Abstract
An observational study was conducted at 18 transplant centers in the United States and Canada to characterize the platelet recovery of patients receiving myeloablative therapy and stem cell transplantation and to determine the clinical variables influencing recovery, determine platelet utilization and cost, and incidence of hemorrhagic events. The study included 789 evaluable patients transplanted in 1995. Clinical, laboratory, and outcome data were obtained from the medical records. Variables associated with accelerated recovery in multivariate models included (1) higher CD34 count; (2) higher platelet count at the start of myeloablative therapy; (3) graft from an HLA-identical sibling donor; and (4) prior stem cell transplant. Variables associated with delayed recovery were (1) prior radiation therapy; (2) posttransplant fever; (3) hepatic veno-occlusive disease; and (4) use of posttransplant growth factors. Disease type also influenced recovery. Recipients of peripheral blood stem cells (PBSC) had faster recovery and fewer platelet transfusion days than recipients of bone marrow (BM). The estimated average 60-day platelet transfusion cost per patient was $4,000 for autologous PBSC and $11,000 for allogeneic BM transplants. It was found that 11% of all patients had a significant hemorrhagic event during the first 60 days posttransplant, contributing to death in 2% of patients. In conclusion, clinical variables influencing platelet recovery should be considered in the design and interpretation of clinical strategies to accelerate recovery. Enhancing platelet recovery is not likely to have a significant impact on 60-day mortality but could significantly decrease health care costs and potentially improve patient quality of life.
DESPITE RECENT advances in cytokine and stem cell technology, platelet transfusions are still required to support patients after myeloablative therapy and marrow (BM) or peripheral blood stem cell (PBSC) transplantation. Platelet transfusions are associated with morbidity related to transfusion reactions, platelet alloimmunization and transfusion-associated viral infections.1-3 In addition, the costs of platelet transfusion support and management of related complications adds to the costs of transplantation. Finally, prolonged thrombocytopenia may place these patients at increased risk of life-threatening hemorrhage.4 5 Effective strategies to enhance platelet recovery could therefore benefit patients undergoing hematopoietic stem cell transplantation (HSCT).
An understanding of the clinical variables influencing platelet recovery would have an impact on clinical practice, as well as on the design and interpretation of clinical trials examining such strategies. Moreover, an estimation of the magnitude and cost of transfusion utilization, as well as the incidence of hemorrhagic complications associated with prolonged thrombocytopenia, may provide insight into the potential benefits of a strategy to enhance platelet recovery.
To characterize the platelet recovery of patients after HSCT and to identify key predictors of recovery, we conducted an observational study at 18 transplant centers in the United States and Canada. Platelet utilization, transfusion practices and costs, and hemorrhagic events after HSCT were examined.
METHODS AND PATIENTS
Study Design
A multicenter, observational cohort study of platelet recovery and utilization in patients after myeloablative therapy and HSCT was conducted.
Patient Population
Patients undergoing autologous, allogeneic, or syngeneic transplantation with PBSC or BM, or both, were eligible. The target population comprised consecutive patients who received a PBSC or BM transplant, or both, during the 4-month site-specific enrollment period (May 1, 1995 to October 11, 1995).
Study Sample
In this study, 980 consecutive patients were screened. A total of 119 patients were excluded because of (1) use of investigational agents that rendered outcome data proprietary (62 patients), (2) refusal to sign informed consent if so required by their institution (37 patients), and (3) data not adequately collected (20 patients).
