Abstract
Optimal numbers of CD34+ cells to be reinfused in patients undergoing peripheral blood progenitor cell (PBPC) transplantation after high-dose chemotherapy are still unknown. Hematologic reconstitution of 168 transplantations performed in patients with lymphoproliferative diseases was analyzed according to the number of CD34+ cells reinfused. The number of days from PBPC reinfusion until neutrophil recovery (>1.0 × 109/L) and unsustained platelet recovery (>50 × 109/L) were analyzed in three groups defined by the number of CD34+ cells reinfused: a low group with less than or equal to 2.5 × 106 CD34+ cells/kg, a high group with greater than 15 × 106 CD34+cells/kg, and an intermediate group to which the former two groups were compared. The 22 low-group patients had a significantly delayed neutrophil (P < .0001) and platelet recovery (P < .0001). The 41 high-group patients experienced significantly shorter engraftment compared with the intermediate group with a median of 11 (range, 8 to 16) versus 12 (range, 7 to 17) days for neutrophil recovery (P = .003), and a median of 11 (range, 7 to 24) versus 14 (range, 8 to 180+) days for platelet recovery (P< .0001). These patients required significantly less platelet transfusions (P = .002). In a multivariate analysis, the amount of CD34+ cells reinfused was the only variable showing significance for neutrophil and platelet recovery. High-group patients had a shorter hospital stay (P = .01) and tended to need fewer days of antibotic administration (P = .12). In conclusion, these results suggest that reinfusion of greater than 15 × 106 CD34+ cells/kg after high-dose chemotherapy for lymphoproliferative diseases further shortens hematopoietic reconstitution, reduces platelet requirements, and may improve patients' quality of life.
THE PLACE OF high-dose chemotherapy with autologous stem cell support has been well established these last years in a number of selected hematologic malignancies.1-5 The use of mobilized peripheral blood progenitor cells (PBPC) has replaced conventional bone marrow transplantation and is nowadays the main source for hematopoietic rescue.6 This change in stem cell source has many reasons, including ease of collection and more rapid hematologic recovery with PBPC compared with bone marrow.7-9 Moreover, PBPC may constitute the only available source of hematopoietic stem cells when bone marrow is hypoplastic because of numerous previous treatments or pelvic irradiation10 and may eventually reduce the risk of tumor cell contamination.11 12
Until a few years ago, mononuclear cell (MNC) count, as well as determination of colony-forming unit–granulocyte-macrophage (CFU-GM) yield, were commonly the most useful indicators of harvest quality.13-15 Unfortunately, this last assay was poorly standardized and poorly reproducible between different laboratories and thus not always comparable from one institution to another.16 The determination by flow cytometry of the subset of peripheral blood cells expressing the CD34 antigen (CD34+ cells) is commonly used today to assess the progenitor cell content and correlates quite well with the progenitors assays.17 Moreover, the CD34+ cell count has been shown to be a good predictor of engraftment kinetics, especially for platelets.18-20 The optimal level of CD34+cell count to be reinfused has still not been well determined, even if some investigators have proposed 2 or 2.5 × 106cells/kg as the minimal threshold for rapid reconstitution,6,21,22 with a greater risk for delayed platelet engraftment under this limit.21,23 However, other factors like prior chemotherapy regimens have also been shown to predict time to engraftment in some reports.24
If an agreement usually exists concerning the need of a minimal number of CD34+ cell count to be reinfused after high-dose chemotherapy, the level of the optimal threshold has not yet been determined. Few trials including large numbers of homogeneous patients are available and the hypothetical benefit of a higher threshold is still unknown. To assess this question, we retrospectively analyzed 168 consecutive high-dose therapy regimens with PBPC transplantations performed for lymphoproliferative diseases between June 1994 and December 1996, and we explored in these patients the role of the amount of CD34+ cells reinfused on hematopoietic reconstitution.
MATERIALS AND METHODS
Patients.
