Abstract
Recent studies of mice deficient in the thrombin receptor, protease-activated receptor 1 (PAR1), provided definitive evidence for the existence of a second thrombin receptor in mouse platelets. We recently identified a new thrombin receptor designated protease-activated receptor 3 (PAR3). The mRNA encoding a mouse homologue of PAR3 was highly expressed in mouse splenic megakaryocytes, making it a good candidate for the missing mouse platelet thrombin receptor. We now report that PAR3 protein is expressed on the surface of mouse platelets and that PAR3 antibodies partially inhibit activation of mouse platelets by thrombin but not U46619, a thromboxane receptor agonist. These observations suggest that PAR3 contributes to mouse platelet activation by thrombin.
ACTIVATION OF PLATELETS by thrombin is thought to be critical for both normal hemostasis and for platelet-dependent arterial thrombosis in unstable angina and myocardial infarction. Thrombin's actions on human platelets are mediated at least in part by protease-activated receptor 1 (PAR1).1-4 Thrombin binds to and cleaves the amino terminal exodomain of this unusual G protein-coupled receptor.1,5-8Receptor cleavage serves to unmask a tethered peptide ligand that binds intramolecularly to the body of the receptor, effecting transmembrane signaling.1,5,9 Synthetic peptides mimicking this tethered ligand sequence act as PAR1 agonists.1,3 Such peptides mimic many of thrombin's actions on human platelets,1-4and PAR1 antibodies partially inhibited activation of human platelets by thrombin.10,11 By contrast, synthetic peptides mimicking the tethered ligand domain of mouse PAR1 did not activate mouse platelets,12-14 and the thrombin responses of platelets from PAR1 knockout and wild-type mice were indistinguishable.14 These observations suggested that PAR1 was not important for thrombin signaling in mouse platelets and prompted a search for a second thrombin receptor responsible for activation of these cells.
We recently cloned a new protease-activated receptor designated PAR3.15 Human PAR3 is clearly a second thrombin receptor; it conferred thrombin-stimulable calcium mobilization when expressed in Xenopus oocytes and phosphoinositide hydrolysis when expressed in COS7 cells. By Northern analysis it was expressed in the heart, liver, pancreas, thymus, small intestine, stomach, lymph node, trachea, adrenal, and bone marrow. By contrast, a putative mouse homologue of PAR3 was highly expressed in megakaryocytes with little expression detected elsewhere. This tissue distribution made mouse PAR3 a strong candidate for the missing mouse platelet thrombin receptor.15 However, thrombin is known to cause rapid increases in intracellular calcium in mouse platelets, but attempts to trigger calcium mobilization and phosphoinositide hydrolysis via mouse PAR3 expressed in Xenopus oocytes or COS7 cells have been unsuccessful thus far (unpublished data). We therefore sought to determine whether PAR3 protein was expressed in mouse platelets and, if so, to assess its functional importance. Polyclonal antisera were raised to a peptide representing the thrombin cleavage site and tethered ligand domains of mouse PAR3. Polyclonal10 and monoclonal11 antibodies to the cognate region of PAR1 were previously shown to partially inhibit activation of PAR1-transfected cells and human platelets by thrombin. We report that PAR3 protein was indeed expressed in mouse platelets and that PAR3 antibodies partially inhibited mouse platelet activation by thrombin, strongly suggesting an important role for PAR3 in this process.
MATERIALS AND METHODS
In situ hybridization.
C57BL/6 mice were anesthetized with pentobarbital and then perfusion-fixed with 4% paraformaldehyde in phosphate-buffered saline. Tissues were dissected free, immersion-fixed in 4% paraformaldehyde overnight, and then embedded in paraffin. Five-micrometer sections were hybridized with a sense or antisense 33P-riboprobe transcribed from full-length mPAR3 cDNA subcloned into pBluescript II SK−.14 The photographic emulsion was developed after 1 week of exposure.
PAR polyclonal antibodies.
