Abstract
The cryptic translocation t(12;21)(p13;q22) has been recently recognized as the most common genetic rearrangement in B-lineage childhood acute lymphoblastic leukemia (ALL). The resulting fusion transcript, termed TEL-AML1, has been associated with an excellent prognosis at initial ALL diagnosis. Hence, we postulated that the incidence of TEL-AML1 fusion should be lower in patients with ALL relapse. To address this assumption and to investigate the prognostic significance of TEL-AML1 expression in relapsed childhood ALL, bone marrow samples of 146 children were analyzed by reverse-transcriptase (RT)-polymerase chain reaction (PCR). All children were treated according to Berlin-Frankfurt-Münster (BFM) ALL relapse trial protocols (ALL-REZ BFM 90-96). Their clinical features and outcome were compared with those of 262 patients who could not be tested due to lack of bone marrow samples. Thirty-two of 146 children with relapsed ALL were TEL-AML1–positive. Four of the negative patients had T-lineage and nine Philadelphia chromosome (Ph1)-positive leukemia. Thus, the incidence ofTEL-AML1 in relapsed Ph1-negative, B-cell precursor ALL is 32 of 133 (24%). The 32 TEL-AML1–positive and 101 negative patients differed significantly with respect to duration of last remission (42.5 v 27 months; P = .0001) and age at initial diagnosis (53.5 v 74 months;P = .0269). At a median follow-up time of 21.5 months, children positive for TEL-AML1 had a significantly (P = .0011) higher probability of event-free survival (EFS; 0.79 v 0.33). The predominant majority of patients had been treated for initial ALL according to German multicenter BFM (108 of 133) or Cooperative ALL study group (CoALL) (19 of 133) frontline protocols. The comparison of tested and not-tested (N = 262) patients showed no significant difference.TEL-AML1 positivity predicted a favorable short-term outcome; long-term results are unknown. Screening for TEL-AML1 should become routine at relapse diagnosis and might be used for therapy stratification in future trials.
CURRENT RISK-BASED treatment regimens apply distinct prognostic factors as criteria when stratifying children with acute lymphoblastic leukemia (ALL) into particular treatment protocol arms with either reduced toxicity (low-risk) or a more intensive approach to disease control (high-risk). In newly diagnosed ALL, universally accepted stratification criteria are age and WBC count at diagnosis,1 but risk assessment is modified by response to therapy2,3 and other predictive factors, such as cytogenetic or molecular findings [hyperdiploidy/hypodiploidy, translocation t(9;22); t(1;19); involvement of 11q23 or their respective molecular products].4-7 In contrast, the main determinants of outcome at relapse of ALL are duration of first remission, immunophenotype of leukemic cells, and site of relapse.8,9 However, also at relapse, genetic features are evolving as important predictors of outcome. The translocation t(9;22) or its molecular counterpart, BCR-ABL fusion transcript, is an independent risk factor at first relapse of childhood ALL associated with an adverse prognosis.10 11
Recently, screening of leukemic cells by molecular techniques has demonstrated that 16% to 32% of newly diagnosed B-cell precursor ALL12-18 have a cryptic translocation t(12;21)(p12;q22) between TEL, a novel member of the ETS-like family of transcription factors (chromosome 12), and the AML1 gene of the AML1/CBFβ (core-binding factor) transcription factor complex (chromosome 21).19,20 In the larger series, the frequency of TEL-AML1 fusion was constantly approximately 25%.13,15,17,18,21 Normal function of AML1 is essential for hematopoiesis,22 and the fusion protein interferes dominantly with AML1-dependent transcription of target genes, thus influencing the regulatory pathway necessary for normal growth and differentiation of hematopoietic cells and contributing to the pathogenesis of leukemia.22-24 TEL and AML1are independently involved in several other translocations in acute and chronic leukemias and myelodysplastic syndromes.25-29
Six studies of children with newly diagnosed ALL treated by risk-directed combination chemotherapy have associated TEL-AML1expression in ALL with an excellent prognosis and a long-term continuous complete remission (CCR).13-18 We assessed the incidence of TEL-AML1–positive ALL at relapse and its prognostic significance.
MATERIALS AND METHODS
Molecular detection of the TEL-AML1 fusion mRNA.
