Abstract
The BEACOPP (bleomycin, etoposide, adriamycin, cyclophosphamide, vincristine, procarbazine, and prednisone) regimen, a rearranged and accelerated version of the standard COPP/adriamycin, bleomycin, vinblastine, and dacarbazine (ABVD) chemotherapy, has been shown to be effective and safe in a previous pilot study for advanced stage Hodgkin’s disease (HD). The present study aimed to determine a maximum practicable dose of three drugs, ie, etoposide, adriamycin, and cyclophosphamide, for which acute toxicities were acceptable and to assess the feasibility of the escalated scheme. Sixty untreated patients with advanced stage HD were enrolled in this study. Radiotherapy was given in 44 patients (73%) after chemotherapy to initial bulk lesions and residual disease. Granulocyte-colony stimulating factor (G-CSF) was given from day 8 to prevent prolonged neutrocytopenia and severe infections. The intended doses of adriamycin, etoposide, and cyclophosphamide in the BEACOPP schedule could be substantially escalated: adriamycin from 25 to 35, cyclophosphamide from 650 to 1,200, and etoposide from 100 to 200 mg/m2. The major toxicities were leukocytopenia and thrombocytopenia with considerable heterogeneity between individual patients. Of 60 patients, 56 (93%) achieved a complete remission (CR). At a median observation of 32 months, the rates of survival and freedom from treatment failure (FFTF) were estimated to be 91% (95% confidence interval 83% to 99%) and 90% (82% to 98%). These results show that a moderate dose escalation of adriamycin, cyclophosphamide, and etoposide of the baseline BEACOPP regimen is feasible. The escalated BEACOPP regimen shows very encouraging results in advanced stage HD and is now being compared in a randomized phase III study with BEACOPP at baseline dose level.
THE TREATMENT OF CHOICE for patients with advanced Hodgkin’s disease (HD) is polychemotherapy. A number of regimens induce complete remission (CR) rates in 70% to 90% of patients.1 However, about one third of those achieving CR will subsequently relapse. For more than 20 years, the MOPP chemotherapy regimen (mustargen, vincristine, procarbazine, and prednisone), introduced by DeVita et al,2 had been considered standard treatment for advanced stages. The analysis of regimens which are not crossreactive with MOPP led to the development of the ABVD scheme (adriamycin, bleomycin, vinblastine, and dacarbacine) by Bonadonna et al.3 Subsequently, several other chemotherapy combinations were analyzed, which did not improve the results of MOPP- and ABVD-like regimens.2
Large randomized studies have compared MOPP or MOPP-like regimens with MOPP alternating with ABVD, and the superiority of alternating MOPP/ABVD over MOPP alone has been demonstrated.3,4 However, it is not yet clear whether MOPP/ABVD is superior to ABVD alone. Hybrid regimens such as MOPP/ABV, in which all cytostatic drugs are given within 8 days, did not show better remission or relapsefree survival rates when compared with the alternating MOPP/ABVD standard therapy or ABVD, but were more toxic.5 6
One possible way of improving treatment results is by dose escalation of effective cytostatic drugs. The relationship between the total dose of chemotherapy and antitumor response has been demonstrated in certain experimental tumor models where the response curve for several cytotoxic drugs is steep in the linear phase.7 A positive correlation between the dose of antineoplastic drugs and response rate was also demonstrated in human tumors in retrospective analyses.2,7 In HD, the dose of vincristine has been shown to affect the results of treatment in the MOPP scheme.8 9Prospective clinical trials proving the role of dose in HD, however, do not exist.
