Abstract
The deficiency of Rh proteins on the red blood cells from individuals of the Rhnull amorph type may be the result of homozygosity for a silent allele at the RH locus. This phenotype is also associated with the lack or reduced expression of glycoproteins (Rh50, CD47, LW, and glycophorin B), which interact with Rh polypeptides to form the multisubunit Rh membrane complex. In this study, we describe two molecular alterations affecting the RHCEgene in two unrelated Rhnull amorph individuals bearing Rh50 and CD47 normal transcripts. The first type of mutation, located at the donor splice-site in intron 4, induced the activation of two cryptic splice-sites within this intron and one such site in exon 4 that all generated aberrant transcripts. The second type of mutation affected the coding region and introduced a frameshift and a premature stop codon resulting in a shorter predicted protein (398 v 417 residues), including a completely different C-terminus of 76 amino acids. This suggests that protein folding and/or protein-protein interaction mediated by the C-terminal domain of the Rh proteins may play a role in the routing and/or stability of the Rh membrane complex.
THE HUMAN RH LOCUS is composed of two highly homologous genes RHD and RHCE in the RhD-positive phenotype, whereas the RHD gene is absent in the Caucasian RhD-negative phenotype.1 The RHD andRHCE genes comprise 10 exons and encode the Rh proteins that carry the D and CcEe antigens, respectively. These proteins are believed to be part of a multisubunit complex in the red blood cell (RBC) membrane together with glycoproteins including Rh50, a member of the Rh protein family,2-4 CD47, glycophorin B, and LW (for review see Cartron,5 Cartron and Agre,6 and Anstee and Tanner7). The Rhnull phenotype is characterized by the lack of all Rh antigens on the RBC membrane and by the absence or severe decrease of the other members of the Rh complex.8-10 These individuals exhibit a mild clinical syndrome called the Rh-deficiency syndrome, characterized by a chronic hemolytic anemia of varying severity, with RBCs showing stomatocytosis and spherocytosis, increased osmotic fragility, altered cation transport, and abnormal phospholipid organization.11-15Rhnull phenotypes are currently distinguished into two types called regulator and amorph (or silent). The regulator type, the most common of Rhnull, is caused by the homozygosity of a rare autosomal suppressor gene (X°r, responsible for the phenotypic suppression of Rh antigen expression on RBCs)unlinked to the RH locus.8,9 Recent studies from our laboratory16 17 (Chérif-Zahar et al, manuscript submitted) have elucidated the molecular basis of this phenotype and identified 6 different mutant alleles of the RH50gene, which is therefore regarded as the most likely candidate suppressor gene.
The amorph type is rare and arises from homozygosity of a silent allele at the RH locus8,9 in which the RHD gene is absent in the individuals studied so far.18,19 In contrast, the RH locus may be composed of one or two genes in the Rhnullregulator phenotype. To date, only 7 cases of Rhnull amorph individuals have been studied at the serological level,8,20 21 yet their molecular defects remained unknown. In the present report, we describe for the first time the molecular alterations affecting the RHCE gene in 2 unrelated Rhnull amorph individuals.
MATERIALS AND METHODS
Blood samples.
The 2 Rhnull samples of the amorph type from German (patient DR) and Spanish (patient DAA and family members) origin, which were described previously,21 22 were collected on EDTA and heparin, respectively, and investigated in Paris. Blood samples from common RhD-positive (CCDee) and RhD-negative (ccddee) phenotypes were obtained from the Institut National de la Transfusion Sanguine (Paris, France).
Flow cytometry and Western blot analysis.
Immunostaining of intact RBCs was performed as described,23using murine monoclonal antibodies (MoAbs) directed against CD47 (MoAb 6H9; gift of Dr M. Telen, Durham, NC) and the Rh50 glycoprotein (2D10; gift of Dr A. vom dem Borne, Amsterdam, The Netherlands). Human monoclonal anti-D, -C, -c, -E, and -e were blood grouping reagents provided by the Centre National de Références sur les Groupes Sanguins (CNRGS; Paris, France). The murine monoclonal anti-LW was provided by Dr H.H. Sonneborn (Biotest, Dreieich, Germany). Fluorescein-conjugated F(ab′)2 fragments of goat antimouse IgG (Immunotech, Marseille, France) were used and the mean fluorescence intensity was determined with a FACScan flow cytometer (Becton Dickinson, San Jose, CA). Determination of antigen site densities was performed with QIFIKIT microbeads coated with variable numbers of mouse Ig molecules (Biocytex, Marseille, France). RBC membrane proteins (60 μg) were separated on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; 10% acrylamide), transferred onto nitrocellulose sheets, and incubated with rabbit anti-Rh protein antibodies MPC4 (1:800 dilution) and MPC8 (1:2,000),24 with anti-Rh50 protein murine MoAb 2D10 (1:1,250) and with an anti-p55 antibody (1:10,000) prepared by immunizing rabbits with synthetic peptides against residues 28 through 47 of the p55 protein.25 Secondary antibodies were antirabbit or antimouse IgG peroxidase-tagged antibodies (Biosys, Compiègne, France) as required. Immunoblots were stained with the ECL chemiluminescent system (Amersham, Bucks, UK).