Data Collection
Patient medical history as well as clinical, laboratory, and outcomes data were obtained from medical records. Patients were followed and data collected through posttransplant day 60. To determine predictors of recovery, two categories of variables were examined. The first were baseline predictors, those pretreatment variables known before the start of myeloablative therapy. The second were time-dependent predictors, variables occurring after the start of myeloablative therapy. Analyses of platelet utilization and cost were based on transfusion data. In this study, 6 U of random donor platelets were defined as equivalent to a single donor transfusion unit. A platelet transfusion event was defined as a single transfusion. Hemorrhagic events were recorded and their severity graded using Cancer and Leukemia Group B criteria (see Table 6). Posttransplant events such as hepatic veno-occlusive disease (VOD), disseminated intravascular coagulation (DIC), thrombotic microangiopathic hemolytic anemia, and graft-versus-host disease (GVHD) were recorded based on the clinical diagnoses made at the individual centers. GVHD was graded using the Seattle criteria.6
Definitions
Platelet recovery to greater than 20,000/μL was defined as the day when the first of two consecutive platelet counts satisfied the following conditions: (1) both platelet counts were greater than 20,000/μL; (2) no platelet transfusion was given on or between the days of the two counts; (3) the second platelet count was at least as high as the first; (4) the two counts were 2 or more days apart or 1 day apart, with the next (third) count greater than or equal to the first count with no intervening platelet transfusions. Patients who died or who were lost to follow-up before achieving recovery were considered censored at the date of last reported platelet count. Neutrophil recovery was defined as the first of 2 consecutive days of an absolute neutrophil count (ANC) of greater than 500/μL. An alternative donor was defined as any donor who was not an HLA-identical sibling. The mononuclear cell count was defined as the number of monocytes and lymphocytes per kilogram; the total nucleated cell count was defined as the number of monocytes, lymphocytes, and polymorphonuclear cells per kilogram.
Estimation of Platelet Transfusion Costs
Platelet costs were based on 1996 charges from the Fred Hutchinson Cancer Research Center (Seattle, WA). A cost of $592 was applied for each single-donor apheresis unit: apheresis ($413), irradiation ($13), transport and handling ($10), tubing ($15), and hospital charges per transfusion ($141). A cost of $475 was applied for each 6-unit random donor platelet concentrate: platelet concentrates ($296) and charges for transport, handling, tubing, and hospital charges per transfusion, as described above.
Statistical Methods
Time to platelet recovery was summarized by transplant type and underlying disease using Kaplan-Meier estimates and log-rank tests. Multivariate proportional hazards models for platelet recovery were fit separately for the two largest transplant subgroups: autologous PBSC and allogeneic BM patients. Models were built in stages with the best baseline predictor model identified first, to which time-dependent predictors were added to obtain the final model. Variable selection at each stage proceeded as follows: all univariate predictors significant at α = .1 were considered candidates for inclusion in the multivariate model which was constructed using stepwise and best-subset selection algorithms. Inclusion of a variable in the first multivariate model required that it be significant at the α = .05 level, or for variables with strong prior evidence of explanatory power, the .1 level. Underlying disease was included in all models to mitigate potential confounding. Missing value indicators were used for predictors with 10% or more missing values to reduce loss of observations during variable selection. Final models were summarized by regression parameter estimates and standard errors and graphically using covariate-adjusted recovery curves. Only those subjects included in the largest diagnosis groups within each transplant class were evaluable for analysis (autologous PBSC-355 patients; allogeneic BM-275 patients). By contrast, all subjects in each transplant class, irrespective of diagnosis were included in analyses of platelet utilization.
Cell contents of grafts were transformed to log10 scale before model building. Time-dependent predictors for clinical events such as GVHD and VOD were parameterized as binary indicators. Temperature was parameterized using two binary indicators for maximum temperature of less than 38°C, 38 to 39°C, or greater than 39°C for each of three time intervals (days 1 to 15, 16 to 30, and 31 to 60).
Neutrophil recovery, as a time-dependent variable, was a powerful predictor of platelet recovery. However, it was not used in multivariate model building because as a marker of impending engraftment it does not provide insight into the causal factors affecting platelet recovery, but rather confounds their effects in a multivariate model.
In this study, the relative risk of a variable was the probability of platelet recovery, at any given time, for a patient having that variable compared with that of a patient not having that variable. For example, if the relative risk of variable A were 1.5, patients having variable A had a 1.5-fold higher probability of platelet recovery, at any given time, than that of patients not having variable A. For continuous variables, we examined the relative risk of the log10 of that variable. For example, if the relative risk of the log10 variable B were 1.5, for every 10-fold increase in variable B, the probability of platelet recovery, at any given time, increased by a factor of 1.5. For the variable of disease, the relative risk of recovery for a given disease was the probability of recovery, at any given time, compared with that of patients having the reference disease. The reference disease chosen for each transplant class was the diagnosis of the largest group of patients.
