Abstract
Platelet factor 4 (PF-4) inhibits angiogenesis in vitro and in vivo. The mechanism of inhibition is poorly understood. We have investigated the mechanism of inhibition by examining the interaction of PF-4 and the fibroblast growth factor-2 (FGF-2)/fibroblast growth factor receptor (FGFR) system. PF-4 inhibited the binding of FGF-2 to high-affinity and low-affinity binding sites in murine microvascular endothelial cells (LEII cells) and proliferation. Maximum inhibition of binding to endothelial FGF receptors was observed at PF-4 concentrations between 5 and 10 μg/mL (half maximum inhibition at 0.6 μg/mL), and proliferation was completely inhibited at 2 μg/mL. At this concentration, PF-4 reduced internalization of125I–FGF-2 by threefold and delayed degradation. To gain insight into the mechanism of inhibition, we have analyzed the interaction of PF-4 with FGF-2/FGFR by using mutant heparan sulfate–deficient Chinese hamster ovary (CHO) cells transfected with the FGFR-1 cDNA (CHOm–FGFR-1) and by examining the direct interaction with FGF-2. In the absence of heparin, PF-4 inhibited binding of 125I–FGF-2 to CHOm–FGFR-1 cells in a concentration-dependent manner, although not completely. In the presence of heparin, PF-4 abolished totally the stimulatory effect of heparin. Furthermore, PF-4 complexed to FGF-2 and inhibited endogenous or heparin-induced FGF-2 dimerization. These results indicate that PF-4 interacts with FGF-2 by complex formation, inhibiting FGF-2 dimerization, binding to FGF receptors, and internalization. This mechanism most likely contributes to the antiangiogenic properties of PF-4.
ANGIOGENESIS INVOLVES the formation of new blood vessels of capillary origin. This phenomenon is tightly controled by a set of factors that include fibroblast growth factor 2 (FGF-2) and vascular endothelial growth factor (VEGF).1,2The angiogenic effects of these molecules are counterbalanced by inhibitory molecules such as angiostatin,3,4endostatin,5 thrombospondin-1 (TSP-1),6 the 16-kD human prolactin fragment (16-kD PRL),7or platelet factor 4 (PF-4).8
FGF-2 mediates its biological activity by binding to specific cell-surface receptors and heparan sulfate proteoglycans (HGSP). HSPG contribute to the binding of FGF-2 to high-affinity receptors by stabilizing the FGF-2/FGF receptor complex, protecting FGF-2 from degradation, or facilitating FGF-2 oligomerization.1 Thus, angiogenesis inhibitors may impair FGF-2 activity by interfering at the level of HSPG/FGF-2/FGF receptor interactions.
PF-4 belongs to the C-X-C chemokine family.9 This family also includes interleukin-8 (IL-8), β-thromboglobulin, neutrophil-activating protein, interferon-inducing protein 10 (IP-10), and melanocyte growth-stimulating activity.9 PF-4 is a 7.8-kD protein of 70 amino acid length.10 It shares homologies in particular with β-thromboglobulin and IL-8 of 51% and 31%, respectively.9, 11 The crystal structure of human PF-4 has been solved to a resolution of 2.4 A by molecular replacement.12 The N-terminal residues form antiparallel β-sheet–like structures. A positively charged ring of lysine and arginine side chains encircles the PF-4 molecule presenting multiple potential sites for heparin binding.
PF-4 exhibits biological activity for several cell types including megakaryocytes,13 leukocytes,14lymphocytes,15 and endothelial cells.8,16 It has been shown that PF-4 inhibits endothelial-cell proliferation, migration, and angiogenesis in vitro and in vivo.8, 16 In addition, PF-4 reduces tumor growth in vivo.17,18Intralesional injection in mice of recombinant human PF-4 inhibited melanoma cell or HCT 116 colon carcinoma cell growth by an angiogenesis-dependent mechanism.17 Furthermore, virally transduced rat glioma cells with a secretable PF-4 cDNA grew slowly in vivo and only formed hypovascular tumors.19 This indicates that the vasculature is the prime target for PF-4. In addition, PF-4 is targeted in vivo to endothelial cells that undergo active angiogenesis.20 PF-4 may also be important as a physiological regulator of FGF activity. Indeed, platelets release during activation an inhibitor of FGF-2 activity.21 It has been shown that this inhibitor is identical to PF-4. Thus, PF-4 may counteract excessive angiogenic factor activity at sites of platelet activation.
The mechanism of PF-4 action is incompletely understood and controversial. Sato et al22 have reported that PF-4 inhibits binding of FGF-2 to low-affinity binding sites and high-affinity receptors in NIH 3T3 fibroblasts. Others only reported competition of PF-4 with FGF-2 binding to low-affinity proteoglycans.23 In agreement with the latter observation, Luster et al24 showed that the IP-10 chemokine is associated with cell surface proteoglycans and that IP-10 binding can be competed by PF-4. In addition, a common heparan sulfate binding site may also be shared with histidine-rich glycoprotein (HRGP) as this molecule, like PF-4, displaces FGF-2 from the extracellular matrix.23 Furthermore, Gengrinowitch et al25reported that VEGF binding to endothelial cell VEGF receptors was inhibited by PF-4. Finally, Gupta and Singh16 showed that PF-4 intervenes at a specific point in the cell cycle by blocking the progression of endothelial cells in S-phase.
In this study, we sought to determine the contribution of PF-4 in the inhibition of FGF-2 activity and its mechanism of action. In a series of systematic studies, we show in particular that PF-4 indeed interferes with FGF-2 binding to high-affinity receptors and inhibits FGF-2 dimerization. The implication of this finding in the context of antiangiogenesis is discussed.
MATERIALS AND METHODS
Cells
Murine lung microvascular endothelial cells (LEII cells; kindly donated by Dr Thomas Maciag, American Red Cross, Rockville, MD) were grown in Dulbecco's modified Eagle's medium (DMEM; GIBCO, Life Technologies, Gaithersburg, MD) containing 10% fetal calf serum (FCS; GIBCO), 1 g/L glucose, 1% glutamin, and 50 IU/mL penicillin/ 50 μg/mL streptomycin at 37°C in a 5% CO2 atmosphere. Heparan sulfate–deficient chinese hamster ovary cells (CHOm–FGFR-1 cells, 745-flg; kindly donated by Dr Avner Yayon, The Weizmann Institute, Rehovot, Israel) were grown in DMEM containing 10% FCS (GIBCO), 1 g/L glucose, and 1% nonessential amino acids at 37°C in a 5% CO2 atmosphere. These cells express less than 5% HGSP. Binding 125I–FGF-2 to low-affinity binding sites (extracted with 20 mmol/L HEPES, 2 mol/L NaCl, pH 7.4) was less than 3% of total specific binding (sum of specifically bound125I–FGF-2 to high-affinity and low-affinity sites).
Cell Proliferation Experiments
Proliferation assays were performed as described.26Briefly, cells were seeded at 20,000 cells on 3.5-cm2dishes in complete DMEM containing 10% FCS, 1% glutamin, and antibiotics. After overnight attachment, the cells were washed once with serum-free DMEM and test medium containing 1% FCS, and the indicated concentrations of FGF-2 or PF-4 were added. Cells were counted at specified days with a Coulter counter (Coultronics, Margency, France).