The synthetic peptides DATVNPR/SFFLRNPSENTFELVPLGDGC (mPAR1 amino acids 35-60 plus carboxyl glycine-cysteine) and AKPTLTIK/SFNGGPQNTFEEFPLSDIEGC (mPAR3 amino acids 30-57 plus carboxyl cysteine) were purified by reverse-phase high-pressure liquid chromatography and conjugated via their carboxyl terminal cysteine to keyhole limpet hemocyanin using m-maleimidobenzoyl-N-hydroxysuccinimide ester (Pierce Chemical Co, Rockford, IL). Antigen conjugation, rabbit immunization, and collection of sera were performed by AnimalPharm Services (Healdsburg, CA) using standard techniques.16 The immune sera used in these studies was collected on day 70 after the primary and 6 booster immunizations. IgG was purified from preimmune and immune sera using protein-A agarose and gel filtration (Pierce Chemical Co). Using enzyme-linked immunosorbent assay (ELISA), PAR3 IgG recognized the PAR3 peptide antigen but not the PAR1 antigen, and PAR1 IgG recognized the PAR1 antigen and not the PAR3 antigen (not shown, but see Fig 2). Binding of PAR1 and PAR3 IgG to the surface of transfected COS7 cells was examined as previously described.17
Binding of PAR3 IgG to the surface of mouse platelets by flow cytometry. Washed platelets from C57BL/6J mice were incubated with preimmune or PAR3 IgG as indicated and then washed and incubated with FITC-goat antirabbit IgG. After three washes, platelets were analyzed by flow cytometry. Each figure represents an analysis of 10,000 events with SSC (side scatter) on the abscissa and FITC fluorescence intensity on the ordinate. The median FITC fluorescence intensities were 3.5 for preimmune IgG and 27.4 for PAR3 IgG. The same population of cells that bound PAR3 IgG bound antibody to the platelet marker CD41; PAR1 IgG showed no significant binding (not shown).
Binding of PAR3 IgG to the surface of mouse platelets by flow cytometry. Washed platelets from C57BL/6J mice were incubated with preimmune or PAR3 IgG as indicated and then washed and incubated with FITC-goat antirabbit IgG. After three washes, platelets were analyzed by flow cytometry. Each figure represents an analysis of 10,000 events with SSC (side scatter) on the abscissa and FITC fluorescence intensity on the ordinate. The median FITC fluorescence intensities were 3.5 for preimmune IgG and 27.4 for PAR3 IgG. The same population of cells that bound PAR3 IgG bound antibody to the platelet marker CD41; PAR1 IgG showed no significant binding (not shown).
Western blotting of platelets and transfected COS7 cells.
Washed mouse platelets were prepared as described below and then resuspended in sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer without reducing agent. These samples were passed through a 27-g needle and then stored frozen as aliquots until analyzed by SDS-PAGE and immunoblot.
COS7 cells (105 per 3.8 cm2 well) were transfected with 0.5 μg of mouse PAR1 or PAR3 cDNA in the mammalian expression vector pBJ1 vector using LipofectAmine Reagent (GIBCO BRL, Grand Island, NY). After 5 hours, the transfection medium was replaced with Dulbecco's modified Eagle's medium (DME H-16) containing 5% fetal bovine serum. After an additional 48 hours, cells were lysed and lysates analyzed by SDS-PAGE (10% acrylamide) and immunoblot. Western blotting was performed using protein-A agarose-purified primary IgGs at the concentration of 1 μg/mL; bound primary antibody was detected using the ECL system (Amersham, Arlington Heights, IL).
Flow cytometry.
Washed mouse platelets were resuspended in platelet buffer (see below) plus 1 μmol/L prostaglandin E1 and 5 mmol/L EDTA and then incubated with 10 μg/mL primary antibodies at 4°C for 1 hour followed by the appropriate fluorescein isothiocyanate (FITC)-conjugated second antibody at 4 μg/mL for 0.5 hour. After three washes, platelets were analyzed using flow cytometry. Rat antimouse CD41 antibody (PharMingen 09911D; PharMingen, San Diego, CA) that recognizes the abundant platelet surface protein αIIb was used as a positive control in these studies.