RESULTS
Patients
Of the 789 evaluable patients, 456 underwent autologous and 333 allogeneic transplantation. Among autologous transplant patients, 355 (78%) received PBSC alone, 64 (14%) received BM alone, and 37 (8%) received both PBSC and BM. Among allogeneic transplant patients, 283 (85%) received BM alone, 43 (13%) received PBSC alone, and 7 (2%) received both PBSC and BM.
For the two largest patient groups, autologous PBSC and allogeneic BM recipients, baseline clinical and laboratory characteristics are shown in Table 1, their time-dependent characteristics, in Table 2. A total of 176 (50%) autologous PBSC recipients and 63 (22%) allogeneic BM recipients were followed for less than 60 days, as described in Table3.
Eighteen recipients of autologous PBSC had undergone a previous stem cell transplant. Fifteen of these patients (83%) underwent two transplants within 6 months of each other as part of a planned treatment approach at one center for patients with multiple myeloma. PBSC collected before the first transplant supported both the first and second transplant. Finally, only patients who demonstrated rapid recovery after the first transplant were eligible to receive a second transplant.
Platelet Recovery—Autologous PBSC Transplants
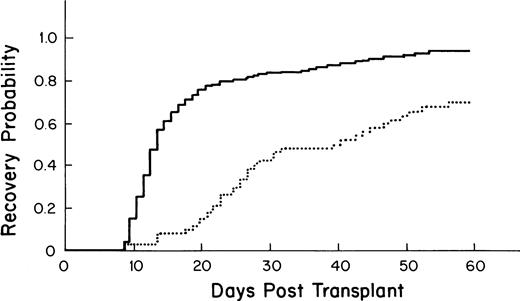
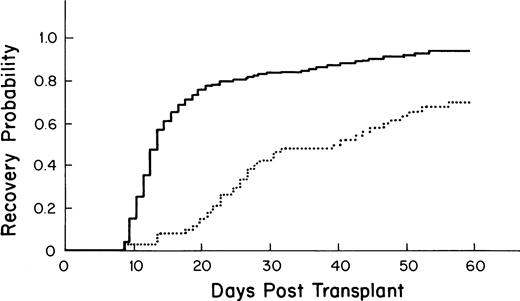
The median time to recovery for the 355 patients who received autologous PBSC was 13 days (95% confidence interval [CI] = 12, 13; Fig 1).
Kaplan-Meier plot of the probability of platelet recovery after an autologous PBSC transplant (—, n = 355) compared with that of an autologous BM transplant (… , n = 64); P= .0001.
Kaplan-Meier plot of the probability of platelet recovery after an autologous PBSC transplant (—, n = 355) compared with that of an autologous BM transplant (… , n = 64); P= .0001.
Univariate analysis.
Variables associated with an accelerated recovery included a higher platelet count at the start of myeloablative therapy, use of chemotherapy for mobilizing stem cells, a higher CD34 count in the graft, and having had a previous stem cell transplant (Table4). Compared with breast cancer and multiple myeloma, all other diseases were associated with a longer time to recovery. Baseline variables associated with slower recovery included the use of a radiation-containing preparative regimen and a higher mononuclear cell count of the graft. For the time-dependent variables, earlier neutrophil recovery was associated with faster platelet recovery. Fever greater than 39°C and the presence of VOD were associated with slower recovery.
Multivariate analysis.
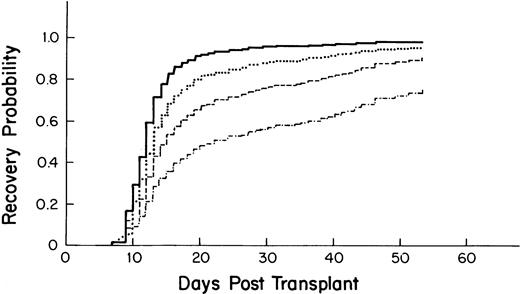
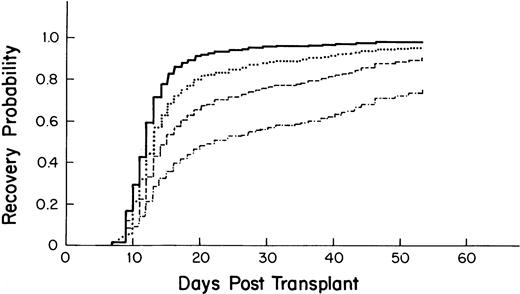
Variables associated with accelerated recovery included a higher CD34 count of the graft (Fig 2), higher platelet count at the start of myeloablative therapy, and having had a previous stem cell transplant (Table 5). Undergoing radiation therapy before the myeloablative regimen was associated with slower recovery. Compared with breast cancer, a diagnosis of leukemia, non-Hodgkin's lymphoma (NHL), multiple myeloma, and Hodgkin's disease (HD) was associated with a longer time to recovery. For the time-dependent variables, posttransplant fever and VOD were associated with a longer time to recovery.