Binding Studies, Cross-Linking to Receptors, Internalization, and Degradation
FGF-2 and PF-4 were labeled with 125I-Na using iodogen (Pierce, Rockford,IL) as a coupling agent according to the manufacturer's indications and according to Moscatelli.27The specific activity of 125I–FGF-2 and125I–PF-4 were 80, 000 to 200,000 cpm/ng and 35,000 to 100,000 cpm/ng, respectively. Binding experiments to high- and low-affinity sites were performed essentially as described by Moscatelli.27 Cells were seeded at 2.5 × 105/cm2, cultured in complete medium onto 3.5-cm2 dishes, and grown for 2 days. Cells were washed twice with ice-cold phosphate buffer saline (PBS) before binding and incubated with the indicated concentrations of 125I–FGF-2 or 125I–PF-4 in DMEM containing 20 mmol/L HEPES (pH 7.4), 0.15% gelatin for 2 hours at 4°C in the presence or absence of 1 μg/mL unlabeled ligand or competitors (FGF-2; PF-4; protamine sulfate [grade III; Sigma, St Louis, MO]; VEGF 165; PDGF BB; EGF). At the end of the incubation period, the cells were washed three times with ice-cold PBS. 125I–FGF-2 or 125I–PF-4 was dissociated from its cellular low-affinity binding sites by two 20-second washes with ice-cold 20 mmol/L HEPES (pH 7.4), 2 mol/L NaCl, and from its high-affinity sites by two 20-second washes with ice-cold 20 mmol/L NaAc (pH 4.0), 2 mol/L NaCl. Bound 125I–FGF-2 was quantified using a Kontron MR 250 γ-counter (Saint-Quentin-Yvelines, France). Nonspecific binding was determined by incubating separate dishes with 125I–FGF-2 and a 100-fold excess of unlabeled ligand. Specific binding was determined by substracting nonspecific binding from total binding.
Cross-linking experiments of 125I–FGF-2 or125I–PF-4 to receptors were performed and analyzed as described by Bikfalvi et al,26 using 0.2 mmol/L Bis(sulfosuccinyl) suberate (BS3; Pierce) in PBS as a coupling agent. The quantity of protein used in each experiment was normalized according to cell number or protein. Samples were run on a 7% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Autoradiographies were done against X-OMAT AR films (Eastman Kodak, Rochester, NY) at −80°C in the presence of an intensifying screen or analyzed by PhosphorImager (Molecular Dynamics, Sunnyvale, CA).
125I–FGF-2 internalization experiments were performed as described by Roghani and Moscatelli.28 LEII cells were incubated with 10 ng/mL 125I–FGF-2 with or without 2 μg/mL PF-4 at 37°C in a 5% CO2 atmosphere. At indicated times, the cells were washed three times with ice-cold PBS, and cell surface–bound material was extracted by washing the cells for 20 seconds twice with 2 mol/L NaCl in 20 mmol/L HEPES (pH 7.4) and twice with 2 mol/L NaCl in 20 mmol/L acetic acid (pH 4). The amount of internalized radioactivity was determined by solubilizing the cells with extraction buffer containing 10% glycerol, 2% SDS, 1.6 mmol/L EDTA in 125 mmol/L Tris-HCL (pH 6.8), and γ-counting. In other experiments, aliquots of cell extracts were separated by electrophoresis on a 15% SDS-PAGE, dried, and exposed to autoradiography or analyzed by PhosphorImager. Furthermore, the medium was incubated overnight at 4°C with trichloroacetic acid (TCA; 12% final concentration) to determine TCA-soluble and precipitable radioactivity.
Complex Formation Between FGF-2 and PF-4
Complex formation in solution.
Ten nanograms per milliliter 125I–FGF-2 and 1μg/mL of PF-4 were incubated in PBS with or without 0.8 mmol/L Ca++and 0.5 mmol/L Mg++ for 1 hour at room temperature (400-μL volume). Subsequently, 1 mmol/L BS3 (final concentration) was added, and the samples were incubated for another 30 minutes. The reaction was stopped by adding extraction buffer (125 mmol/L Tris-Cl, pH 7.4, 10% glycerol, 1.6 mmol/L EDTA, 2% SDS, and 2% 2-β mercaptoethanol) from a 5× concentrated stock solution. Samples were then run on 12% or 15% SDS-PAGE, and the dried gels were analyzed by PhosphorImager or autoradiography.
Immobilization of FGF-2 onto surfaces and binding experiments.
Binding of 125I–PF-4 to FGF-2– or VEGF 165–coated wells was performed as described. Ninety-six–well enzyme-linked immunosorbent assay (ELISA) plates were coated with 15 ng FGF-2/well in buffer A (1 mmol/L EDTA, 20 mmol/L K2HPO4, 10 mmol/L KH2PO4, 150 mmol/L NaCl) in a volume of 50 μL. After 2 hours of incubation at room temperature, the plates were washed five times with buffer B (10 mmol/L Tris-HCl, pH 7.2, 150 mmol/L NaCl, 0.1% Tween 20). The wells were subsequently incubated again with buffer A, which contained an additional 0.1% gelatin, and washed again after 1 hour with buffer B five times. The FGF-2–coated wells were then incubated in buffer A with different125I–PF-4 concentrations and competitors at 37°C for 1 hour. At the end of the incubation period, the wells were washed five times with buffer B. Surface-associated 125I–PF-4 was then extracted with 200 μL of 0.2 mol/L NaOH and counted in a γ-counter. In some experiments, wells were preincubated for 1 hour with 200 ng/well (4 μg/mL) heparin in buffer A. At the end of the preincubation period, the wells were washed twice before the addition of 125I–PF-4. To determine nonspecific binding, separate wells were not coated with FGF-2 and only preincubated with buffer A before the initiation of binding. Specific binding was determined by substracting nonspecific binding from total binding.
FGF-2 Dimerization Experiments
FGF-2 dimerization was studied according to the method described by Ornitz et al.29 Briefly, 5 ng 125I–FGF-2 and 500 ng unlabeled FGF-2 were incubated for 1 hour at room temperature with or without the indicated concentrations of heparin and/or PF-4 in PBS in a final volume of 45 μL. At the end of the incubation period, 5 μL BS3 (0.1 mmol/L final concentration) was added for another 30 minutes of incubation. The reaction was stopped by adding SDS-sample buffer from a 5× concentrated stock solution. The samples were boiled and run on a 12% or 15% SDS-PAGE. The gels were analyzed by PhosphorImager or autoradiography.
For all the experiments outlined above, autoradiograms or PhosphorImager results were analyzed by a public domain NIH Image Program developed at the US National Institutes of Health and available from the Internet by anonymous FTP form zippy.nimh.nih.gov or by using a Bio Profil V 6.0 scanner with Bio 1 D software (Vilber Lourmat, Paris).
RESULTS
PF-4 Inhibits Binding of FGF-2 to Endothelial Cells, Internalization, Degradation, and Biological Activity
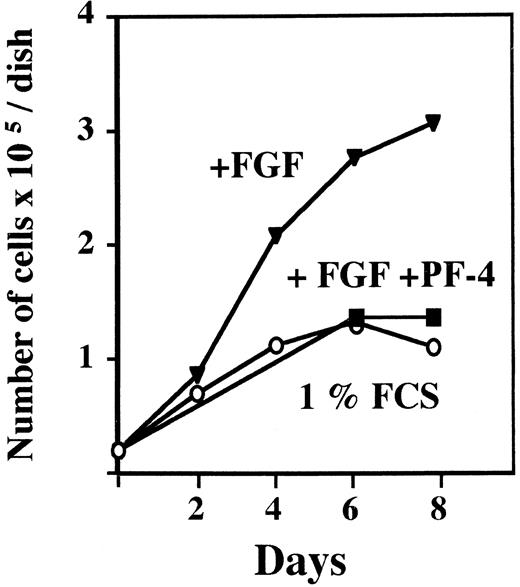
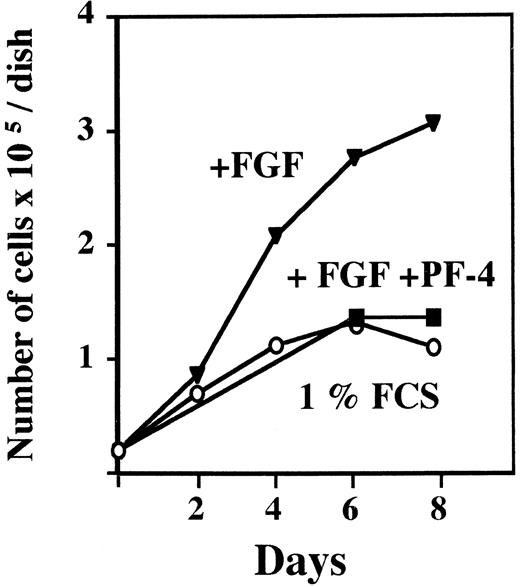
We first performed proliferation experiments to ascertain the potency of our PF-4 preparation. Murine lung capillary endothelial cells (LEII cells) were incubated with PF-4 and a fixed concentration of FGF-2 (10 ng/mL), and the cell number was estimated at specified days by cell counting. After 6 days, the cell number was increased by FGF-2 treatment alone threefold. The cell number in the presence of FGF-2 and PF-4 (2 μg/mL) was identical to the number in unstimulated control cells. Thus, PF-4 at 2 μg/mL inhibited completely endothelial cell growth induced by FGF-2 (Fig 1).