To study dose escalation in HD, two main questions have to be addressed: (1) which group of patients may profit from dose-escalated therapy? (2) To what extent can the doses of key drugs be safely escalated within the limits of a predefined rate of toxicities? Despite several attempts, no subgroup of patients with a very high risk of treatment failure (who may be candidates for primary high-dose chemotherapy with stem cell support) could be identified in HD. Thus, the question remained whether moderate dose escalation applied to all advanced-stage patients is possible with defined tolerable toxicities and whether this strategy could improve treatment results. A mathematical model of lymphoma growth and chemotherapy effects has been recently developed.10 The model was based on assumptions of an exponential tumor growth, chemotherapy sensitivity, the potential treatment efficacy as a function of total dose, and a net treatment efficacy at the end of treatment. It extended the simple, well-known model of chemotherapy by incorporating heterogeneity concerning tumor growth and chemosensitivity. The model allows estimation of the distribution of latency times (time until the tumor can be clinically detected) and the distribution of chemosensitivity in a patient population. The model was fitted to the data of 705 patients of stage IIIB/IV HD of the German Hodgkin’s Lymphoma Study Group (GHSG). It predicted that moderate dose escalation of 30% may lead to a potential benefit of 10% to 15% in tumor control at 5 years.
To prepare a series of studies on the role of moderate dose escalation in the treatment of advanced stage HD, the GHSG initiated a phase II study with a new scheme: BEACOPP in baseline dose.11 The BEACOPP regimen incorporates most active drugs of COPP/ABVD regimen, ie, adriamycin, cyclophosphamide, vincristine, procarbazine, bleomycin, and prednisone (Table 1). Etoposide was added, because it was assumed to have a high activity in HD and can be escalated substantially. Time schedule of application was rearranged in that (1) all cytotoxic drugs were given within 8 days and recycled after 21 days. The rescheduling of drugs allows a longer therapy-free interval with a better regeneration of hematopoiesis.11
In a first phase-II trial, BEACOPP was applied at baseline level (doses for adriamycin, cyclophosphamide, and etoposide were 25, 650, and 100 × 3 mg/m2, respectively) without granulocyte colony-stimulating factor (G-CSF). Thirty patients were recruited for this study, and 29 were evaluable for response and toxicity. Toxicities were tolerable with no treatment-related deaths. Of 29 patients, 27 reached CR. At a median follow-up of 40 months, the freedom from treatment failure (FFTF) rate was 89%.11
The objective of the present study was to escalate the doses of adriamycin, cyclophosphamide, and etoposide up to a predefined rate of acceptable hematotoxicity. The dose of adriamycin was increased at a fixed level to 35 mg/m2, whereas the doses of cyclophosphamide and etoposide were increased stepwise. This was done in an adaptive strategy to identify a maximum practicable dose level, which could be safely applied with a predefined rate of maximal hematologic and nonhematologic toxicities. G-CSF was given from day 8 to reduce neutropenia and the rate of infections.
MATERIALS AND METHODS
Patient eligibility and pretreatment diagnosis.
Patients between 16 and 65 years of age with biopsy-confirmed HD in untreated stages IIB and IIIA were eligible for this study if they had one of the following risk factors: extranodal involvement or large mediastinal mass (more than one third of thoracic diameter), massive spleen involvement, high erythrocyte sedimentation rate (ESR) (over 30), or more than three involved lymph node sites). Patients in stages IIIB and IV with or without risk factors were also eligible. Exclusion criteria included a positive human immunodeficiency virus (HIV) test, pregnancy, creatinine-clearance below 60 mL/minute, white blood cell (WBC) count less than 3,000/μL, platelet count less than 100,000/μL, serum bilirubin greater than 2 mg/dL, concurrent infections, and severe cardiac, pulmonary, or cerebral dysfunction. Each patient provided written informed consent.
Pretreatment evaluation included medical history and physical examination, complete blood count, liver and renal functional tests; ESR, chest x-ray, abdomen ultrasound, chest, abdominal and pelvic computed tomography (CT), and bone marrow biopsy. A liver biopsy was performed in 42 patients. For analysis of toxicity, echocardiography and lung functional tests were also performed. Staging laparotomy was not required to enter the study and was performed in five cases only.
Treatment protocol.
Patients were scheduled to receive eight cycles of chemotherapy. Chemotherapy was administered as described in Table 1. G-CSF (filgrastim, 300 μg for patients <70 kg body weight, 480 μg for patients >70 kg, subcutaneously [SC] once daily) was applied from day 8 for at least 3 days. G-CSF application was stopped when leukocytes exceeded 2,000/μL for 3 days after nadir or when leukocytes exceeded 50,000/μL once. The following cycle was applied on day 21 if leukocytes were above 2,500/μL and platelets above 80,000/μL.