Probability of platelet recovery after autologous PBSC transplantation by CD34 stem cell count as derived from the multivariate model. – · —, 1 × 106 cells/kg; ––, 2.5 × 106 cells/kg; ···, 5.0 × 106 cells/kg; —, 10.0 × 106cells/kg).
Probability of platelet recovery after autologous PBSC transplantation by CD34 stem cell count as derived from the multivariate model. – · —, 1 × 106 cells/kg; ––, 2.5 × 106 cells/kg; ···, 5.0 × 106 cells/kg; —, 10.0 × 106cells/kg).
Platelet Recovery—Allogeneic BM Transplants
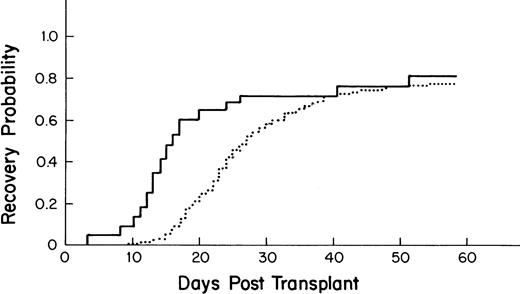
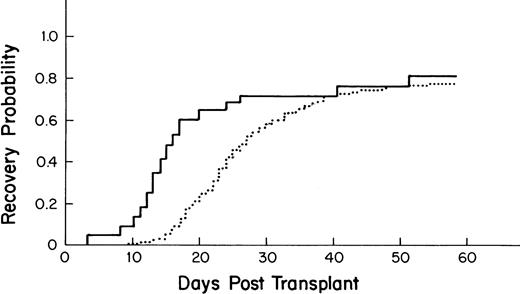
The median time to recovery for the 275 allogeneic BM transplant patients was 27 days (95% CI-25, 30; Fig3).
Kaplan-Meier plot of the probability of platelet recovery after an allogeneic PBSC transplant (—, n = 43) compared with that of an allogeneic BM transplant (···, n = 275); P = .001.
Kaplan-Meier plot of the probability of platelet recovery after an allogeneic PBSC transplant (—, n = 43) compared with that of an allogeneic BM transplant (···, n = 275); P = .001.
Univariate analysis.
Variables associated with an accelerated recovery included use of a graft from an HLA-identical sibling donor and a higher total nucleated cell count of the graft (Table 4). Compared with acute myeloid leukemia (AML), all other diseases were associated with a shorter time to recovery. Baseline variables associated with slower recovery included any manipulation of the marrow graft, T-cell depletion, and the use of a radiation-containing preparative regimen. For the time-dependent variables, earlier neutrophil recovery was associated with faster platelet recovery. The use of posttransplant growth factors, as well as the presence of fever, VOD, and GVHD, was associated with slower recovery.
Multivariate analysis.
The use of an HLA-identical sibling donor was strongly associated with accelerated recovery (Table 5). Although not reaching statistical significance, a higher total nucleated cell count of the graft was associated with more rapid recovery. Patients with AML and myelodysplastic syndromes had slower recovery than patients with chronic myeloid leukemia. For the time-dependent variables, fever, VOD, and the use of posttransplant growth factors were associated with a longer time to recovery.
Platelet Recovery and Utilization by Stem Cell Source
Recipients of autologous PBSC had faster recovery (median recovery day 13; 95% CI = 12, 13) than that of recipients of autologous BM (median recovery day 40; [27,49] as shown in Fig 1(P = .0001). Similarly, recipients of allogeneic PBSC had faster recovery (median recovery day 16; [14,20]) than that of recipients of allogeneic BM (median recovery day 27; [25,30]), as shown in Fig 3 (P = .001).