Reverse transcription coupled with polymerase chain reaction (PCR) amplification.
Reticulocyte RNAs from 30 mL of peripheral blood of Rhnullpatients were extracted by selectively lysing RBCs with the Orskov reaction.26 One microgram of RNAs was reverse transcribed in a total volume of 33 μL using the First Strand cDNA synthesis kit (Pharmacia, Uppsala, Sweden) following the manufacturer's instructions. Five microliters of cDNA products were then subjected to PCR in 50 mmol/L KCl, 10 mmol/L Tris (pH 8.3), 0.01% gelatin, 0.2 mmol/L of the four dNTPs, 50 pmol of each primer, and 2.5 U ofTaq polymerase (Perkin-Elmer-Cetus, Norwalk, CT). PCR conditions were as follows: denaturation for 5 minutes at 94°C followed by denaturation for 1 minute at 94°C, annealing for 1 minute at 55°C, and extension 1 minute and 30 seconds at 72°C for 30 cycles. Relevant PCR fragments were purified on a 1% low melting agarose gel followed by a Wizard PCR preps minicolumns (Promega, Madison, WI) and subcloned into a PCR II vector using the TA cloning kit (Invitrogen, Leek, Netherlands).
Genomic DNA analysis.
DNA was prepared from peripheral leukocytes by the SDS/proteinase K extraction procedure.27 Exon/intron junctions of theRHCE gene from patient DAA were amplified and directly sequenced. The list of the primers used is given in Table 1. The experimental conditions for PCR were as described above, except that the extension step was for 30 seconds at 72°C. Long-range PCR was used to amplify exon 4-exon 6 and exon 6-exon 7 DNA fragments and was performed in a total volume of 50 μL containing 350 μmol/L dNTPs, 120 ng of each primer, 300 ng of genomic DNA, 2.5 U of enzyme mix (Taq and Pwo DNA polymerases) from the Expand long template PCR system (Boehringer Mannheim) and 5 μL of 10× PCR buffer (500 mmol/L Tris-HCl, pH 9.2, 160 mmol/L (NH4)2SO4, 17.5 mmol/L MgCl2). Amplification conditions were as follows: 2 minutes at 94°C (1 cycle); 10 seconds at 94°C, 30 seconds at 65°C, and 4 minutes at 68°C (10 cycles); 10 seconds at 94°C, 30 seconds at 65°C, and 4 minutes at 68°C with additional 20 seconds of elongation for each cycle (15 cycles); and a final elongation step of up to 7 minutes at 68°C.
A 200-bp fragment, encompassing exon 4-intron 4 junction, from DAA and her family members was obtained using primer P (Table 1) and the antisense primer 5′-TCTGAGCCATTCTGCTCAGCC-3′. PCR conditions were as follows: denaturation for 5 minutes at 94°C and 30 cycles of 30 seconds at 94°C, 30 seconds at 58°C, and 30 seconds at 72°C. A 135-bp fragment, encompassing exon 7 from DR sample, was obtained with sense primer 5′-TGTGTTGTAACCGAGT-3′ and antisense primer 5′-ACATGCCATTGCCG-3′ after 30 cycles as follows: 30 seconds at 94°C, 30 seconds at 50°C, and 30 seconds at 72°C. PCR products were purified through a microcon ultrafiltration membrane (Amicon, Epernon, France).
Ribonuclease protection assays.
