Abstract
Granule major basic protein (MBP) is expressed exclusively in eosinophils, basophils, and placental trophoblasts. To identify thecis-elements and transcription factors involved in regulating MBP expression, we subcloned 3.2 kb of sequence upstream of the exon 9 transcriptional start site (P2 promoter) and serial 5′ deletions into the pXP2 luciferase reporter vector. An 80% decrement in promoter activity was obtained when MBP sequences between bp −117 to −67 were deleted. To identify transcription factors that bind to and transactivate through the bp −117 to −67 region, we first compared the upstream genomic sequences of human and murine MBP; a potential GATA binding consensus site was conserved in the 50-bp region between the two genes. To determine which GATA proteins bind this consensus site, we performed electrophoretic mobility shift assays (EMSAs), which showed that both GATA-1 and GATA-2 can bind to this consensus site. To determine the functionality of this site, we tested whether GATA-1 and GATA-2, either individually or in combination, can transactivate the MBP promoter in the Jurkat T cell line. Cotransfection with a GATA-1 expression vector produced 20-fold augmentation of MBP promoter activity, whereas GATA-2 had no activity. In contrast, combined cotransfection of GATA-1 and GATA-2 decreased the ability of GATA-1 to transactivate the MBP promoter by approximately 50%. Our results provide the first evidence for a GATA-1 target gene in eosinophils, a negative regulatory role for GATA-2 in MBP expression, and possibly eosinophil gene transcription in general during myelopoiesis.
EOSINOPHILS, primarily tissue-dwelling granulocytes that constitute 1% to 5% of normal circulating leukocytes, play an important cytotoxic effector role in host immune defenses against multicellular helminth parasites and in immune responses to certain malignancies. Eosinophils are recognized to be potent inflammatory effector cells that contribute to the pathogenesis of a wide variety of allergic diseases and idiopathic syndromes, especially asthma and the hypereosinophilic syndrome. The recognition of eosinophil involvement in the inflammatory pathogenesis of allergic, parasitic, and certain neoplastic diseases has generated considerable interest in characterizing the molecular basis for the commitment and differentiation of myeloid progenitors to the eosinophil lineage in the greater context of myeloid and hematopoietic development.
Eosinophil differentiation from hematopoietic stem cells and myeloid progenitors is regulated primarily by cytokines interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-5.1-3 Although IL-3 and GM-CSF each allow proliferation of eosinophil progenitors, IL-5 is specifically involved in their terminal differentiation, functional activation, and prolonged survival in tissues.4 IL-5 has been shown to support the proliferation and terminal differentiation of eosinophilic precursors in vitro in clonal cell culture assays and to activate mature eosinophils for enhanced effector functions.3 Furthermore, IL-5 has been shown to play a major role in eosinophilopoiesis in vivo.5-7 Although IL-3 and GM-CSF participate in the proliferation and differentiation of stem cells to the multipotential myeloid and eosinophil progenitor pools, and homeostatic levels of eosinophil differentiation in mice, IL-5 is an eosinophil-specific cytokine that is required for both the terminal differentiation and amplification of eosinophil development as well as eosinophilic inflammatory responses to proceed.8
The molecular basis for the commitment of myeloid progenitors to the eosinophil lineage has not been determined. It is generally believed that specific combinations of transcription factors determine the lineage fate of hematopoietic progenitors.9 Promoter regions of genes expressed specifically in immature myeloid cells have been shown to be regulated by a number of factors including PEBP2/CBFs, C/EBPs, PU.1, and c-Myb.10-12 Furthermore, the synergy in transcription of promoters activated by GATA-1, Sp1, EKLF (erythroid Krüppel-like factor), SCL/tal-1, and NF-E2 plays an important role in regulating activity of erythroid promoters.13-16 These insights and understanding have been obtained through detailed analyses of the promoters for genes expressed selectively in the myeloid or erythroid lineages.
Eosinophils contain a number of enzymatic and nonenzymatic inflammatory and cytotoxic mediators in their secondary (specific) granules. These highly cationic proteins include the ribonucleases eosinophil-derived neurotoxin (EDN, RNS2), eosinophil cationic protein (ECP, RNS3), eosinophil peroxidase (EPO, the eosinophil counterpart of neutrophil myeloperoxidase), and the granule major basic protein (MBP). In addition, the Charcot-Leyden crystal (CLC) protein (eosinophil lysophospholipase/galectin-10) is a major cytosolic, granule, and nuclear protein. We and others have examined the 5′ promoter regions of these granule protein genes for consensus transcription factor binding sites that could be relevant to their coordinate expression during eosinophil differentiation and granulogenesis. We have previously shown increased expression of mRNA for the GATA-binding proteins GATA-1, -2, and -3 during eosinophil differentiation of myeloid leukemic cell lines and constitutive expression in mature blood eosinophils and basophils.17 Based on these findings, we hypothesized that GATA-binding proteins play an important role in eosinophil gene regulation, differentiation, and maturation. However, functional characterization of the promoters for the genes encoding EPO,18 CLC,19 20 and EDN21,22 has thus far failed to provide direct evidence for transcriptional regulation by GATA-binding proteins.
To identify transcription factors involved in regulating the commitment and differentiation of hematopoietic progenitors toward the eosinophilic lineage, and the coordinate expression of genes encoding specific granule proteins in this process, we have analyzed thecis-acting element and DNA-binding proteins that may regulate the expression of gene encoding MBP. Our findings indicate a major positive regulatory role for GATA-1 and negative regulatory role for GATA-2 in MBP gene transcription, providing the first evidence for a GATA-regulated target gene in the eosinophil lineage.
MATERIALS AND METHODS
Cell cultures.
The eosinophil-committed subline of the HL-60 promyelocytic leukemic cell line, HL-60-C15 (a gift of Dr Steven Fishkoff, Lederle Laboratory, New York), was maintained in RPMI 1640 supplemented with 10% fetal bovine serum (FBS; JRH Biosciences, Lenexa, KS), 2 mmol/L L-glutamine, and passaged twice weekly.23 HL-60-C15 cells (2 × 105 cells/mL) were induced with 0.5 mmol/L n-butyrate (Sigma) for 48 hours as previously described.17Another eosinophil-committed cell line (YJ-11), established by Yuji Yamaguchi (manuscript submitted), was also maintained in RPMI 1640 supplemented with 8% FBS. Other myeloid and nonmyeloid cell lines used in these studies including the basophilic KU812, myelomonocytic U937, T-lymphocytic Jurkat, and the trophoblast cell lines, BeWo, NUC-1, Jeg-3, and Jar (a gift of Dr Shigeru Saito, Department of Obstetrics and Gynecology, Nara Medical University), were maintained in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% FBS.
Nuclear run-on transcription assay.
Nuclear run-on assays were performed as previously described.24 The following DNAs were used to prepare slot blots: a 0.85-kb fragment containing the complete coding region of MBP in pUC12; a full-length cDNA of c-myc in pcDNAIneo; a 1.6-kb fragment of human CD11b cDNA; a full-length cDNA of human myeloperoxidase (MPO) in pcDNAIneo; a full-length cDNA of human β-actin in pBluescriptII KS(−); a 1.9-kb EcoRI/SalI fragment containing the human 18S rRNA cDNA in pBR322 (kindly provided by Dr Howard A. Young, Laboratory of Molecular Immunoregulation, NCI, Bethesda, MD); and pBR322 vector alone. Autoradiograms were exposed at −80°C with an intensifying screen.
Northern blot analysis.
Total RNA was prepared from uninduced and butyrate-induced HL-60-C15 cells by the guanidium isothiocyanate method.25 The RNA (15 to 20 μg/lane) was denatured in formamide-formaldehyde and electrophoresed in 1% agarose-formaldehyde gels. The RNA was then transferred to Hybond-N nylon membranes (Amersham International plc, Buckinghamshire, UK) and the blots probed sequentially with full-length cDNA of MBP26 and 18S rRNA27 cDNA for internal control of RNA loading. Hybridization with the random-primed DNA probes was performed at 42°C in 50% formamide, 6× standard saline citrate (SSC), 0.2% Ficoll-polyvinylpyrrolidone (PVP), and 0.1% sodium dodecyl sulfate (SDS). Filters were washed twice in 2× SSC with 0.2% SDS at 53°C for 30 minutes and twice in 0.2× SSC with 0.2% SDS at 55°C for 30 minutes. Autoradiography was performed at −80°C with Kodak XAR-5 film (Eastman Kodak, Rochester, NY). The filter was then stripped and rehybridized to an 18S rRNA probe.
Primer extension.
