdR uptake. Supernatants from 48-hour cultures of activated T cells failed to activate resting B cells, indicating that helper function was contact dependent (not shown). EBV-specific CD8+ T cells express low levels of gp39 (CD40) ligand upon activation by PMA/ionomycin, but the extent to which B-cell activation is dependent on gp39 expression by CD8+ T cells is not known; these studies are in progress.
DISCUSSION
The CD8+ T cell has long been regarded as an MHC class I-restricted effector cell that secretes IFNγ and TNFα/β and is predominantly cytotoxic, most notably against virus-infected target cells. However, recent reports have lent substance to the proposal that CD8+ T-cell responses exhibit considerable functional diversity and may be assigned to type 1 and type 2 subsets that broadly parallel the classical Th1 and Th2 subsets of CD4+ T cells.18 24-27 In this report, we present evidence that the CD8+ T-cell response to EBV is also functionally diverse and that CD8+ T-cell effector functions are not limited to virus-specific cytotoxicity.
Two-color flow cytometric analysis of intracellular cytokine expression at the single-cell level by EBV-specific polyclonal CD8+ T cells showed that IFNγ expression was a common denominator and further showed the existence of CD8+ T-cell subsets that expressed both IFNγ and IL-4. For two of three donors, the IFNγ+/IL-4+ subset was the major subset (58% of JTC CD8+ T cells and 59% of MJC CD8+ T cells). A high frequency of IFNγ+/IL-4+double-positive CD8+ T cells was associated with expression of IL-10 by the antigen-presenting LCL, suggesting that cytokine expression by the LCL may, at least in part, influence the phenotype of the responding T cells. We also found that EBV-specific CD8+ T cells expressed IL-13, in two instances (JTC and KR) at a higher frequency than IL-4 (78% and 79% IL-13+v 58% and 28% IL-4+, respectively). IL-13 shares many of the characteristics of IL-4, including the ability to induce proliferation and differentiation of B cells activated by T-cell gp39 (CD40 ligand) interaction with B-cell CD40.28 In this context, it is notable that IL-13 expression is enhanced by cyclosporin A (CsA), whereas IL-4 expression is inhibited.29 IL-13 expression by EBV-specific CD8+ T cells may thus contribute to B-cell activation and potential EBV reactivation from latency in CsA-immunosuppressed transplant patients and may consequently play a role in the development of EBV-associated oligoclonal and polyclonal posttransplant lymphoproliferative disorders.
Although analysis of intracellular cytokine expression provided strong evidence for functional diversity within the CD8+ T-cell response to EBV, with a significant proportion of responding cells capable of expressing type 2 cytokines, the possibility remained that the IL-4– and IL-13–expressing CD8+ T cells were no more than nonspecific bystanders and that specificity for EBV resided solely within the IFNγ+/IL-4− subset, which has a classical type 1 phenotype and would be expected to be strongly cytotoxic. To address this problem and to gain a correlation of cytotoxic function with patterns of cytokine synthesis, we undertook an extensive clonal analysis of the CD8+ T-cell response to EBV. Functional analysis of 27 EBV-specific CD8+ T-cell clones showed wide variability in cytotoxicity against autologous LCL. Those clones that exhibited little or no cytotoxicity were nevertheless found to mount strong HLA-restricted proliferative responses against autologous LCL, thus confirming the specificity of the response. It is not known whether these clones fail to lyse autologous LCL by virtue of low TCR affinity, as described by Hill et al,17 or whether noncytotoxicity is related to CD8+ T-cell type 2 subset differentiation; the two possibilities are not mutually exclusive.
A significant inverse relationship between cytotoxicity and the level of IL-4 secretion was observed, although all the clones secreted IL-4 detectable by ELISA. In contrast, only 10 of 25 clones tested secreted IL-10 upon activation, with the 11 most strongly cytotoxic clones failing to secrete detectable levels of IL-10. These results suggest that IL-10 secretion, rather than IL-4 secretion, may more accurately define a type 2 CD8+ T-cell response with reduced cytotoxic function against EBV, a conclusion that is consistent with the finding that IL-10 is a powerful modulator of type 1 T-cell responses, through inhibition of IL-12 synthesis by accessory cells.30-32 A similar inverse relationship between IL-10 secretion and cytotoxic function has been described for mouse CD8+ T cells. Inoue et al33 note that functionally assigned suppressor CD8+ T-cell clones produced IL-10 upon stimulation with anti-CD3, whereas IL-10 was not expressed by CD8+ cytotoxic T-cell clones.