Platelet aggregation, ATP secretion, and calcium mobilization.
Washed platelets were prepared from C57BL/6 mouse and resuspended at the concentration of 6 × 108/mL in platelet buffer (20 mmol/L Tris-HCl, pH 7.4, 140 mmol/L NaCl, 2.5 mmol/L KCl, 1 mmol/L MgCl2, 1 mg/mL glucose, and 0.2% bovine serum albumin). Platelet aggregation and ATP secretion were evaluated in the platelet buffer with 2 mmol/L CaCl2 and Chromolume (Chrono-log Corp, Havertown, PA) using a dual channel lumiaggregometer (Chrono-log Corp) as changes of light transmittance or chemiluminescence, respectively.14
For calcium mobilization studies, platelets were washed once with platelet buffer and then incubated in the same buffer plus 1 mmol/L EDTA and 4 μg/mL Fura-2 AM for 30 minutes at 37°C. Platelets were then pelleted at 500g for 10 minutes and resuspended in platelet buffer.14 EGTA (0.1 mmol/L) was added at zero time in each scan to block aggregation. In all of the platelet experiments shown, IgGs were added 5 minutes before the zero time at a final protein concentration of 60 μg/mL.
RESULTS AND DISCUSSION
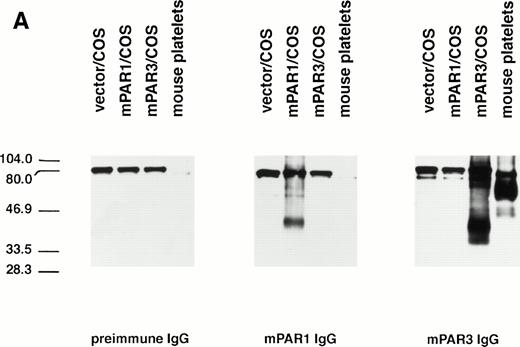
The specificity of the PAR3 and PAR1 peptide antisera used in these studies was first characterized by immunoblot. Mouse PAR3 IgG recognized several bands in PAR3-transfected COS7 cells but not in PAR1-transfected COS7 cells or in untransfected cells (Fig 1 and data not shown); PAR3 preimmune IgG did not recognize these bands. The major bands recognized by PAR3 IgG migrated at approximately 40 and 90 kD. The transfection-dependence of these bands and their recognition by immune but not preimmune IgG suggests that they almost certainly represent PAR3 protein; their aberrant migration is at least in part due to different levels of glycosylation (D.Z. and S.R.C., unpublished results). Mouse PAR1 IgG recognized a protein in PAR1-transfected COS7 cells, but not in PAR3-transfected or untransfected cells (Fig 1).
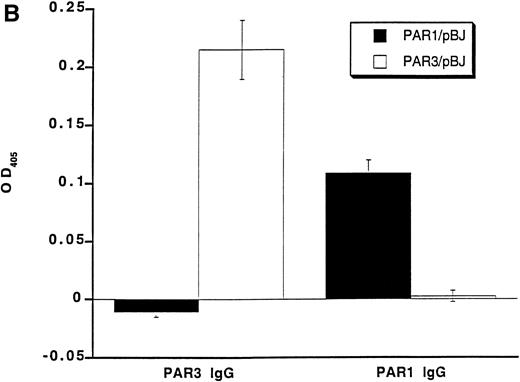
Recognition of PARs by immunoblot and cell surface binding. (A) Immunoblotting of platelet and PAR-transfected COS7 cell lysates with PAR antisera. COS7 cells were transfected with plasmids directing expression of mouse PAR1 or PAR3 or with empty vector. Transfected COS7 lysates and lysates of washed mouse platelets were analyzed by SDS-PAGE and immunoblot with PAR1, PAR3, or preimmune IgG (see the Materials and Methods). Preincubation of the PAR1 or PAR3 IgG with the peptide antigen to which each was raised (final peptide concentration, 3.3 nmol/L; overnight at 4°C) ablated their ability to recognize PAR1 or PAR3, respectively (not shown). This experiment was replicated four times. (B) Selective recognition of PARs on the COS7 cell surface. COS7 cells transfected with mPAR1 or mPAR3 expression vectors or with empty vector were fixed briefly with 2% paraformaldehyde, incubated with PAR1 or PAR3 IgG (60 μg/mL) as indicated, and then washed extensively. Antibody bound to the surface of unpermeabilized cells was detected using horseradish peroxidase-conjugated second antibody by cell surface ELISA.17 Data shown are the mean ± SEM (n = 4); binding to empty expression vector-transfected cells (0.14 OD units for PAR3 IgG; 0.08 for PAR1 IgG) was subtracted. This experiment was replicated twice.