Total RNA was extracted from HL-60-C15 cells by homogenizing the cells in 4 mol/L guanidine isothiocyanate followed by sedimentation through 5.35 mol/L CsCl. Poly(A)+ RNA was separated by Oligotex-dT30 (Takara Shuzo, Kyoto, Japan) and digested with RNAse-free DNAse I for 30 minutes at 37°C. The primer extension followed the method of Kong et al28 and Li et al.29 Briefly, DNAse-free RNA samples were annealed with the primer 5′-CCACCCAGAGACCTTCCTGG-3′ by heating to 70°C and cooling slowly to 50°C. Primer extension was performed at 50°C for 15 minutes in a final volume of 40 μL of 50 mmol/L Tris (pH 8.0), 50 mmol/L KCl, 5 mmol/L MgCl2, 5 mmol/L dithiothreitol (DTT), 52.5 ng/mL bovine serum albumin (BSA), 7 U/mL RNAsin, 0.03 mmol/L each of dATP/dGTP/dTTP, 0.03 mmol/L [α-32P] dCTP, and 10 U of AMV reverse transcriptase. A further 10 U of reverse transcriptase was added and the reaction was continued for another 15 minutes. After phenol/chloroform extraction and ethanol precipitation, the primer extension product was redissolved in water plus sequencing stop solution and electrophoresed onto a 6% polyacrylamide/8 mol/L urea gel. The gel was fixed, dried, and exposed to x-ray film.
Plasmids for transient transfections.
The promoterless luciferase plasmid pXP2 was used for all promoter studies. A 3.2-kb BamHI/BglII human MBP genomic fragment was filled in with Klenow enzyme and subcloned into theSma I site of the pXP2 promoterless luciferase plasmid. The resulting construct contained approximately 3.2 kb of 5′-flanking DNA and extended 3′ to bp +47. Unidirectional deletions of the 3.2-kb/MBP-luciferase construct were prepared using Exonuclease III as described.30 Dideoxysequencing using Sequenase (Amersham International plc) was performed to identify the positions of the Exonuclease III (Promega, Madison, WI) end points.
Expression vectors for human GATA-1 and human GATA-2 under control of the elongation factor-1α promoter, pEF-GATA1 and pEF-GATA2, were prepared by cloning the human GATA-1 or GATA-2 cDNAs inserted into pEF-BSSHII, a modified version of the expression vector pEF-BOS.31 Plasmids expressing murine C/EBPα, human C/EBPβ, and murine C/EBPδ under control of the human elongation factor-1α promoter, pEF-mC/EBPα, pEF-hC/EBPβ, and pEF-mC/EBPδ, respectively, were described previously.32
Transient transfections.
DNA was prepared and cells transfected by electroporation as previously described using 1.5 × 107 cells in 500 μL with minor modification.18 Briefly, the HL-60-C15 cells were electroporated at 280 V, 960 μF, and the YJ, KU812, Jurkat, HeLa, Raji, and U937 cell lines at 300, 280, 250, 150, 280, and 300 V, 960 μF, respectively, conditions previously optimized for these lines. Luciferase activity in cell lysates prepared 4 to 6 hours posttransfection was measured as relative light units (RLU) using a Lumat LB9501 luminometer (Berthold GmbH & Co KG, Bad Wildbad, Germany). Cell extracts were prepared in 500 μL of 1% Triton X-100, 25 mmol/L Gly-gly, 15 mmol/L MgSO4, 4 mmol/L EGTA, 1 mmol/L dithiothreitol (DTT), and 100-μL aliquots of these extracts (equivalent to 1.5 × 106 cells) were analyzed for luciferase activity. Cotransfection with a cytomegalovirus-human growth hormone (pCMV-hGH) plasmid or CMV-βGal plasmid was used for normalization of transfection efficiency among the different cell lines, different plasmid DNA preparations, and individual transfection experiments. Growth hormone production or β-galactosidase activity was measured by a commercially available radioimmunoassay kit (Nichols Institute Diagnostics, San Juan Capistrano, CA) or β-galactosidase enzyme assay kit (Promega Co, Madison, WI), respectively. Individual transfection experiments were repeated at least three times, and the results are shown as mean RLU/ng of hGH/mL or RLU/mU of β-galactosidase/mL (±SEM).
Electrophoretic mobility shift assay (EMSA).
COS cells transfected with the GATA-1 or GATA-2 expression vectors, pEF-GATA1 and pEF-GATA2, were obtained 24 hours after electroporation with these plasmids. Nuclear extracts were prepared as described33 and protein concentrations were determined using the Bradford assay (BioRad, Richmond, CA). Nuclear extracts (8 to 12 μg) were incubated with radiolabeled oligonucleotides (0.1 to 1 ng) and then subjected to electrophoresis as described previously.18 An anti–GATA-1 monoclonal antibody (MoAb) for supershift analysis was obtained commercially (Santa Cruz Biotechnology, Santa Cruz, CA). Reactions were electrophoresed at 14 V/cm on 6% polyacrylamide gels cast in 0.5× TBE at 4°C. EMSAs were performed with the following oligonucleotides (a mutated GATA consensus site is underlined): −93/−58 bp (WT) (5′-AAGTGATGAAATGGTCCTTATCAGCCTTGCTATCTC-3′), −93/−58 bpΔ GATA (5′-AAGTGATGAAATGGTCCGGCGACGCCTTGCTATCTC-3′).
Reverse transcription-polymerase chain reaction (RT-PCR).
We used two oligonucleotide primers for GATA-1 (A; 5′-GGAGCCCTCTCAGCTCAGC-3′, B; 5′-GCCACCAGCTGGTCCTTCAG-3′)17and human β-actin oligonucleotides (C; 5′-GTCGTCGACAACGGCTCCGG-3′, D; 5′-AAGGTGTGGTGCCAGATTTTCTCCA-3′)34 as the internal control. Primers were synthesized using a DNA synthesizer (Model 381A; Applied Biosystems Inc, Foster, CA). The synthesized length of cDNA fragment for human GATA-1 and human β-actin was 470 bp and 220 bp, respectively. The isolated RNA was reverse-transcribed in a total volume of 20 μL in buffer containing 50 mmol/L Tris-HCl (pH 8.3), 75 mmol/L KCl, 3 mmol/L MgCl2, 10 mmol/L DTT, a 10 mol/L dNTP mixture, 100 pmol random hexamer oligonucleotides (Promega, Madison, WI), and 7.5 U of AMV reverse transcriptase (Promega). Double-stranded DNA was then synthesized from the cDNA with 1 U of Thermus aquaticus (Taq) polymerase (Promega) and two pairs of oligonucleotides (human GATA-1 and human β-actin), using 25 PCR cycles on a Perkin-Elmer Cetus PCR 1000 Thermocycler (Perkin-Elmer-Cetus, Norwalk, CT). Each cycle included denaturation at 94°C for 1 minute, reannealing of the primers and fragment at 55°C for 2 minutes, and polymerization at 72°C for 2 minutes.
Southern blot analysis of PCR amplified human GATA-1.
A portion of each sample was separated by electrophoresis on a 5% polyacrylamide gel, then transferred to a Hybond-N membrane (Amersham). Samples immobilized on the membrane were hybridized to a32P-labeled probe of human GATA-1 or human β-actin cDNA at 42°C in 50% formamide, 6× SSC, 0.2% Ficoll-PVP, 0.1% SDS. The filter was washed twice in 2× SSC containing 0.2% SDS at 55°C for 30 minutes and 0.2× SSC with 0.2% SDS at 57°C for 60 minutes, then autoradiographed for 12 hours using an intensifying screen.
RESULTS
Expression of mRNA encoding MBP is transcriptionally upregulated during eosinophilic differentiation of HL-60-C15 cells.
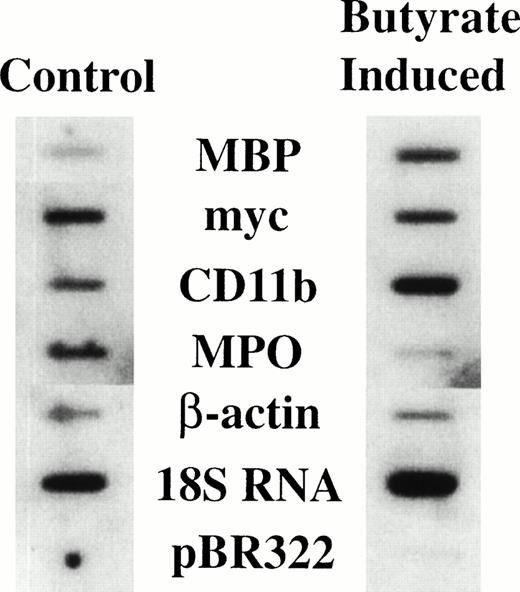
It has previously been shown that treatment of HL-60-C15 cells with n-butyrate induces eosinophilic differentiation and granulation of the cell line,17 with a concomitant increase in steady-state levels of mRNAs encoding all the major eosinophil granule cationic proteins (ECP, EDN, EPO, and MBP) and the CLC protein.19For EPO and CLC protein we have shown by nuclear run-on analysis that the increased levels of steady-state mRNA are in part transcriptionally mediated during n-butyrate induction of HL-60-C15 eosinophilic differentiation.18 19 To determine whether MBP expression is likewise transcriptionally regulated during eosinophilic differentiation, nuclear run-on analysis was performed on both uninduced and 48-hour n-butyrate–induced HL-60-C15 cells (Fig 1). The transcription rate for the MBP gene increased fivefold with n-butyrate–induced eosinophilic differentiation over the constitutive rate in uninduced cells. The transcriptional rate of the CD11b gene increased marginally whereas myeloperoxidase (MPO) and myc transcription decreased during eosinophilic differentiation. These results indicate that MBP gene expression is transcriptionally upregulated during eosinophilic differentiation of the HL-60-C15 cell line, in contrast to the downregulation of genes (eg, MPO) specifically expressed during neutrophil and monocyte development.