Between June 1994 and December 1996, 153 patients (97 men, 56 women) with a median age of 49 years (range, 15 to 66) were treated at the Centre Hospitalier Lyon-Sud with high-dose therapy and PBPC transplant for lymphoproliferative diseases (Table 1). Approval was obtained from the Institutional Review Board for these studies. Informed consent was provided according to the Declaration of Helsinki. Patients were selected for intensive chemotherapy because of first line treatment failure for Hodgkin's disease (HD) or non-Hodgkin's lymphoma (NHL), because of NHL with two or three unfavorable prognostic criteria according to the age-adjusted International Prognosis Index (IPI) at diagnosis,25 or because of stage 3 multiple myeloma (MM). Additional inclusion criteria included an Eastern Cooperative Oncology Group (ECOG) performance status less than 2 and evidence of adequate hepatic, renal, and cardiac function.
Among the 153 patients analyzed, 15 were treated twice with high-dose therapy. Two patients (one NHL and one HD) received two PBPC transplantations because of relapse more than 1 year after a first intensification, whereas 13 patients received a sequential intensification with two PBPC reinfusions (Table 1). Considering that the main aim of this study was to evaluate the influence of the CD34+ cell count in the harvest on engraftment, we took into account in this analysis the total of the 168 PBPC transplantations performed during this period. However, for response and survival assessment, each sequential intensification was analyzed only once. In both patients who received a second PBPC transplantation because of a relapse after a first intensification, only the first PBPC transplant was considered for response and survival evaluation.
Disease status was assessed at harvest with physical examination and computerized tomography (CT) scan. Bone marrow biopsy was performed in all but three patients who had a previous bone marrow involvement. When patients had no previous history of tumor cell bone marrow involvement and achieved partial or complete remission before transplant, bone marrow was considered as being not involved at time of transplant. Bone marrow infiltration by lymphoma was designated as minimal when tumor cells represented less than 10% of the biopsy.
Mobilization regimen and collection of PBPC.
PBPC were collected in 109 patients after high-dose chemotherapy consisting of cyclophosphamide 5.25 g/m2 alone or cyclophosphamide 4.5 g/m2 plus etoposide 450 mg/m2, the cytostatic agents being given in both regimens in three divided doses over 24 hours, followed by the administration of a hematopoietic growth factor, granulocyte colony-stimulating factor (G-CSF), or GM-CSF. Thirty-seven patients were mobilized with standard chemotherapy and growth factor, generally consisting in the last course of induction regimen and seven patients had a mobilization with G-CSF alone. When a second mobilization procedure was needed, the second harvest was collected after mobilization with G-CSF alone at doses of 5 to 20 μg/kg/d.
Leukaphereses usually started on the first day when leukocyte count in the peripheral blood reached 1.0 × 109/L. Since June 1996, daily measurements of peripheral blood CD34+ cells were performed and leukaphereses started when this count reached 20/μL. When platelet count was less than 30 × 109/L on the day before the first leukapheresis, patients were transfused with irradiated platelets. When mobilization was performed with hematologic growth factor alone, leukaphereses started on the fifth day of their administration. Leukaphereses were achieved with continuous-flow blood cell separators, Baxter CS3000 (Baxter Healthcare Ltd, Berkshire, UK) or COBE Spectra (COBE Laboratories Ltd, Gloucester, UK). The total volume processed in each leukapheresis was between 2 to 3 blood mass volume at a flow rate of 40 to 60 mL/minute. Aphereses were usually performed until enough cells were collected to ensure the harvest of 2.5 × 106 CD34+ cells/kg to be reinfused, given an eventual loss of 20% of the cells after thawing. The median number of leukaphereses per harvest was three (range, 1 to 6). The final product was then cryopreserved in the patient's serum with 10% dimethyl sulfoxide (DMSO) in the vapor phase of liquid nitrogen.
Measurement of progenitor cell content.