A chimeric probe encompassing 149 nucleotides (nt) between positions −1 and 148 of the RhIXb cDNA28 and 270 nt between positions 162 and 431 of the Rh50 cDNA2 was constructed using an experimental strategy previously described.29 In a first step, the two cDNA regions were amplified using sense primer Rp1 5′-GATGAGCTCTAAGTACCCGCGG-3′ and antisense primer Rp25′-CATATGCTAGCATCGATGTTAACCTTGATAGGATGCCACGAGC-3′ for Rh cDNA and sense primer Rp35′-GTTAACATCGATGCTAGCATATGCC AAGATGTACATGTTATGA-3′ and antisense primer Rp45′-ATGATCAGCATTTGGGTGGGGCTCG-3′ for Rh50 cDNA. In a second step, the PCR products were mixed and amplified with primers Rp1 and Rp4. The chimeric PCR product was cloned into a pCR II vector using the TA cloning kit (Invitrogen). From the linearized recombinant plasmids, antisense RNA probe was synthesized using [α-32P]-UTP (800 Ci/mmol; NEN, Boston, MA) in an in vitro transcription system (Riboprobe Core System; Promega). The full-length transcript was purified from a 5% acrylamide, 8 mol/L urea gel with 0.5 mol/L ammonium acetate, 1 mmol/L EDTA, and 1% SDS. The Rh-Rh50 RNA probe (1.5 × 105cpm) was hybridized with 25 μg of total reticulocyte RNAs overnight at 52°C and digested with RNase A/T1 mixture, following the manufacturer's instructions (RPAII; Ambion, Austin, TX). The protected RNA fragments were separated through a 5% denaturing polyacrylamide gel. The autoradiograph was digitized by a StudioStar AGFA scanner and the hybridizing signals were quantified using the public domain NIH Image program (developed at the US National Institutes of Health, Bethesda, MD).
DNA sequencing.
Sequencing was performed on an automated ALF express sequencer (Pharmacia) using either the Cy5 Autoread sequencing kit (Pharmacia) with plasmid DNA or the Thermo Sequenase sequencing kit (Amersham) for direct sequencing of PCR products, following the manufacturer's instructions.
RESULTS
Cell surface expression of the antigens and proteins from the Rh membrane complex.
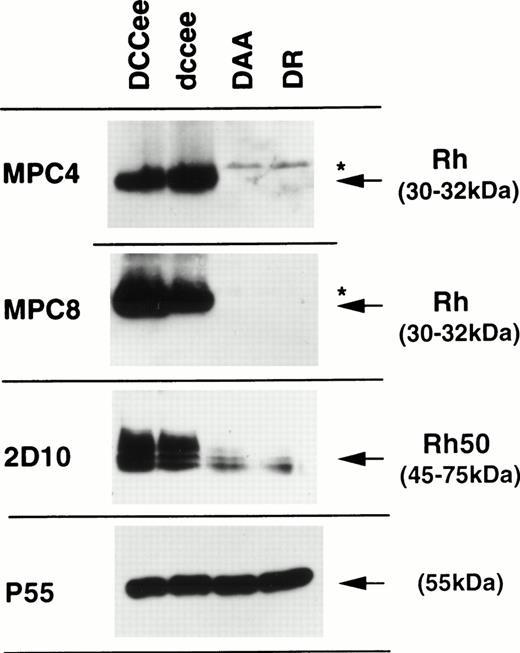
RBC samples from the two Rhnull patients, DAA and DR, were analyzed by flow cytometry with MoAbs specific for Rh (D, C, c, E, and e) and LW antigens, as well as MoAbs directed against Rh50 and CD47 (Table 2). Samples from DAA and DR did not react with anti-Rh and anti-LW reagents and reacted only weakly with Rh50 (∼25%) and with CD47 (<10%) as compared with controls. The absence of Rh protein in DAA and DR was also confirmed by Western blot analysis (Fig 1). Antibodies MPC4 and MPC8, that recognize residues 224-233 and 408-416 of the Rh protein,24 respectively, did not react with the membrane proteins of the two Rhnull samples. In contrast, a small amount of Rh50 glycoprotein was detected in RBCs from these patients with the MoAb 2D10. All samples exhibited normal amounts of protein p55, used as a control, which is a palmitoylated peripheral membrane protein present in all RBCs except those from patients deficient in protein 4.1 and glycophorin C.30 Previous serological and Western blot analyses of the DAA family members showed that Rh, LW, and Rh50 proteins were normally expressed on their RBCs.21
Immunostaining of RBC membrane proteins from erythrocytes of DAA and DR patients. RhD-positive (DCCee) and RhD-negative (dccee) membrane proteins were used as controls. The Rh50 glycoprotein carries an N-linked polylactosylaminyl carbohydrate chain2 and is shown as a band of 45- to 75-kD. Antibodies (see the Materials and Methods) are identified on the left-hand side. Stars indicate nonspecific bands occasionally seen from sample to sample and masked in controls due to high intensity of the Rh-specific band.
Immunostaining of RBC membrane proteins from erythrocytes of DAA and DR patients. RhD-positive (DCCee) and RhD-negative (dccee) membrane proteins were used as controls. The Rh50 glycoprotein carries an N-linked polylactosylaminyl carbohydrate chain2 and is shown as a band of 45- to 75-kD. Antibodies (see the Materials and Methods) are identified on the left-hand side. Stars indicate nonspecific bands occasionally seen from sample to sample and masked in controls due to high intensity of the Rh-specific band.
Southern blot analysis.