Nuclear run-on assay of HL-60-C15 cells induced with n-butyrate. Nuclear run-on transcription analysis was performed with nuclei isolated from uninduced and 48-hour n-butyrate (0.5 mmol/L) induced HL-60-C15 cells. Equivalent numbers of counts were hybridized to duplicated filters containing equimolar amounts of the indicated probes.
Nuclear run-on assay of HL-60-C15 cells induced with n-butyrate. Nuclear run-on transcription analysis was performed with nuclei isolated from uninduced and 48-hour n-butyrate (0.5 mmol/L) induced HL-60-C15 cells. Equivalent numbers of counts were hybridized to duplicated filters containing equimolar amounts of the indicated probes.
The P2 promoter in the 5′ region of MBP gene is specific for the eosinophil lineage.
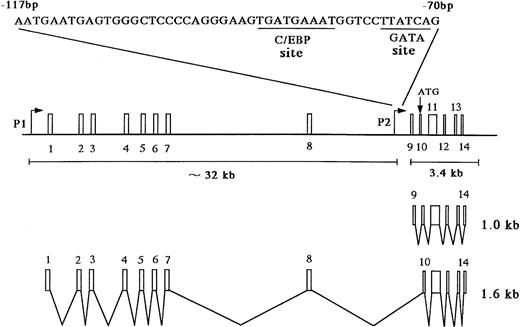
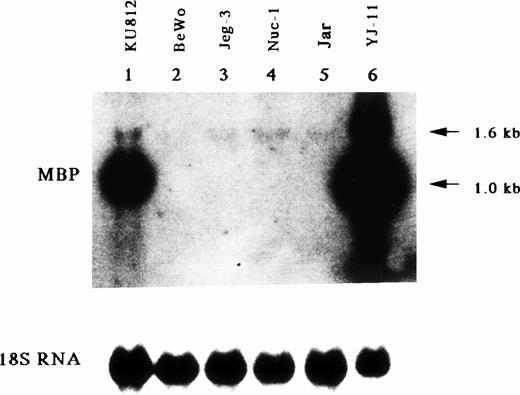
The MBP gene contains a total of 14 exons, including nine upstream exons (exons 1-9) comprising the 5′ untranslated region and five coding exons (exons 10-14) (see Fig 3A).29,35 Two transcripts, major 1.0-kb and minor 1.6-kb messages, are observed in developing and mature eosinophils. Although the 1.6-kb mRNA transcript is derived from exons 1-8 and exons 10-14, the 1.0-kb mRNA transcript is derived from exon 9 and the coding exons, consistent with a previous report (see Fig3A).26 Exons 8 and 10 are separated by a 9-kb, noncoding region containing exon 9, the only upstream exon of the 1.0-kb transcript. Li et al29 have reported that the 1.6 and 1.0 kb mRNAs arise by differential splicing of alternate MBP transcripts from the distal and proximal promoters, respectively, located 32 kb apart in the genomic DNA. The distal promoter that drives expression of the 1.6-kb transcript and the proximal promoter that drives expression of the 1.0-kb transcript have been designated the P1 and P2 promoters, respectively (see Fig 3A).29 Human MBP has been shown to be expressed in both basophils36 and placental cells37 in addition to eosinophils. Therefore, we have determined which promoter, P1 or P2, is specific for the eosinophil and basophil lineages by analyzing expression of the 1.6-kb and 1.0-kb MBP transcripts in eosinophilic, basophilic, and trophoblastic cell lines by Northern blot analysis (Fig 2). In eosinophilic and basophilic cell lines, YJ-11 and KU812, respectively, the major MBP mRNA transcript was 1.0 kb, with only minor expression of the 1.6-kb transcript. In contrast, only the 1.6-kb transcript was observed in all trophoblastic cell lines examined. These results suggest that the P2 promoter driving the 1.0-kb mRNA transcript is specific for the eosinophil and basophil lineages whereas the P1 promoter is specifically used for placental trophoblast lineage expression.
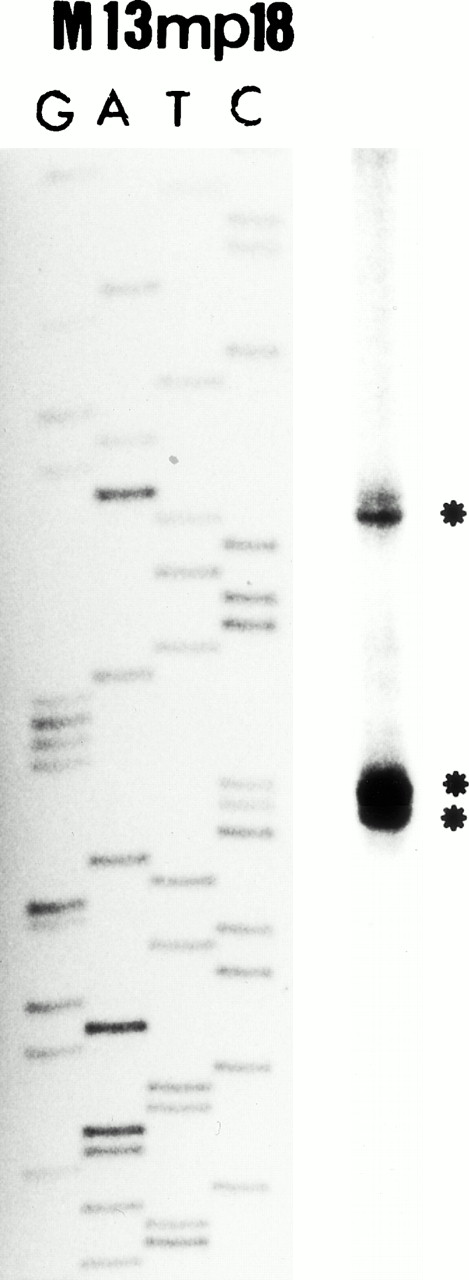
(A) Map of the human MBP gene and the positively acting cis-elements of MBP P2 promoter. The structure of the MBP gene was based on the study of Li et al.29 (B) Identification of the transcriptional start site of the MBP gene (P2). Ten micrograms of poly(A)+ RNA from YJ-11 cells was subjected to primer extension and the resulting radiolabeled DNA product was electrophoresed on a 6% denaturing polyacrylamide gel next to M13mp18 DNA-sequence size markers.
(A) Map of the human MBP gene and the positively acting cis-elements of MBP P2 promoter. The structure of the MBP gene was based on the study of Li et al.29 (B) Identification of the transcriptional start site of the MBP gene (P2). Ten micrograms of poly(A)+ RNA from YJ-11 cells was subjected to primer extension and the resulting radiolabeled DNA product was electrophoresed on a 6% denaturing polyacrylamide gel next to M13mp18 DNA-sequence size markers.
Northern blot analysis for mRNA encoding eosinophil granule MBP in various leukemic cell lines. Lane 1, human basophilic leukemia cell line, KU812; lanes 2 through 5, human choriocarcinoma cells, BeWo (lane 2), Jeg-3 (lane 3), Nuc-1 (lane 4), and Jar (lane 5); lane 6, human eosinophil-committed leukemic cell line, YJ-11. The blot was sequentially probed with an MBP cDNA and an 18S rRNA cDNA probes to control for RNA loading.
Northern blot analysis for mRNA encoding eosinophil granule MBP in various leukemic cell lines. Lane 1, human basophilic leukemia cell line, KU812; lanes 2 through 5, human choriocarcinoma cells, BeWo (lane 2), Jeg-3 (lane 3), Nuc-1 (lane 4), and Jar (lane 5); lane 6, human eosinophil-committed leukemic cell line, YJ-11. The blot was sequentially probed with an MBP cDNA and an 18S rRNA cDNA probes to control for RNA loading.
Identification of the transcriptional start site in the P2 promoter region of MBP gene.
The above results suggested that the 5′ region upstream of exon 9, ie, the putative P2 promoter region, is specific for the eosinophil lineage. Therefore, we have defined the transcriptional start site in the MBP P2 promoter region using primer extension analysis. As shown in Fig 3B, one minor and two major bands (start sites) were detected. We have designated the adenine residue located 78 bp upstream of the end of exon 9, observed as the first major band in the primer extension analysis (Fig 3B), as the major transcriptional start site for the P2 promoter of MBP gene.