RNA isolation and reverse transcription (RT) have been described elsewhere.10 Nested polymerase chain reaction (PCR) of cDNA of TEL-AML1 fusion14 and c-ABL-cDNA integrity control10 were performed as published previously, with the only difference that a duplex-PCR permitting the simultaneous coamplification of cDNA of TEL-AML1 and c-ABL was established. Duplex-PCR was performed with a DNA-Engine PTC-200 (MJ-Research, Watertown, MA). Cycle times and temperatures for denaturation, annealing, and synthesis for external and internal PCRs were as follows: initial denaturation, 94°C, 6 minutes; 61°C, 30 seconds, 72°C, 45 seconds, and 94°C, 60 seconds, 30 cycles; final elongation, 72°C, 10 minutes. Each experiment was repeated at least once and included no template cDNA as a negative control. Positive results were confirmed by analyzing at least one additional sample independently and by sequencing of the PCR products by an ABI 377 automated sequencer (Applied Biosystems, Foster City, CA).
Patients and treatment.
Bone marrow samples from 146 unselected children and adolescents were obtained at ALL relapse diagnosis. All patients were treated according to the ALL-REZ BFM 90-96 relapse trials of the Berlin-Frankfurt-Münster (BFM) study group.8 30Written informed consent was obtained from the patients or their guardians. The ALL-REZ BFM studies were approved by the institutional review boards of the Freie Universität Berlin and Humboldt-University at Berlin. To affirm that the tested samples were representative for relapsed childhood ALL, all patients that had been treated on the same protocols but were not tested were used as the control group. The definition of therapy groups for trial ALL-REZ BFM 96 is provided in Table 1.
Data analysis.
Statistical analysis was performed using the SAS Software (SAS Institute, Cary, NC), version 6.11 for Windows (Microsoft, Redwood, USA). The analysis entailed univariate statistics, Wilcoxon rank-sum tests, Fisher's exact test,31 and survival analysis using the Kaplan Meier method.32 The level of significance was set to .05.
Event-free survival (EFS) was computed from date of remission to the last date patients were reported in continuous remission or the date of an adverse event. In case of nonresponse to therapy or death during induction, EFS was set to 0. EFS was censored at the date of bone marrow transplantation (BMT) for survival analyses showing results of chemotherapy only. In cases of autologous BMT, EFS was censored with the date of BMT for analyses presenting results of chemotherapy and allogeneic BMT.33
RESULTS
Molecular detection of the TEL-AML1 fusion mRNA.
Among 146 analyzed samples, 32 were found to beTEL-AML1–positive. Corresponding to different fusions with theAML1 gene, two variant TEL-AML1 RT-PCR amplification products were detected.12,14 34 The longer PCR amplification product (269 bp), which resulted from fusion of exon 5 ofTEL with exon 2 of AML1, was predominantly detected (29 of 32 TEL-AML1–positive cases). The shorter amplified product (230 bp), resulting from fusion of TEL exon 5 with exon 3 ofAML1, was observed in the remaining 3 of 32 cases. This shorter PCR product was also simultaneously amplified in the otherTEL-AML1–positive samples, although with lower intensity than the longer fragment. DNA sequencing of the 230-bp product show that exon 2 of AML1 (39 bp) was omitted by alternative splicing in these cases. Bone marrow samples of 114 patients were negative forTEL-AML1.
Patients and treatment.
TEL-AML1 fusion transcripts were detected in relapsed Philadelphia chromosome (Ph1)-negative, B-cell precursor ALL only. Among 114 TEL-AML1–negative patients, there were nine Ph1-positive patients and four with T-cell immunophenotype. Since both Ph1 positivity and T-lineage are known to be independent risk factors in relapsed childhood ALL, these patients were excluded from further analysis.8 11Thus, the study population was restricted to 133 patients with relapsed Ph1-negative, B-cell precursor ALL, and the incidence ofTEL-AML1 was 32 of 133 (24%). Accordingly, the control group included only patients with Ph1-negative, B-cell precursor relapsed ALL (N = 262), who could not be tested due to lack of bone marrow samples. The characteristics of all 395 patients are listed in Tables 2 and 3 and shown in Fig1.
Boxplots of continuous variables by patients group. Age, age at initial diagnosis (months); Age(R), age at relapse diagnosis (months); Dur R, duration of remission (months); Log (PBC), logarithm (base 10) of (peripheral blast cell count [1/μL] + 1); Log (WBC), logarithm (base 10) of (WBC count [1/μL] + 1); OT, observation time = (today − date of relapse diagnosis) (months); n, not tested (n = 262); −, TEL-AML1–negative (n = 101); +, TEL-AML1–positive (n = 32). Boxes represent the first (25%) and third (75%) quartile of the distribution; cross-line denotes median; lower whisker, the 5th, and the upper whisker, the 95th percentile.