Earlier reports by us and others in which the Rh cDNA probe was hybridized with genomic DNA from patients DAA and DR, digested withHindIII18 and BamHI,19respectively, have shown that both patients carry only the RHCEgene. Moreover, no gross alteration seemed to affect the RH locus, as deduced by comparison to the hybridization pattern of the RhD-negative control. Hybridization of the same probe, on EcoRI andBamHI restriction digests of DAA DNA, and on HindIII and EcoRI digests of DR DNA led to the same conclusions (not shown).
Detection of the mutation in the RHCE gene from patient DAA.
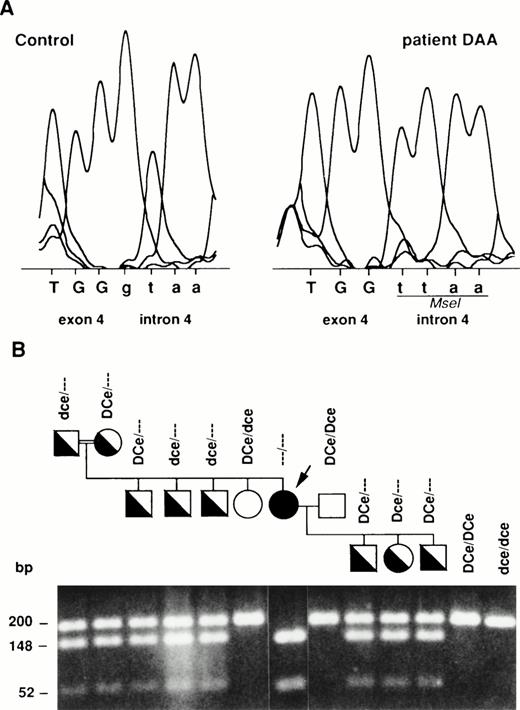
We have previously shown that no mutation was present either in the Rh cDNA or in the proximal promoter of the unique RHCE gene from patient DAA.18 This raised the possibility that the silentRH allele in DAA may be caused by a splice-site mutation. Consequently, we have amplified and sequenced all exon-intron junctions from exon 1 to exon 7 of the RHCE gene using the primer-pairs described in Table 1. A g-to-t transversion was identified at the invariant 5′ donor splice-site of intron 4 (Fig 2A). This mutation was predicted to create a novel Mse I restriction site in genomic DNA. TheMse I polymorphism was used to test the inheritance of the splice-site mutation in the DAA family by a PCR-restriction fragment length polymorphism (RFLP) assay (Fig 2B). Consistent with the genotype of each family member, only the 200-bp genomic fragments from DAA's husband and sister remained undigested, whereas it was cleaved into two fragments of 148 and 52 bp in the propositus, thus indicating that DAA was homozygous for the mutation. All other samples exhibited a heterozygous pattern consisting of both uncleaved and cleaved amplification products.
Mutation of the RHCE gene in DAA and its inheritance in the family. (A) The g nucleotide of the 5′ donor splice-site of intron 4 from the normal RHCE gene in the RhD-negative control is replaced by a t in DAA. The Mse I site created by this mutation is shown. Intronic sequences are in lowercase. (B) Tree of family DAA and Mse I restriction pattern of the genomic PCR product encompassing the exon 4-intron 4 junction are shown. The “silent” RH chromosome segregating in the family is indicated by “- - -”. Solid symbols refer to homozygous (DAA propositus, arrow) or heterozygous individuals for the silent RH gene. Open symbols refer to wild-type RH genes. The 200-bp amplification product is digested with Mse I and is cleaved into two fragments of 148 and 52 bp in the mutated gene. PCR products from RhD-positive (DCe/DCe) and RhD-negative (dce/dce) controls were uncleaved.
Mutation of the RHCE gene in DAA and its inheritance in the family. (A) The g nucleotide of the 5′ donor splice-site of intron 4 from the normal RHCE gene in the RhD-negative control is replaced by a t in DAA. The Mse I site created by this mutation is shown. Intronic sequences are in lowercase. (B) Tree of family DAA and Mse I restriction pattern of the genomic PCR product encompassing the exon 4-intron 4 junction are shown. The “silent” RH chromosome segregating in the family is indicated by “- - -”. Solid symbols refer to homozygous (DAA propositus, arrow) or heterozygous individuals for the silent RH gene. Open symbols refer to wild-type RH genes. The 200-bp amplification product is digested with Mse I and is cleaved into two fragments of 148 and 52 bp in the mutated gene. PCR products from RhD-positive (DCe/DCe) and RhD-negative (dce/dce) controls were uncleaved.
Analysis of the RhCE transcript in Rhnull patients.