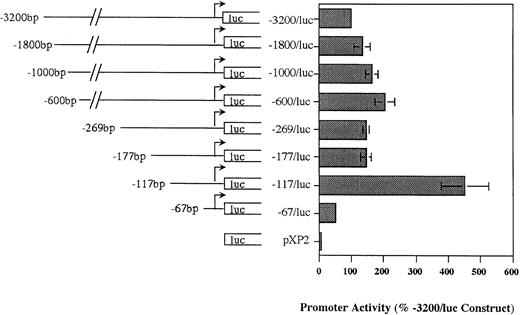
Deletion analysis of the MBP P2 promoter demonstrates the presence of positive and negative regulatory elements.
Our Northern analysis of the MBP transcripts expressed in eosinophil and basophil versus trophoblast cell lines indicated that the putative P2 promoter (region immediately upstream of exon 9) should be specific for the eosinophil and basophil lineages. We analyzed the ability of this region to drive reporter gene (luciferase) activity in HL-60-C15 cells transiently transfected with various MBP P2 promoter constructs in the promoterless pXP2 expression vector to determine whether this region was functionally active and to locate the cis-element(s) required for MBP promoter activity. An MBP genomic fragment containing 3.2 kb of sequence upstream of the exon 9 transcriptional start site was subcloned into the pXP2 luciferase vector. When transiently transfected into HL-60-C15 cells, the −3.2-kb/MBP-luciferase (luc) promoter construct reproducibly expressed greater than 50-fold more luciferase activity than the promoterless pXP2 control. To localize regulatory elements in the MBP P2 promoter, a series of eight deletion mutants were produced from the −3.2-kb/MBP construct using exonuclease III and the PCR. No significant decrease in promoter activity was observed when sequences between −3.2 kb and −177 bp were deleted (Fig 4). Further deletion of the region between bp −177 and −117 resulted in a threefold increase in activity. In contrast, deletion of additional sequence to bp −67 produced a significant, 75% decrease in promoter activity when compared with the bp −117 construct. These results suggest that basal MBP promoter activity is controlled by both negative and positive elements in the bp −177 to −117 and bp −117 to −67 segments of the gene, respectively.
Functional activity of pXP2-MBP (P2) luciferase constructs in the eosinophilic HL-60-C15 cell line. Promoter activities of additional 5′ deletion mutants of the MBP-pXP2 construct in HL-60-C15 cells. Fifteen micrograms of each MBP-pXP2 construct was transfected along with 10 μg of pBluescript II KS(−) as carrier DNA. Luciferase activities have been normalized for the amount of hGH produced by cotransfection with a control CMV-hGH expression vector. Corrected RLU for each construct are shown as the percent activity relative to the mean activity of the longest 3.2-kb MBP (P2) promoter construct. The mean ± SEM for three replicate experiments is shown.
Functional activity of pXP2-MBP (P2) luciferase constructs in the eosinophilic HL-60-C15 cell line. Promoter activities of additional 5′ deletion mutants of the MBP-pXP2 construct in HL-60-C15 cells. Fifteen micrograms of each MBP-pXP2 construct was transfected along with 10 μg of pBluescript II KS(−) as carrier DNA. Luciferase activities have been normalized for the amount of hGH produced by cotransfection with a control CMV-hGH expression vector. Corrected RLU for each construct are shown as the percent activity relative to the mean activity of the longest 3.2-kb MBP (P2) promoter construct. The mean ± SEM for three replicate experiments is shown.
Lineage-specificity of the MBP P2 versus P1 promoters.
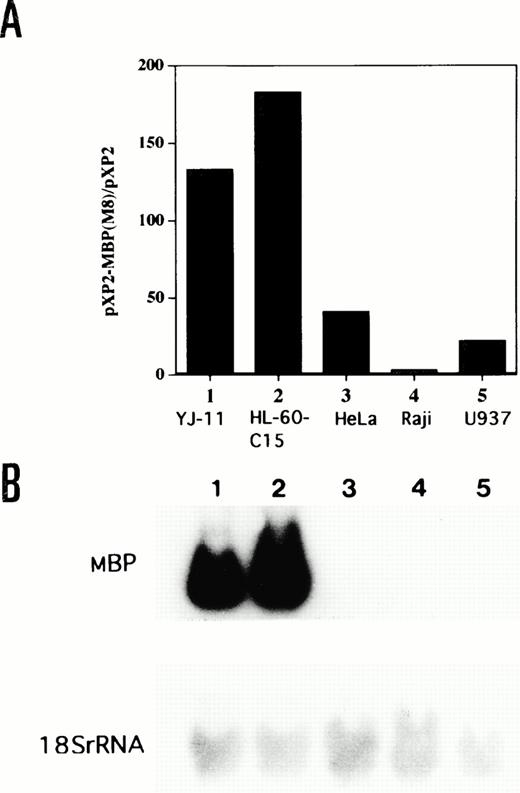
The lineage specificity of the MBP P2 promoter was assessed by transfecting the −117 bp/MBP-pXP2 construct into eosinophilic (YJ-11 and HL-60-C15), nonhematopoietic (HeLa), lymphoid (Raji), and myelomonocytic (U937) cell lines (Fig 5A). As shown by Northern blot analysis in Fig 5B, MBP mRNA expression was observed in YJ-11 and HL-60-C15 cells, but not in HeLa, Raji, and U937 cells. The MBP promoter was approximately 4 to 7 times less active in HeLa, Raji, and U937 cells than in HL-60-C15 cells (Fig 5A). To compare the specificity and activity of the P2 versus P1 MBP promoters in the eosinophil lineage, we also prepared a pXP2 construct containing the MBP P1 promoter (MBP-P1-pXP2). An MBP genomic fragment containing 285 bp of sequence upstream of the transcriptional start site for exon 1 was subcloned into the promoterless pXP2 vector. The MBP-P1-pXP2 construct was essentially inactive compared with the pXP2 control in transient transfections of the YJ-11 and HL-60-C15 eosinophil cell lines (data not shown). These results suggest that the MBP P1 promoter is inactive in the eosinophil lineage and that usage of the P2 promoter predominates for MBP expression during eosinophil development.
(A) Comparative activity of the MBP P2 promoter in eosinophil (YJ-11, HL-60-C15), myelomonocytic (U937), lymphoid (Raji), and nonhematopoietic (HeLa) cell lines. Fifteen micrograms of the −117/MBP-luc promoter construct was transfected, along with 10 μg of pBluescript II KS(−) as carrier DNA, into each cell line. Promoter (luciferase) activities of the −117/MBP-luc construct were normalized based on the measurement of growth hormone expression from a control cotransfected CMV-hGH expression vector in the various cell lines. (B) Northern blot analysis for mRNA encoding eosinophil granule MBP in the various cell lines. Fifteen micrograms of total RNA isolated from the indicated human cell lines was hybridized to the MBP cDNA. Lane 1, YJ-11; lane 2, HL-60-C15; lane 3, HeLa; lane 4, Raji; and lane 5, U937. Hybridization of the same filter to a 18S rRNA probe is shown as control for amounts of RNA.
(A) Comparative activity of the MBP P2 promoter in eosinophil (YJ-11, HL-60-C15), myelomonocytic (U937), lymphoid (Raji), and nonhematopoietic (HeLa) cell lines. Fifteen micrograms of the −117/MBP-luc promoter construct was transfected, along with 10 μg of pBluescript II KS(−) as carrier DNA, into each cell line. Promoter (luciferase) activities of the −117/MBP-luc construct were normalized based on the measurement of growth hormone expression from a control cotransfected CMV-hGH expression vector in the various cell lines. (B) Northern blot analysis for mRNA encoding eosinophil granule MBP in the various cell lines. Fifteen micrograms of total RNA isolated from the indicated human cell lines was hybridized to the MBP cDNA. Lane 1, YJ-11; lane 2, HL-60-C15; lane 3, HeLa; lane 4, Raji; and lane 5, U937. Hybridization of the same filter to a 18S rRNA probe is shown as control for amounts of RNA.
GATA-1 and GATA-2 bind to a GATA consensus site in the MBP P2 promoter.