The percentage of CD34+ cells in thawed apheresis suspensions was determined using three parameter flow cytometry using side scatter, CD34-phycoerythrin (PE) and CD45 fluorescein isothiocyanate (FITC), as previously reported by Donaldson et al.26 Briefly, 50 μL of each suspension was incubated with PE-conjugated anti-CD34 monoclonal antibody (MoAb) (Human Progenitor Cell Antigen-2 [HPCA-2]; Becton Dickinson, Oxford, UK) and FITC-conjugated anti-CD45 MoAb (Becton Dickinson) for 30 minutes at 4°C. A control sample was labelled with PE-conjugated IgG1 and FITC-conjugated IgG1 MoAb. After incubation, red blood cells were lysed with fluorescence-activated cell sorting (FACS) lysing solution (Becton Dickinson) and washed twice with phosphate-buffered saline. Flow cytometry was performed using the FACScan (Becton Dickinson, Mountain View, CA). Initial gating to exclude CD45 negative events was performed and only CD34+, CD45+ events with low side scatter were considered to be progenitor cells. In the CD45+ gate, 50,000 events were acquired. The absolute numbers of CD34+ hematopoietic progenitor cells were calculated by multiplying the percentage of positive cells by the total nucleated cell content, as determined by conventional automated cell counting (Minos STX, ABX International, Paris, France).
Before August 1995, the clonogenic assays for CFU-GM were not uniform in all patients. After this period, clonogenic assays were performed using the Methocult SFH 4435 (Terry Fox Laboratories, Vancouver, Canada). Cells were seeded at 40 × 103 cells/plate on each of two culture dishes (3 × 35 mm) and the dishes were incubated at 37°C in a 5% CO2 humidified incubator. The growth of CFU-GM was scored on day 14.
Conditioning regimen.
Fifty-nine patients (35%) received total body irradiation (TBI)-containing conditioning regimens, which consisted of cyclophosphamide 120 mg/kg, etoposide 900 mg/m2, and 10 Gy fractionated TBI in 49 cases and melphalan 140 mg/m2combined with 10 to 12 Gy fractionated TBI in 10 cases. A total of 109 patients (65%) received conditioning regimens without TBI, which consisted of carmustine 300 mg/m2, etoposide 800 mg/m2, aracytine 800 mg/m2, and melphalan 140 mg/m2 (BEAM) in 68 patients, melphalan 200 mg/m2 in 18 patients, ICE (ifosfamide 12 g/m2, carboplatine 1,500 mg/m2, and etoposide 1,500 mg/m2) in 18 patients, and other regimens in five patients. PBPC were reinfused 2 days after the last day of chemotherapy in BEAM or melphalan regimens and 3 days after ICE therapy. In case of cyclo/etoposide/TBI or melphalan/TBI, PBPC were reinfused on the last day of irradiation. All but two patients received a hematopoietic growth factor (G- or GM-CSF) after PBPC transplant, which was discontinued when neutrophil count was greater than 1.0 × 109/L.
Toxicity.
Hematologic toxicity was defined from the day of the PBPC reinfusion (day 0). The time to absolute neutrophil count (ANC) greater than 0.1, 0.5, or 1.0 × 109/L was defined as the number of days from day 0 for the neutrophils to increase and to be stable at least 3 days over these values. The time to platelets over 20, 50, or 100 × 109/L was defined as the number of days for platelets to be stable over these values without any transfusions. Platelets were routinely transfused in case of thrombopenia under 20 × 109/L or when hemorrhage occurred and consisted mainly of single donor apheresis product. Transfusions of red blood cell units were performed when hemoglobin values decreased to less than 80 g/L, or above this limit when symptomatic anemia occurred. The total number of blood products administered was registered during the entire hospital stay and during the 2 months after reinfusion of PBPC. Duration of hospital stay was determined from day 0 until discharge of the patient from the sterile unit or from any specialized medical unit. Antibiotherapy was given for any clinical or microbiological infection, or for a persistent undocumented fever above 38°C.
Response.
Response assessment was evaluated at harvest, then 3 months after transplant with clinical examination, CT scan, bone marrow aspirate, and biopsy. A complete response (CR) was defined as the disappearance of all sites of disease including bone marrow. Partial response (PR) was defined as a reduction of more than 50% in the product of the two largest diameters of each measurable lesion, or as the persistence of bone marrow infiltration as the only residual site of disease. Stable disease (SD) was defined as a reduction of less than 50% in these measures. Progressive disease (PD) was defined as the appearance of a new lesion or as the increase in size of any preexistent lesion.
