Abstract
The human blood coagulation factor VIII C2 domain (Ser2173-Tyr2332) contains an epitope recognized by most polyclonal inhibitory anti-factor VIII alloantibodies and autoantibodies. We took advantage of the differential reactivity of inhibitory antibodies with human and porcine factor VIII and mapped a major determinant of the C2 epitope by using a series of active recombinant hybrid human/porcine factor VIII molecules. A series of five C2-specific human antibodies and a murine anti-factor VIII monoclonal antibody, NMC-VIII/5, inhibited a hybrid containing a substitution of porcine sequence for Glu2181-Val2243 significantly less than human factor VIII. In contrast, four of the five patient antibodies and NMC-VIII/5 inhibited a hybrid containing a substitution of porcine sequence for Thr2253-Tyr2332 equally well as human factor VIII. Thus, a major factor VIII inhibitor epitope determinant is bounded by Glu2181-Val2243 at the NH2-terminal end of the C2 domain. Because C2 inhibitors block the binding of factor VIII to phospholipid and von Willebrand factor, for which binding sites have been localized to Thr2303-Tyr2332, these results imply that the segment bounded by Glu2181-Val2243 also is involved in these macromolecular interactions.
INHIBITORY ANTIBODIES (inhibitors) to factor VIII (FVIII) either develop as alloantibodies in approximately 25% of hemophilia A patients after FVIII infusions or as autoantibodies in nonhemophiliacs.1 The characterization of the epitopes recognized by these inhibitors has been the subject of several investigations.2-10 The approximately 300-kD FVIII molecule contains homologous A and C regions that define an A1-a1-A2-B-a2-A3-C1-C2 domainal sequence.11 12 The 36-residue a1 peptide is proteolytically cleaved by factor IXa or activated protein C, whereas the 41-residue a2 peptide is proteolytically cleaved by thrombin or factor Xa. Although FVIII is synthesized as a single-chain molecule, it circulates in heterodimeric form because of proteolysis within the B domain. FVIII heavy and light chains are designated A1-a1-A2-B and a2-A3-C1-C2, respectively.
Inhibitor neutralization assays indicate that antibodies to epitopes in the A2, a2-A3-C1, and C2 domains are responsible for the anticoagulant activity in most inhibitor plasmas.8 Patients can have antibodies that recognize any combination of these epitopes. Additionally, the epitope pattern can vary with time.13 The epitopes recognized by inhibitors from alloantibody and autoantibody patients appear to be identical, despite their different immunological backgrounds. However, the epitope pattern differs in that autoantibodies are more likely to recognize single epitopes, whereas alloantibodies usually recognize multiple epitopes.8
FVIII participates in several macromolecular interactions that are potential targets for disruption by inhibitory antibodies. It binds to and circulates with von Willebrand factor (vWF) as an inactive precursor. It is activated by thrombin or factor Xa, which cleaves FVIII at Arg372, between the A1 and A2 domains; at Arg740, between the A2 and B domains; and at Arg1689, which releases the a2 peptide from the light chain.14 FXa catalyzes additional cleavages within the A1, A2, and A3 domains.14,15 Thrombin-activated FVIII (FVIIIa) is an A1-a1/A2/A3-C1-C2 heterotrimer.14 16-19 It functions in the intrinsic pathway of blood coagulation by binding to both factor IXa, phospholipid, and possibly FX and promoting the activation of FX by FIXa.
The release of the a2 peptide is necessary for FVIII to dissociate from vWF. This is a requisite step in blood coagulation, because the binding of FVIII to phospholipid membranes and to vWF are mutually exclusive.20-22 vWF promotes release of FVIII into the circulation and increases its circulatory lifetime.23-26 However, it is not required for the activation of FVIII or the function of FVIIIa. In principle, inhibitors also could act by blocking the interaction of FVIII with vWF and destabilize FVIII in vivo. However, this is not a common mechanism of action.
A2 inhibitors bind to the FVIIIa/FIXa/phospholipid membrane complex and inhibit FX activation noncompetitively.27a2-A3-C1 inhibitors have not been as thoroughly characterized, but may inhibit the binding of FVIIIa to FIXa.28 C2 inhibitors inhibit the binding of FVIII to phospholipid,29 which is consistent with the finding that phospholipid protects FVIII from inactivation by some inhibitors.29,30 Additionally, C2 inhibitors inhibit the binding of FVIII to vWF,31,32 suggesting that vWF and phospholipid bind to common or overlapping sites on FVIII. However, thea2 region is necessary for binding of FVIII to vWF, but not to phospholipid.33-38 Thus, phospholipid and vWF binding involve distinct sites in FVIII. The interplay between the binding of FVIII to phospholipid, vWF, and C2 inhibitors implies a complexity to C2 macromolecular interactions that is poorly understood.