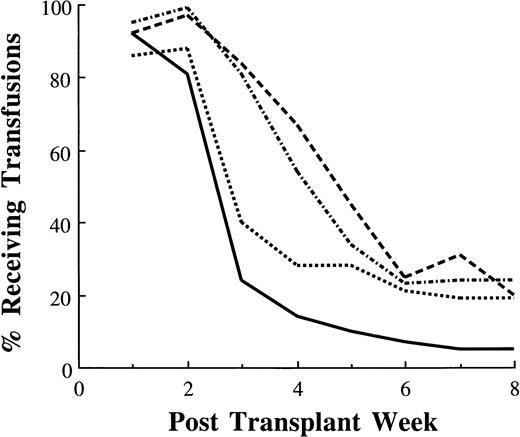
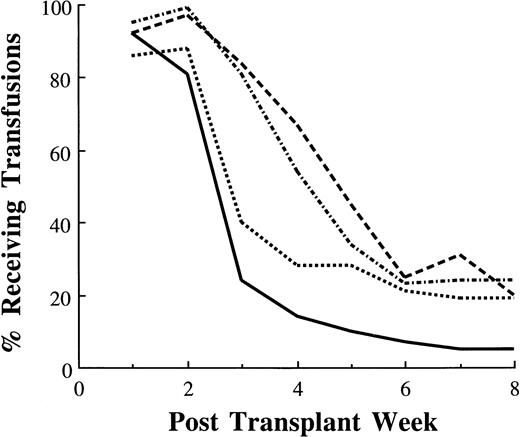
Eighty-four percent of those undergoing autologous and 81% of those undergoing allogeneic BM transplants required transfusions 3 weeks after transplant (Fig 4). By contrast, 24% of autologous and 40% of allogeneic PBSC transplant recipients required transfusions 3 weeks after transplant. At week 8, 20% of autologous and 24% of allogeneic BM recipients were still transfusion dependent compared with 5% and 19% of those who received autologous and allogeneic PBSC, respectively.
Percentage of patients receiving platelet transfusions after transplantation: autologous PBSC (—, n = 355); autologous BM (---, n = 64); allogeneic PBSC (····, n = 43); allogeneic BM (–·–, n = 283).
Percentage of patients receiving platelet transfusions after transplantation: autologous PBSC (—, n = 355); autologous BM (---, n = 64); allogeneic PBSC (····, n = 43); allogeneic BM (–·–, n = 283).
Platelet Transfusion Practices—The Platelet Trigger
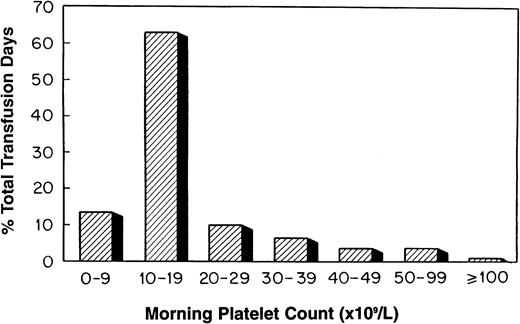
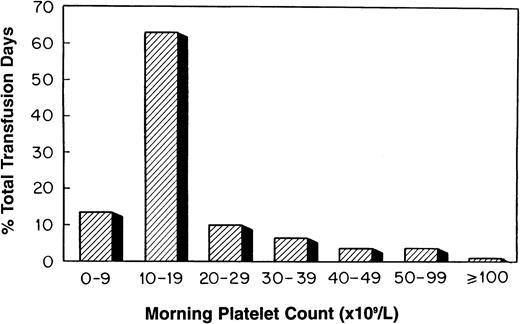
Platelets were transfused prophylactically at all 18 transplant centers. The platelet count routinely used to “trigger” such a transfusion varied by center (≤10,000/μL, 4 centers; ≤15,000/μL, one center; ≤20,000/μL, 12 centers; and ≤30,000/μL, one center). Platelet transfusion triggers were raised for such clinical circumstances as bleeding, fever, anticoagulation, surgical procedures, and the presence of GVHD. Platelet counts were routinely checked once or greater than once daily by 12 and 6 centers, respectively. The distribution of platelet transfusion days by morning platelet count is shown in Fig 5. The platelet count on 63% of platelet transfusion days was 10,000 to 19,000/μL. By contrast, a morning platelet count of less than 10,000/μL occurred on 14% of the platelet transfusion days.