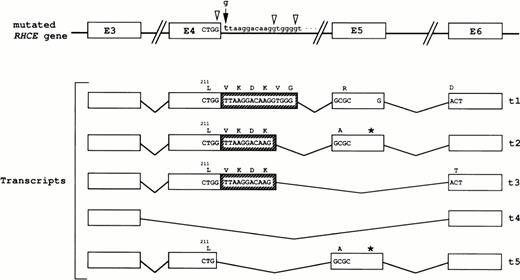
The Rh cDNAs in patient DAA were amplified between nt 337 and nt 939 using primers L and M (Table 1), which were deduced from exon 3 and exon 6 of the RHCE sequence, respectively. A major expected product of 603 bp and a minor product of 330 bp (corresponding to the amplified region of a spliceoform lacking exons 4 and 531,32 ) were observed in the RhD-negative control, whereas 4 PCR products were amplified in DAA sample (not shown). These cDNA fragments were cloned and sequenced. Three abnormal transcripts were identified (Fig 3) that most likely arise from activation of two cryptic sites: transcript t1 included the first 16 nucleotides of intron 4, whereas transcripts t2 and t3 contained the first 11 nucleotides. Transcript t3 differed from t2 by the loss of the nucleotide region of exon 5. The smaller transcript, t4, resulted from the skipping of both exons 4 and 5 nucleotide regions and corresponded to a spliceoform already known in common phenotypes31 32(ie, the 330-bp PCR product in the RhD-negative sample; see above). The predicted consequence of the new boundary between exon 4 and exon 5 (or exon 6) is the insertion of either 6 or 4 residues after leucine 211 (Fig 3). The nucleotide inclusions in transcripts t1 and t2 introduced a frameshift creating premature termination codons 314 and 99 nt downstream from these insertions, respectively. The abnormal predicted proteins are 321 (t1) and 231 (t2) amino acids long and both contain the first 211 residues of the normal Rh protein, which comprises 417 residues. The reading frame was not altered in transcripts t3 and t4, the size of their predicted proteins being 364 and 312 amino acids, respectively.
RHCE gene mutation and transcripts in patient DAA. The invariant g residue at the donor splice-site in intron 4 is mutated to t. The three cryptic splice-sites are indicated by open arrowheads. The schematic representation of the exons 3 to 6 (E) and introns in the mutated gene is shown (not to scale), as well as those of the amplified regions (see text) of 5 different transcripts (t1 through t5). The first 16 and 11 nucleotides of intron 4 (hatched boxes) were included in t1 and t2/t3 transcripts, respectively. The 6 and 4 amino acid residues inserted in the corresponding predicted proteins are indicated. The smaller transcript, t4, resulted from the skipping of both exons 4 and 5 nucleotide regions. Transcript t5 differs from the normal RhCE cDNA only by the deletion of the last G nucleotide of exon 4. The approximate positions of stop codons in t2 and t5 transcript are indicated by stars.
RHCE gene mutation and transcripts in patient DAA. The invariant g residue at the donor splice-site in intron 4 is mutated to t. The three cryptic splice-sites are indicated by open arrowheads. The schematic representation of the exons 3 to 6 (E) and introns in the mutated gene is shown (not to scale), as well as those of the amplified regions (see text) of 5 different transcripts (t1 through t5). The first 16 and 11 nucleotides of intron 4 (hatched boxes) were included in t1 and t2/t3 transcripts, respectively. The 6 and 4 amino acid residues inserted in the corresponding predicted proteins are indicated. The smaller transcript, t4, resulted from the skipping of both exons 4 and 5 nucleotide regions. Transcript t5 differs from the normal RhCE cDNA only by the deletion of the last G nucleotide of exon 4. The approximate positions of stop codons in t2 and t5 transcript are indicated by stars.
Interestingly, the neighboring nucleotide, upstream from the splicing mutation in intron 4 of the RHCE gene in patient DAA, is a guanine, thus creating a third potential splice-site (Fig 3). Indeed, computer splice-site prediction, using the scoring method of Shapiro and Senapathy,33 identified this new site as the third highest score after the two sites used in transcripts t1 and t2/t3. To test whether this potential splice-site was activated in vivo, we performed an RT-PCR assay on RNA reticulocytes from patient DAA and a control, using the sense primer 5′-ATGCTGGCGCCCTCTTCT-3′ and the antisense primer M (Table 1). The sense primer should correspond to the potential exon 4-exon 5 boundary of a hypothetical transcript if the third cryptic splice-site were used. A PCR product of the expected size (321 bp) was observed in both DAA and control samples (not shown). This result confirmed the existence in DAA of an additional transcript (t5) that differs only by the deletion of a G nucleotide at position 635 from the normal RhCE cDNA (ie, the last nucleotide of exon 4; Fig 3). In addition, by sequencing the Rh cDNA region between nucleotides −19 and 680, we have demonstrated that the silent RH gene in DAA was an RHce allele (not shown).