All EBV-specific CD8+ T-cell clones secreted IFNγ upon activation, but we found a strong and statistically highly significant inverse relationship between IFNγ secretion and cytotoxicity. Two distinct subsets were observed, the first of which expressed high levels of IFNγ but was relatively noncytotoxic, whereas the second subset was highly cytotoxic but produced lower levels of IFNγ. Although unexpected, in view of the known cross-regulatory functions of IFNγ and IL-10,32,34 the coincidence of high IFNγ and IL-10 secretion by human CD8+ clones has also been described by others.35
Since the discovery of polarized Th1 (type 1) and Th2 (type 2) subsets of CD4+ T cells, there has been a long-standing practice of pigeonholing T-cell responses, and particularly T-cell clones, as being of a type 1 or a type 2 phenotype. Although our intracellular cytokine assays on polyclonal EBV-specific CD8+ T cells suggest the existence of discrete, but overlapping, subsets, clonal analysis clearly shows that many intermediate and apparently contradictory phenotypes exist. Classical type 1 and type 2 T-cell subsets have previously been defined by exclusive expression of IFNγ or IL-4, respectively, but simultaneous expression of IL-4 and IFNγ by human CD4+ and CD8+ T cells has been described by several investigators,24,26 36 and all 27 of the MJC CD8+ T-cell clones described in this report secreted both IFNγ and IL-4. Furthermore, four of the five MJC CD8+clones shown to activate resting B cells (a type 2 T-cell function) were also strongly cytotoxic (a characteristic of type 1 T cells).
The observation that EBV-specific CD8+ T cells can provide help for activation of resting B cells is the major finding of this report. As the site of EBV latency in vivo is the resting B cell, it is probable that activation and subsequent differentiation of latently infected B cells will result in reactivation of EBV lytic cycle replication.37,38 Experiments in SCID mice engrafted with human PBL (SCID/hu mice), showing that the presence of T cells is required for the development of EBV-induced lymphoproliferative disease and tumors,39,40 strongly support the proposal that T-cell help for B-cell activation is a necessary and critical step in EBV pathogenesis. Furthermore, the work of Veronese et al39demonstrated that CD8+ T cells were capable of fulfilling the necessary helper function for development of EBV-induced tumors in SCID/hu mice, an observation that supports the proposal that helper, or type 2, CD8+ T cells are capable of playing a significant role in the pathogenesis of EBV-associated disease. Expression of IL-10 by EBV-specific CD8+ T cells may further contribute to the pathogenesis of EBV-associated lymphoproliferative disorders. In addition to IL-4 and IL-13, both of which promote B-cell activation and proliferation, IL-10 is a potent growth and differentiation factor for activated B cells41 and has recently been described as a growth factor for EBV-transformed LCL.42 Moreover, expression of type 2 cytokines, particularly IL-10, may antagonize type 1 T-cell responses30-32 34 and thus modulate cytotoxic T-cell control of latent EBV infection.
On the basis of these findings, we would argue that the conventional view of the role of CD8+ T cells in EBV infection merits reappraisal. In contrast with the consensus that EBV-specific CD8+ T cells are primarily cytotoxic and fulfill an immunosurveillance role, our results have raised the possibility that EBV-specific CD8+ T cells may also contribute to the pathogenesis of EBV disease. Because the resting B cell is the principal site of EBV latency37,38 43 and B-cell activation leads to viral lytic cycle activation, we propose that EBV-specific type 2 CD8+ T-cell responses have the potential to reactivate latent viral infection and may thus play a major role in the development of EBV-associated lymphoproliferative disorders and lymphoma in immunocompromised individuals with impaired EBV-specific cytotoxic T-cell responses. Analysis of the phenotype and function of EBV-specific CD8+ T-cell responses in the various lymphoproliferative diseases and lymphomas associated with EBV may thus contribute to our understanding of the pathogenesis and immune control of these disorders.
ACKNOWLEDGMENT
The authors thank Melaney Gee and Jim Crouch for skilled technical assistance. Statistical analysis was performed by Dan Ayers (Biostatistics Office, Arkansas Cancer Research Center, Little Rock, AR).
Supported by National Institutes of Health Grants No. CA 63931, CA 73556, and AG 09822 and by a grant from the Arkansas Science and Technology Authority. R.R. is a Special Fellow of the Leukemia Society of America.
Address reprint requests to Martin J. Cannon, PhD, Department of Microbiology and Immunology, Mail Slot 511, University of Arkansas for Medical Sciences, 4301 W Markham, Little Rock, AR 72205.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.