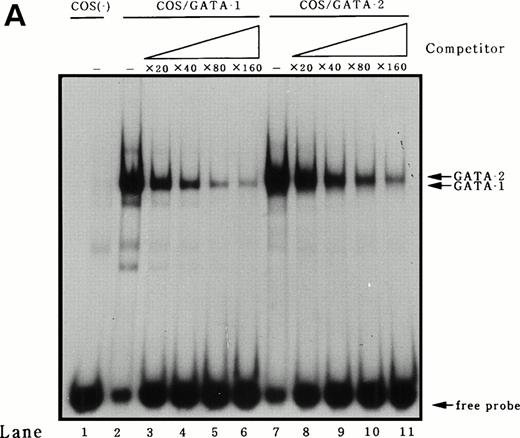
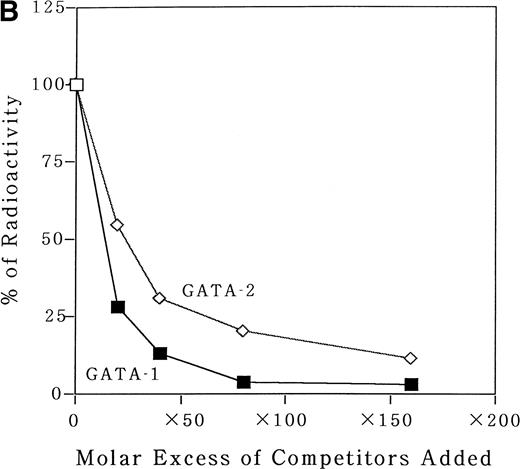
To identify transcription factors that bind to and transactivate the 50-bp positively acting region from bp −117 to −67, we first compared the upstream genomic sequences for human35 and murine MBP.38 A potential GATA binding consensus site was conserved in this region in both genes (Fig 3A). It has been reported that both GATA-1 and GATA-2 play crucial roles in the differentiation of myeloid cells.9 To address the question of whether GATA-1 or GATA-2 binds to the potential GATA-binding site in the MBP P2 promoter, EMSAs were performed. An EMSA with nuclear extracts from COS-7 cells which were transiently transfected with either GATA-1 or GATA-2 expression vectors and those from a human eosinophil-committed leukemia cell line, YJ-11, and an oligonucleotide spanning the MBP P2 promoter from position bp −93 to −58, showed specific complex formation for GATA-1 or GATA-2 (Fig6A and B, lanes 2 and 6). The DNA-protein complex formed by the nuclear extracts YJ cells with the oligonucleotides containing GATA-binding site of the MBP P2 promoter represents GATA-1 or GATA-2 proteins (Fig 6A, lane 5). Formation of the GATA-1 or GATA-2 complexes could be blocked by an excess of the unlabeled “self” oligonucleotides (Fig 6A, lanes 2, 4, and 6; and Fig 6B, lanes 3 and 7), but was not blocked by a mutated GATA consensus site oligonucleotide (Fig 6B, lanes 4 and 8). Furthermore, a small but definite supershift of the GATA-1 complex was obtained by the addition of an anti–GATA-1 antibody to the EMSA binding reaction (Fig 6B, lane 5). To determine whether or not there was a difference in the binding affinities of GATA-1 and GATA-2 for the MBP GATA consensus site, competition experiments were performed using a labeled oligonucleotide from bp −93 to −58 and nuclear extracts from COS-7 cells transfected with the GATA-1 or GATA-2 expression vectors. Unlabeled oligonucleotide competitors were added in increasing molar amounts to the binding reactions. These competitive DNA binding studies showed that the binding of the oligonucleotide with GATA-1 was more efficiently competed than that of GATA-2 (Fig7A). Quantitation of the competition efficiency indicated that the binding affinity of GATA-1 for the GATA consensus site in the MBP P2 promoter was approximately twofold greater than that of GATA-2 (Fig 7B).
Identification of GATA-1 or GATA-2 binding to the MBP promoter by gel-shift assay. (A) A double-stranded MBP promoter oligonucleotide extending from position −93 to −58 bp was end-labeled with [γ-32P] ATP and incubated with 1 μg of double-stranded poly(dI-dC) in the presence of 12 μg nuclear proteins from COS 7 cells which were transiently transfected with GATA-1 (lanes 1 and 2) or GATA-2 (lanes 3 and 4) expression vectors, and in the presence of 12 μg nuclear extracts from YJ-11 cells (lanes 5 and 6). A 50-fold molar excess of each unlabeled oligonucleotide (Competitors), identical to the probe in the binding reaction, was added (lanes 2, 4, and 6). The arrow represents GATA-1– or GATA-2–specific bands, which are inhibited by unlabeled oligonucleotides spanning bp −93 to −58 of MBP P2 promoter region. (B) A double-stranded MBP promoter oligonucleotide extending from position −93 to −58 bp was end labeled with [γ-32P] ATP and incubated with 1 μg of double-stranded poly(dI-dC) in the presence of 8 μg nuclear proteins from COS 7 cells (lane 1), those from COS 7 cells which were transiently transfected with GATA-1 (lanes 2 through 5), or GATA-2 (lanes 6 through 8) expression vectors. The following unlabeled double-stranded competitor oligonucleotides were added at a 100-fold molar excess over the probe oligonucleotide: MBP bp −93 to −58 (lanes 3 and 7) and MBP mutated GATA-consensus site oligonucleotide (lanes 4 and 8). In lane 5, anti–GATA-1 antibody was added to the reaction mixture.
Identification of GATA-1 or GATA-2 binding to the MBP promoter by gel-shift assay. (A) A double-stranded MBP promoter oligonucleotide extending from position −93 to −58 bp was end-labeled with [γ-32P] ATP and incubated with 1 μg of double-stranded poly(dI-dC) in the presence of 12 μg nuclear proteins from COS 7 cells which were transiently transfected with GATA-1 (lanes 1 and 2) or GATA-2 (lanes 3 and 4) expression vectors, and in the presence of 12 μg nuclear extracts from YJ-11 cells (lanes 5 and 6). A 50-fold molar excess of each unlabeled oligonucleotide (Competitors), identical to the probe in the binding reaction, was added (lanes 2, 4, and 6). The arrow represents GATA-1– or GATA-2–specific bands, which are inhibited by unlabeled oligonucleotides spanning bp −93 to −58 of MBP P2 promoter region. (B) A double-stranded MBP promoter oligonucleotide extending from position −93 to −58 bp was end labeled with [γ-32P] ATP and incubated with 1 μg of double-stranded poly(dI-dC) in the presence of 8 μg nuclear proteins from COS 7 cells (lane 1), those from COS 7 cells which were transiently transfected with GATA-1 (lanes 2 through 5), or GATA-2 (lanes 6 through 8) expression vectors. The following unlabeled double-stranded competitor oligonucleotides were added at a 100-fold molar excess over the probe oligonucleotide: MBP bp −93 to −58 (lanes 3 and 7) and MBP mutated GATA-consensus site oligonucleotide (lanes 4 and 8). In lane 5, anti–GATA-1 antibody was added to the reaction mixture.
Analysis for the binding affinity of GATA-1 and GATA-2 to the GATA consensus site in the MBP promoter by EMSA. (A) Competitor with the unlabeled oligonucleotides was added using increasing amounts of 0 to 160-fold molar excess as indicated. A double-stranded MBP promoter oligonucleotide extending from bp −93 to −58 was end-labeled and incubated with 1 μg of poly (dI-dC) in the absence of nuclear extract (lane 1) or in the presence of 8 μg of nuclear extracts from COS 7 cells which was transfected with GATA-1 (lanes 2 through 6) or GATA-2 (lanes 7 through 11) expression vectors. (B) Quantitation of the competition efficiency of the MBP promoter oligonucleotide for GATA-1 or GATA-2 binding. The radioactivity of each band was quantitated with a BAS-2000II radioanalysis imaging system (Fujix, Tokyo, Japan).
Analysis for the binding affinity of GATA-1 and GATA-2 to the GATA consensus site in the MBP promoter by EMSA. (A) Competitor with the unlabeled oligonucleotides was added using increasing amounts of 0 to 160-fold molar excess as indicated. A double-stranded MBP promoter oligonucleotide extending from bp −93 to −58 was end-labeled and incubated with 1 μg of poly (dI-dC) in the absence of nuclear extract (lane 1) or in the presence of 8 μg of nuclear extracts from COS 7 cells which was transfected with GATA-1 (lanes 2 through 6) or GATA-2 (lanes 7 through 11) expression vectors. (B) Quantitation of the competition efficiency of the MBP promoter oligonucleotide for GATA-1 or GATA-2 binding. The radioactivity of each band was quantitated with a BAS-2000II radioanalysis imaging system (Fujix, Tokyo, Japan).
GATA-1, but not GATA-2, transactivates the MBP P2 promoter.