In this study, we took advantage of the limited cross-reactivity of human and porcine FVIII and constructed a series of active recombinant hybrid human/porcine FVIII molecules to map C2 inhibitor epitopes by homolog scanning mutagenesis. We previously used this method to map a major determinant of the A2 inhibitor epitope to Arg484-Ile508.10 Our results indicate that C2-specific antibodies recognize a region bounded by Glu2181-Val2243 in the NH2-terminal half of the C2 domain.
MATERIALS AND METHODS
Materials.
Citrated hemophilia A and normal pooled human plasmas were purchased from George King Biomedical, Inc (Overland Park, KS). Heparin-Sepharose was purchased from Sigma Chemical Co (St Louis, MO). Human vWF was purified as described previously.37 Murine monoclonal anti-FVIII antibody, ESH5, was purchased from American Diagnostica (Greenwich, CT). Highly purified, albumin-free human recombinant FVIII was a gift from Baxter Corp (Duarte, CA) and was stored in aliquots at 0.45 mg/mL at −80°C. Alkaline phosphatase-conjugated goat antimouse antibody was purchased from Bio-Rad (Hercules, CA). Fetal bovine serum, geneticin, penicillin, streptomycin, Dulbecco’s modified Eagle’s medium-F12 (DMEM-F12) medium, and AIM-V medium were purchased from Life Technologies, Inc (Gaithersberg, MD). Taq DNA polymerase was purchased from Promega (Madison, WI). Vent DNA polymerase was purchased from New England Biolabs (Beverley, MA). Pfu DNA polymerase and the phagemid pBlueScript II KS− were purchased from Stratagene (La Jolla, CA). Synthetic oligonucleotides were purchased from Life Technologies or Cruachem, Inc (Sterling, VA). Restriction enzymes were purchased from New England Biolabs or Promega. 5′-Phosphorylated primers were used when polymerase chain reaction (PCR) products were produced for cloning purposes. Nucleotide (nt) numbering of oligonucleotides used as primers for PCR amplification of porcine FVIII cDNA or genomic DNA uses the human FVIII cDNA as reference.39 A FVIII expression vector, designated HB−/ReNeo, was a gift from Biogen, Inc (Cambridge, MA). HB−/ReNeo contains ampicillin and geneticin resistance genes and a human FVIII cDNA that lacks the entire B domain, defined as the Ser741-Arg1648 cleavage fragment produced by thrombin. To simplify mutagenesis of FVIII C2 domain cDNA, which is at the 3′ end of the FVIII insert in ReNeo, a Not I site was introduced two bases 3′ to the stop codon of HB−/ReNeo (Fig1) by splicing-by-overlap extension (SOE) mutagenesis.40This construct is designated HB−/ReNeo/Not I.
Recombinant FVIII constructs. Amino acid numbering refers to mature, full-length human FVIII.39 The parent human B-domainless FVIII construct (HB−) lacks residues 741-1648. Porcine B-domain deficient FVIII (PSQ) contains a 14-residue sequence (SQ) corresponding to Ser741-Asn745, Pro1640-Arg1648 in human FVIII, instead of the B domain. Shaded regions correspond to areas of porcine substitution. In constructs HP23-HP28, these boundaries are defined by amino acid residues of human-porcine nonidentity at amino acid residues 2181, 2199, 2207, 2243, 2253, 2311, and 2321.
Recombinant FVIII constructs. Amino acid numbering refers to mature, full-length human FVIII.39 The parent human B-domainless FVIII construct (HB−) lacks residues 741-1648. Porcine B-domain deficient FVIII (PSQ) contains a 14-residue sequence (SQ) corresponding to Ser741-Asn745, Pro1640-Arg1648 in human FVIII, instead of the B domain. Shaded regions correspond to areas of porcine substitution. In constructs HP23-HP28, these boundaries are defined by amino acid residues of human-porcine nonidentity at amino acid residues 2181, 2199, 2207, 2243, 2253, 2311, and 2321.
Total RNA was isolated by acid guanidinium thiocyanate-phenol-chloroform extraction.41 cDNA was synthesized from mRNA using Moloney murine leukemia virus reverse transcriptase (RT) and random hexamers according to instructions supplied by the manufacturer (First-Strand cDNA Synthesis Kit; Pharmacia Biotech, Uppsala, Sweden). Plasmid DNA was purified using a Qiagen Plasmid Maxi Kit (Qiagen, Inc, Valencia, CA). PCR reactions were done using a Hybaid OmniGene thermocycler using Taq, Vent, or Pfu DNA polymerases, unless otherwise indicated. PCR products were gel-purified, precipitated with ethanol, and ligated into plasmid DNA using T4 DNA ligase (Rapid DNA ligation kit; Boehringer Mannheim, Indianapolis, IN). Insert-containing plasmids were used to transform Escherichia coli Epicurean XL1-Blue cells. All novel FVIII DNA sequences generated by PCR were confirmed by dideoxy sequencing using an Applied Biosystems 373a automated DNA sequencer and the PRISM dye terminator kit (Applied Biosystems, Foster City, CA).