Recognition of PARs by immunoblot and cell surface binding. (A) Immunoblotting of platelet and PAR-transfected COS7 cell lysates with PAR antisera. COS7 cells were transfected with plasmids directing expression of mouse PAR1 or PAR3 or with empty vector. Transfected COS7 lysates and lysates of washed mouse platelets were analyzed by SDS-PAGE and immunoblot with PAR1, PAR3, or preimmune IgG (see the Materials and Methods). Preincubation of the PAR1 or PAR3 IgG with the peptide antigen to which each was raised (final peptide concentration, 3.3 nmol/L; overnight at 4°C) ablated their ability to recognize PAR1 or PAR3, respectively (not shown). This experiment was replicated four times. (B) Selective recognition of PARs on the COS7 cell surface. COS7 cells transfected with mPAR1 or mPAR3 expression vectors or with empty vector were fixed briefly with 2% paraformaldehyde, incubated with PAR1 or PAR3 IgG (60 μg/mL) as indicated, and then washed extensively. Antibody bound to the surface of unpermeabilized cells was detected using horseradish peroxidase-conjugated second antibody by cell surface ELISA.17 Data shown are the mean ± SEM (n = 4); binding to empty expression vector-transfected cells (0.14 OD units for PAR3 IgG; 0.08 for PAR1 IgG) was subtracted. This experiment was replicated twice.
The anti-PAR3 peptide IgG also bound to the surface of unpermeabilized COS7 cells expressing PAR3 but not to COS7 cells expressing PAR1; the converse was found for the PAR1 peptide IgG (Fig 1). Thus, by both immunoblot and surface binding studies, the PAR3 and PAR1 peptide antisera recognized PAR3 and PAR1, respectively, with no evidence for cross-reactivity.
PAR3 and PAR1 IgGs were next used to assess expression of PAR3 and PAR1 protein by mouse platelets. By immunoblot of mouse platelet lysate, PAR3 recognized a broad band centered at a molecular weight of approximately 60 kD and minor bands at approximately 50 kD. The upper band probably represents fully processed glycosylated PAR3 and the lower band precursor and partially glycosylated forms. These bands were not detected by PAR1 IgG or by PAR3 preimmune IgG. PAR3 IgG also bound to the surface of mouse platelets as assessed by flow cytometry (Fig 2); PAR1 IgG showed little or no binding above background. These observations strongly suggest that PAR3 protein is indeed expressed by mouse platelets and are consistent with previous in situ hybridization experiments showing PAR3 mRNA in mouse splenic megakaryocytes15 and with recent studies confirming PAR3 mRNA expression in megakaryocytes from mouse bone marrow (data not shown). In contrast to the case with PAR3, PAR1 IgG failed to recognize a specific band in mouse platelets. The absence of detectable PAR1 protein in mouse platelets is consistent with its apparent lack of functional importance in these cells.12-14
Given the ability of PAR3 peptide IgG to bind specifically to the surface of PAR3-transfected COS7 cells and to mouse platelets, we next determined whether this IgG could block thrombin signaling in mouse platelets. Thrombin triggered rapid increases in cytoplasmic calcium in washed mouse platelets. The EC50 for this response was approximately 0.5 nmol/L (data not shown). Preincubation of mouse platelets with PAR3 IgG, but not preimmune IgG, markedly attenuated calcium mobilization by 0.5 nmol/L thrombin (Fig 3). By contrast, PAR3 IgG did not attenuate responses to an EC50 concentration the thromboxane receptor agonist U46619 (Fig 3).