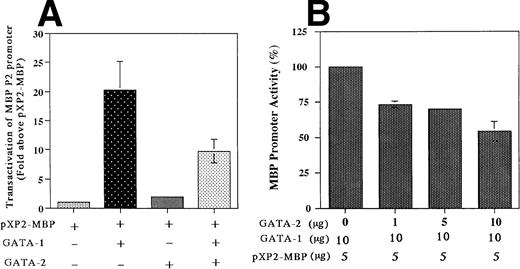
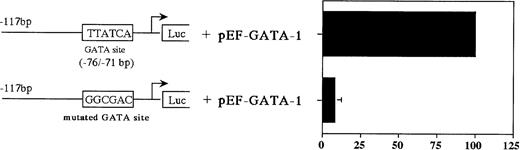
The results described above indicated that both GATA-1 and GATA-2 are capable of binding to the GATA consensus site (bp −76 to −71), albeit with somewhat different affinities. To determine whether this GATA binding site is functional, we analyzed the ability of GATA-1 and GATA-2, either individually or in combination, to transactivate the MBP P2 promoter in the GATA-1 and GATA-2 negative Jurkat cell line. Cotransfection with the GATA-1 expression vector alone produced a 20-fold increase in MBP P2 promoter activity, whereas GATA-2 alone had no effect, but did decrease GATA-1 transactivation by 50% in the combined cotransfection (Fig 8A). In addition, the combined cotransfection of GATA-2 along with GATA-1 decreased the ability of GATA-1 to transactivate the MBP promoter in a dose-dependent fashion (Fig 8B). These findings indicate that GATA-2 acts as a competitive inhibitor for GATA-1 in the transactivation of the MBP promoter. Constructs containing the intact MBP P2 promoter with the GATA motif (bp −76 to −71), and a mutated promoter with a 6-bp linker substitution for the GATA-binding site (bp −76 to −71), were cotransfected into the GATA-1 negative Jurkat cell line along with the human GATA-1 expression vector pEF-GATA-1 (Fig9). Mutation of the GATA-binding site in the context of the most active bp −117/pXP2 MBP P2 promoter construct resulted in a 90% loss of promoter activity in response to GATA-1 transactivation (Fig 9). These results show the functional importance of GATA-1 in regulating MBP promoter activity and likely MBP gene expression.
Transactivation of the MBP P2 promoter by GATA-1 and GATA-2 (A) and competitive inhibition by GATA-2 (B). Transactivation of the MBP P2 promoter (bp −117/MBP-pXP2) in Jurkat cells, in which neither GATA-1 nor GATA-2 transcription factors are expressed. The T-lymphocytic Jurkat cell line was transfected by the electroporation method with 15 μg of the MBP P2 promoter construct −117 MBP-luc along with one of the following expression constructs: pXP2-MBP (control), 20 μg of pEF-BOS (the control plasmid containing elongation factor promoter without cDNAs); pXP2-MBP + GATA-1, 10 μg of pEF-hGATA-1 and 10 μg of EF-BOS; pXP2-MBP + GATA-2, 10 μg of pEF-hGATA-2 and 10 μg of pEF-BOS; pXP2-MBP + GATA-1 + GATA-2, 10 μg of pEF-hGATA-1 and 10 μg of pEF-hGATA-2. Luciferase activity was measured 24 hours after transfection and normalized for transfection efficiency based on the activity of a cotransfected β-galactosidase expression vector (CMV-βGAL). Data are shown as the mean of three independent experiments (±SEM).
Transactivation of the MBP P2 promoter by GATA-1 and GATA-2 (A) and competitive inhibition by GATA-2 (B). Transactivation of the MBP P2 promoter (bp −117/MBP-pXP2) in Jurkat cells, in which neither GATA-1 nor GATA-2 transcription factors are expressed. The T-lymphocytic Jurkat cell line was transfected by the electroporation method with 15 μg of the MBP P2 promoter construct −117 MBP-luc along with one of the following expression constructs: pXP2-MBP (control), 20 μg of pEF-BOS (the control plasmid containing elongation factor promoter without cDNAs); pXP2-MBP + GATA-1, 10 μg of pEF-hGATA-1 and 10 μg of EF-BOS; pXP2-MBP + GATA-2, 10 μg of pEF-hGATA-2 and 10 μg of pEF-BOS; pXP2-MBP + GATA-1 + GATA-2, 10 μg of pEF-hGATA-1 and 10 μg of pEF-hGATA-2. Luciferase activity was measured 24 hours after transfection and normalized for transfection efficiency based on the activity of a cotransfected β-galactosidase expression vector (CMV-βGAL). Data are shown as the mean of three independent experiments (±SEM).
Mutations of the GATA-binding sites diminish the MBP P2 promoter activity. Jurkat cells were cotransfected with either pXP2-MBP containing a wild-type GATA-binding site, or pXP2-MBP containing a mutated GATA-binding site, bp −76 to −71, along with 10 μg of pEF-hGATA-1. The error bar represents the SEM for three independent experiments. Luciferase activity was normalized to β-galactosidase activity produced from a cotransfected CMV-βGal plasmid.
Mutations of the GATA-binding sites diminish the MBP P2 promoter activity. Jurkat cells were cotransfected with either pXP2-MBP containing a wild-type GATA-binding site, or pXP2-MBP containing a mutated GATA-binding site, bp −76 to −71, along with 10 μg of pEF-hGATA-1. The error bar represents the SEM for three independent experiments. Luciferase activity was normalized to β-galactosidase activity produced from a cotransfected CMV-βGal plasmid.
Transcriptional activation of the MBP P2 promoter by C/EBP family members.
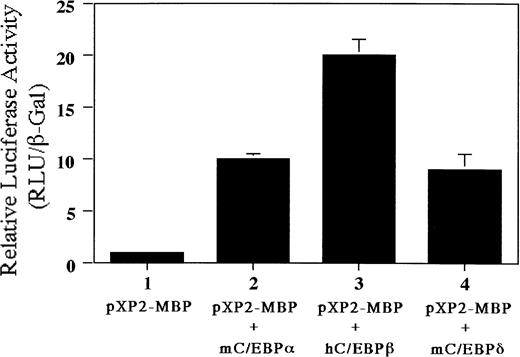
We have identified a C/EBP consensus binding site only 7 bp upstream of the functional GATA-binding site in the MBP P2 promoter. Therefore, we addressed whether C/EBP families (C/EBPα, C/EBPβ, and C/EBPδ) can transactivate the MBP P2 promoter. As shown in Fig10, luciferase activity was increased 10-fold by cotransfecting the plasmids expressing C/EBPα or C/EBPδ, while cotransfection with the C/EBPβ expression vector produced a 20-fold increase. These data indicate that C/EBP family members and GATA-1 may be able to cooperatively activate MBP gene expression.
Transactivation of the MBP P2 promoter by C/EBPα, C/EBPβ, and C/EBPδ. Ten micrograms of pXP2 or pXP2-MBP was transfected into Jurkat cells along with 10 μg of pEF-mC/EBPα, pEF-hC/EBPβ, or pEF-mC/EBPδ. pCMV-βGal was included as an internal control. Luciferase and β-galactosidase activities were assayed 24 hours later. Data are shown as the mean of three independent experiments (±SEM).
Transactivation of the MBP P2 promoter by C/EBPα, C/EBPβ, and C/EBPδ. Ten micrograms of pXP2 or pXP2-MBP was transfected into Jurkat cells along with 10 μg of pEF-mC/EBPα, pEF-hC/EBPβ, or pEF-mC/EBPδ. pCMV-βGal was included as an internal control. Luciferase and β-galactosidase activities were assayed 24 hours later. Data are shown as the mean of three independent experiments (±SEM).
Induction of GATA-1 mRNA expression during IL-5–induced eosinophil differentiation of normal human bone marrow progenitors.