Dose levels of adriamycin, cyclophosphamide, and etoposide are given in Table 1. The individual level was planned for all eight cycles except if the patient had a toxic event, in which case, a dose reduction was performed (see below). A toxic event occurring in one treatment cycle was defined as a WBC less than 1,000/μL for more than 4 days and/or a platelet count less than 25,000/μL and/or fever/infection World Health Organization (WHO) grade 4 and/or mucositis WHO grade 4.
We developed a generalized version of the up-and-down method of Storer12 adapted to multiple cycle chemotherapy and simultaneous treatment of patients.13 In addition to baseline level, six dose levels were also specified (Table 1). A patient was selected for an individual dose level by the study coordination center starting at level 1. The initial dose level for the subsequent patient was determined on the basis of each toxicity result (yes or no) of the previously treated patients. A toxic event at a given dose level reduced the actual dose level by 1. When two patients treated at a given level were recorded to have no toxic events, the actual level for the subsequent patient was increased by 1. At least two cycles of chemotherapy without toxic events had to elapse in two patients before assignment was made to the next level. Thus, the procedure approximated the maximum practicable level with a 33% probability of toxic events per cycle.
If a treatment delay of 1 to 2 weeks or a toxic event had occurred, a dose reduction of one level was performed for the individual patient for all subsequent cycles. In case a second toxic event or treatment delay occurred in the subsequent cycle, the dose was reduced to the baseline level; if the event occurred not in the subsequent, but in a later cycle, the dose level was reduced by two levels. If a therapy-related delay of more than 2 weeks occurred, a reduction to the baseline level was performed. If a therapy-related delay of more than a week occurred at baseline level, the doses of cyclophosphamide, adriamycin, procarbazine, and etoposide were reduced to 75%.
Consolidative radiotherapy.
Bulky disease areas (initially more than 5 cm measured by CT scan) were irradiated with 30 Gy; residual disease that appeared enlarged (> 2cm) clinically or by CT scan after eight cycles of chemotherapy was treated with 40 Gy.
Response assessment and follow up.
All patients had a physical examination, complete blood cell count, blood chemistry including ESR, CT scans of involved areas after four cycles and eight cycles of chemotherapy, and after radiotherapy. Follow-up examinations were performed within the first 2 years in 3-month intervals, at years 3 and 4 in 4-month intervals, and from year 5 in 6-month intervals.
Treatment was documented after each cycle of chemotherapy and after radiotherapy. Documentation included dose schedule, dose given, and toxicities. All data were carefully checked by two data managers and a physician. The success of treatment was determined by restaging 4 weeks after chemotherapy and 4 to 8 weeks after the termination of the protocol treatment. Restaging consisted of a controlled and careful documentation of all initial disease manifestations by adequate clinical and histologic methods. CR was defined as the disappearance of all clinical disease manifestations for at least 4 weeks; partial remission (PR) was defined as the reduction in all disease localizations by at least 50% as detected by the products of perpendicular diameters. Residual disease (>2cm) with suspected active disease after chemotherapy was allocated for radiotherapy; residual disease after chemo- and radiotherapy was considered as CRR (CR with residual lesion), which was to be observed, but not treated further.
Biometry.
Dose intensity was calculated as total dose per m2 divided by the duration of the entire therapy in weeks. Because the “last day of therapy” was not documented as such, this was taken to be 21 days after the beginning of the last cycle.
FFTF was defined as the time from the start of therapy to the first of the following events: death, progressive disease, non-CR status at the end of the protocol treatment or relapse. Survival times were obtained and included all deaths, whether disease-related or not. Kaplan-Meier estimates are given for the probabilities of survival beyond a given time.
A statistical model for the probability of a toxic event in a given BEACOPP cycle as a function of dose level was fitted to the toxicity data. The probability of toxic response was assumed to increase logistically (ie, in an S-shaped curve) with increasing dose. The “random effects” model allows for patient heterogeneity in susceptibility to toxicity. The influence of the parameters dose level, sex, and cycle was analyzed.