Effect of PF-4 on endothelial cell proliferation. LEII cells were seeded at 20,000 cells/dish. After overnight attachment, the test medium was added and cells were counted every other day for 8 days for dishes without PF-4 and at day 6 and 8 for dishes with PF-4. (○) 1% FCS; (▾) 1% FCS + 10 ng/mL FGF-2; (▪) 1% FCS + 10 ng/mL FGF-2 + 2 μg/mL PF-4. The figure depicts a representative experiment done in duplicates (data points as mean; standard deviation (SD) values: 0 < SD < 0.25).
Effect of PF-4 on endothelial cell proliferation. LEII cells were seeded at 20,000 cells/dish. After overnight attachment, the test medium was added and cells were counted every other day for 8 days for dishes without PF-4 and at day 6 and 8 for dishes with PF-4. (○) 1% FCS; (▾) 1% FCS + 10 ng/mL FGF-2; (▪) 1% FCS + 10 ng/mL FGF-2 + 2 μg/mL PF-4. The figure depicts a representative experiment done in duplicates (data points as mean; standard deviation (SD) values: 0 < SD < 0.25).
We then investigated the effect of PF-4 on the binding of FGF-2 to endothelial cells. LEII cells were incubated with 10 ng/mL125I–FGF-2 and increasing concentrations of PF-4 (1 to 10,000 ng/mL). Binding to low- and high-affinity sites was performed as indicated in Materials and Methods. FGF-2 binding to low-affinity sites or high-affinity binding sites was inhibited in a concentration-dependent manner by PF-4 (Fig2A and B). Maximum inhibition of FGF-2 binding to low-affinity binding sites or high-affinity sites (FGF receptors) was reached at 5 to 10 μg/mL PF-4 with half maximum inhibition at 0.72 and 0.6 μg/mL, respectively. FGF-2 binding was not competed by any of the following agents: human VEGF 165, platelet-derived growth factor BB (PDGF BB), or epidermal growth factor (EGF). To ascertain the inhibitory effect of PF-4 on FGF-2 binding, cross-linking of 125I–FGF-2 to cell-surface receptors in the presence of PF-4 was performed (Fig 2C). Cross-linked material was detected after SDS-PAGE and autoradiography. The intensity of the cross-linked material was decreased threefold (34% of control; Fig 2C, lane 1) in the presence of PF-4 as estimated by NIH Image Program analysis.
(A through C) Effect of PF-4 on 125I–FGF-2 binding to endothelial cells and cross-linking. LEII cells (500,000 cells/dish) were incubated at 4°C with 10 ng/mL125I–FGF-2 and increasing concentrations of PF-4 in the absence or presence of 1 μg/mL unlabeled FGF-2.125I–FGF-2 bound to high-affinity (A) or low-affinity binding sites (B) was determined as indicated in Materials and Methods. Nonspecific binding is indicated in black bars. Specific binding (ng/106 cells) is shown as inset. (C) Cross-linking of 125I–FGF-2 to receptors in the presence or absence of 10 μg/mL PF-4 or unlabeled ligand. (A and B) Representative experiment done in duplicates (data points as mean; SD values: [A] 0 < SD < 0.8; [A, inset] 0 < SD < 0.03; [B] 0.13 < SD < 0.8).
(A through C) Effect of PF-4 on 125I–FGF-2 binding to endothelial cells and cross-linking. LEII cells (500,000 cells/dish) were incubated at 4°C with 10 ng/mL125I–FGF-2 and increasing concentrations of PF-4 in the absence or presence of 1 μg/mL unlabeled FGF-2.125I–FGF-2 bound to high-affinity (A) or low-affinity binding sites (B) was determined as indicated in Materials and Methods. Nonspecific binding is indicated in black bars. Specific binding (ng/106 cells) is shown as inset. (C) Cross-linking of 125I–FGF-2 to receptors in the presence or absence of 10 μg/mL PF-4 or unlabeled ligand. (A and B) Representative experiment done in duplicates (data points as mean; SD values: [A] 0 < SD < 0.8; [A, inset] 0 < SD < 0.03; [B] 0.13 < SD < 0.8).
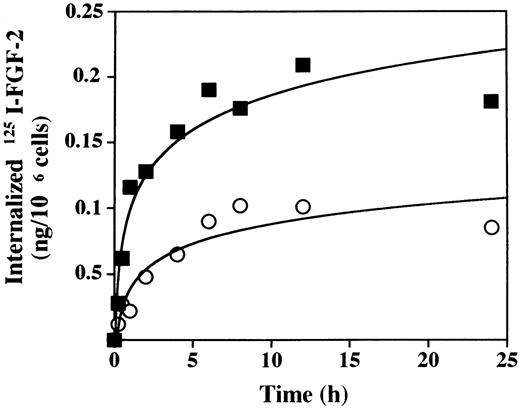
We next studied the effect of PF-4 on internalization and degradation of FGF-2. To measure FGF-2 internalization, we incubated LEII cells with 10 ng/mL 125I–FGF-2 in the presence or absence of 2 μg/mL PF-4 at 37°C over a 24-hour period. Analysis of membrane-bound and intracellular 125I–FGF-2 was performed as indicated in Materials and Methods. As depicted in Fig 3, long-term internalization of125I–FGF-2 was strongly inhibited by PF-4. In the absence of PF-4, maximum internalization was 0.209 ng125I–FGF-2/106 cells. This value decreased to 0.102 ng 125I–FGF-2/106 cells when the experiment was performed in the presence of PF-4. The initial rate of internalization after 1 hour was reduced approximately threefold (R2 = 0.037 ng/106 cells/h) in the presence of PF-4 when compared with control (R1 = 0.116 ng/106 cells/h). When the medium was precipitated with trichloro-acetic acid (TCA) and analyzed for TCA-soluble and precipitable radioactivity, we found that PF-4 decreased moderately the amount of the TCA-soluble radioactivity, although the kinetics of release were similar (data not shown). In addition, we examined the patterns of the 125I–FGF-2 degradation fragments in cell extracts (data not shown). Three degradation fragments of 15 kD, 10 kD, and 6 kD were detected. In the absence of PF-4, 125I–FGF-2 was almost completely converted into the 15-kD, 10-kD, and 6 kD form at 8 hours. In the presence of PF-4, the appearance of the 125I–FGF-2 degradation fragments was delayed and the conversion of the 18-kD FGF-2 into the 15-kD, 10-kD, and 6-kD forms was only observed after 12 hours. This indicated that the conversion of 18-kD FGF-2 into lower molecular weight FGF-2 forms is delayed by PF-4.
Internalization of 125I–FGF-2 in the presence of PF-4. For internalization experiments, LEII cells (500,000 cells/dish) were incubated at 37°C with 10 ng/mL125I–FGF-2 in the presence (○) or absence (▪) of 2 μg/mL PF-4 or 1 μg/mL unlabeled ligand. At the indicated time points, cell surface–bound and internalized 125I–FGF-2 were determined as indicated in Materials and Methods. The figure depicts a representative experiment done in duplicates (data points as mean; SD values: 0 < SD < 0.03 ).
Internalization of 125I–FGF-2 in the presence of PF-4. For internalization experiments, LEII cells (500,000 cells/dish) were incubated at 37°C with 10 ng/mL125I–FGF-2 in the presence (○) or absence (▪) of 2 μg/mL PF-4 or 1 μg/mL unlabeled ligand. At the indicated time points, cell surface–bound and internalized 125I–FGF-2 were determined as indicated in Materials and Methods. The figure depicts a representative experiment done in duplicates (data points as mean; SD values: 0 < SD < 0.03 ).