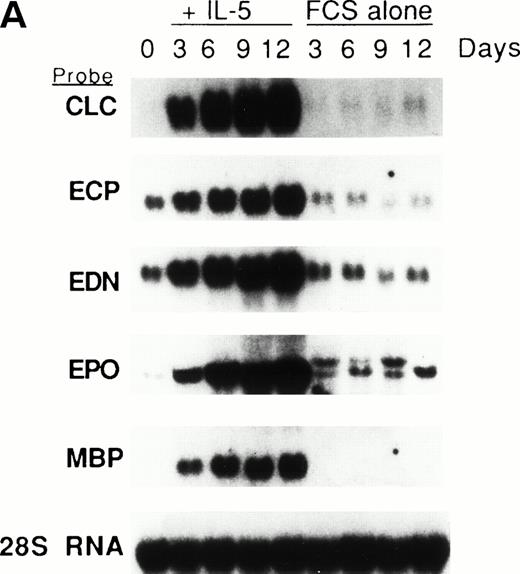
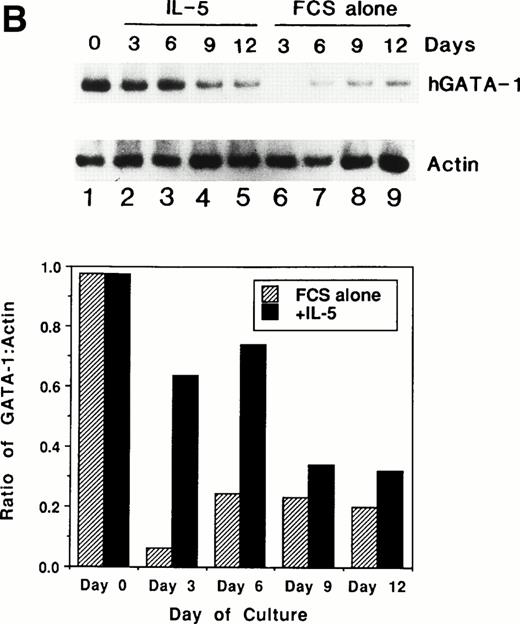
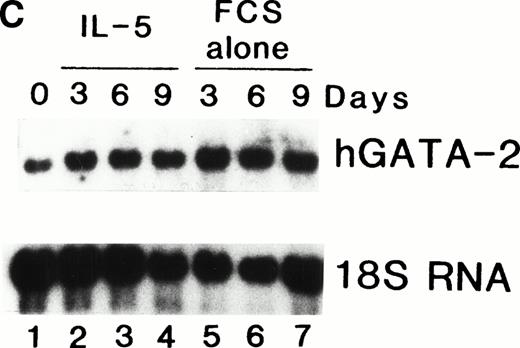
We previously showed that GATA-1 mRNA expression was upregulated in both the HL-60-C15 and HL-60(3 + C5) cell lines when n-butyrate or BCGF-II (as a source of IL-5), respectively, was used to induce eosinophilic differentiation.17 Results from our experiments demonstrating GATA-binding protein transactivation of the MBP P2 promoter suggest that GATA-1 plays an important role in regulating aspects of eosinophil-lineage commitment, differentiation, and gene regulation. To characterize the expression of the GATA-binding proteins during eosinophil development from normal bone marrow (BM)–derived myeloid progenitors, we assessed the steady-state levels of GATA-1 and GATA-2 mRNAs in normal human BM mononuclear cells stimulated to differentiate into eosinophils with IL-5. As shown by Northern blot analysis in Fig 11A,expression of mRNAs for all the major eosinophil granule-associated proteins, including MBP, increased significantly in BM progenitors cultured with IL-5 for 3 days, with a continued increase throughout the culture period. We used this ex vivo system for inducing eosinophil development of myeloid progenitors to analyze the expression of mRNAs encoding the GATA-binding proteins. A semiquantitative RT-PCR method was used for mRNA expression of GATA-1 and a Northern blot analysis was used for that of GATA-2. As shown in Fig 11B, GATA-1 expression decreased markedly from day 0 to day 3 in the absence of IL-5. In contrast, GATA-1 mRNA levels persisted in the presence of IL-5 for the duration of the culture period. These findings suggest that GATA-1 expression is maintained during IL-5–stimulated terminal differentiation of eosinophils from BM myeloid progenitors. On the other hand, GATA-2 mRNA was expressed constitutively in normal human BM mononuclear cells both in the presence and absence of exogenous IL-5 (Fig 11C).
(A) Northern blot analyses of mRNA for eosinophil granule proteins in normal human bone marrow (hBM) cells cultured with IL-5. hBM cells were cultured in the presence or absence of the supernatant (10% vol/vol) from X63Ag8-653-mIL-5 myeloma cells as a source of IL-5. Total RNA was obtained at days 0, 3, 6, 9, and 12 of culture. Each lane contains 8 μg of total RNA. Exposure to x-ray film was for 18 hours. (B) mRNA expression for hGATA-1 in normal hBM cells cultured with IL-5. Southern blot analysis of RT-PCR products amplified from 1 μg of total RNA extracted from hBM cells cultured in the presence or absence of the supernatant from X63Ag8-653-mIL-5 myeloma cells as a source of IL-5. Total RNA was obtained at days 0 (lane 1), 3 (lanes 2 and 6), 6 (lanes 3 and 7), 9 (lanes 4 and 8), and 12 (lanes 5 and 9) of culture. The blot was probed simultaneously with hGATA-1 cDNA and β-actin as an internal control. Each lane contains 1/10th of the amplification products from each time point. (C) Northern blot analysis of mRNA for hGATA-2 in hBM cells stimulated with (IL-5) or without (FCS alone) the supernatant from X63Ag8-653-mIL-5 myeloma cells as a source of IL-5. Total RNA was obtained at days 0, 3, 6, and 9 of culture. Each lane contains 3.5 μg of total RNA.
(A) Northern blot analyses of mRNA for eosinophil granule proteins in normal human bone marrow (hBM) cells cultured with IL-5. hBM cells were cultured in the presence or absence of the supernatant (10% vol/vol) from X63Ag8-653-mIL-5 myeloma cells as a source of IL-5. Total RNA was obtained at days 0, 3, 6, 9, and 12 of culture. Each lane contains 8 μg of total RNA. Exposure to x-ray film was for 18 hours. (B) mRNA expression for hGATA-1 in normal hBM cells cultured with IL-5. Southern blot analysis of RT-PCR products amplified from 1 μg of total RNA extracted from hBM cells cultured in the presence or absence of the supernatant from X63Ag8-653-mIL-5 myeloma cells as a source of IL-5. Total RNA was obtained at days 0 (lane 1), 3 (lanes 2 and 6), 6 (lanes 3 and 7), 9 (lanes 4 and 8), and 12 (lanes 5 and 9) of culture. The blot was probed simultaneously with hGATA-1 cDNA and β-actin as an internal control. Each lane contains 1/10th of the amplification products from each time point. (C) Northern blot analysis of mRNA for hGATA-2 in hBM cells stimulated with (IL-5) or without (FCS alone) the supernatant from X63Ag8-653-mIL-5 myeloma cells as a source of IL-5. Total RNA was obtained at days 0, 3, 6, and 9 of culture. Each lane contains 3.5 μg of total RNA.
DISCUSSION
We have functionally characterized the P2 promoter for the human eosinophil granule MBP gene, which is used exclusively during the differentiation of myeloid progenitors to the eosinophil granulocyte lineage. We have previously reported that the expression of two other eosinophil genes encoding eosinophil peroxidase (EPO) and Charcot-Leyden crystal protein (lysophospholipase/galectin-10) is transcriptionally regulated during butyrate-induced eosinophilic differentiation of HL-60-C15 cells.18,19 As shown in this study, expression of the MBP gene is also transcriptionally regulated during eosinophil differentiation. In addition, expression of the mRNA encoding the eosinophil granule-associated proteins, MBP, EPO, ECP, and EDN, increases along with eosinophil development and then decreases with maturation of the cells.39 These results suggest that transcription factors involved in the coordinate regulation of the eosinophil granule protein genes may be associated with the process of commitment and differentiation of hematopoietic progenitors toward the eosinophilic lineage.
We have demonstrated the capacity of GATA-1 to bind to and transactivate the MBP P2 promoter. These results provide the first evidence for a GATA-1 target gene in the eosinophil lineage. We have previously suggested that gene transcription during eosinophil and basophil hematopoietic development is regulated in part by members of the GATA-binding family of transcription factors.17 In particular, eosinophils, basophils, and eosinophil-differentiated myeloid cell lines were shown to express GATA-1 and GATA-3 in an inducible fashion.17 In this study we have shown that GATA-1 expression is sustained in human BM cells stimulated with IL-5. Although GATA-1 expression induced by the addition of IL-5 in ex vivo system of human BM cells may be a phenomenon in an artificial system, the increased expression of GATA-1 has been observed not only in the BM cells stimulated with IL-5 but also in the cell line capable of differentiating toward the eosinophil lineage (data not shown). Kulessa et al40 have shown that stable clones containing peroxidase-positive granules characteristic of eosinophils develop from Myb-Ets–transformed myeloblasts after transfection with a GATA-1 expression vector. They speculate that lineage determination by GATA-1 depends in part on its intracellular levels. The multipotential cell line 416B with the highest level of GATA-1 expression exhibited megakaryocytic features, whereas cell lines with approximately fourfold lower levels displayed properties consistent with eosinophil differentiation.40 These results suggest that GATA-1 is one of the transcription factors involved in regulating the commitment and differentiation of myeloid progenitors to the eosinophil lineage. Based on previously published reports, the GATA-binding proteins are speculated to be expressed in the various hematopoietic lineages as follows: GATA-1 in erythrocytes, megakaryocytes, mast cells, eosinophils, and basophils; GATA-2 in mast cells, megakaryocytes, erythrocytes, monocytes, neutrophils, eosinophils, and basophils; GATA-3 in T cells, mast cells, and eosinophils; and GATA-4 in an adult T-cell leukemia cell line, ATL-16T.41 As noted above, we have shown that mRNAs for GATA-1, GATA-2, and GATA-3 are expressed in mature eosinophils.17 Our current results indicate that GATA-1 can independently transactivate the MBP P2 promoter, but that GATA-2 has the capacity to compete for GATA-1 binding and to inhibit GATA-1 transactivation by approximately 50%. We have also shown that eosinophilic cell lines and human BM-derived progenitors express mRNAs for GATA-1 and GATA-2, with constitutive expression of GATA-2 and inducible expression of GATA-1 during eosinophil differentiation.17 The hematopoietic expression of GATA-2 in these systems overlaps with that of GATA-1, although GATA-2 is also expressed more widely. During erythroid differentiation, it has been shown that the expression of GATA-2 decreases as GATA-1 expression increases.42 Furthermore, the forced expression of GATA-2 arrests erythroid differentiation.43 These findings suggest that GATA-2 functions as a transdominant negative regulator for hematopoietic myeloid progenitors capable of differentiating toward the eosinophil lineage.