RESULTS
Patient characteristics.
Table 2 lists the clinical characteristics of the 60 patients recruited. Median age was 31 years with a range between 16 and 62. Thirteen percent of the patients were in stage IIB and had additional risk factors, either large mediastinal mass, massive spleen, or extranodal involvement. Fifty percent were in stage III and 37% in stage IV, respectively. Thus, most patients had both advanced stage disease and additional clinical risk factors such as bulky disease (68%), a large mediastinal mass (25%), extranodal involvement (48%) including bone, skin, pleura, and lung. Among patients with stage IV (n = 21), there were 10 patients with liver involvement, six with infiltration in the bone marrow, eight with lung infiltration, and six with bone infiltration. The histologic subtype in most patients was nodular sclerosis (NS, 80%), followed by mixed cellularity (MC, 15%), whereas lymphocyte predominant (LP) subtype was rare (2%).
Dose escalation within the BEACOPP scheme.
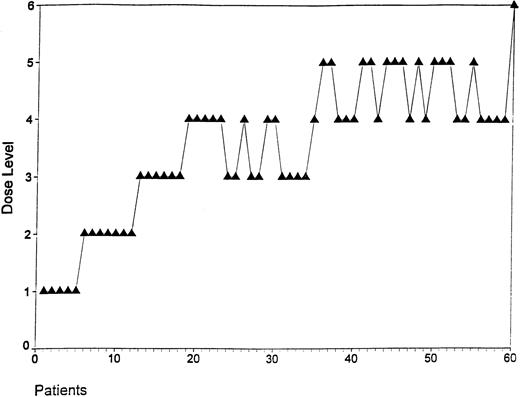
The evolution of dose levels of this adaptive escalation study is given in Fig 1. Because the information about whether a toxic event had occurred was not always available at the beginning of the treatment for an individual patient, more than three patients were recruited at levels 1 and 2.
Evolution of dose levels in the BEACOPP scheme. Each dot represents the initial dose level of a patient recruited in the study. Dose escalation in the cohort of patients was performed as described.
Evolution of dose levels in the BEACOPP scheme. Each dot represents the initial dose level of a patient recruited in the study. Dose escalation in the cohort of patients was performed as described.
The intended dose levels of cyclophosphamide and etoposide were compared with the actual given levels achieved on average in all cycles (Fig 2). Intended dose refers to the initial dose level in the first cycle for each patient and to the intended course duration of 8 × 21 days. Actual dose intensities refer to the total given dose and to the actual duration of the course of all given cycles (last cycle taken as 21 days). Given dose intensities of cyclophosphamide and etoposide were lower than intended dose intensities, because (1) not all cycles were repeated after 21 days and (2) reduction of dose levels was performed in some patients due to toxicities. Sixty-eight percent of cycles were given after 21 to 25 days, and 85% of cycles were given within 30 days. Although the intended dose intensities could not be reached, the given dose intensities increased parallel to the intended levels. G-CSF administration was documented in 66% of all cycles, with a mean duration of administration of 7.4 days (maximum, 23 days).
Comparison of the intended dose levels of cyclophosphamide and etoposide with the actual given levels achieved on average in all cycles. Intended dose refers to the initial dose level in the first cycle for each patient and to the intended course duration of 8 × 21 days. Actual dose intensities refer to the total given dose and to the actual duration of the course of all given cycles (last cycle taken as 21 days). Patients separated according to sex and arranged in order of increasing initial dose level. (▴) Given DI of C; (•) given DI of E; (▴) intended DI of C; (•) intended DI of E.
Comparison of the intended dose levels of cyclophosphamide and etoposide with the actual given levels achieved on average in all cycles. Intended dose refers to the initial dose level in the first cycle for each patient and to the intended course duration of 8 × 21 days. Actual dose intensities refer to the total given dose and to the actual duration of the course of all given cycles (last cycle taken as 21 days). Patients separated according to sex and arranged in order of increasing initial dose level. (▴) Given DI of C; (•) given DI of E; (▴) intended DI of C; (•) intended DI of E.