The distribution of platelet transfusion days by morning platelet count for all stem cell transplant patients (n = 789).
The distribution of platelet transfusion days by morning platelet count for all stem cell transplant patients (n = 789).
Financial Impact of Platelet Transfusions
The 789 evaluable patients had 10,626 platelet transfusion events: 8,794 (83%) single donor and 1,832 (17%) random donor. Using conservative cost estimates, the average 60-day platelet cost per patient for the two largest patient groups was approximately $4,000 for autologous PBSC transplants and $11,000 for allogeneic BM transplants. The number of platelet transfusion events by both disease and transplant class is shown in Table 6.
Hemorrhagic Events
Among the 789 evaluable patients, 143 hemorrhagic events of moderate or greater severity occurred in 89 patients, or 11% of the study population (Table 7). Most events occurred in patients undergoing allogeneic transplantation (78%), and before platelet recovery (89%). The median (range) time of hemorrhage from the date of stem cell infusion was 19 days (0 to 60). The major site of bleeding was genitourinary. In this regard, many of such events were related to chemotherapy-induced cystitis. Genitourinary prophylaxis routinely used by centers included mesna alone (1 center), hydration alone (6 centers), and both mesna and hydration (11 centers). The second most common site of bleeding was gastrointestinal. In this regard, 9 centers routinely used prophylactic H2 blockers. Most events (66%) occurred when the morning platelet count was greater than 20,000/μL. Sixteen patients, or 2% of the entire study population, died from a hemorrhagic event.
DISCUSSION
The time to platelet recovery after myeloablative therapy and HSCT is influenced both by baseline and time-dependent clinical variables. For autologous PBSC recipients, the CD34 content of the graft was the most significant variable associated with faster recovery. As both lineage-committed and uncommitted stem cells express CD34, its strength as a predictor was expected and confirms the findings of others.7-11 The impact of the other variables identified in the multivariate model on recovery were independent of the CD34 content of the graft. The pretreatment platelet count may act as a surrogate marker for the quality of the stem cell graft and microenvironment. The effect of disease may be related to differences in prior therapy and their impact on stem cells or to inherent biologic differences in the stem cell/microenvironment compartments. The influence of prior local radiation therapy may be the result of toxic effects of radiation on stem cells and marrow microenvironment.12 Finally, the association of faster recovery for recipients of a second autologous PBSC transplant is not intuitively obvious. Of the 18 patients who had a prior HSCT, however, 15 were from a single institution, all having a diagnosis of multiple myeloma, with the second transplant a planned part of the treatment strategy. Therefore, the impact of a prior HSCT on platelet recovery is probably attributable to confounding variables related to the clinical course and treatment plan of these patients, rather than to a true biologic effect.
After allogeneic BM transplantation, patients with an HLA-identical sibling donor recovered faster than did those transplanted from other alternative donors. Alternative donors have increased disparities with their recipient at the major histocompatibility complex (MHC), minor histocompatibility complex, or both. Recipients of marrow from alternative donors have a higher incidence of GVHD.13,14GVHD has been associated with shortened platelet survival and delayed platelet recovery.15,16 However, the variable “HLA-identical sibling donor,” and not GVHD, was found significant in the multivariate model, suggesting that histocompatibility differences, independent of the development of GVHD, influence recovery. Such differences are associated with an increased risk of graft rejection.13,17 The resistance to engraftment in this setting might affect platelet production, even though neutrophil recovery occurs. The other baseline predictor of recovery for allogeneic marrow recipients was disease. The reason for this is unclear, but a direct effect of disease, or its prior treatment, on the marrow microenvironment might affect platelet recovery, because marrow stroma in large part remains of host origin after an allogeneic BM transplant.18
The time-dependent variables influencing platelet recovery included fever, VOD and the use of growth factors for allogeneic BM recipients. In this study, fever was used as a surrogate for the presence of infection. Thrombocytopenia has been reported as a complication of both infection and VOD and is attributed, in part, to shortened platelet survival.16,19,20-22 Direct platelet damage by bacterial products, platelet adhesion to injured endothelium, platelet adsorption of circulating immune complexes, and platelet autoantibody production may all contribute to enhanced platelet destruction. In addition, endogenous cytokines that are increased during infection and hepatic VOD may result in an increased consumption of platelets. In both preclinical and clinical studies, the administration of some recombinant cytokines results in a reduction of circulating platelets and, where studied, this is related to increased platelet consumption.23 24 Whatever the cause, the newly engrafted marrow may not be able to compensate for the increased platelet destruction associated with these transplant complications, resulting in slower platelet recovery.