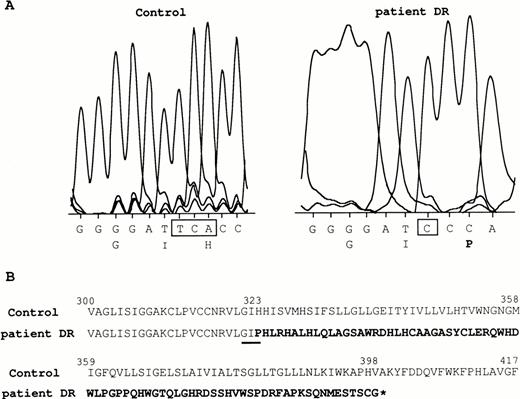
Total RNAs of patient DR were reverse transcribed and the entire RhCE cDNA coding sequence was amplified using primers J and K (Table 1). Sequence analysis showed that the TCA nucleotides at positions 966-968 in the normal cDNA were replaced by a C in DR (Fig 4A). This introduced a frameshift after the isoleucine 322 codon and resulted in a premature stop codon at nt 1,197-1,199. The predicted truncated protein is 398 amino acids long (instead of 417) and includes 76 novel residues at the C-terminus (Fig 4B). The mutations were localized in exon 7 of the RHCEgene and can be detected by DNA restriction analysis, because a newBamHI site is created. DR patient was shown to be homozygous for these mutations by BamHI cleavage after PCR amplification of exon 7 (not shown). Moreover, sequence analysis confirmed that the silent gene in DR is derived from an RHCe allele, as previously determined by Pst I RFLP analysis.19
RHCE cDNA mutation in patient DR. (A) The TCA nucleotide sequence, present in the RhD-negative control, is mutated to C in patient DR. The nearby nucleotide and amino acid sequence surrounding the mutation are shown. (B) The mutation introduces a frameshift that results in a completely different C-ter region (in bold) from amino acid residue 323 of the predicted shorter protein (star indicates the stop codon in the corresponding nucleotide sequence). The amino acid sequence shown in (A) is underlined.
RHCE cDNA mutation in patient DR. (A) The TCA nucleotide sequence, present in the RhD-negative control, is mutated to C in patient DR. The nearby nucleotide and amino acid sequence surrounding the mutation are shown. (B) The mutation introduces a frameshift that results in a completely different C-ter region (in bold) from amino acid residue 323 of the predicted shorter protein (star indicates the stop codon in the corresponding nucleotide sequence). The amino acid sequence shown in (A) is underlined.
Analysis of the Rh50 and CD47 transcripts in Rhnullpatients.
Rh and Rh50 proteins, together with CD47, are crucial proteins of the Rh membrane complex. Indeed, the absence of Rh and Rh50 proteins results in a severe reduction or absence of expression of the other members of the complex. Moreover, no molecular defects are presently known for CD47. In contrast, other members of the Rh complex (eg, LW and GPB) seem to be dispensable.5 Therefore, the entire coding regions of the Rh502 and CD4734 cDNAs were amplified from reticulocyte RNA of DAA and DR patients, as previously described.16 PCR products of 1,265 bp and 1,009 bp from Rh50 and CD47 cDNAs, respectively, were obtained, cloned, and sequenced. No mutation was detected in either cDNA (not shown).
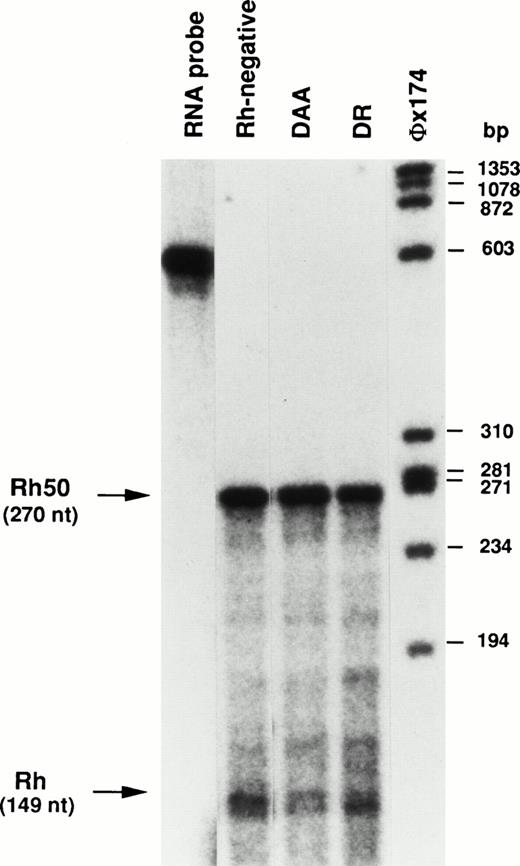
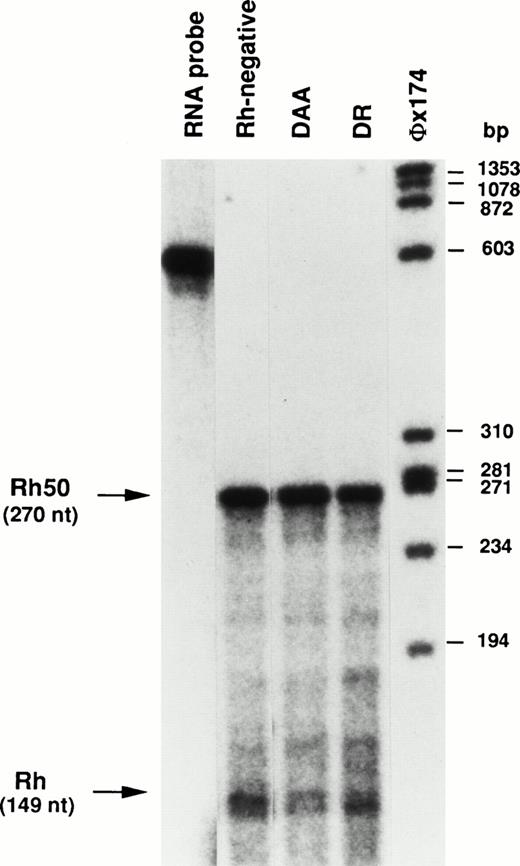
Analysis of Rh and Rh50 transcripts by ribonuclease protection assay.