Radiotherapy.
A total of 44 patients (73%) received radiotherapy for initial bulky disease alone (n = 12), residual disease alone (n = 2), both bulky and residual disease (n = 28), or erroneously without either documented bulky or residual disease (n = 2). Two patients terminated chemotherapy before remission status could be determined, and one could not be irradiated due to severe toxicity after chemotherapy, from which he died.
Toxicities.
Table 3 lists the frequency of toxicities (WHO grade III and IV) observed in 452 cycles. Major toxicities included leukocytopenia, thrombocytopenia, and anemia. Leukocytopenia and thrombocytopenia occured more frequently at dose levels 4 to 6 as compared with levels 1 to 3. However, the high percentage of leukocytopenia (71% to 76%) was not accompanied by a similar rate of infections, which only occurred in 2% of all cycles. Platelet substitutions were performed in 7.5% and erythrocyte substitutions in 23% of cycles. Nausea, gastrointestinal toxicities, pain, and respiratory toxicities were reported with low frequencies.
When the rate of toxic events (as defined above) was compared in individual patients, it appeared that there was substantial heterogeneity (Fig 3). First, female patients suffered more frequently from toxic events than male patients (P < .0001). Second, the rate of toxic events increased steadily from the second to the eighth cycle, which suggests a cumulative effect (P < .001). However, the first cycle was significantly more toxic than the following two cycles. Third, toxic events occurred in some patients during several successive cycles, although these patients had been subsequently treated at lower dose levels or at baseline level. The statistical analysis also showed a significant effect of dose level (P < .01). The probability of toxic events increased with dose level, giving a maximum practical dose at level 4. At this level, the probability of a toxic event was about 33% averaging over all treatment cycles.
Occurence of toxic events in individual patients treated at different dose levels. (▪) Toxic response; (○) no toxicity.
Occurence of toxic events in individual patients treated at different dose levels. (▪) Toxic response; (○) no toxicity.
Treatment results.
After chemotherapy alone, clinical CR was reported in 25 and PR in 30 of 60 patients. All patients in PR received additional radiotherapy at residual sites. In addition, initial bulky disease was irradiated. After consolidating radiotherapy, 56 of 60 patients (93%) were in CR (Table 4). The four patients who did not reach a CR were as follows: one patient was treated initially with two cycles of BEACOPP and was in PR. Due to toxicities, he was then treated with two cycles COPP/ABVD and went into CR (considered as failure). One patient developed a progressive lymphoma during therapy, which was diagnosed as a non-Hodgkin’s lymphoma (NHL) and died of lymphoma. One patient died of septicemia during therapy. One patient stopped treatment and died of HD.
Furthermore, two patients developed acute leukemia after chemotherapy: one patient was treated originally at level 4 and developed myelodysplastic syndrome 18 months after the beginning of chemotherapy. Ten months later, a secondary leukemia was diagnosed. A second patient initially treated at level 3 developed a secondary leukemia 35 months after chemotherapy and died of leukemia. One patient developed a colon carcinoma 15 months after treatment and died. So far, six of 60 patients died due to relapse of HD (n = 1), progression (n = 1), toxicity (n = 1), development of a NHL (n = 1), secondary leukemia (n = 1), and colon carcinoma (n = 1).
At a median observation of 32 months, FFTF and overall survival rates were estimated to be 91% (95% confidence interval 83% to 99%) and 90% (82% to 98%). Kaplan-Meier plots are given in Fig 4.
Estimates of FFTF and SV for patients treated with BEACOPP. FFTF, freedom from treatment failure; SV, overall survival. (□) FF; (◊) 7 failed/60; (□) SV; (+) 6 dead/60.
Estimates of FFTF and SV for patients treated with BEACOPP. FFTF, freedom from treatment failure; SV, overall survival. (□) FF; (◊) 7 failed/60; (□) SV; (+) 6 dead/60.
DISCUSSION
Role of dose escalation in the treatment of HD.