Inhibitory effect of PAR3 IgG on α-thrombin–induced calcium mobilization in mouse platelets. Mouse platelets were loaded with fura-2 and cytosolic free Ca2+ concentration [Ca2+]i (nmol/L) was measured fluorometrically.14 Extracellular calcium was chelated to prevent platelet aggregation. Platelets were preincubated with the indicated IgG preparations for 5 minutes at 37°C and then transferred to a fluorometer at room temperature. α-Thrombin (0.5 nmol/L) or U46619 (3 μmol/L) was added at approximately 100 seconds. The data shown are representative of three experiments with thrombin and two experiments with U46619.
Inhibitory effect of PAR3 IgG on α-thrombin–induced calcium mobilization in mouse platelets. Mouse platelets were loaded with fura-2 and cytosolic free Ca2+ concentration [Ca2+]i (nmol/L) was measured fluorometrically.14 Extracellular calcium was chelated to prevent platelet aggregation. Platelets were preincubated with the indicated IgG preparations for 5 minutes at 37°C and then transferred to a fluorometer at room temperature. α-Thrombin (0.5 nmol/L) or U46619 (3 μmol/L) was added at approximately 100 seconds. The data shown are representative of three experiments with thrombin and two experiments with U46619.
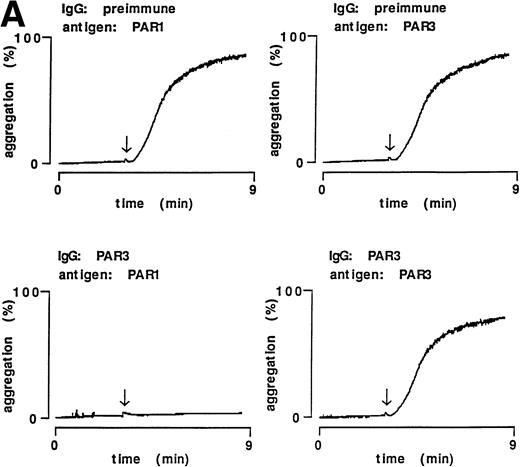
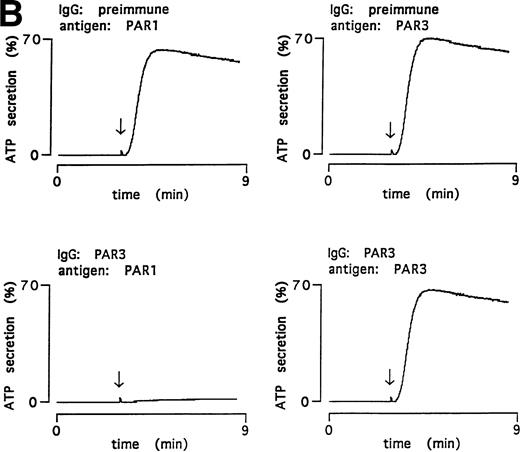
PAR3 IgG also inhibited thrombin-triggered ATP secretion and aggregation in mouse platelets in response to 0.5 nmol/L thrombin (Fig 4); preimmune IgG was without activity. Preincubation with PAR3 antigen ablated the inhibitory activity of PAR3 IgG preparations; preincubation with the cognate PAR1 antigen had no effect. These data strongly suggest that the inhibitory effects of PAR3 IgG on thrombin signaling were mediated by antibodies that recognize PAR3.
Inhibitory effect of PAR3 IgG on mouse platelet responses to α-thrombin and ablation of inhibition by the peptide antigen. α-Thrombin–induced (0.5 nmol/L) platelet aggregation (A) and secretion (B) are shown. IgGs were preincubated with peptide antigens for 5 minutes at 37°C. Platelets were incubated with this mixture (final concentration of IgG, 60 μg/mL; antigen, 0.1 μmol/L) for 5 minutes at 37°C; responses were then analyzed in a lumiaggregometer. α-Thrombin was added at approximately 3 minutes (arrow). Responses were expressed as the percentage of maximum, with 100% defined as the response induced by 10 nmol/L α-thrombin in platelets incubated with preimmune IgG only. The latter had no effect on platelet responses. The data shown were replicated in two separate experiments.