Boxplots of continuous variables by patients group. Age, age at initial diagnosis (months); Age(R), age at relapse diagnosis (months); Dur R, duration of remission (months); Log (PBC), logarithm (base 10) of (peripheral blast cell count [1/μL] + 1); Log (WBC), logarithm (base 10) of (WBC count [1/μL] + 1); OT, observation time = (today − date of relapse diagnosis) (months); n, not tested (n = 262); −, TEL-AML1–negative (n = 101); +, TEL-AML1–positive (n = 32). Boxes represent the first (25%) and third (75%) quartile of the distribution; cross-line denotes median; lower whisker, the 5th, and the upper whisker, the 95th percentile.
The age at initial ALL diagnosis was lower in theTEL-AML1–positive group (Table 3 and Fig 1); the oldest positive patient was 10.6 years at initial ALL diagnosis. However, the age at relapse diagnosis was similar in all groups. Thus, the duration of remission was significantly higher in the TEL-AML1–positive group (Fig 1 and Tables 2 and 3). It is noteworthy that all fiveTEL-AML1–positive patients who presented with a second ALL relapse were late relapses (Table 1). Nonetheless, two very early and four early relapses could be found in the TEL-AML1–positive group. Initial treatment was similar in the vast majority ofTEL-AML1–tested patients. One hundred eight of 133 children were treated according to BFM2,35 and 19 of 133 according to Cooperative ALL study group (CoALL)36frontline protocols (Table 2). Only one of theTEL-AML1–positive patients with an early relapse had been treated according to an East European protocol.
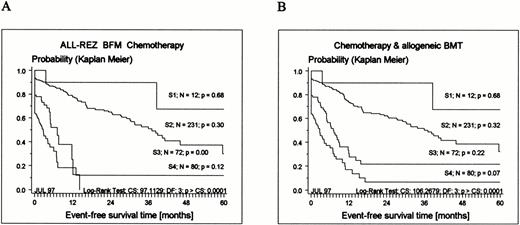
According to their classification as late relapses, mostTEL-AML1–positive patients are currently stratified into strategic (S) therapy groups S1 and S2 (Tables 1 and 2). The significant difference of EFS of all patients by therapy group is shown in Fig 2.
Kaplan-Meier estimates of EFS related to strategic therapy groups S1 to S4 (for definition, see Table 1) for 395 patients enrolled in the ALL-REZ BFM protocols. (A) Results of chemotherapy only (BMT censored); (B) chemotherapy and allogeneic BMT included (see Materials and Methods). CS, chi-square; DF, degrees of freedom.
Kaplan-Meier estimates of EFS related to strategic therapy groups S1 to S4 (for definition, see Table 1) for 395 patients enrolled in the ALL-REZ BFM protocols. (A) Results of chemotherapy only (BMT censored); (B) chemotherapy and allogeneic BMT included (see Materials and Methods). CS, chi-square; DF, degrees of freedom.
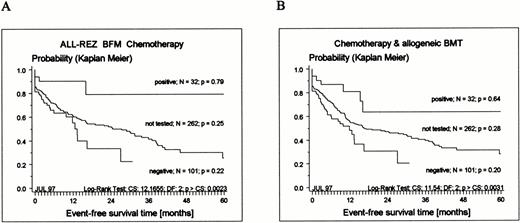
The observation time of tested and not-tested patients was statistically significantly different (Fig 1 and Table 3). The median observation time of the not-tested group was 48 months, and 50% of all adverse events occurred within 26 months after relapse diagnosis (Fig3). Since the median observation time of the tested group was 21.5 months, response to therapy, frequency of BMT, and frequency of events in remission cannot be regarded as estimate of the final outcome. However, a clear pattern regarding outcome emerged (Fig 3). TEL-AML1–positive patients had an excellent prognosis compared with TEL-AML1–negative patients. Assuming that TEL-AML1–positive and –negative patients were equally distributed among tested and not-tested patients, there should be no differences across both groups. In fact, there were no significant differences between tested and not-tested patients regarding sex, age, duration of last remission, and time point of relapse, WBC and peripheral blast cell count, number of relapses, site, stratification into therapy groups, response to therapy, and frequency of BMT. Consequently, the EFS of the not-tested group should be between the EFS of negative and positive patients. Indeed, the probability of EFS of not-tested patients disjoins the EFS of positive and negative patients (Fig 3).