The role of dose escalation in HD is not yet clear. A retrospective analysis showed that the total dose of mustargen, vincristine, and procarbazine correlated with the CR rate.8 9 Prospective clinical studies analyzing the role of dose escalation, however, have not yet been performed.
The predominant dose-limiting toxicity of many combination chemotherapy regimens is myelosuppression, particularly neutrocytopenia. G-CSF promotes the proliferation and differentiation of neutrophil precursors and enhances the effector functions of mature neutrophils in vitro and in vivo.14 It has been shown that G-CSF can lead to a reduced duration of neutrocytopenia, reduction in the number of febrile days, a reduced incidence of infections, and as a result, fewer days on antibiotics.15 16 Furthermore, clinical trials have shown that G-CSF facilitates the delivery of cytotoxic chemotherapy and leads to fewer treatment delays or dose reduction.
The use of hematopoietic growth factors may allow a significant escalation of dose intensity when a regimen is used with myelosuppression as the dose-limiting side effect. In a randomized study in NHL, patients treated with chemotherapy plus G-CSF achieved a greater dose intensity than control patients without growth factor.16 The role of G-CSF in dose escalation, however, is still controversial and previous studies have reported both positive and negative results with respect to the efficacy of colony-stimulating factors.15-18 Interestingly, none of these studies could demonstrate a significant increase in tumor control or overall survival.
Dose escalation within the BEACOPP scheme.
The BEACOPP scheme was originally designed to study the role of a moderate dose escalation in advanced stage HD in a comprehensive way. A parametric model was developed to describe tumor growth and chemotherapy effects and was fitted to data from more than 700 patients treated with COPP/ABVD or COPP/ABV/IMEP in the HD6 study of the GHSG. The model predicted that a moderate dose increase by about 30% of the standard chemotherapy doses would raise the CR rate by about 15%.10
The standard COPP/ABVD scheme has a number of disadvantages in studying the role of dose escalation of key cytotoxic drugs. Therefore, the GHSG developed a new regimen, which contains the most active drugs in the same doses as COPP/ABVD. Dacarbazine was replaced by etoposide, and vinblastine was omitted. Etoposide was incorporated because studies showed a single-agent response rate of 25% in refractory HD.19 In addition, etoposide has been used successfully in a variety of second-line regimens in HD and is an essential part of the commonly used high-dose myeloablative regimens BEAM (BCNU, etoposide, cytosine arabinoside and melphalan) and CVB (cyclophosphamide, etoposide and BCNU).20 21
The schedule within the BEACOPP scheme was rearranged so that all major myelosuppressive drugs were applied within the first 3 days of the cycle. G-CSF was introduced from day 8 to reduce prolonged leukocytopenia. The BEACOPP scheme was planned to be repeated at day 21 (as compared with day 28 in COPP/ABVD), which resulted in a dose intensification by approximately 30%.
In a phase-II study presented elsewhere,11 30 patients in advanced stage HD were treated with the BEACOPP regimen at baseline level without G-CSF application. Twenty-five of 30 patients received more than 80% of the planned dosage of drugs. Twenty-seven of 29 evaluable patients reached a CR. The FFTF rate was 89% at a median follow-up time of 40 months.
The major result of the present study is that a moderate yet potentially effective dose escalation of three major cytotoxic drugs is possible at controlled levels of tolerable toxicities. The dose increase is in the order of magnitude required for testing the model prediction of a 10% to 15% outcome improvement.13
Toxicities of BEACOPP.
Although transient leukocytopenias grade 3 to 4 were more common with BEACOPP than detected with COPP/ABVD, there were only a few serious infections. Whether this low incidence is due to the prophylactic application of G-CSF is not clear. Toxic events in female patients occurred more frequently than in male patients, and a considerable cumulative effect was detected after multiple cycles.