The relative abundance of Rh and Rh50 transcripts in patients DAA and DR was determined by ribonuclease protection assay (RPA). The ratio between the hybridization signals of the Rh and Rh50 protected fragments (149 nt and 270 nt, respectively, see the Materials and Methods) in Rhnull patients was compared with that in control samples (Fig 5). The Rh50 signal was normalized according to the higher number of U residues (radioactively labeled) present in the Rh50 protected fragment (70 U residues v 35 U residues in the Rh fragment). The Rh/Rh50 transcript ratio was 0.74 ± 0.05 (n = 7) in the control. This value is equivalent to that obtained in patient DR (0.75). In contrast, the Rh/Rh50 ratio is clearly lower in patient DAA (0.41).
Ribonuclease protection analysis of RhCE and Rh50 mRNAs. Total reticulocyte RNAs (25 μg) protected fragments of 149 nt and 270 nt from Rh and Rh50, respectively. The ratio between Rh and Rh50 hybridizing signals is equivalent in the Rh-negative control and in DR, whereas it is clearly lower in DAA (see text). The chimeric Rh-Rh50 RNA probe was 568 nt (see the Materials and Methods). A radiolabeledHae III digest of phage øX174 was used as size marker.
Ribonuclease protection analysis of RhCE and Rh50 mRNAs. Total reticulocyte RNAs (25 μg) protected fragments of 149 nt and 270 nt from Rh and Rh50, respectively. The ratio between Rh and Rh50 hybridizing signals is equivalent in the Rh-negative control and in DR, whereas it is clearly lower in DAA (see text). The chimeric Rh-Rh50 RNA probe was 568 nt (see the Materials and Methods). A radiolabeledHae III digest of phage øX174 was used as size marker.
DISCUSSION
In the present study, we have elucidated the molecular defects leading to the Rhnull phenotype of the amorph type, which is caused by the presence, at the homozygous state, of a silent allele at the RH locus, in 2 unrelated patients (DAA21 and DR22). We have identified two types of mutation affecting the unique RHCE gene present in the genome of these patients, which both resulted in the lack of expression of the Rh antigens on the RBC membrane.
Patient DAA carries a g-to-t mutation at the 5′ donor splice-site of intron 4 (Fig 2A). Analysis of Rh transcripts indicated that at least three cryptic splice-sites are activated as a result of this mutation (Fig 3). Two of them generated transcripts incorporating 16 and 11 nucleotides of intron 4, respectively. These insertions cause premature stop codons in t1 and t2 transcripts (in the transcribed regions corresponding to exon 7 and exon 5, respectively), but not in transcript t3, because the skipping of the exon 5 region restores the reading frame. Transcript t4 is detected in all phenotypes by RT-PCR.31,32 The activation of the third cryptic splice-site generates transcript t5. The existence of this transcript may account for the inconsistency with our previous data describing a normal Rh transcript in DAA.18
Rh proteins in DAA sample were not detectable by Western blot analysis with MPC4 and MPC8 antibodies (Fig 1), as well as with the MPC1 antibody that recognizes the N-ter region.18 21Consequently, it is presumed that either the abnormal transcripts and/or the putative truncated proteins may be degraded within the cells. In addition, RPA analysis suggested that the Rh/Rh50 transcript ratio is lower in patient DAA as compared with the Rh-negative control (Fig 5), possibly due to the instability of the aberrant Rh transcripts.