Inhibitory effect of PAR3 IgG on mouse platelet responses to α-thrombin and ablation of inhibition by the peptide antigen. α-Thrombin–induced (0.5 nmol/L) platelet aggregation (A) and secretion (B) are shown. IgGs were preincubated with peptide antigens for 5 minutes at 37°C. Platelets were incubated with this mixture (final concentration of IgG, 60 μg/mL; antigen, 0.1 μmol/L) for 5 minutes at 37°C; responses were then analyzed in a lumiaggregometer. α-Thrombin was added at approximately 3 minutes (arrow). Responses were expressed as the percentage of maximum, with 100% defined as the response induced by 10 nmol/L α-thrombin in platelets incubated with preimmune IgG only. The latter had no effect on platelet responses. The data shown were replicated in two separate experiments.
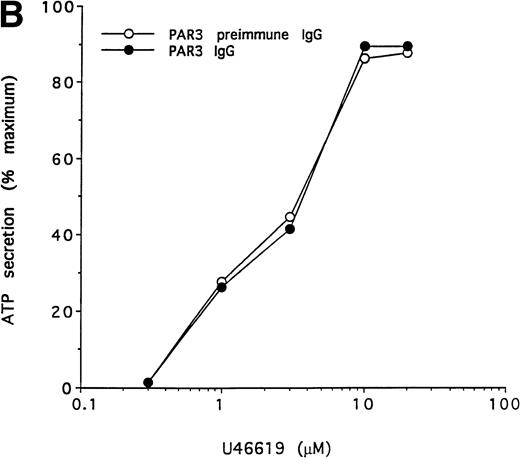
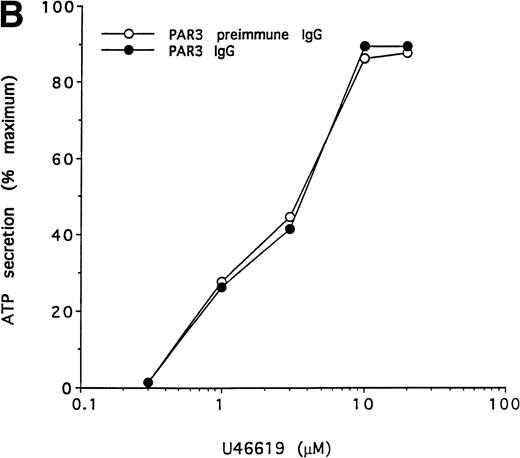
To further test the specificity of the PAR3 IgG preparation's inhibitory actions, we compared its effects on the concentration-response curves for thrombin and U46619 using platelet secretion as an endpoint (Fig 5). PAR3 IgG reliably right-shifted the concentration-response to thrombin. At thrombin concentrations at or below EC50, thrombin-induced secretion was virtually ablated. By contrast, PAR3 IgG had no effect on platelet secretion induced by U46619, even at sub-EC50agonist concentrations. Mouse PAR1 IgG had no effect on thrombin signaling and did not enhance the inhibitory effect of PAR3 IgG (Fig 5and data not shown). Taken together, these observations strongly suggest that PAR3 signaling is necessary for normal activation of mouse platelets by thrombin.
Effect of PAR3 IgG on mouse platelet ATP secretion in response to α-thrombin or U46619. Washed platelets were incubated with PAR3 IgG (60 μg/mL) for 5 minutes at 37°C and then stimulated with the indicated concentration of α-thrombin (A) or U46619 (B). The maximum response to each concentration of agonist was quantitated and expressed as a percentage of the response of platelets incubated with preimmune IgG only to 10 nmol/L α-thrombin. (In separate experiments, preimmune IgG had no significant effect on platelet responses.) Each point represents a single aggregometry tracing. The data shown were replicated in two separate experiments.