Effect of PF-4 on FGF-2 Binding in Cells Deficient of Heparan Sulfates and Expressing FGFR-1
To assess more accurately the interaction of PF-4 with the FGF-2/FGF receptor system, we chose to study the interaction of PF-4 and FGF-2 with FGFR-1. We used mutant CHO cells deficient of heparan sulfates that express FGFR-1 (CHOm–FGFR-1). These cells have been used for the analysis of heparin requirement in FGF-2 binding.28-31 We investigated the effect of PF-4 on 125I–FGF-2 binding in the absence and presence of heparin in this cell type. In the absence of heparin, PF-4 inhibited FGF-2 binding in a concentration-dependent manner (Fig 4A). Half maximum inhibition was reached at a PF-4 concentration of 0.85 μg/mL and maximum inhibition at 5 to 10 μg/mL. Maximum inhibitory PF-4 concentrations reduced 125I–FGF-2 binding to approximately 50%. The effect of PF-4 on heparin-induced 125I–FGF-2 was investigated in two ways. First, when binding was performed in the presence of increasing heparin concentrations (1 to 1,000 ng/mL),125I–FGF-2 binding was totally blocked at all heparin concentrations by 10 μg/mL of PF-4 (Fig 4B). Second, in the presence of a fixed concentration of heparin (50 ng/mL) and increasing concentrations of PF-4 (0.01 to 5 μg/mL), PF-4 abolished the effect of heparin on 125I–FGF-2 binding at 1 μg/mL with a half maximum inhibition at 0.4 μg/mL (Fig 4C). Finally, cross-linking of125I–FGF-2 to FGFR-1 was inhibited by PF-4 in the absence or presence of 50 ng/mL heparin (Fig 4D). Heparin by itself increased the intensity of the cross-linked material approximately twofold as estimated by the NIH imager program. In the absence of heparin, PF-4 decreased 125I–FGF-2 binding twofold (40% of unstimulated control). Furthermore, the stimulatory effect of heparin on the cross-linking of 125I–FGF-2 to FGFR-1 was totally abrogated by PF-4.
(A through D) Effect of PF-4 on the binding of125I–FGF-2 to receptors in cells deficient of heparan sulfates and expressing FGFR-1 (CHOm–FGFR-1), and cross-linking. CHOm–FGFR-1 cells (500,000 cells/dish) were incubated with 10 ng/mL125I–FGF-2 with or without PF-4, 50 ng/mL heparin, or 1 μg/mL unlabeled ligand. Binding of 125I–FGF-2 to receptors or cross-linking was performed as indicated in Materials and Methods. (A) Effect of increasing concentrations of PF-4 on high-affinity binding of 125I–FGF-2 to receptors in the absence of heparin. (B) Effect of increasing concentrations of heparin on 125I–FGF-2 binding in the presence (•) or absence (○) of 10 μg/mL PF-4. (C) Effect of increasing concentrations of PF-4 on 125I–FGF-2 binding in the presence or absence of 50 ng/mL heparin. (D) Cross-linking of125I–FGF-2 to receptors in the presence or absence of 10 μg/mL PF-4 or heparin. The differences in the amounts of125I–FGF-2 bound between the different experiments reflect clonal variability in FGFR-1 expression. (A through C) Representative experiments done in duplicates (data points as mean; SD values: [A] 0 < SD < 0.04; [B] 0.01 < SD < 0.3; [C] 0.005 < SD < 0.09).
(A through D) Effect of PF-4 on the binding of125I–FGF-2 to receptors in cells deficient of heparan sulfates and expressing FGFR-1 (CHOm–FGFR-1), and cross-linking. CHOm–FGFR-1 cells (500,000 cells/dish) were incubated with 10 ng/mL125I–FGF-2 with or without PF-4, 50 ng/mL heparin, or 1 μg/mL unlabeled ligand. Binding of 125I–FGF-2 to receptors or cross-linking was performed as indicated in Materials and Methods. (A) Effect of increasing concentrations of PF-4 on high-affinity binding of 125I–FGF-2 to receptors in the absence of heparin. (B) Effect of increasing concentrations of heparin on 125I–FGF-2 binding in the presence (•) or absence (○) of 10 μg/mL PF-4. (C) Effect of increasing concentrations of PF-4 on 125I–FGF-2 binding in the presence or absence of 50 ng/mL heparin. (D) Cross-linking of125I–FGF-2 to receptors in the presence or absence of 10 μg/mL PF-4 or heparin. The differences in the amounts of125I–FGF-2 bound between the different experiments reflect clonal variability in FGFR-1 expression. (A through C) Representative experiments done in duplicates (data points as mean; SD values: [A] 0 < SD < 0.04; [B] 0.01 < SD < 0.3; [C] 0.005 < SD < 0.09).
We compared the effect of PF-4 on 125I–FGF-2 binding in CHOm–FGFR-1 cells with that of protamine sulfate. Protamine sulfate at maximum inhibitory concentrations (20 to 100 μg/mL) decreased the binding of 125I–FGF-2 to FGF receptors in CHOm–FGFR-1 cells by approximately 80% in the absence of heparin. In addition, the inducing effect of heparin on 125I–FGF-2 binding was also inhibited by protamine sulfate (data not shown).
Taken together, these results indicate that PF-4 significantly inhibits FGF-2 binding to FGFR-1 in the absence of heparin. In addition, PF-4 also abrogates the inducing effect of heparin on FGF-2 binding.
Binding of PF-4 to Endothelial Cells and Cells Deficient of Heparan Sulfates and Expressing FGFR-1
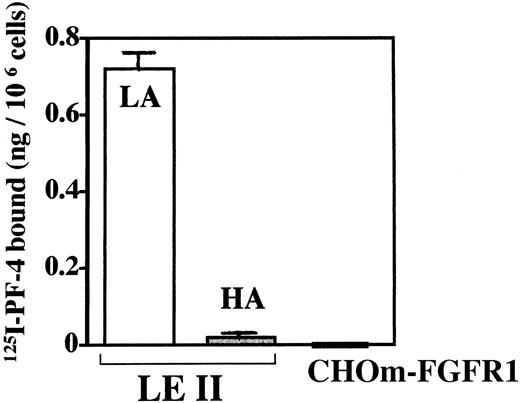
To investigate whether PF-4 by itself is able to bind to endothelial cells or CHOm–FGFR-1 cells, we performed binding experiments with125I–PF-4 (Fig 5).125I–PF-4 bound to high- or low-affinity binding sites was extracted as indicated in Materials and Methods. As indicated in Fig 5,125I–PF-4 significantly bound to low-affinity binding sites (up to 0.72 ng/106 cells) but only very weakly to high-affinity binding sites (0.015 to 0.03 ng/106 cells). In addition, no significant binding to CHOm–FGFR-1 cells could be detected. Furthermore, when 125I–PF-4 was cross-linked to LEII or CHOm–FGFR-1 cells, no cross-linked material was visible (data not shown). These results indicate that PF-4 binds significantly to low-affinity binding proteoglycans but not to high-affinity FGFR-1. In addition, specific high-affinity PF-4 receptors were not detected in LEII or CHOm–FGFR-1 cells.
Binding of 125I–PF-4 to endothelial cells and cells deficient of heparan sulfates and expressing FGFR-1. LEII cells or CHOm–FGFR-1 cells (500,000 cells/dish) were incubated with125I–PF-4 in the presence or absence of unlabeled ligand and binding to high-affinity (HA, cell surface–bound125I–PF-4 extracted with 2 mol/L NaCl buffer at pH 4) or low-affinity sites (LA, cell surface–bound 125I–PF-4 extracted with 2 mol/L NaCl buffer at pH 7.4) were analyzed as indicated in Materials and Methods. The binding of the figure depicts representative experiments done in duplicates (data points as mean + SD).