Statistical analysis.
Distributions of clinical characteristics or variables among patient subgroups as defined by the number of CD34+ cells reinfused were compared using the Pearson chi-squared test. Patients who had not achieved a platelet count of 20, 50, or 100 × 109/L at day 180 were designated as 180+ in the median range. Patients who died before engraftment were censored on the date of death and were designated on the graphs by a plus sign. Rates of neutrophil and platelet recovery were estimated using the product-limit method of Kaplan-Meier and compared using a log-rank test. Associations between prognostic factors and hematopoietic recovery were evaluated using a log-rank test. Multivariate analyses were performed using the Cox hazards regression model. The supportive care was graded using the number of red blood cells and platelet units transfused, the length of antibiotic administration, and hospital stay, and the differencies among subgroups were assessed using the Mann-Whitney U test.
RESULTS
Patient characteristics.
Of the 168 transplantations performed, three groups of patients were constituted according to the number of CD34+ cells reinfused (Table 1). First, the upper quartile of the distribution of CD34+ cells reinfused was arbitrarily identified and included 41 patients (24%) who received greater than or equal to 15 × 106 CD34+cells/kg. This group was designated as the high CD34+group. Patients who did not receive the number considered as a safe threshold (>2.5 × 106 CD34+cells/kg) were then identified. These 22 patients (13%) who were still intensified because of the adverse prognosis of their disease constituted the low CD34+ group. The remaining 105 patients (63%) composed the intermediate CD34+ group and received greater than 2.5 × 106, but less than 15 × 106 CD34+ cells/kg. The intermediate CD34+ group was then considered as the reference group to which either the low CD34+ group or the high CD34+ group were respectively compared.
Table 2 shows the most important characteristics of patients at transplant and their distribution according to the reinfused CD34+ cell count. When compared with the intermediate group, the low group was characterized by a significantly higher number of patients who received at least 6 months of chemotherapy (73% v 45%, P = .02), and by more patients previously treated with fludarabine (27% v 9%,P = .01). When compared with the intermediate group, less patients in the high group had received at least three previous chemotherapy regimens (27% v 49%, P = .02). No other variables related to previous treatment and to disease status at transplant were significantly different in the three patient subgroups.
The proportion of patients receiving TBI-containing regimens did not differ significantly between the three groups (P = .44). After reinfusion of PBPC, growth factors (G-CSF in 107 patients, GM-CSF in 59 patients) were started at day 1 (n = 31), day 7 (n = 130), or later (n = 5) for a median time of 6 days (range, 1 to 17). Two patients did not receive any growth factor. Onset of hematopoietic growth factor administration was not significantly different in these three subgroups. However, when compared with the intermediate group, patients reinfused with a low number of CD34+ cells had a longer period of growth factor administration (median of 9 days v 6 days, P = .009), while the difference was not significant when compared with the high CD34+ group (median, 5 days;P = .18).
PBPC reinfused.
Considering the total group of patients, the median count of MNC and CD34+ cells reinfused were respectively 3.6 × 108/kg and 6.86 × 106/kg (Table 2). The median number of CFU-GM, evaluated for the last 100 patients with the Methocult assay was 114 × 104/kg. The median numbers of CD34+ cells reinfused in the three subgroups were respectively 1.5, 5.85, and 21.6 × 106/kg, whereas interestingly, the median numbers of MNC reinfused were very similar.
Hematopoietic recovery.