Effect of PAR3 IgG on mouse platelet ATP secretion in response to α-thrombin or U46619. Washed platelets were incubated with PAR3 IgG (60 μg/mL) for 5 minutes at 37°C and then stimulated with the indicated concentration of α-thrombin (A) or U46619 (B). The maximum response to each concentration of agonist was quantitated and expressed as a percentage of the response of platelets incubated with preimmune IgG only to 10 nmol/L α-thrombin. (In separate experiments, preimmune IgG had no significant effect on platelet responses.) Each point represents a single aggregometry tracing. The data shown were replicated in two separate experiments.
The failure of mouse PAR1 agonists to activate mouse platelets,12-14 the lack of an effect of the PAR1 knockout on mouse platelet activation,14 and our inability to detect PAR1 protein or alter platelet thrombin responses with mouse PAR1 antibodies all suggest that PAR1 plays little or no role in mouse platelet activation. Does the observation that inhibition of mouse platelet activation by PAR3 IgG is overcome at high thrombin concentrations imply the existence of yet another thrombin receptor? We previously showed that a human PAR1 IgG inhibited human platelet activation by thrombin and that this inhibition was overcome at high thrombin concentrations.10 The same phenomenon was observed for signaling via PAR1 heterologously expressed in Xenopus oocytes, a system in which all thrombin signaling requires the exogenous receptor.1 10 The ability of high concentrations of thrombin to overcome the blocking antibody may simply be due to competition; the irreversibility of the proteolytic activation mechanism versus the reversibility of antibody binding might favor thrombin in this regard. It is thus not necessary to invoke the existence of yet another mouse platelet thrombin receptor to explain available data. However, it is critical to point out that neither do these data exclude the existence of such a receptor. The presence or absence of persistent thrombin responses in platelets derived fromPar3g −/− mice will be definitive in this regard.
Synthetic peptides that mimic the tethered ligand domains of PAR1 and PAR2 function as agonists for these receptors. Unfortunately, this was not the case for human and mouse PAR3.15 It is thus not possible to use a PAR3 tethered ligand peptide to determine whether PAR3 activation is sufficient for activation of mouse platelets.
Previous studies showed that antibodies to human PAR1's thrombin cleavage site and agonist peptide domain inhibited PAR1 signaling.10 11 The present study provides similar data for antibodies to the cognate domain of PAR3. Antibodies that bind to the protease cleavage site and/or tethered ligand domain of protease-activated receptors may thus be generally useful for inhibiting signaling by PAR2 or perhaps as yet undiscovered members of this budding receptor subfamily.
In summary, PAR3 mRNA and protein are expressed in mouse megakaryocytes and platelets, and PAR3 antibodies partially inhibit thrombin responses in mouse platelets. These results strongly suggest that PAR3 plays an important role in activation of mouse platelets by thrombin. This result motivates investigation of mouse PAR3 signaling in platelets and begs the question of why mouse PAR3 has thus far not shown signaling in transfected COS7 cells and other systems. Our data supporting a role for PAR3 in mouse platelet activation also motivate generation of aPar3g −/− mouse. If viable, this mouse will either provide evidence for the existence of yet another platelet thrombin receptor or will constitute a valuable system for determining the relative importance of thrombin signaling in various models of thrombosis. Lastly, the finding that mouse PAR3 appears to play an important signaling role prompts studies to define the role of human PAR3. Using Northern blot analysis, the tissue distribution of human PAR3 appears to be distinct from that of mouse PAR3.14Studies to identify which human cell types express PAR3 are needed. In particular, it is unknown whether PAR3 contributes to human platelet function. Human PAR3 IgG did not inhibit thrombin responses in human platelets and did not enhance the inhibition seen with human PAR1 IgG (H.I. and S.R.C., unpublished results), but such negative results must be interpreted with caution. Nonetheless, given the dichotomy in the roles of PAR1 in human versus mouse platelets,14 it would not be surprising if PAR3 also served distinct roles in mice and humans. How such distinct tissue-specific roles for PAR1 and perhaps PAR3 might have evolved remains an interesting question.