It has been reported that NF-M, the chicken homolog of C/EBPβ, induces eosinophil differentiation in a multipotent progenitor cell line transformed by the Myb-Ets oncoprotein.44 We have identified a C/EBP consensus binding site only 7 bp upstream of the functional GATA-binding site in the MBP P2 promoter, and shown that cotransfection with C/EBPα, C/EBPβ, and C/EBPδ expression vectors effectively transactivates the MBP P2 promoter (Fig 10). These findings suggest that the family of C/EBPs, in particular C/EBPα and β, may also be associated with eosinophil differentiation. Of interest, C/EBPα null (knock-out) mice have recently been shown to have impaired eosinophil development.45 Müller et al44 speculate that the balance between three transcription factors, c-Myb, GATA-1, and C/EBPβ, plays a decisive role in hematopoietic lineage determination. They suggest that in the multipotent precursor cell line (MEP), the expression of GATA-1 in the absence of C/EBPβ results in differentiation toward the thrombocytic and erythroid lineages, the expression of C/EBPβ in the absence of GATA-1 drives differentiation toward the myelomonocytic lineage, whereas the expression of both factors allows differentiation toward the eosinophilic lineage.44 We have obtained results demonstrating expression of both GATA-1 and C/EBPβ concomitant with eosinophilic differentiation of the HT93A cell line, which can differentiate from uncommitted blastic cells toward eosinophil and neutrophil lineages after treatment with retinoic acid (data not shown). From our results to date, we conclude that transcription factors involved in fulfillment of the eosinophilic differentiation program likely include GATA-1, C/EBP(α and β), and c-Myb. Recent studies of mice lacking functional GATA-1, C/EBPβ, and c-Myb genes have shown impaired hematopoietic development of erythroid, mast cell, and certain myeloid lineages.45-47 However, the eosinophil lineage has yet to be evaluated in any of these knockout mice. However, recent analysis of the eosinophil lineage in C/EBPα null mice has demonstrated impaired eosinophil development (complete absence of eosinophils) along with impaired G-CSF signaling and neutrophil development.45 Based on our current findings, we would predict that eosinophil development is impaired wholly or in part in mice also lacking expression of the GATA-1. In the absence of more profound effects on eosinophil development, we would expect that expression of certain key eosinophil genes such as MBP should be lacking in GATA-1 null mice. The regulation of hematopoietic differentiation from lineage commitment through terminal maturation is likely determined by combinations of transcription factors, their cofactors, as well as interactions among the factors. Continued elucidation of the transcriptional mechanisms that regulate eosinophil development and expression of eosinophil granule-derived mediators of inflammation and tissue damage such as MBP may provide new targets for drug development and therapeutic intervention in eosinophil-induced inflammation in allergic diseases.
ACKNOWLEDGMENT
We thank Gerald J. Gleich for providing the MBP genomic gene; Kenji Kishi for a gift of KU812 cells; Shigeru Saito for a gift of trophoblast cell lines; Daniel G. Tenen for providing myc, CD11b, and MPO cDNAs; and Shizuo Akira for providing the human C/EBPβ cDNA.
Supported by Grants-in-Aid from the Ministry of Education, Science and Culture of Japan (to Y.Y.) and from the National Institutes of Health, USA (AI33043) and the W.M. Keck Foundation (to S.J.A.).
Address reprint requests to Yuji Yamaguchi, MD, Department of Cell Differentiation, Institute of Molecular Embryology and Genetics, Kumamoto University School of Medicine, Honjo 2-2-1, Kumamoto, 860 Japan.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.

![Fig. 6. Identification of GATA-1 or GATA-2 binding to the MBP promoter by gel-shift assay. (A) A double-stranded MBP promoter oligonucleotide extending from position −93 to −58 bp was end-labeled with [γ-32P] ATP and incubated with 1 μg of double-stranded poly(dI-dC) in the presence of 12 μg nuclear proteins from COS 7 cells which were transiently transfected with GATA-1 (lanes 1 and 2) or GATA-2 (lanes 3 and 4) expression vectors, and in the presence of 12 μg nuclear extracts from YJ-11 cells (lanes 5 and 6). A 50-fold molar excess of each unlabeled oligonucleotide (Competitors), identical to the probe in the binding reaction, was added (lanes 2, 4, and 6). The arrow represents GATA-1– or GATA-2–specific bands, which are inhibited by unlabeled oligonucleotides spanning bp −93 to −58 of MBP P2 promoter region. (B) A double-stranded MBP promoter oligonucleotide extending from position −93 to −58 bp was end labeled with [γ-32P] ATP and incubated with 1 μg of double-stranded poly(dI-dC) in the presence of 8 μg nuclear proteins from COS 7 cells (lane 1), those from COS 7 cells which were transiently transfected with GATA-1 (lanes 2 through 5), or GATA-2 (lanes 6 through 8) expression vectors. The following unlabeled double-stranded competitor oligonucleotides were added at a 100-fold molar excess over the probe oligonucleotide: MBP bp −93 to −58 (lanes 3 and 7) and MBP mutated GATA-consensus site oligonucleotide (lanes 4 and 8). In lane 5, anti–GATA-1 antibody was added to the reaction mixture.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/9/10.1182_blood.v91.9.3447/3/m_blod40926006aw.jpeg?Expires=1768040995&Signature=X0vCJvjN~Pdbu6Te9GNAWS8M4l3na1I1K1G1X2QNAjkKmu9TLOraGaP6BPbKv~sOcXsfN~1Exw9oQF~E6rA4rOO6TooKMDihSfEhS3sZN8YtTKv5Nun8C3p5bhMIgkk-21uQkvEfSBhK2wQdzZyKt9-DRpvui2qfvqEdKfvB8HfsVD1Zd46MQ-bFSql-Hf7HLCb~dG1x28Y~jVPWD9IiYeyNCwe7lWyg4Q3M0d6tku2ayCon4WmHk3Q0g7PNH-8U3bzhSv4GLPXIsj5zdvE8Zp8dQCM~-dsBOo7tP1cHlju1EJvjHWr34x9MU4ry4kbo2OAN~MOeG~Ke7qxU~O6X1Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 6. Identification of GATA-1 or GATA-2 binding to the MBP promoter by gel-shift assay. (A) A double-stranded MBP promoter oligonucleotide extending from position −93 to −58 bp was end-labeled with [γ-32P] ATP and incubated with 1 μg of double-stranded poly(dI-dC) in the presence of 12 μg nuclear proteins from COS 7 cells which were transiently transfected with GATA-1 (lanes 1 and 2) or GATA-2 (lanes 3 and 4) expression vectors, and in the presence of 12 μg nuclear extracts from YJ-11 cells (lanes 5 and 6). A 50-fold molar excess of each unlabeled oligonucleotide (Competitors), identical to the probe in the binding reaction, was added (lanes 2, 4, and 6). The arrow represents GATA-1– or GATA-2–specific bands, which are inhibited by unlabeled oligonucleotides spanning bp −93 to −58 of MBP P2 promoter region. (B) A double-stranded MBP promoter oligonucleotide extending from position −93 to −58 bp was end labeled with [γ-32P] ATP and incubated with 1 μg of double-stranded poly(dI-dC) in the presence of 8 μg nuclear proteins from COS 7 cells (lane 1), those from COS 7 cells which were transiently transfected with GATA-1 (lanes 2 through 5), or GATA-2 (lanes 6 through 8) expression vectors. The following unlabeled double-stranded competitor oligonucleotides were added at a 100-fold molar excess over the probe oligonucleotide: MBP bp −93 to −58 (lanes 3 and 7) and MBP mutated GATA-consensus site oligonucleotide (lanes 4 and 8). In lane 5, anti–GATA-1 antibody was added to the reaction mixture.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/91/9/10.1182_blood.v91.9.3447/3/m_blod40926006bw.jpeg?Expires=1768040995&Signature=wvRRYOSNTOFuaIHIFvVU5TDTEng97i8v5h2dJoqfVbXCn44Fm8hNDsQd5b9ay1V~06Z83yejXYAZb5XAbf2CYvSgS4O538dA-oVWxV30pO~16HeuLg8U7nddXDgpWLP-nE9doLk834pPohJN-2b1D2vFFoC35WoM7Bn75uBk9twfzN-aLk4eWOmujx4e3qX-8OC8vBb-GnLlSBoD9SzX0fWny9qm8cMonzcE01toki2YDlxiw2gr-091DRSgPaYqTmfgpcoTFPoS0nAtoYANrhmhRCYX8VhExy~aROxvsJd5LVMOefk0MPy63M4XdKqzS1qfZx9bqtfaCocz5T5alg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)