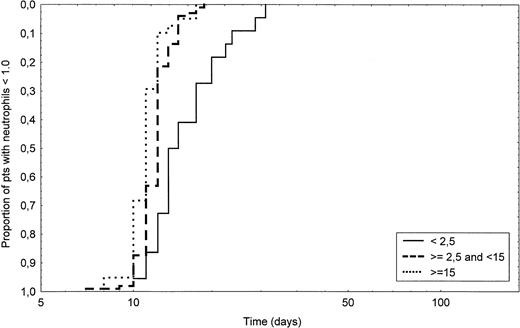
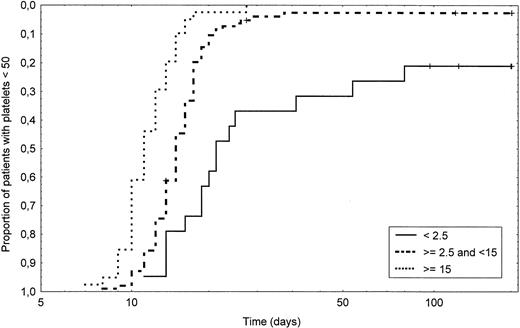
Table 3 shows for all patients and for the three CD34+ cell dose subgroups the median time, range, 10th and 90th percentile of neutrophil and platelet recovery. The median time to achieve an ANC greater than 0.1, 0.5, or 1.0 × 109/L was respectively 10, 11, and 12 days. In the low CD34+ group patients, the median time to achieve a neutrophil count greater than 1.0 × 109/L was 1 day longer as compared with the intermediate-group patients, a difference which is still highly significant (P < .0001). When compared with the intermediate CD34+ group, higher CD34+cell dose allowed to significantly further shorten neutrophil engraftment by 1 day (P = .003). The probability of achieving an ANC greater than 1.0 × 109/L according to the three CD34+ cell counts is shown in Fig 1. As shown in Table 3, when the thresholds of 0.1 and 0.5 ANC were considered, the differences between the groups were essentially similar and significant. The median time to achieve an unsustained platelet count greater than 20, 50, or 100 × 109/L was respectively 10, 14, and 17 days for all patients. The difference for the three thresholds was highly significant between each group (P< .0001). Figure 2 shows the probability of achieving a platelet count greater than 50 × 109/L according to the CD34+ cell count reinfused. Moreover, 81% of the intermediate-group patients achieved a platelet count greater than 100 × 109/L at day 30 as compared with 28% of the low and 97% of the high-group patients (P < .0001 and =.01, respectively). When considering patients evaluated at day 100 (n = 141), significantly more patients failed to recover 50 or 100 × 109/L platelets in the low group as compared with the intermediate group (20% v 2% and 60% v 18%,P = .002 and .0001, respectively). When comparing the high and the intermediate groups, the proportion of patients below the 50 or 100 × 109/L platelet thresholds was 0% versus 2% and 3% versus 14% (P = .4 and .058, respectively).
Time to recovery to an ANC greater than 1.0 × 109/L in intermediate CD34+ group patients (dashed line) compared with low CD34+ group patients (solid line) (P < .0001) and with high CD34+group patients (dotted line) (P = .003). Of note, the time is shown on a logarithmic scale.
Time to recovery to an ANC greater than 1.0 × 109/L in intermediate CD34+ group patients (dashed line) compared with low CD34+ group patients (solid line) (P < .0001) and with high CD34+group patients (dotted line) (P = .003). Of note, the time is shown on a logarithmic scale.
Time to recovery to a platelet count greater than 50 × 109/L in intermediate CD34+ group patients (dashed line) compared with low CD34+ group patients (solid line) (P < .0001) and with high CD34+group patients (dotted line) (P < .0001). Of note, the time is shown on a logarithmic scale.
Time to recovery to a platelet count greater than 50 × 109/L in intermediate CD34+ group patients (dashed line) compared with low CD34+ group patients (solid line) (P < .0001) and with high CD34+group patients (dotted line) (P < .0001). Of note, the time is shown on a logarithmic scale.
Factors influencing hematopoietic recovery.
When comparing the low and intermediate groups, CD34+ cell dose was in univariate analysis a significant factor (P < .0001) influencing neutrophil recovery (Table 4). The other significant variables were the duration of cumulative treatments before harvest (P = .003), the line of treatment (P = .04), and the previous use of fludarabine (P = .03), whereas alkylating agents exposure was of borderline significance (P = .059). The CD34+ cell dose was the only variable significantly influencing platelet recovery (P < .0001). These five variables were then considered for multivariate regression analysis. When comparing the low and intermediate-group patients, CD34+ cell dose remained the only independent variable significantly influencing neutrophil recovery (P = .02).
When comparing the high group and intermediate groups, the CD34+ cell dose was the only variable showing significance in univariate analysis for neutrophil and platelet engraftment (P= .003 and < .0001, respectively), and multivariate analysis was therefore not performed.