The use of granulocyte colony-stimulating factor or granulocyte-macrophage colony-stimulating factor (G-CSF or GM-CSF) after allogeneic BM transplantation was associated with a decreased probability of platelet recovery. Growth factors have not previously been shown to have an adverse effect on platelet recovery after an allogeneic transplant, although one study did show a trend for slower recovery in patients who received GM-CSF.25-27 The effect of growth factors on platelet recovery in the present study may be related to the reasons that growth factors were started rather than to a true biologic effect on recovery.
Platelet recovery for recipients of autologous and allogeneic PBSC was significantly faster than that of patients who received autologous and allogeneic BM transplants, respectively. Such differences in the kinetics of recovery may be due to differences in CD34 content and subset composition between peripheral blood and marrow.28Alternatively, differences may be related to the 5 to 10-fold larger number of cells that are infused during PBSC transplants.29
Using estimates of the number of transplants performed in the United States and Canada during 1995, we estimate conservative 60-day costs of platelet transfusions to be approximately $22 million for autologous PBSC transplants and $44 million for allogeneic marrow transplants annually.30 These estimates do not include the additional costs incurred for HLA matching, ABO and cross-matching and CMV serotyping of platelets nor the costs involved in the management of transfusion and hemorrhagic-related complications. A strategy to enhance platelet recovery and decrease the need for platelet transfusions could thus have a significant impact on the costs of HSCT.
Despite prevention of hemorrhage as the primary reason for the routine administration of platelet transfusions, a detailed analysis of hemorrhagic events after stem cell transplantation has previously been lacking. In this study, the incidence of clinically significant bleeding was 11% and that of hemorrhagic death 2%. Whereas most events occurred before platelet recovery, they generally occurred when the morning platelet count was greater than 20,000/μL. This finding suggests that clinical events that occur during the early posttransplant period, such as mucositis, GVHD, cystitis, and infection, may be more important predictors of hemorrhage than platelet count. Indeed, using a lower platelet count to “trigger” the prophylactic transfusion of platelets would result in fewer transfusions per patient, hence decreasing transfusion risks and costs. These advantages would be offset if the risk of hemorrhagic complications were to increase; however, recent studies suggest this not to be the case.5 31
As with any study that is epidemiologic in nature, limitations exist that need to be considered. The subjects in this study were derived from large academic transplant centers and therefore reflect their investigational interests and clinical practice. As such, the results of this study may not be applicable to HSCT done in smaller community settings. In addition, we did not capture all potential clinical variables that might affect recovery. The incorporation of such variables to the model could alter the significance of the variables described in this study. Finally, the methodology used to assess colony-forming unit granulocyte-macrophage (CFU-GM) number and CD34 positivity was not standardized in this study. Furthermore, an analysis of CD34 subsets, which could potentially be more predictive of recovery, was not performed.
The results of this study do, however, suggest that clinical trials of strategies to enhance platelet recovery should control for baseline pretransplant variables that influence recovery. The interpretation of the results of such clinical trials should include an analysis of time-dependent events after transplantation that influence platelet recovery. The relatively low incidence of life-threatening hemorrhage in this study suggests that enhancing recovery may not have a significant effect on 60-day mortality. Therefore, enhancing platelet recovery will likely be of clinical benefit if it results in a decrease in platelet transfusions, leading to a decrease in transfusion-related morbidity, health care costs, and an improved quality of life for patients.
APPENDIX
EPIDEMIOLOGY OF PLATELET RECOVERY STUDY GROUP
Supported by a Grant from Genentech, Inc.
Address reprint requests to Steven H. Bernstein, MD, Roswell Park Cancer Institute, Elm and Carlton Sts, Buffalo, NY 14263.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.