Binding of 125I–PF-4 to endothelial cells and cells deficient of heparan sulfates and expressing FGFR-1. LEII cells or CHOm–FGFR-1 cells (500,000 cells/dish) were incubated with125I–PF-4 in the presence or absence of unlabeled ligand and binding to high-affinity (HA, cell surface–bound125I–PF-4 extracted with 2 mol/L NaCl buffer at pH 4) or low-affinity sites (LA, cell surface–bound 125I–PF-4 extracted with 2 mol/L NaCl buffer at pH 7.4) were analyzed as indicated in Materials and Methods. The binding of the figure depicts representative experiments done in duplicates (data points as mean + SD).
PF-4 Complexes With FGF-2
We next set out to examine whether PF-4 complexes with FGF-2. We examined this by performing cross-linking experiments of FGF-2 and PF-4 and by directly studying the interaction of these molecules immobilized onto surfaces.
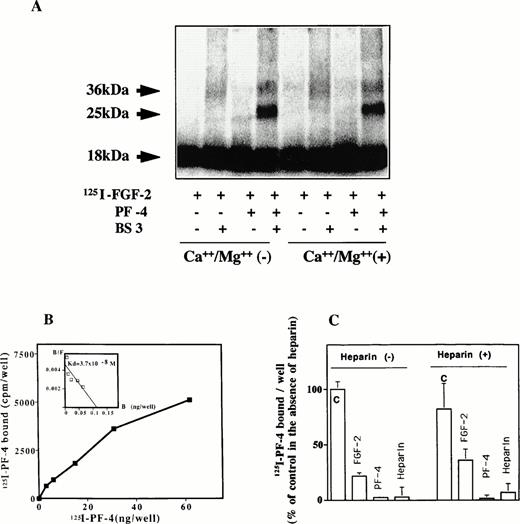
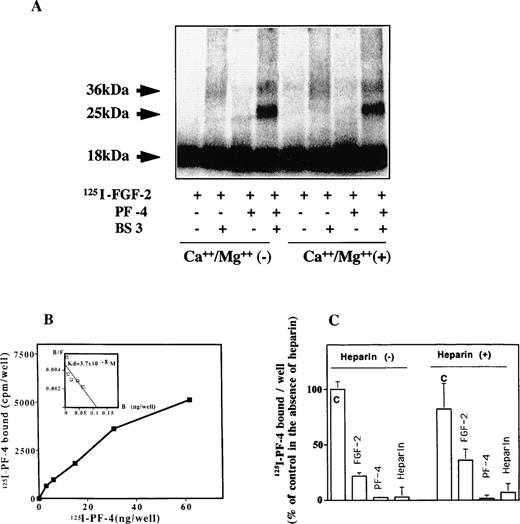
125I–FGF-2 was incubated with PF-4 at room temperature for 20 minutes and cross-linked by using BS3 as a cross-linking agent (Fig 6A). The cross-linked material was subsequently analyzed by SDS-PAGE and autoradiography or PhosphorImager. In the presence of the cross-linking agent, two bands of 36 kD and of 25 kD were observed. The 36-kD band corresponded to endogenous FGF-2 dimers and was visible in the absence of PF-4, although weakly under our experimental conditions. With 10 ng/mL125I–FGF-2 and 1 μg/mL PF-4 in solution, an additional 25-kD band was revealed by autoradiography. Under the experimental conditions, scanning analysis indicated relative intensities of the 25 kD or 36 kD of 20% and 8%, respectively (72% for the remaining 18-kD FGF-2 band). To study the effect of divalent cations, the cross-linking reactions were performed in the absence and presence of 0.8 mmol/L Ca++ and 0.5 mmol/L Mg++. Divalent cations did not influence the appearance or the intensity of the 25-kD cross-linked material. As a control, the cross-linking reaction was performed with bovine serum albumin (BSA) instead of PF-4. Complex formation was not observed between BSA and 125I–FGF-2, even at 10 μg/mL BSA.
(A through C) Complex formation of PF-4 with FGF-2. (A) Cross-linking experiments in solution. One microgram of PF-4 and 10 ng125I–FGF-2 were incubated in 400 μL PBS in the presence or absence of 0.8 mmol/L Ca++ and 0.5 mmol/L Mg++ for 1 hour. Subsequently, 1 mmol/L BS3was added for another 30 minutes. At the end of the incubation period, extraction buffer was added and aliquots were loaded onto a 12% SDS-PAGE. The dried gel was analyzed by PhosphorImager or autoradiography. (B) Binding of 125I–PF-4 to cell surface immobilized FGF-2. Fifteen nanograms per 50 microliters of FGF-2 was adsorbed onto the surface of a 96-well ELISA plate and incubated with different concentrations of 125I–PF-4. Binding was performed and analyzed as indicated in Materials and Methods. Scatchard plot is shown as inset. The figure depicts an representative experiment done in triplicates (data points as mean; SD values: 24 < SD < 780). (C) Competion of 125PF-4 binding to FGF-2. Wells were coated with FGF-2 at 15 ng/50 μL. Some of the wells were preincubated with 200 ng heparin. Plates were washed twice with buffer A to remove unbound heparin. Others wells received buffer alone. Ten nanograms125I-PF-4, with or without 5 μg PF-4, 5 μg FGF-2, or 50 ng heparin, was added to the wells. Binding was performed and analyzed as indicated in Materials and Methods. The figure depicts a representative experiment done in triplicates (data points as mean + SD). 100% corresponds to 7,000 cpm.
(A through C) Complex formation of PF-4 with FGF-2. (A) Cross-linking experiments in solution. One microgram of PF-4 and 10 ng125I–FGF-2 were incubated in 400 μL PBS in the presence or absence of 0.8 mmol/L Ca++ and 0.5 mmol/L Mg++ for 1 hour. Subsequently, 1 mmol/L BS3was added for another 30 minutes. At the end of the incubation period, extraction buffer was added and aliquots were loaded onto a 12% SDS-PAGE. The dried gel was analyzed by PhosphorImager or autoradiography. (B) Binding of 125I–PF-4 to cell surface immobilized FGF-2. Fifteen nanograms per 50 microliters of FGF-2 was adsorbed onto the surface of a 96-well ELISA plate and incubated with different concentrations of 125I–PF-4. Binding was performed and analyzed as indicated in Materials and Methods. Scatchard plot is shown as inset. The figure depicts an representative experiment done in triplicates (data points as mean; SD values: 24 < SD < 780). (C) Competion of 125PF-4 binding to FGF-2. Wells were coated with FGF-2 at 15 ng/50 μL. Some of the wells were preincubated with 200 ng heparin. Plates were washed twice with buffer A to remove unbound heparin. Others wells received buffer alone. Ten nanograms125I-PF-4, with or without 5 μg PF-4, 5 μg FGF-2, or 50 ng heparin, was added to the wells. Binding was performed and analyzed as indicated in Materials and Methods. The figure depicts a representative experiment done in triplicates (data points as mean + SD). 100% corresponds to 7,000 cpm.
We next set out to examine the direct interaction of PF-4 and FGF-2 using a solid-phase binding assay (Fig 6B). Ninety-six–well ELISA plates were coated with 15 ng FGF-2, and binding of125I–PF-4 was performed as indicated in Materials and Methods. When increasing concentrations of 125I–PF-4 were added to the wells, concentration-dependent binding to FGF-2 was observed. The affinity for PF-4 binding to FGF-2 calculated from the data above was high with an estimated kd of 3.7 × 10−8 mol/L. We next investigated the competition of direct binding between FGF-2 and PF-4. PF-4, FGF-2, and heparin were tested for competing with binding of 125I–PF-4 to FGF-2 (Fig 6C). The experiments were peformed under two conditions. In one set of experiments, the wells were coated with 15 ng FGF-2 and preincubated with 200 ng/well (4 μg/mL) heparin. In another set of experiments, preincubation was done with buffer only. When preincubation was done with buffer only, 125I–PF-4 binding was strongly inhibited by 5 μg FGF-2 (21% of control), 5 μg PF-4 (5% of control), and 50 ng heparin (10% of control). In heparin-preincubated wells, 125I–PF-4 bound still to FGF-2–coated wells, and binding was also inhibited by these competitors. The competion of the binding by FGF-2 was slightly less effective in heparin-preincubated wells (31% of control). This may suggest that the heparin binding domain of FGF-2 is only partially involved in the association between PF-4 and FGF-2 and that other domains are also implicated.