ACKNOWLEDGMENT
The authors thank Dr JoAnn Trejo for her help with computer art, Linda Prentice for paraffin sections, Prof Henry Bourne (UCSF) for critical reading of this manuscript, and Prof John Fenton II (Albany Medical College, Albany, NY) for purified α-thrombin.
H.I. and D.Z. contributed equally to this work.
Supported by the Daiichi Research Center at the University of California, San Francisco, and by National Institutes of Health Grants No. HL44907, DK50267, and HL59202. D.Z. was supported by NRSA HL09256. A.J.C. was supported by KO8 HL03234.
Address reprint requests to Shaun R. Coughlin, MD, PhD, HSE-1300, University of California, San Francisco, 505 Parnassus Ave, San Francisco, CA 94143-0130.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.


![Fig. 3. Inhibitory effect of PAR3 IgG on α-thrombin–induced calcium mobilization in mouse platelets. Mouse platelets were loaded with fura-2 and cytosolic free Ca2+ concentration [Ca2+]i (nmol/L) was measured fluorometrically.14 Extracellular calcium was chelated to prevent platelet aggregation. Platelets were preincubated with the indicated IgG preparations for 5 minutes at 37°C and then transferred to a fluorometer at room temperature. α-Thrombin (0.5 nmol/L) or U46619 (3 μmol/L) was added at approximately 100 seconds. The data shown are representative of three experiments with thrombin and two experiments with U46619.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/11/10.1182_blood.v91.11.4152/4/m_blod41122003x.jpeg?Expires=1769085523&Signature=yUHzhn6pOGakZlGN9qsY~u9rimotp-G1plIAlqz9yFZeIzJAsD1UvtzhFf7VPMbCy8~yTz6vgN6xFaFbwkTr4lLF2dHU061R8CaEZe3SbHPdrNQ0shAZZmowHOhWrCqLKWv5Y4oAR~SXhhhFRJxZFNhjvNBOyy-wPHFgZt5Hkmca2Nwu2kmZgyIqIuIzQy9wJpSBYlR1j-8mmbQyAQTndic4RE~6wiGHpozZLZxacvdLvZCAU3Y907EUk1Z1bss2xobEz1KpB7gvF9bjQq3yAF8Cwb8sse~Z72hn4IKdTvv0LIVxUzjMdwR2DLnGYcX7xNOAsutiA~czzhhs2kHxlA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)





![Fig. 3. Inhibitory effect of PAR3 IgG on α-thrombin–induced calcium mobilization in mouse platelets. Mouse platelets were loaded with fura-2 and cytosolic free Ca2+ concentration [Ca2+]i (nmol/L) was measured fluorometrically.14 Extracellular calcium was chelated to prevent platelet aggregation. Platelets were preincubated with the indicated IgG preparations for 5 minutes at 37°C and then transferred to a fluorometer at room temperature. α-Thrombin (0.5 nmol/L) or U46619 (3 μmol/L) was added at approximately 100 seconds. The data shown are representative of three experiments with thrombin and two experiments with U46619.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/11/10.1182_blood.v91.11.4152/4/m_blod41122003x.jpeg?Expires=1769085524&Signature=DklCydO6seR-kt1UL~J-65DPd9itZHcG0qh~vfZnXBjIBAxd7RoCK~~zCyY7ox8QUE51zg6CnlNWjLLZazScPYL~eZAxLOrA6hBqQWNJ-FqtxMsfWDLOMqYi2GJ9rd-Ij4BZxlM3RYXsAi~2WMiZfpPfs-1Pbq6XWm15Dkk3wGQ73ICMag8MdWFykBW4BbYmx0D1b~Lu8R4wnqcU4CT71Ck2UigRxtasIau58P96gn2Mz8PydRJ8Ejw3KEWojbaTj5YfwzGfJRoTCQ3WDLa0A1M4e8svVEK7o9w7Lw8gNBVTfUbBO7lGaA2HGWx5sKdEZ8IhYMkDVa3YiQTBCDDBkA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)