FVIII assays.
The activity of recombinant FVIII proteins was measured by one-stage clotting assay42 using either a manual tilt tube assay or an ST4 BIO Coagulation Instrument (Diagnostica Stago, Parsippany, NJ). One unit of FVIII is defined as the activity in 1 mL of pooled normal citrated human plasma. FVIII inhibitor titers were measured using the Bethesda assay.43 To measure the inhibitor activity against recombinant FVIII, human B-domainless FVIII, porcine B-domain–deficient FVIII, or hybrid human porcine B-domainless FVIII were added to hemophilia A plasma to a final concentration of 0.8 to 1.2 U/mL. One Bethesda unit (BU) is defined as the amount of inhibitory activity that produces 50% inhibition of FVIII activity in the one-stage assay.
Construction of a hybrid FVIII expression vector, HP20, containing the porcine C2 domain.
A porcine FVIII cDNA corresponding to the 3′ end of the C1 domain and all of the C2 domain was cloned into pBluescript by RT-PCR from spleen total RNA using primers based on known porcine FVIII cDNA sequence.44 This construct and HB−/ReNeo were used as templates to construct a human C1-porcine C2 fusion product in pBlueScript by SOE mutagenesis. The C1-C2 fragment in this plasmid was removed with Apa I and Not I and ligated into Apa I/Not I-cut HB−/ReNeo/Not I to produce HP20/ReNeo/Not I.
Construction of hybrid human/porcine FVIII molecules containing porcine C2 domain substitutions.
HB−/ReNeo/Not I and HP20/ReNeo/Not I were used as templates for a series of SOE mutagenesis constructions that produced hybrid human/porcine cDNAs HP23, HP24, HP25, HP26, HP27, and HP28 in ReNeo/Not I (Fig 1). Because of sequence identity between human and porcine FVIII, we define boundaries of porcine substitutions by the first amino acids that differ between human and porcine FVIII at the NH2-terminal and C-terminal ends of the insertions. These boundaries in HP23, HP24, HP25, HP26, HP27, and HP28 correspond to human FVIII residues Thr2253-Gln2311, Glu2181-Val2243, Thr2253-Met2321, Glu2181-Met2199, Lys2207-Val2243, and Lys2207-Met2321, respectively.
Construction of B-domain deleted hybrid human/porcine FVIII containing the porcine light chain (HP18).
The human FVIII light chain consists of amino acid residues Asp1649-Tyr2332. The corresponding residues in the porcine FVIII cDNA were substituted for this region of HB− to produce a hybrid human/porcine FVIII molecule designated HP18. This was performed by substituting a PCR product corresponding to porcine a2region, the A3 domain, the C1 domain, and part of the C2 domain for the corresponding region in HP20. To facilitate constructions, a synonymousAvr II site was introduced into nt 2273 at the junction of the A2 and A3 domains of HP20 by SOE mutagenesis.
Construction of B-domain–deleted hybrid human/porcine FVIII containing the porcine signal peptide, A1 domain, and A2 domain (HP22).
The human FVIII signal peptide, A1 domain, and A2 domains consist of amino acid residues Met(−19)-Arg740. The corresponding residues in the porcine FVIII cDNA were substituted for this region of HB− to produce a molecule designated HP22. Additionally, a synonymous Avr II site was introduced into nt 2273 at the junction of the A2 and A3 domains of HP22 by SOE mutagenesis. HP22 was constructed by fusion of a porcine signal peptide-A1-partial A2 fragment in pBlueScript44 with a B-domainless hybrid human/porcine FVIII containing the porcine A2 domain, designated HP1.9
Construction of porcine B-domain–deleted FVIII and a porcine B-domain–deficient FVIII, PSQ.
A Spe I/Not I fragment of HP18/BS (+Avr II) was digested with Avr II/Not I and ligated into AvrII/Not I-digested HP22/BS (+Avr II) to produce a construct PB−/BS (+Avr II), which consists of the porcine FVIII lacking the entire B domain. PB−was cloned into ReNeo by ligating an Xba I/Not I fragment of PB−/BS (+Avr II) into HP22/ReNeo/Not I (+Avr II).