The analysis of factors influencing hematopoietic recovery was essentially similar when other thresholds for platelet (20 or 100 × 109/L) or neutrophil (0.1 or 0.5 × 109/L) recovery were considered (not shown).
Supportive care and clinical toxicity.
Table 5 shows the influence of the amount of CD34+ cells reinfused on patients' supportive care. The median number of red blood cell transfusions was not significantly different between the three subgroups. In contrast, patients in the intermediate CD34+ group required a median number of two platelet transfusions, as compared with three for the low group and one for the high-group patients (P = .001 and .002, respectively). When compared with the intermediate group, the median time of antibiotic administration was 2 days longer in the low group and 1 day shorter in the high group (P = .009 and .12, respectively). Furthermore, when compared with the intermediate group, low-group patients had a longer hospital stay, whereas high-group patients had a significantly shorter hospital stay (P = .0002 and .01, respectively).
Considering all other nonhematologic toxicities, we did not observe any significant differences between the three subgroups of patients (not shown), except for infections that occured within the 30 days after PBPC transplantation in 41% of the low group as compared with 21% of the intermediate-group patients (P = .05).
We observed four treatment-related deaths. In the low CD34+group, one patient reinfused with 1.62 × 106CD34+ cells/kg died of intracerebral bleeding at day 97. In the intermediate CD34+ group, three patients died of treatment-related toxicity, two early (one of fatal cardiac failure and one after venoocclusive disease) and one at day 105 of septicemia.
Response and survival.
Three months after transplant, 150 patients were assessable for response and three had died. Ninety-six patients (63%) achieved a CR, 30 (20%) a PR, three (2%) had a SD and 21 patients (14%) relapsed. At the time of analysis, 48 patients (31%) had progressed. The median follow-up for these patients was 14 months, but it was at least 6 months for all patients without relapse. To assess the potential influence of the CD34+ cell count reinfused on disease outcome, we examined in each group the rate of early relapses defined as disease progression within 6 months after the reinfusion of PBPC. Among the 24 patients who progressed during this period, we observed four early relapses (18%) in the low CD34+ group as compared with 15 (14%) in the intermediate CD34+ group (P = .89) and five (12%) in the high CD34+ group (P = .79).
DISCUSSION
This report analyzes the influence of the amount of reinfused CD34+ cells on hematologic recovery in 168 intensified patients. Among them, 22 (13%) received less than or equal to 2.5 × 106 CD34+ cells/kg, a level lower than our planned threshold, whereas 41 (24%) received CD34+ cell doses greater than 15 × 106/kg.
The present study confirms that patients who received less than or equal to 2.5 × 106 CD34+ cells/kg had significantly later hematopoietic engraftment when compared with those receiving greater than 2.5 × 106 cells/kg. In a trial including 61 patients, Haas et al18 had already shown that a dose of at least 2.5 × 106 CD34+cells/kg was required for a safe engraftment. Bensinger et al19 have proposed the same minimal threshold, but suggested that a higher limit value could be better for platelet recovery. Other investigators have recommended a minimal threshold of 2 × 106 CD34+ cells/kg to provide rapid engraftment.21,22 Only a small study found no correlation between CD34+ cell count and platelet recovery, but consisted of no more than two patients receiving less than 2.5 × 106 CD34+ cells/kg.27 In the present study, a significant proportion of patients reinfused with less than or equal to 2.5 × 106 CD34+ cells/kg experienced delayed hematologic recovery, particularly significant for platelets, with a median time that was 5 days longer to achieve an unsustained platelet count greater than 50 × 109/L. Even if an absolute value cannot be determined, the definition of a minimal threshold is probably clinically helpful, considering that 95% of the present patients receiving more than 2.5 × 106 CD34+ cells/kg achieved an ANC greater than 1.0 × 109/L in less than 15 days and an unsustained platelet count greater than 20 × 109/L in less than 14 days. However, as already reported,23 this data confirms that PBPC transplant is still feasible with moderate toxicities even for patients with lower CD34+ cell dose in harvest. This option must therefore always be weighed with the patient considering the potential benefit provided by intensified treatment, as compared with the increased risk of delayed platelet engraftment and longer hospitalization.