The association of PF-4 with FGF-2 was not specific for FGF-2 alone, because PF-4 was able to bind surface immobilized VEGF 165 (data not shown).25 However, as already reported, PF-4 did not bind to insulin, transferrin, or VEGF 121.25 Furthermore, binding of PF-4 to BSA was not observed (data not shown).
PF-4 Inhibits FGF-2 Dimerization
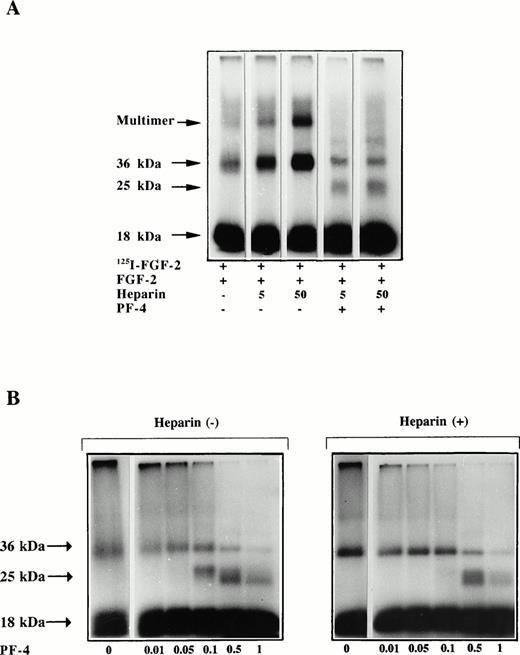
We next investigated the effect of PF-4 on FGF-2 dimerization (Fig 7). The dimerization experiments were performed according to the technique described by Ornitz et al.29 In this procedure, 125I–FGF-2 and unlabeled FGF-2 are incubated together to enhance the dimerization signal. As seen in Fig 7A, not only was the appearance of FGF-2 dimers stimulated by heparin with this method, but so was that of FGF-2 multimeric complexes. When 5 ng 125I–FGF-2 and 500 ng unlabeled FGF-2 were incubated with increasing heparin concentrations and a fixed PF-4 (1 μg) concentration (50 μL incubation volume), FGF-2 dimerization was strongly inhibited. The intensity of the 36-kD band as estimated by the NIH Image Program was reduced by PF-4 threefold to fourfold (32% and 25% of control) at all heparin concentrations. In addition, the high molecular weight complex that represents most likely FGF-2 multimers was also totally inhibited by PF-4. Moreover, in the presence of PF-4 additional cross-linked material of 25-kD size was visible. This signal possibly corresponds to FGF-2/PF-4 heterodimeric complexes. The specificity of the cross-linking reaction was assessed by incubating labeled FGF-2, unlabeled FGF-2, and heparin with increasing concentrations of BSA (100 ng/mL to 4 mg/mL) and then performing the cross-linking reaction. Even at very high BSA (4 mg/mL) concentrations, no effect on FGF-2 dimerization and no complex formation between BSA and FGF-2 was observed (data not shown).
(A and B) Effect of PF-4 on FGF-2 dimerization. Five nanograms 125I–FGF-2 and 500 ng unlabeled FGF-2 were incubated in 45 μL PBS with or without heparin and specified PF-4 concentration. After 1 hour of incubation, 5 μL cross-linking reagent was added and the samples were incubated for further 30 minutes. At the end of the incubation period, extraction buffer was added and the samples were boiled and loaded on a 12% SDS-PAGE. The dried gels were analyzed by autoradiography. (A) Effect of increasing heparin concentrations (ng) on FGF-2 dimer formation in the presence of 1 μg PF-4. (B) Concentration dependency of the effect of PF-4 (μg) on FGF-2 dimerization in the absence (left panel) or presence (right panel) of 50 ng heparin.
(A and B) Effect of PF-4 on FGF-2 dimerization. Five nanograms 125I–FGF-2 and 500 ng unlabeled FGF-2 were incubated in 45 μL PBS with or without heparin and specified PF-4 concentration. After 1 hour of incubation, 5 μL cross-linking reagent was added and the samples were incubated for further 30 minutes. At the end of the incubation period, extraction buffer was added and the samples were boiled and loaded on a 12% SDS-PAGE. The dried gels were analyzed by autoradiography. (A) Effect of increasing heparin concentrations (ng) on FGF-2 dimer formation in the presence of 1 μg PF-4. (B) Concentration dependency of the effect of PF-4 (μg) on FGF-2 dimerization in the absence (left panel) or presence (right panel) of 50 ng heparin.
We then investigated the effect of increasing PF-4 concentrations on FGF-2 dimer formation. In the absence of heparin, increasing amounts of PF-4 (10 to 1,000 ng) gradually reduced the appearance of the 36-kD band. PF-4 at 1 μg inhibited significantly the intensity of the 36-kD band. In parrallel, the 25-kD band was visible at PF-4 amounts of 0.1 μg and higher. Similarily, in the presence of heparin, increasing PF-4 amounts also inhibited the intensity of the 36-kD band with the appearance of the 25-kD band at 0.5 μg and higher.
DISCUSSION
We undertook a systematic study to clarify the inhibitory mechanism of PF-4 on FGF-2 activity in endothelial cells. The results reported herein indicate that PF-4 interferes with FGF-2 and FGF receptors by inhibiting FGF dimer formation, binding, internalization, and degradation. This is based on the following observations: (1) PF-4 inhibited binding to low-affinity binding sites and to high-affinity FGF receptors in endothelial cells and biological activity, (2) PF-4 inhibited FGF-2 internalization and delayed degradation, (3) PF-4 inhibited FGF-2 binding in CHOm–FGFR-1 in the absence of heparin, (4) PF-4 abrogated the stimulatory effect of heparin on FGF-2 binding in CHOm–FGFR-1 cells, (5) FGF-2 complexes with PF-4, and (6) PF-4 inhibited residual and heparin-induced FGF-2 dimerization.
PF-4 inhibited high- and low-affinity binding of FGF-2 to murine capillary endothelial cells. These data are reinforced by cross-linking of 125I–FGF-2 to FGF receptors that showed a strong decrease in the intensity of the cross-linked material when the experiment is performed in the presence of PF-4. This is in agreement with the results reported by Sato et al,22 who also reported inhibition of FGF-2 binding to high- and low-affinity binding sites in murine 3T3 fibroblasts. In contrast to these observations, others only described competition of FGF-2 binding to low-affinity proteoglycans.23 The reasons for these differences are not known at the present time. The nature of the low-affinity binding proteoglycan has recently been investigated. Luster et al24have reported that PF-4 but not other members of the chemokine family inhibited IP-10 binding to proteoglycans. Thus, these data may indicate a common proteoglycan binding site for IP-10 and PF-4. We found that PF-4 inhibited endothelial cell proliferation at maximum concentrations of 2 μg/mL. This is in agreement with the data of Gupta and Singh et al,16 who also reported inhibition within this PF-4 concentration range. These authors used bovine retinal microvascular endothelial cells but also endothelial cells from large vessels (human aorta, fetal bovine heart, and human umbilical cord) in their experiments. The concentration of PF-4 required for half maximum inhibition was in the range of 1 to 3 μg/mL for these different cell types. Furthermore, these concentrations are physiologically significant because platelets may generate locally in vivo very high PF-4 concentrations ranging between 2.5 to 25 μg/mL.32PF-4 concentrations that inhibited maximally FGF-2 proliferation decreased FGF-2 internalization by threefold and delayed the appearance of FGF-2 degradation fragments. This indicates that in addition to impairment of FGF-2 binding, internalization of FGF-2 is strongly inhibited by PF-4, and this at a PF-4 concentration that did not completely inhibit FGF-2 binding to LEII cells.