In human FVIII, the presence of a 14 amino acid segment, SerPheSerGlnAsn-ProProValLeuLysArgHisGlnArg, corresponding to B-domain residues Ser741-Asn745, Pro1640-Arg1648, in place of the B domain produces a molecule designated r-VIII SQ.45 This molecule reportedly is expressed more efficiently in mammalian cell culture compared with B-domain–deleted FVIII.45 The cDNA coding for this segment was inserted into the corresponding region of PB−/BS (+Avr II) by SOE mutagenesis and then cloned into ReNeo, producing a product PSQ/ReNeo/Not I (+Avr II).
Expression of recombinant FVIII molecules.
All transfected cell lines were maintained in DMEM-F12 containing 10% fetal bovine serum, 50 U/mL penicillin, and 50 μg/mL streptomycin. Fetal bovine serum was heat-inactivated at 56°C for 1 hour before use. HB−/ReNeo, PSQ/ReNeo/Not I (+AvrII), and hybrid human/porcine FVIII cDNAs in ReNeo initially were transfected into COS-7 cells to confirm that active protein could be expressed. They were then stably transfected into BHK cells and selected for geneticin resistance as described previously,9except that expressing cells were maintained in growth medium containing 600 μg/mL geneticin. Cells from Corning T-75 flasks (Corning, Corning, NY) grown to confluence were transferred to Nunc triple flasks (Nunc, Glostrup, Denmark) in medium containing 600 μg/mL geneticin and grown to confluence. The medium was removed and replaced with serum-free, AIM-V medium without geneticin. FVIII expression was monitored by coagulation assay and 100 to 150 mL of medium was collected once daily for 4 to 5 days. Typical expression levels were 300 U (∼60 μg) FVIII per 200 mL of medium per day. Pooled medium was stored at 4°C in 0.05% (wt/vol) sodium azide.
Recombinant hybrid FVIII molecules and PSQ were partially purified and concentrated from the growth medium by heparin-Sepharose chromatography. Heparin-Sepharose (10 mL) was equilibrated with 0.075 mol/L NaCl, 10 mmol/L HEPES, 2.5 mmol/L CaCl2, 0.005% Tween-80, and 0.02% sodium azide, pH 7.40. Medium (100 to 200 mL) from expressing cells was applied to the heparin-Sepharose, which then was washed with 30 mL of equilibration buffer without sodium azide. FVIII was eluted with 0.65 mol/L NaCl, 20 mmol/L HEPES, 5 mmol/L CaCl2, and 0.01% Tween-80, pH 7.40, and was stored at −80°C. The yield of FVIII coagulant activity was typically 50% to 75%.
PSQ also was purified to homogeneity from serum-free medium using W3-3 antiporcine FVIII immunoaffinity chromatography and MonoQ high-performance liquid chromatography (HPLC) using chromatography conditions as described previously for plasma-derived porcine FVIII.46 The specific activity of PSQ was 18,300 U per A280 based on the human FVIII standard curve.
Recombinant FVIII was quantitated using a sandwich enzyme-linked immunosorbent assay (ELISA) in which human vWF was used to capture FVIII and antihuman FVIII murine MoAb, ESH5, was used for detection. ESH5 was selected because it binds the FVIII heavy chain (A1 or A2 domain) and is unlikely to be influenced by mutations in the C2 domain.
Wells in a Corning Immobilon microtiter plate were coated with 6 μg/mL vWF in 0.15 mol/L NaCl, 10 mmol/L sodium phosphate, and 0.05% sodium azide, pH 7.4 (PBS-N) overnight at room temperature. The plate was washed with H2O, blocked by submersion for 1 hour in PBS-N, 0.05% Tween-20, and 0.05% nonfat dry milk, and then washed again with H2O. Samples containing recombinant human FVIII standards (0 to 200 ng/mL) or test recombinant proteins were added and incubated for 1 hour at 37°C. Samples were performed in duplicate and two dilutions on the standard curve were made for each test recombinant protein. After washing the wells with 0.15 mol/L NaCl, 20 mmol/L HEPES, 5 mmol/L CaCl2, and 0.01% Tween-80, pH 7.4 (HCT), 1 μg/mL ESH5 in HCT was added for 1 hour at 37°C. Wells were washed with HCT and goat antimouse alkaline phosphatase-conjugated antibody, diluted 1:500 in HCT, was added for 1 hour at 37°C. After washing with HCT, the wells were developed by the addition ofp-nitrophenylphosphate (Bio-Rad) for 1 hour according to the instructions supplied by the manufacturer. The reaction was stopped by the addition of 0.4 mol/L NaOH and absorbances at 405 nm were read in a microplate reader. The molecular masses of human B-domain–containing and the B-domainless constructs differ (∼250 kD v 160 kD, respectively). ELISA readings were corrected accordingly to calculate the concentration of test constructs in mass units.