The most interesting point of this report is to indicate that after excluding the patients reinfused with less than or equal to 2.5 × 106 CD34+ cells/kg from the analysis, patients reinfused with a very high CD34+ cell dose showed better hematopoietic engraftment and especially faster platelet recovery. Until now, no study had clearly shown that reinfusion of a high number of CD34+ cells could bring an additional clinical benefit, as compared with the reinfusion of an adequate progenitor cell count. Kiss et al20 reported in 27 patients the existence of a threshold effect between rapid and slow engraftment of 5 × 106 CD34+ cells/kg. In a large study including heterogeneous patients with various diseases, Weaver et al28 also defined an optimal CD34+ cell dose of more than 5.0 × 106/kg and have shown a 95% probability of rapid engraftment above this limit. Moreover, this study suggested a dose-effect relationship for platelets up to doses of 10 × 106 CD34+ cells/kg, a finding also reported by others.29-31 However, in many of these studies, the group of patients receiving a higher number of CD34+cells was compared with another group that included several patients receiving less than optimal numbers of CD34+ cells. In the present study, the median time for an unsustained platelet count greater than 20 × 109/L in the high CD34+group was only 8 days, all patients achieving this count at 13 days. The dose-effect relationship between CD34+ cell dose and platelet recovery was also valid for the thresholds of 50 and 100 × 109/L platelets. When considering long-term platelet recovery, a higher proportion of patients recovered 100 × 109/L platelets at day 100 in the high group when compared with the intermediate group.
In the high CD34+ group patients, the benefit was also clear for platelet requirements, with a median number of one transfusion and a very narrow range, only one patient needing three platelet transfusions. Moreover, high CD34+ cell count allowed to further shorten the duration of hospitalization and tended to decrease the number of days with antibiotics. This reduction in supportive care due to more rapid platelet recovery might allow significant cost savings. Of note, the number of aphereses performed to obtain the PBPC harvest was not significantly different in both groups (not shown).
While CD34+ cell dose appears as the strongest predictor of engraftment, several other conditions such as duration of previous treatments and number of chemotherapy regimens have been reported to favor delayed neutrophil or platelet recovery.24 28 In the present study comprising only patients with lymphoid malignancies, CD34+ cell dose remained, after multivariate analysis, the only independent variable significantly influencing either neutrophil or platelet engraftment, whatever the patient group.
Some data have shown that tumor cells could be mobilized in peripheral blood after chemotherapy and G-CSF,32 and that tumor cells may be found in the CD34+ population in follicular lymphoma and MM.33 34 Even if the biologic significance of PBPC tumor cell contamination remains uncertain, the benefit of the reinfusion of large cell numbers might be questionable, considering the putative risk of tumor cell reinfusion. In the present study, however, the administration of high CD34+ cell doses did not correlate with a higher rate of early relapses within the 6 months after PBPC transplantation.
In conclusion, the present study suggests that compared with the reinfusion of adequate CD34+ cell count defined as PBPC harvest containing more than 2.5 × 106CD34+ cells/kg, the reinfusion of more than 15 × 106 CD34+ cells/kg after high-dose chemotherapy further shortens hematopoietic engraftment. Indeed, it markedly improves platelet independence and seems to shorten the duration of hospitalization. The hypothesis that reinfusion of high CD34+ cell count is cost effective and may contribute to a better quality of life has to be analyzed in further studies. It will also be interesting to investigate whether high CD34+ cell doses improve long-term hematopoietic recovery, as late impairment of progenitor cell compartment has been documented in patients after autologous transplantation.35 36
Supported by the Comité Départemental du Rhône de la Ligue contre le Cancer (Lyon, France). N.K. was supported by the Communauté de Travail des Alpes Occidentales (COTRAO) and by the Fondation Michel Clavel (Lyon, France).
Address reprint requests to Gilles Salles, MD, Service d'Hématologie, Centre Hospitalier Lyon-Sud, 69495 Pierre-Benite Cedex, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.