To explain the inhibitory effect of PF-4 on FGF-2 binding and activity, several mechanisms may be considered. First, PF-4 may only compete with FGF-2 for the availability of heparan sulfates that have been shown to be important in FGF-2 activity. Second, PF-4 may directly bind FGF-2 receptors and compete for binding at a receptor level. Third, PF-4 may complex to FGF-2 and inhibit FGF-2 dimer formation. We therefore undertook a systematic study to analyze the mechanism of inhibition by PF-4.
In a first series of experiments we used CHO cells deficient of heparan sulfates and transfected with FGFR-1. We observed an inhibitory effect of increasing PF-4 concentrations on FGF-2 binding with maximum inhibition between 5 and 10 μg/mL, and this in the absence of heparin. When binding was performed in the presence of heparin, PF-4 completely abrogated the stimulatory effect of heparin on FGF-2 binding. Two conclusions may be drawn from these data. First, FGF-2 is clearly able to bind significantly to this cell type in the absence of heparin. This observation is in agreement with Roghani et al33 who also found significant FGF-2 binding to CHOm–FGFR-1 cells in the absence of heparin. Second, the inhibitory action of PF-4 certainly involves more than only an antiheparin effect as PF-4 already impedes FGF-2 binding in the absence of heparin. This may also suggest that, in addition to the heparin binding domain, other PF-4 domains are also implicated.
None of the studies reported so far was able to identify a specific cell-surface receptor for PF-4.9 PF-4 only bound to IL-8 receptors in leukocytes when modified at the N-terminus.14,34 Furthermore, IP-10 binding to endothelial cells can be competed by PF-4, but not by other members of the chemokine family.24 These binding sites are dependent on the presence of cell surface HGSP. Accordingly, we only detected significant low-affinity binding of PF-4 to endothelial cells. Furthermore, cell surface receptors for PF-4 could not be detected by cross-linking of 125I–PF-4 to endothelial cells. Finally, there was clearly no direct interaction between PF-4 and FGFR-1 because125I–PF-4 failed to bind to CHOm–FGFR-1 cells. Thus, these results indicate that PF-4 does not inhibit FGF-2 binding by directly associating with FGFR-1 and blocking access of the ligand to FGF receptors.
FGF-2 is able to associate, besides with cell surface FGF receptors, with a number of molecules including soluble or matrix-bound FGF receptors35; proteoglycans,36 including perlecan,37 glypican I,38 glypican III,39 and ryudocan40; and α2-macroglobulin.41 We found that PF-4 associated with FGF-2. When 125I–FGF-2 was incubated with PF-4 in solution, a complex of 25 kD was detected by cross-linking. This may correspond to an FGF-2/PF-4 heterodimer of one molecule FGF-2 and a PF-4 monomer. Higher molecular weight complexes were not detected on 12% PAGE with this method. Solid phase-binding assays indicated concentration-dependent binding of 125I–PF-4 to FGF-2 that occured with high affinity. 125I–PF-4 binding to immobilized FGF-2 could be competed by FGF-2, heparin, and PF-4. Furthermore, when the plates were preincubated with 4 μg/mL heparin,125I–PF-4 still associated to surface immobilized FGF-2. In addition, FGF-2 competed a little less with 125I–PF-4 binding, albeit significantly. This may suggest that in addition to the heparin-binding domains, other FGF-2 domains may be involved in the interaction with PF-4.
In the present model of FGF receptor activation, FGF-2 dimer formation is required to induce FGF receptor dimerization and activation.42 It has been reported that heparin is important for FGF-2 dimer formation. However, the magnitude of the heparin effect is still a matter of debate.29-31,33,42-44We found that PF-4 inhibited residual (endogenous) and heparin-induced FGF-2 dimer formation. The formation of FGF-2 oligomers was also abrogated by PF-4. This correlates with the inhibition of FGF-2 binding in LEII and CHOm–FGFR-1 cells and of biological activity. This is also in agreement with recent nuclear magnetic resonance (NMR) studies that indicate that FGF receptor activation absolutely requires the formation of FGF-2 dimers.43 It is to note that we observed residual binding of FGF-2 to FGFR-1 in CHOm–FGFR-1 cells at PF-4 concentrations that almost completely inhibited FGF-2 dimerization. This fraction is approximately 50% of total binding in absence of heparin and of approximately 25% of heparin-induced binding to FGFR-1. This suggests that binding but not receptor activation is, to a some extent, FGF-2 dimer-independent.
What are the domains implicated in the interaction between PF-4 and FGF-2? PF-4 contains several domains potentially implicated in its activity. The C-X-C motif is located at the PF-4 N-terminus. It has been reported that cleavage of this sequence that retains the peptide 17-70 will dramatically increase the inhibitory activity of PF-4.45 PF-4 17-70 may occur as a natural peptide in leucocytes produced by a leucocyte elastase.45 The major heparin-binding domain is localized at the C-terminus between amino acid 58-70. Maione et al46 have modified this sequence to eliminate the lysine residues needed for heparin binding but to retain the amphipatic α-helical structure of the carboxyterminus. These modified molecules retained full antiangiogenic activity. Another study suggested that a loop containing Arg-20, Arg-22, His-23, and Thr-25, as well as Lys-46 and Arg-49, is also involved in heparin binding.47 Site-directed mutagenesis and heparin binding indicates that the arginines residues are especially important. We are currently examining the FGF-2 and PF-4 domains implicated in the interaction between these two molecules. Preliminary data indicate that the heparin-binding domain in FGF-2 or PF-4 is not the only one implicated and is alone insufficient to account for the biological effects of PF-4 toward FGF-2.
In the light of the results reported here, the following model of PF-4 inhibition is suggested. Spontaneous FGF-2 dimer formation or heparin-induced FGF-2 dimer formation is inhibited by PF-4. The inhibition of FGF-2 dimerization may result from complex formation of FGF-2 with PF-4 that empedes the successful association of two FGF-2 molecules. This in turn inhibits FGF receptor dimerization, FGF-2 dimer-dependent binding, receptor activation, and internalization. A number of molecules, including endogenous angiogenesis inhibitors, are able to interfere with FGF-2 binding to receptors and biological activity.48,49 It is possible that several of these are acting by a similar extracellular mechanism providing a trap for angiogenic molecules, no longer allowing successful receptor activation. During the preparation of this manuscript, Taraboletti et al50 reported binding of TSP-1 to FGF-2. More interestingly, Miao et al51 showed that a synthetic heparin-mimicking nonsulfated polyanionic aromatic compound (RG-13577) of 5-kD size inhibited FGF-2 activity and dimerization. Our data indicate that this mechanism also applies to endogenous angiogenesis inhibitors.
ACKNOWLEDGMENT
The authors thank Dr David Moscatelli (New York University Medical Center, New York) and Dr Sophie Javerzat (Growth Factor and Cell Differentiation Laboratory, University Bordeaux I) for critically reading of the manuscript, Dr Daniel B. Rifkin (Department of Cell Biology, New York University Medical Center) for the gift of recombinant FGF-2, Dr Thomas Maciag (American Red Cross, Rockville, MD) for providing LEII cells, Dr Avner Yayon (The Weizmann Institute, Rehovot, Israel) for providing CHOm–FGFR-1 cells, Jean Amiral (Serbio, Les Ulis, France) for providing recombinant human PF-4, and Dr Jean Plouet (CNRS, Toulouse) for providing recombinant VEGF. The authors also thank Jean-François Comps for his help in the preparation of the figures.
Supported by grants from la Ligue Nationale Contre le Cancer, l'Association pour la Recherche sur le Cancer (ARC), la Région Aquitaine, le Pôle Médicament Aquitaine, la Fondation GEFLUC, la Fondation Simone et Cino del Duca, and le MSR (to A.B.).