The specific activity of recombinant FVIII was calculated from coagulation assay and ELISA determinations and expressed in terms of units per milligram. The following results were obtained: HB−, 4,900; HP20, 8,000; HP23, 3,900; HP24, 7,400; HP25, 7,100; HP26, 8,500; HP27, 7,000; and HP28, 15,000. The uncertainty of each measurement, which is based on duplicate determination in the coagulation assay and quadruplicate determination in the ELISA assay, is approximately 20%. Thus, the apparent specific activity of most of the hybrids is significantly higher than that of HB−. Whether this difference is true or represents a small systematic error in the ELISA method, the results indicate that C2 hybrids are at least as active as human FVIII and thus are suitable for epitope mapping analysis.
Patient plasmas.
Citrated human plasmas from five inhibitor patients (HR, LK, AA, RvR, and YA) were used either without further purification (HR, RvR, and YA) or as IgG preparations (LK and AA). Inhibitor IgG was prepared as described previously.5 The inhibitors in HR, LK, AA, and RvR antibodies were specific for the C2 domain as judged by antibody neutralization assays performed as described previously.8Briefly, inhibitor plasmas were incubated with increasing concentrations of recombinant human A2 domain, recombinant human C2 domain, or purified plasma-derived human FVIII light chain, which contains the A3, C1, and C2 domains. The residual inhibitor was measured using the Bethesda assay. In all plasmas, the inhibitor titer was neutralized by less than 10% by A2 and by greater than 79% by C2 or FVIII light chain. There was no difference between neutralization of HR, LK, AA, and RvR antibodies by C2 and FVIII light chain. In contrast, YA plasma was neutralized by less than 10% by A2, by 62% by C2, and by greater than 95% by light chain. This finding indicates that most of the inhibitor activity of YA is directed toward the C2 domain, but there is additional activity directed toward the A3 or C1 domains.
RESULTS
We constructed a series of hybrid B-domainless human/porcine FVIII cDNAs, human B-domainless FVIII, and a porcine B-domain–deficient FVIII (Fig 1), stably expressed them from BHK cells in serum-free medium, and partially purified them using heparin-Sepharose chromatography. The hybrid molecules contained substitutions of the porcine A3/C1/C2 domains, C2 domain only, or porcine substitutions within the C2 domain. The specific coagulant activities of the hybrids based on an ELISA were equal to or greater than that of recombinant human B-domainless FVIII, as described in Materials and Methods, indicating that they were suitable for epitope mapping studies.
The recombinant FVIII molecules were added to hemophilia A plasma, and their inhibition by five anti-human C2 antibodies (HR, LK, AA, YA, and RvR) and the murine MoAb NMC-VIII/5 (Table1) was measured using the Bethesda assay. NMC-VIII/5 was selected for study because it binds the C2 domain and, like human C2 inhibitors, inhibits the binding of FVIII to phospholipid and to vWF.47The C2-specificity of HR, LK, AA, and YA was previously identified by the observation that they were neutralized by greater than 78% by soluble, recombinant human C2 domain.8 RvR is greater than 95% neutralized by recombinant C2.48
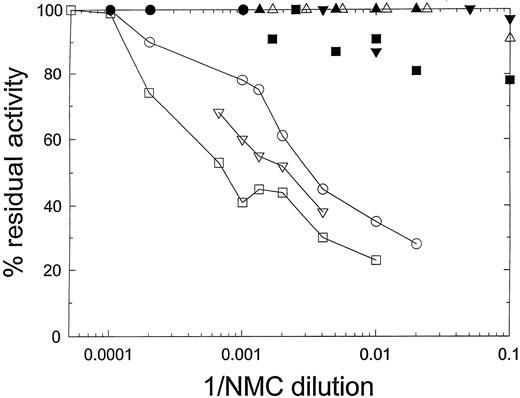
The Bethesda titer of the NMC-VIII/5 IgG preparation toward human B-domainless FVIII, determined by the concentration at which it produced 50% inhibition of its coagulant activity, was 260 BU/mL (Fig 2). NMC-VIII/5 did not detectably inhibit HP18 or HP20, confirming its C2 specificity. The Bethesda titer of NMC-VIII/5 toward HP24 was less than 10 BU/mL. By comparison with Fig 1, this indicates that residues Glu2181-Val2243 contain a major determinant of the NMC-VIII/5 epitope. The Bethesda titer of NMC-VIII/5 toward HP27 and HP28 also was below the limits of detection, indicating that the regions bounded by residues Glu2181-Met2199 and Lys2207-Val2243 each contribute to NMC-VIII/5 binding.
Inhibition of hybrid human/porcine FVIII by murine MoAb NMC-VIII/5. HB− or hybrid FVIII was diluted into hemophilia A plasma to a final concentration of 0.8 to 1.2 U/mL and then incubated for 2 hours with the indicated dilutions of NMC-VIII/5 antibody. Residual FVIII activity was measured as described in Materials and Methods. (○) HB−; (•) HP20; (▿) HP23; (▾) HP24; (□) HP25; (▪) HP26; (▵) HP27; (▴) HP28.