Kaplan-Meier estimates of EFS for 133 children with relapsed ALL tested for expression of TEL-AML1 transcripts and 262 not-tested patients treated according to ALL-REZ BFM protocols. (A) Results of chemotherapy only (BMT censored); (B) chemotherapy and allogeneic BMT included (see Materials and Methods).
Kaplan-Meier estimates of EFS for 133 children with relapsed ALL tested for expression of TEL-AML1 transcripts and 262 not-tested patients treated according to ALL-REZ BFM protocols. (A) Results of chemotherapy only (BMT censored); (B) chemotherapy and allogeneic BMT included (see Materials and Methods).
There was only one significant difference between tested and not-tested patients: the frequency of “other” frontline therapy protocols. Nineteen of these patients had been treated according to other (ie, non-BFM) West European protocols and 21 according to East European protocols.
DISCUSSION
The TEL-AML1 fusion represents the most frequent gene fusion arising from a cryptic translocation in childhood ALL.13-15,17,18 21 In the cited studies, the clinical characteristics of children with a TEL-AML1–positive ALL were consistent with respect to B-cell precursor immunophenotype, age between 1 and 10 years, and mostly WBC count less than 50,000/μL, features which are generally associated with a low risk and good prognosis.
A significant association between the frequency of relapse andTEL-AML1 status was found in the studies reported by McLean et al,15 Rubnitz et al,17 and, recently, Borkhardt et al.18 In these reports, the probability of EFS at 5 years for TEL-AML1–positive patients was as high as 91% to 100%, whereas the probability of EFS of negative patients ranged from 65% to 79%. A similar trend was observed by Nakao et al14and Shurtleff et al.13
Given these promising reports, we determined the incidence, clinical features, and outcome of TEL-AML1–positive patients in childhood relapsed ALL. Unexpectedly, the incidence was 32 of 146 (21.9%) in unselected patients. All positive patients had relapsed B-cell precursor ALL, and all but one were younger than 10 years at initial ALL diagnosis. The patients who were positive at the time of relapse had a significantly longer duration of remission than negative patients. The duration of remission was the most important independent risk factor at relapse diagnosis. A very early relapse carried a 5.9 times higher risk ratio, and an early relapse a 3.8 times higher risk ratio for a subsequent relapse compared with late relapses (for definition of relapse time points, see Table 1).8 9Consequently, the majority of TEL-AML1–positive patients were found in therapy groups with a better prognosis (Tables 1 and 3).
The outcome of TEL-AML1–positive patients was significantly better than the outcome of negative patients. This is of particular importance for the decision on high-risk BMT procedures. Yet, it remains to be verified whether TEL-AML1 is an independent risk factor for relapsed childhood ALL.
The similar frequency of TEL-AML1 positivity in relapsed and newly diagnosed ALL appears to be in contrast with published data associating the presence of TEL-AML1 fusion in newly diagnosed ALL with a good prognosis, and these findings emphasize the necessity of molecular diagnostics both at initial diagnosis and at relapse of ALL to clearly evaluate short-term and long-term results of chemotherapeutic regimens. The majority of the tested TEL-AML1patients with relapsed ALL had initial treatment according to BFM or the similar CoALL ALL frontline study. The incidence ofTEL-AML1 positivity in initial B-cell precursor ALL of children enrolled in the multicenter BFM2,35 or Associazione Italiana Ematologia Oncologia Pediatrica (AIEOP)37 ALL frontline trials has been assessed by Borkhardt et al.18 In this partly prospective, partly retrospective study, the frequency ofTEL-AML1–positive B-cell precursor ALL was 22.5% (63 of 217 children) and 29.4% (99 of 337 children), respectively. The estimated EFS rate of the retrospectively analyzed population was 90.1% and 79% at 4 years for the TEL-AML1–positive and –negative patients. Although only three of the TEL-AML1–positive have suffered a relapse, in contrast to 27 of the negative patients, the observation time is too short to predict long-term outcome.
Two hypotheses might explain the same frequency of TEL-AML1fusion detected in newly diagnosed and relapsed ALL. TEL-AML1positivity is associated with prolonged disease-free intervals. In agreement with the aforementioned BFM and AIEOP data,18Rubnitz et al17 reported a probability of EFS of 91% at 5 years for TEL-AML1–positive patients. However, the life-table curves presented show a clear trend for the occurrence of late relapses in this group.17 Thus, the same incidence ofTEL-AML1 positivity at relapse indicates that relapse is delayed, but not decreased in these patients. The median duration of remission of relapsed TEL-AML1–positive patients was 42.5 months (Table 3). Therefore, an observation period of 4 or 5 years may be too short to assess final outcome. This is different in relapsed childhood ALL: more then 50% of all events occurred within 26 month after diagnosis (see the not-tested group in Fig 3).