The most serious late complication of HD therapy is the development of a second cancer. In this study, four patients developed secondary malignancies: two leukemias 28 months and 35 months after therapy, one high-grade NHL 8 months and one colon carcinoma 31 months after therapy. The overall risk of acute myeloid leukemia after HD ranges from 0.5% to 2% per year for the first 10 years.22Alkylating agents are probably associated with leukemia. There may also be an enhanced risk for secondary leukemia and lymphomas after treatment with etoposide. However, most cases of epipodophyllotoxin-associated leukemia have occurred at a cumulative etoposide doses significantly greater than those used in our study and in patients who received the drug on a weekly or biweekly schedule.23,24 The most commonly observed second cancers in patients with HD are solid tumors, which are related to the use of radiotherapy and which develop in up to 13% of patients after 15 years.22 Thus, the final evaluation of chronic toxicities requires a very long follow-up time.
We have as yet no data on fertility following BEACOPP, but it is expected that, as with COPP/ABVD, a high proportion of male patients will be sterile.25
Results of BEACOPP in comparison to other regimens.
The CR, FFTF, and survival rates of the BEACOPP chemotherapy in combination with adjuvant radiotherapy are very encouraging. When compared with the standard COPP/ABVD regimen of the GHSG used in a similar cohort of patients, the results compare favorably and show a significantly higher response and FFTF rate. Most interestingly, the rate of patients with primary progressive disease who have a very unfavorable prognosis is 16% in COPP/ABVD, but less than 2% in the present BEACOPP study. Whether adjuvant radiotherapy to bulky sites and residual disease is necessary is unknown. A recent meta-analysis indicates that combined modality treatment in patients with advanced HD has a significantly inferior long-term survival outcome than chemotherapy alone, if chemotherapy is given over an appropriate number of cycles.26
Reports from randomized trials suggest that ABVD alone is equally effective to MOPP/ABVD and to hybrid MOPP/ABV.4,6 In addition, ABVD has only moderate toxicities with respect to fertility and secondary leukemia in contrast to MOPP.6 However cardiopulmonary toxicities of ABVD could be considerable.27
Recently, other promising regimens have been developed such as Stanford V, in which a brief chemotherapy regimen including doxorubicin, vinblastine, mechlorethamine, vincristine, bleomycin, etoposide, and prednisone plus consolidative radiotherapy for most patients was applied over 12 weeks. Treatment results showed an actuarial 3-year failure-free survival rate of 87%, a survival rate of 96%, and no treatment-related deaths.28 Because this trial was performed at a single institution, prospective randomized multicenter trials are required to compare efficacy and toxicities of Stanford V and BEACOPP with standard protocols.
Conclusions.
Taken together, the results of this study show that with controlled tolerable toxicities, the doses of adriamycin, etoposide, and cyclophosphamide of the 21-day BEACOPP regimen can be escalated considerably in patients with advanced HD. Dose-escalated BEACOPP chemotherapy with additional radiotherapy is safe and shows promising tumor control. In the HD9 study of the GHSG, the BEACOPP scheme at dose level 4 with G-CSF is being currently compared with standard COPP/ABVD and with BEACOPP at baseline dose without G-CSF. To date, more than 900 patients have been recruited in this prospective randomized trial.
Participating study centers: Münster, Med. Klinik (P. Koch); Köln, Med. Klinik (H. Tesch, B. Lathan, M. Sieber, V. Diehl); Göttingen, Med. Klinik (G. Wulf); Homburg, Med. Klinik (M. Pfreundschuh); Kiel, Med. Klinik (N. Schmitz); Stuttgart, Städt. Klinik (K. P. Schalk); Lübeck, Med. Klinik (G. Schwieder); Freiburg, Med. Klinik (G. Dölken); Mannheim, (P. Worst); Nürnberg, Klinik V (U. Bruntsch); Essen-Werden, Ev. KH (T. Tirier); München, Klinik re. d. Isar (U. Müller).
Responsibilities: Data monitoring, M. Sieber, H. Nisters-Backes; biometry, M. Loeffler, D. Hasenclever; Writing committee, H. Tesch, J. Franklin, D. Hasenclever, M. Loeffler.
Supported by a grant of the Deutsche Krebshilfe, Bonn, Germany.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to V. Diehl, MD, Klinik I für Innere Medizin, Universität Köln, 50924 Köln, Germany.