Inhibition of hybrid human/porcine FVIII by murine MoAb NMC-VIII/5. HB− or hybrid FVIII was diluted into hemophilia A plasma to a final concentration of 0.8 to 1.2 U/mL and then incubated for 2 hours with the indicated dilutions of NMC-VIII/5 antibody. Residual FVIII activity was measured as described in Materials and Methods. (○) HB−; (•) HP20; (▿) HP23; (▾) HP24; (□) HP25; (▪) HP26; (▵) HP27; (▴) HP28.
In contrast, NMC-VIII/5 was a potent inhibitor of HP23 and HP25. The Bethesda titers determined from the curves shown in Fig 2 were 530 BU/mL and 1,060 BU/mL for HP23 and HP25, respectively. This finding suggests that residues Thr2253-Met2321 do not contribute significantly to the binding of NMC-VIII/5 to FVIII. We cannot exclude the possibility that amino acid residues that are identical between human and porcine FVIII are present in this region that bind antibody. However, the major reduction in inhibitor activity toward HP24 and the lack of reduction in activity toward HP25 indicate that most of the binding energy associated with NMC-VIII/5 is directed against the region bounded by residues Glu2181-Val2243.
The Bethesda titers for HP23 and HP25 actually were significantly higher than that for human B-domain–deleted FVIII. Although the mechanism for this behavior is not clear, one possibility is that the human FVIII segment bounded by residues Thr2253-Met2321 unfavorably influences the NMC-VIII/5 binding. Thus, the unfavorable interaction would be relieved by insertion of the homologous porcine sequence.
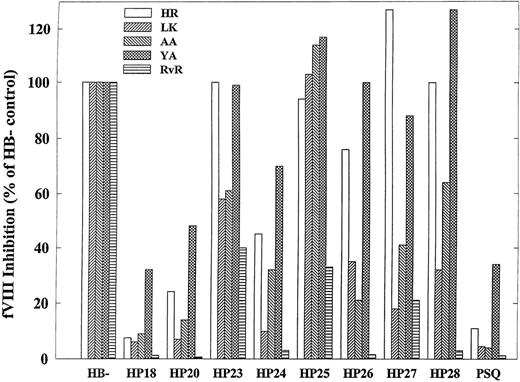
Inhibition studies using human antibodies were performed similarly (Fig 3). In addition to the hybrid human/porcine FVIII constructs described in Fig 1, the inhibition of a porcine B-domain–deficient FVIII by human antibodies was measured. The cross-reactivity levels of the antibodies, defined as the ratio of the Bethesda titers of porcine and human FVIII, were 11%, 5%, 4%, less than 1%, and 34% for HR, LK, AA, YA, and RvR, respectively (Table 2). Calculating the difference of the Bethesda titers of HP18 and porcine B-domain–deficient FVIII and dividing by the human B-domainless FVIII titer is a measure of non-FVIII light chain, presumably anti-A2, antibodies. These antibodies accounted for 5% of the AA titer and were negligible in the other antibodies (Table 2). Calculating the difference of the Bethesda titers of HP20 and HP18 and dividing by the human B-domainless FVIII titer is a measure of antibodies to thea2-A3-C1 region. LK and RvR a2-A3-C1 antibodies were negligible. They accounted for 17%, 4%, and 16% of the activity of HR, AA, and YA, respectively (Table 2). Thus, at least 80% of the activity of all of the antibodies was directed against the C2 domain, which is consistent with the antibody neutralization experiments.
Inhibition of hybrid human/porcine FVIII by human anti-FVIII antibodies. The inhibition of FVIII constructs by antibodies from patients HR, LK, AA, YA, and RvR was measured using the Bethesda assay as described in Materials and Methods.
Inhibition of hybrid human/porcine FVIII by human anti-FVIII antibodies. The inhibition of FVIII constructs by antibodies from patients HR, LK, AA, YA, and RvR was measured using the Bethesda assay as described in Materials and Methods.
Similar to NMC-VIII/5, all five antibodies inhibited HP24 less than human B-domainless FVIII. Calculating the difference of the Bethesda titers of HP24 and HP20 and dividing by the human B-domainless FVIII titer is a measure of C2 antibody activity outside the 2181-2243 region. It was 21%, 3%, 18%, 2%, and 22% for HR, LK, AA, YA, and RvR, respectively. This indicates that, like NMC-VIII/5, the human antibodies recognize residues Glu2181-Val2243 as a major determinant of C2 inhibitor epitope.