More speculative is the hypothesis that the high incidence ofTEL-AML1 fusion at relapse might be the consequence of a new genetic alteration, possibly induced by the preceding treatment, and the “leukemic relapse” may be in fact a second malignancy.AML1 has been reported to be involved in treatment-related secondary leukemia.38 39 This observation is supported by the capacity of DNA topoisomerase II inhibitors to induce a reproducible site-specific double-strand DNA cleavage in susceptible regions of AML140 or MLL41genes, thus, possibly producing an initial step in the pathogenesis of chromosomal translocations and the subsequent development of leukemias. Prospective molecular analyses of leukemic cells at first presentation and at relapse will be required to further elucidate this hypothesis.
In any case, TEL-AML1 status identifies a subgroup of children with B-cell precursor ALL who achieve long-term disease-free intervals with current therapeutic regimens either after first or second round of chemotherapy, and also in case of a second relapse. In contrast, Ph1- or BCR-ABL-positive ALL, which occurs at a frequency of 12% in relapsed childhood B-cell precursor ALL and now constitutes the second most frequent fusion gene, is associated with a dismal prognosis and outcome.10 11 Although the underlying biologic mechanisms remain to be resolved, these findings emphasize the importance of molecular screening to identify not only unfavorable, but also favorable subsets of ALL for stratification of patients to appropriate treatment arms within risk-adapted clinical trials, and to tailor therapy accordingly in future therapeutic strategies.
ACKNOWLEDGMENT
The critical comments of Stephen Sallan, MD, Dana-Farber Cancer Institute, Boston, MA, are greatly appreciated. Immunophenotyping was performed by W.-D. Ludwig, MD, PhD, Department of Medical Oncology/Molecular Biology, Max-Delbrück-Center, Humboldt-University at Berlin, Berlin, Germany. The technical assistance of Tillmann Taube, Serge Dragon, Claudia Hanel, Gisela Götze, and Gabriele Schmitt is gratefully acknowledged, as well as Andrea Kretschmann's help with data preparation of the ALL-REZ BFM relapse studies. The support of the physician and nursing staffs of the participating ALL-REZ BFM centers for providing bone marrow samples is appreciated.
Supported by a grant from the Deutsche Leukämie Forschungshilfe—Aktion für krebskranke Kinder e. V., Bonn, Germany, and by grants from the Deutsche Krebshilfe, Bonn, Germany.
Address reprint requests Karlheinz Seeger, MD, PhD, Department of Pediatric Oncology/Hematology, Mail drop Forschungshaus 2.0412, Charité-Virchow-Klinikum, Humboldt-University at Berlin, 1 Augustenburger Platz, Berlin, Germany, 13353.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 1. Boxplots of continuous variables by patients group. Age, age at initial diagnosis (months); Age(R), age at relapse diagnosis (months); Dur R, duration of remission (months); Log (PBC), logarithm (base 10) of (peripheral blast cell count [1/μL] + 1); Log (WBC), logarithm (base 10) of (WBC count [1/μL] + 1); OT, observation time = (today − date of relapse diagnosis) (months); n, not tested (n = 262); −, TEL-AML1–negative (n = 101); +, TEL-AML1–positive (n = 32). Boxes represent the first (25%) and third (75%) quartile of the distribution; cross-line denotes median; lower whisker, the 5th, and the upper whisker, the 95th percentile.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/5/10.1182_blood.v91.5.1716/3/m_blod4051501.jpeg?Expires=1764981661&Signature=LxES1VLd7bYLs8alS69xUnw~n7wOkfIOAAgwwRzjDHMTDAvrFdFy0IIP7YjTkT~8tOVU3D3H4HreQdEgGCTtGAqw5Sf-81df2hMQTRfLhdGHUUr0LUaa2tAY2QFD00k2WNEGl31GnYsDpsT01ytVDyAE360wtv~N-60j3P3OP49Z8L6lVwFvwFNXPC6mpre2WRxND3XNzMTt92ou5kU86wDdqOXoz0ZwcSDugqLaKd1qz7qB4Lu5c0qex7g8X8kSoLCl~qrZPdy92chIb4X8WJZrK21GF3shkaCpLBR7fNSDu~ZPZ796~iov73f3kw-EXJ~OhtTY5TWx-o-VdySnPg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)