Address reprint requests to Andreas Bikfalvi, MD, PhD, Laboratoire des Facteurs de Croissance et de la Differenciation Cellulaire, Bâtiment de Recherche Biologie Animale, Avenue des Facultés, 33405 Talence, France.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 2. (A through C) Effect of PF-4 on 125I–FGF-2 binding to endothelial cells and cross-linking. LEII cells (500,000 cells/dish) were incubated at 4°C with 10 ng/mL125I–FGF-2 and increasing concentrations of PF-4 in the absence or presence of 1 μg/mL unlabeled FGF-2.125I–FGF-2 bound to high-affinity (A) or low-affinity binding sites (B) was determined as indicated in Materials and Methods. Nonspecific binding is indicated in black bars. Specific binding (ng/106 cells) is shown as inset. (C) Cross-linking of 125I–FGF-2 to receptors in the presence or absence of 10 μg/mL PF-4 or unlabeled ligand. (A and B) Representative experiment done in duplicates (data points as mean; SD values: [A] 0 < SD < 0.8; [A, inset] 0 < SD < 0.03; [B] 0.13 < SD < 0.8).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/9/10.1182_blood.v91.9.3289/3/m_blod40909002w.jpeg?Expires=1769558507&Signature=NTJAj1~qC2HpuuYKarZQd-uvQiMXieh-BOTjLs2OfMoB5Wj7T8nMS3LH97YUaAOcRfBUK4XzdVzC7IPmjL2JYVEA-05fIvq5SQEHfS-RurwtBwQE4nFvf-WSS0rg0r1d0-YB7lRpGGMjxcs~n2Fnlp4FaMqSD6rsBs340t7ZYgM5ghnWiGEO3fNdS4YmO3ms6fkLMLAj17stnuGFxv41bp-YvwOQIpWFWp3iaWq8oQyClAjVd74yd7VbusDXjkW~kKgsiWOacccUFmdo29VxoCMFaCUvH13J55K-rtufHnkiiuoUCXG9FIx9ecUCP3YUR7juFHjc1gq82iRxakIDOg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 4. (A through D) Effect of PF-4 on the binding of125I–FGF-2 to receptors in cells deficient of heparan sulfates and expressing FGFR-1 (CHOm–FGFR-1), and cross-linking. CHOm–FGFR-1 cells (500,000 cells/dish) were incubated with 10 ng/mL125I–FGF-2 with or without PF-4, 50 ng/mL heparin, or 1 μg/mL unlabeled ligand. Binding of 125I–FGF-2 to receptors or cross-linking was performed as indicated in Materials and Methods. (A) Effect of increasing concentrations of PF-4 on high-affinity binding of 125I–FGF-2 to receptors in the absence of heparin. (B) Effect of increasing concentrations of heparin on 125I–FGF-2 binding in the presence (•) or absence (○) of 10 μg/mL PF-4. (C) Effect of increasing concentrations of PF-4 on 125I–FGF-2 binding in the presence or absence of 50 ng/mL heparin. (D) Cross-linking of125I–FGF-2 to receptors in the presence or absence of 10 μg/mL PF-4 or heparin. The differences in the amounts of125I–FGF-2 bound between the different experiments reflect clonal variability in FGFR-1 expression. (A through C) Representative experiments done in duplicates (data points as mean; SD values: [A] 0 < SD < 0.04; [B] 0.01 < SD < 0.3; [C] 0.005 < SD < 0.09).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/9/10.1182_blood.v91.9.3289/3/m_blod40909004w.jpeg?Expires=1769558507&Signature=2VOZTYItYdrgtI1TU~sCp~I6LQxZDAL~YHdRuA29dysLZiqF63HMK4ywDz-2~mkfUFcWo3Md1VIZR9NKyQ732Rg6drSoKnoLCouP5xQBBO8aJIJw6awVAO7Sc3aIBnXDrSxoXBQYBZQ454Oq0N~wt9W1MhiZiTBG10U4ZmqgY416fg1a~DmGahzYvatir5XjeEwezQJV7mCeFliHI0fytC1RYMDZ8id2Fv1Un9Z7A1K-8RE3YKyHLa8WjSxIp5gRgL5bsbjbIY97x3VXtB-BybGUZH~0PKxTLXUk0yYHzVtFnkJoRuQTDugFhbv38jy1GGolm7DD3FQiup3VknUkEw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 2. (A through C) Effect of PF-4 on 125I–FGF-2 binding to endothelial cells and cross-linking. LEII cells (500,000 cells/dish) were incubated at 4°C with 10 ng/mL125I–FGF-2 and increasing concentrations of PF-4 in the absence or presence of 1 μg/mL unlabeled FGF-2.125I–FGF-2 bound to high-affinity (A) or low-affinity binding sites (B) was determined as indicated in Materials and Methods. Nonspecific binding is indicated in black bars. Specific binding (ng/106 cells) is shown as inset. (C) Cross-linking of 125I–FGF-2 to receptors in the presence or absence of 10 μg/mL PF-4 or unlabeled ligand. (A and B) Representative experiment done in duplicates (data points as mean; SD values: [A] 0 < SD < 0.8; [A, inset] 0 < SD < 0.03; [B] 0.13 < SD < 0.8).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/9/10.1182_blood.v91.9.3289/3/m_blod40909002w.jpeg?Expires=1770131536&Signature=XUUc4mlEbBUm46wyzuWEd11fkCHZmBl5L4-Vjp8hd5MMamzPLwgbu1gl136u98QIJByb76BSo5SJh9vaFYWut2JebpeAPu4ZhFkFGRLGc7sNBNlTrS4RGGzJR-cXk3NTGHafikuEm0Yxt6Jpf6mjlaUQdp59Ceie3lQPsS2R1860ME7aZ-wSTr43tRVyL3xCiFzOq-8sUR8wnaIKeUIOX1BPurtBZMyX5Jb6W7H9tIrPL-v~93FJ1hbE8NGvftwKqgWMj2qEVwO0inLvbqqmFA9UjRgm2081uF~aUvyu0t-gy8E8ZLcYq7PJXqknfCtozpaMtuhRXlCbWK~ODpxsDw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. (A through D) Effect of PF-4 on the binding of125I–FGF-2 to receptors in cells deficient of heparan sulfates and expressing FGFR-1 (CHOm–FGFR-1), and cross-linking. CHOm–FGFR-1 cells (500,000 cells/dish) were incubated with 10 ng/mL125I–FGF-2 with or without PF-4, 50 ng/mL heparin, or 1 μg/mL unlabeled ligand. Binding of 125I–FGF-2 to receptors or cross-linking was performed as indicated in Materials and Methods. (A) Effect of increasing concentrations of PF-4 on high-affinity binding of 125I–FGF-2 to receptors in the absence of heparin. (B) Effect of increasing concentrations of heparin on 125I–FGF-2 binding in the presence (•) or absence (○) of 10 μg/mL PF-4. (C) Effect of increasing concentrations of PF-4 on 125I–FGF-2 binding in the presence or absence of 50 ng/mL heparin. (D) Cross-linking of125I–FGF-2 to receptors in the presence or absence of 10 μg/mL PF-4 or heparin. The differences in the amounts of125I–FGF-2 bound between the different experiments reflect clonal variability in FGFR-1 expression. (A through C) Representative experiments done in duplicates (data points as mean; SD values: [A] 0 < SD < 0.04; [B] 0.01 < SD < 0.3; [C] 0.005 < SD < 0.09).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/9/10.1182_blood.v91.9.3289/3/m_blod40909004w.jpeg?Expires=1770131536&Signature=VTQnnI0u4UkPIuGeJni6xXhDzEIrvf-Trh1bZk-fHrx7ZMB9jHoil~gk29EDrZdFJhuxiG1LaJHRogyzG68Sittd1~MH2GLgOffY~Vd6Yhtpja3gMPH~71Muqdx7~zf76tswn-2EkOqZfqv9oAgkKoRt65zuT2-RS25MthWlx0rl~6eC2eQLaWej7~lYPbXnQ7TAGbSbAdzlmhJZdVKmq1u~eLBa17Fn3RVvbPtV3YX-qw81axqrH-DA~LMW~p9hhns9NBE6ziKbDCoD~OXF~2BF2sLMszTW6RFr-4xXurE~l2QBBjEBe63NO~vu1ifH~2n0oQeW~IaIUZkJNMZnqw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)