The complementary mutant to HP24 is HP25. The inhibition of HP25 by HR, LK, AA, and YA was similar to human B-domainless FVIII. Thus, C2 antibodies directed to a region COOH-terminal to residue 2253 were not identified in these patients. In contrast, RvR showed significant reactivity to this region, although less so than the region bounded by residues Glu2181-Val2243. Curiously, LK and AA, but not HR, RvR, and YA, inhibited HP23 less than HP25. A possible explanation for this is that human amino acid residues in the region bounded by residues Gln2311-Met2321 make an unfavorable interaction with LK and AA.
Inhibition of HP26, HP27, and HP28 by LK, AA, and RVR was reduced compared with human B-domainless FVIII, indicating that, as with NMC-VIII/5, the regions bounded by residues Glu 2181-Met2199 and Lys2207-Val2243 both contribute to antibody binding. In contrast, HR and YA were relatively potent inhibitors of HP26, HP27, and HP28. These antibodies were significantly more cross-reactive with porcine FVIII. A possible explanation of these results is that HR and YA have strong interactions with amino acid residues that are identical between human and porcine FVIII in the Ser2173-Thr2253 region in addition to interactions that are human specific.
DISCUSSION
By using a series of active recombinant hybrid human/porcine FVIII molecules, we have mapped a major determinant of an epitope recognized by five human antibodies and by a murine MoAb, NMC-VIII/5, to the NH2-terminal half of the C2 domain. The antibodies used in this study were selected because most of their anticoagulant activity is neutralized by recombinant C2 domain.8 The C2-specificity of the antibodies was confirmed in the present study by showing that they inhibit HP20, which contains a porcine C2 domain substitution (Ser2173-Tyr2332), less than human FVIII. Additionally, HP24, which contains substitution of a porcine sequence corresponding to residues Glu2181-Val2243, is inhibited less than human FVIII by all of the antibodies. This establishes residues Glu2181-Val2243 as a major determinant of a C2 epitope recognized by these antibodies (Fig 4).
Sequence alignment of the C2 domains of human and porcine FVIII. The region containing the C2 epitope identified in this study is underlined.
Sequence alignment of the C2 domains of human and porcine FVIII. The region containing the C2 epitope identified in this study is underlined.
This result was unexpected, because several lines of evidence have implicated the COOH-terminal end of the C2 domain as a target for FVIII inhibitors. Inhibitory C2 antibodies block the binding of FVIII to phospholipid.29 Synthetic peptides corresponding to mature FVIII C2 domain residues Thr2303-Ile2317, Tyr2305-Tyr2332, Ile2308-Glu2322, Ala2318-Tyr2332,49Thr2303-Tyr2332,32 and Thr2303-Leu232450inhibit the binding of FVIII to phospholipid. This finding suggests that the binding sites on FVIII for phospholipid and C2 antibodies overlap and are at the COOH-terminal end of the C2 domain. Furthermore, NMC-VIII/5, which also inhibits the binding of FVIII to phospholipid,47 binds the Thr2303-Tyr2332 peptide,7 directly implying that at least part of its epitope is at the COOH-terminal end of the C2 domain. The C2 peptides corresponding to this region bind phospholipid with micromolar affinity,32,49,50 which is three orders of magnitude lower than the interaction of native FVIII with phospholipid.51This low affinity could be due to a conformational equilibrium of the peptides that favors an inactive state. However, it is also possible that additional regions in FVIII, such as the region identified in this study, are necessary for high-affinity binding to phospholipid.
Several observations suggest that the C2 domain is involved in binding vWF at a site that overlaps the phospholipid binding site. vWF and phospholipid compete for binding to FVIII.20-22 A 34-kD, monomeric, proteolytic fragment of vWF, which corresponds to amino acid residues Ser1-Arg272 of the 2050 amino acid residue subunit of the mature homopolymeric vWF multimer, also inhibits the binding of FVIII to phospholipid.52 This makes it less likely that the competition between vWF and phospholipid is merely due to steric hindrance. Additionally, NMC-VIII-5 inhibits the binding of FVIII to vWF as well as to phospholipid.47 Finally, the Thr2303-Tyr2332 synthetic FVIII peptide inhibits the binding of FVIII to vWF.32
The interaction of vWF with C2 has been studied directly by showing that recombinant C2 binds vWF.38 The affinity of this interaction is approximately 150-fold lower than that of vWF binding to FVIII light chain (a2-A3-C1-C2), which contains all of the binding energy for binding to vWF. Additionally, fragments of 14 kD (Met1672-Glu1794) and 67 kD (Asp1795-Tyr2332) generated byStaphylococcal aureus protease cleavage of the FVIII light chain each bind vWF, albeit with the lower affinity observed for C2. This is consistent with observations that have indicated that the a2 region (Glu1649-Arg1689) is necessary for high-affinity binding of FVIII to vWF33-37 but not to phospholipid. However, when the 14-kD/67-kD complex is not dissociated, its affinity for vWF is the same as that of intact FVIII light chain. These results establish the presence of at least two distinct vWF binding sites within the FVIII light chain, both of which are required for maximal binding.