These results show that the silent allele in DAA, which corresponds to a dce haplotype, is caused by the splice-site mutation in intron 4. This allele can be detected by PCR-RFLP in the family and comparison of the Rh genotypes and the restriction profiles is consistent with the mendelian inheritance of the mutated allele, which is present in double dose in the patient DAA.
Patient DR was previously identified as Rhnull of amorph type by serologic family studies.22 In this patient, the aberrant Rh transcript is brought about by a TCA → C nucleotide change in exon 7 of the RHCE gene (Fig 4A). This was the only alteration found in the Rh cDNA, and it is therefore likely to account for the lack of the expression of Rh antigens in this patient. In fact, no Rh protein was detected by Western blot analysis with antipeptide against the 224-233 amino acids region (MPC4; Fig 1). Unfortunately, we were unable to follow the inheritance of this mutation by RFLP analysis, because samples from the DR family were not available.
The mutation in the RHCE gene of patient DR introduced a frameshift in the transcript, but there is no obvious reason why this message, which is correctly spliced, should not be translated into an Rh protein variant. In addition, RPA analysis showed that the ratio between Rh and Rh50 transcripts in DR is comparable to that in the Rh-negative control (Fig 5). The predicted translation product is a 398- (v 417) amino acid protein, including a completely different C-terminus of 76 amino acids (Fig 4B), and is organized into 10 transmembrane domains, instead of 12, as anticipated from hydropathy analysis.35 The putative mutant protein may therefore have a different protein folding as compared with wild-type Rh proteins, which may alter its routing to the cell surface. Alternatively, the lack of cell surface expression may result from a defective protein-protein interaction normally mediated by the carboxyterminal end of the Rh proteins.
Protein-protein interactions between Rh and Rh50 are believed to be essential for the assembly and/or the transport of the Rh complex to the membrane.36,37 Previous investigations suggested that these interactions may involve the N-ter regions of the two proteins.36 However, the results concerning the DR mutated Rh protein and recent data on the mutations affecting the Rh50 gene in Rhnull regulator phenotypes16(Chérif-Zahar et al, manuscript submitted) point to a critical role of the C-terminus of both proteins in this process.
Finally, evidence is gathering about the respective role of Rh and Rh50 proteins in the expression of the Rh complex. On the one hand, in Rhnull regulator phenotypes, in which the RH50 gene is altered but the RH genes are normal, both the Rh50 and Rh proteins are lacking at the RBC surface.16 Further information comes from the analysis of 1 Rhmod individual (variant of Rhnull regulator8) in which only the Rh50 protein is altered by a single amino acid change, with the Rh protein being normal.16 In this patient, the low amount of Rh50, detectable by flow cytometry and Western blot analyses, may result in only trace amounts of Rh protein, which could be detected just by absorption elution of anti-Rh antibodies.8 These observations suggest that the Rh50 protein is indeed required for the transport of the Rh proteins to the membrane (although its eventual role in the stability of the Rh complex cannot be excluded).
On the other hand, in the Rhnull amorph phenotypes studied here, in which the RH genes themselves are altered, but theRH50 gene is normal, the Rh proteins are not detectable by flow cytometry and Western blot analyses, whereas a certain amount of the Rh50 protein can be clearly seen by these techniques. These data imply that some Rh50 protein is able to reach the membrane even in the absence of detectable Rh protein. Further support to this hypothesis comes from the study of the erythroleukemic K562 cells that express the Rh50 protein,38 about 60 to 90 × 103copies (our unpublished data), as compared with the 180 to 220 × 103 copies on adult RBCs.16However, K562 cells either lack39 or express a very low amount of Rh proteins detectable with some selected anti-D only38 (our unpublished data). These findings suggest that Rh proteins may be partially dispensable for the routing of Rh50 to the membrane. However, optimal transport and/or assembly of the proteins of the Rh membrane complex may require the presence of the Rh proteins, which may play a role in affecting the stability of the complex, possibly through its interaction with some components of the membrane skeleton.
ACKNOWLEDGMENT
The authors thank Pascal Bailly (INTS, Paris, France) for the gift of the anti-p55 antibody.
Supported in part by the EC Contract No. BMH4-CT96-1545 and Ortho Clinical Diagnostics.
Address reprint requests to Jean-Pierre Cartron, PhD, INSERM U76, Institut National de la Transfusion Sanguine, 6 rue Alexandre Cabanel, 75015 Paris, France; e-mail: cartron@infobiogen.fr.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
© 1998 by the American Society of Hematology.