The results of previous studies implicating the COOH-terminal end of the C2 domain in the binding of FVIII to inhibitory antibodies, phospholipid, and vWF can be reconciled with the results of the present study if the NH2-terminal and COOH-terminal sequences of the C2 domain are close to one another and form a single epitope. This may be the case, because residues Cys2174-Cys2326, which are at opposite ends of the C2 domain, are disulfide bonded.53
Epitope mapping studies of human inhibitors that bind C2 and the anti-C2 MoAb NMC-VIII/5 have been performed by immunoblotting analysis of recombinant C2 domain deletion polypeptides expressed in E coli.7,47 The parent polypeptide used in these studies corresponded to FVIII residues Ala1974-Tyr2332, which include the COOH-terminal 47 residues of the A3 domain and all of the C1 and C2 domains. Nested NH2-terminal and COOH-terminal deletions of the C2 domain of this polypeptide were made to determine their effect on antibody binding. Epitopes within the C2 domain or near each terminus could be identified, but not those that spanned both the NH2-termini and COOH-termini, because the disulfide bonds were reduced before immunoblotting. The binding of NMC-VIII/5 to the C2 domain was abolished by all NH2-terminal deletions and by all COOH-terminal deletions except deletion of Ala2328-Tyr2332,47 suggesting that both regions were directly or indirectly crucial for antibody binding and that the epitope was localized to Asp2170-Glu2327.
The epitope of the murine anti-FVIII MoAb ESH8, which does not interfere with either vWF or phospholipid binding of FVIII, was similarly localized to Val2248-Gly2285.7 Therefore, this region of the C2 domain is probably not involved in binding to either vWF or phospholipid. Six inhibitors that were at least partially neutralized by soluble, recombinant C2 domain were also studied. One epitope was identical to that of NMC-VIII/5. Four additional epitopes were localized to regions Asp2170-Ser2312, Thr2303-Ser2312, Ala2218-Ser2312 (two plasmas), and Val2248-Ser2312. The common epitope endpoint of Ser2312 suggests that the residues NH2-terminal to this region are either required for binding by antibodies present in five of the six plasmas or its deletion indirectly prevents antibody binding elsewhere. The requirements for antibody binding to residues near the NH2-terminus of the C2 domain were more heterogeneous. Two epitopes included residues beginning with Asp2170, suggesting that the entire NH2-terminal region was important for binding of less frequent antibodies. The other NH2-termini included Trp2203, Ala2218 (two plasmas), and Val2248.
The present study suggests that inhibitor antibodies that bind COOH-terminal to Val2248 are infrequent compared with those that bind NH2-terminal to this region because the former were present in only one of five plasmas (RvR). In contrast, the earlier immunoblotting studies suggested that antibody binding is eliminated by deletion of specific regions of either the NH2-terminus or COOH-terminus. An important consideration in reconciling these results is that immunoblotting detects all antibodies, whereas hybrid human/porcine FVIII experiments detect only inhibitors. It is known that inhibitor plasmas contain both inhibitory and noninhibitory antibodies.54 Because immunoblotting is performed with denatured, reduced proteins, they may bind a different set of antibodies than the human/porcine FVIII hybrids. An important advantage of using hybrid human/porcine FVIII molecules to map inhibitor epitopes is that they have FVIII activity, which assures that loss of antibody binding is not due to protein denaturation. However, the method cannot identify determinants of epitopes that are identical between human and porcine FVIII. Thus, common amino acid residues that contribute to antibody binding the COOH-terminal region of the C2 domain may not be detected by this method.
Coagulation factor V is homologous to FVIII and contains a A1-A2-B-A3-C1-C2 domain sequence. Ortel et al55 expressed a series of hybrid factor V/FVIII proteins that contained substitutions of FVIII in the factor V C2 domain. Using these hybrids and an inhibitory patient autoantibody to factor V that blocks the binding of factor V to phospholipid, they mapped the phospholipid binding epitope to the NH2-terminal third of the C2 domain. This result is consistent with the results of our study and suggests that the NH2-terminal region of the C2 domain of both factors V and VIII contains an immunodominant epitope that is important for phospholipid binding.
Supported by National Institutes of Health Grants No. R01-HL46215 (P.L.) and P50-HL44336 (D.S.) and by a Hemophilia of Georgia research fellowship (H.M.T.).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Pete Lollar, MD, 1639 Pierce Dr, Room 1003 Woodruff Memorial Bldg, Emory University, Atlanta, GA 30322; e-mail:jlollar@emory.edu.