APPROXIMATELY 10,000 autologous hematopoietic stem cell (HSC) transplants are performed worldwide each year for malignant diseases.1 Results of randomized trials in recent years suggest that high-dose chemotherapy followed by infusion of autologous HSCs can offer prolonged disease-free survival in hematologic malignancies including non-Hodgkin’s lymphoma in relapse,2 acute myelogenous leukemia,3 and multiple myeloma.4Similarly encouraging results have been seen in the treatment of solid tumors.5 6
After transplantation, reconstitution of bone marrow (BM) consists of two distinct phenomena, numerical recovery of BM cellular elements on the one hand and functional recovery of cellular interactions on the other.
Although reappearance of neutrophils and platelets is often considered the endpoint of hematologic recovery after intensive chemotherapy and stem cell transplantation, this ignores the second arm of BM recovery, that of immunological reconstitution. In fact, functional recovery of lymphoid and immune effector cells occurs very gradually, and reconstitution of normal humoral and cellular immunity may take a year or more.
Immune reconstitution involves several components of the immune response. These include (1) reappearance of functional B cells, (2) thymic and extra-thymic T-cell development, (3) reconstitution of effector cells including cytotoxic T cells and natural killer (NK) cells, and (4) efficient antigen presentation to reconstitute the pretransplantation immune repertoire. This restoration of immune function is not merely experimental. It may have direct clinical implications: Immediately after the administration of intensive cytotoxic drugs, minimal tumor burden is presumed to be present, providing potentially ideal circumstances to eliminate residual disease altogether by immunotherapeutic means. In this review, several strategies that could lead to enhancement of cellular immune function to take particular advantage of posttransplantation minimal residual disease will be discussed. In addition, the potential to accelerate immune reconstitution and the effect that might have in the therapy of malignant disease will be considered.
Although there are similarities in immune reconstitution after allo-BM transplantation (BMT)7 8 and autologous HSCs, allo-BMT involves graft-versus-host disease (GVHD) and the use of immunosuppressive therapy to control it, both of which interfere in the early developmental stages of immune reconstitution. Autologous HSC transplantation that entails neither GVHD nor immunosuppressive drugs presents more direct insight into the factors involved in immune reconstitution after grafting.
B CELLS
B-Cell Regeneration and Ig VH Gene Expression
Normal B-cell differentiation is accompanied by a set of preprogrammed steps of Ig gene rearrangement and by successive acquisition and loss of differentiation level-specific surface molecules.9 In both its Ig gene rearrangements and in phenotypic expression, B-cell recovery after transplantation appears to recapitulate normal B-cell ontogeny.10 The relative and absolute numbers of circulating cells expressing CD19 and CD20, two markers of mature B cells, are decreased during the first 3 months following transplantation.11,12 Thereafter, the numbers of such cells increase to a plateau at 6 to 9 months. CD23 (the low-affinity receptor for the Fc portion of IgE) and CD38 (the ecto-enzyme of nicotinamide (NAD) glycohydrolase that acts as an adhesion/homing receptor and is involved in intracellular calcium homeostasis) are strongly expressed on the circulating B cells of neonates and B cells from cord blood, whereas in adults, CD23+ CD38+B cells are a minor subset. During the first year postengraftment, the majority of circulating B cells carry the CD23+, CD38+ undifferentiated phenotype.12Furthermore, as in neonates, the percentage of mIgM+ and mIgD+ B cells is high in autologous transplant recipients. Taken together, this suggests that the majority of posttransplantation circulating B cells are poorly differentiated.
Consistent with the notion that autologous BMT (ABMT) ontology follows developmental ontogeny, ABMT recipients have a significant increase in the B-cell precursor marker CD10 in the BM as early as 1 month after grafting which persists for at least 1 year and precedes repopulation of the peripheral blood mature B cells by 1 to 2 months.13 CD10 (also known as CALLA) is a highly conserved neural endopeptidase transiently expressed on early B progenitors before the appearance of heavy μ chain in the cytoplasm and is re-expressed after activation by antigen. Nevertheless, the phenotype of circulating B cells posttransplantation differs from BM B cells because CD10 is expressed on only a negligible fraction of peripheral B cells.10
In recipients of CD19+ cell-depleted BM, the immunophenotypic features of the resulting BM B-cell precursor populations are similar to those of fetal liver or fetal BM-derived B-cell precursors. As in normal fetal development, the expression of CD10 and CD19 antigens in posttransplantation BM appears to precede expression of other antigens characteristic of normal B-cell ontogeny.14 As might be expected, in patients who received ABMT for B-cell malignancies purged with anti–B-cell monoclonal antibodies (MoAbs), B-cell recovery is further delayed; at 3 months, only 50% of engrafted patients attain normal percentages of CD20+ B cells.15
Ig gene rearrangements after ABMT, like phenotypic markers, suggest retracing of B-cell ontogeny. Posttransplant Ig gene rearrangements are consistent with wide B-cell polyclonality rather than a narrow oligo or restricted clonality.13 However, Ig VH gene family usage does differ from the normal adult distribution during the first 3 months after both allo-BMT and ABMT.16 Following allo-BMT, the VH repertoire resembles that expressed by the early normal fetal BM with a relative increase in the VH2, VH4, VH5, and VH6 gene families. After ABMT, the same general pattern is observed with a decreased expression of the largest family VH3, offset by a relative increase of the much smaller VH4 and VH5 families.16 This preferential expression of these small VH gene families mimics the pattern seen during normal B-cell ontogeny.17
By 90 days after BMT, VH3 and VH4 gene usage is indistinguishable from that of normal adults, suggesting that the Ig repertoire may have normalized by then.16 However, even after attaining adult level of VH gene family usage, the rearranged VH genes exhibit much less somatic mutations in BMT recipients than seen in rearrangements of normal adults.18 This may point to a block in antigen-selected affinity maturation of antibody and/or a maturation arrest in B-cell differentiation after Ig V gene rearrangement and may have implications in failures to attain high-specific, high-affinity antibodies in posttransplant vaccinations.
Origin of Posttransplant B Cells
After ABMT or autologous peripheral blood stem cell transplantation (ABSCT), B cells regenerate from several sources: (1) B cells of the transplant recipient which survived the pretransplantation chemotherapeutic intensification treatment; such cells may be seeded in the BM, lymph nodes, or spleen; (2) B cells present in the graft; (3) hematopoietic stem cell progenitors in the transplant that differentiate after grafting in the recipient; and (4) residual recipient stem cells. Because allogeneically transplanted cells can be readily traced, allo-BMT provides direct insight into the origin of B cells in the transplant recipient. Transfer of humoral immunity has been documented from donor to recipient, including immunity to tetanus, varicella, diphteria, influenza virus, cytomegalovirus, hepatitis B virus, and human immunodeficiency virus,19-23 suggesting that functional B cells are passively transferred by transplantation. The corollary of adoptive transfer of immunity is that active immunization of BM donors might serve to reduce the incidence of infection in recipients of allo-BMT and that immunization before intensification might likewise be considered in ABMT and ABSCT recipients. Immunization of ABMT patients before BM harvests with either protein vaccines such as tetanus toxoid or conjugated carbohydrate vaccines such as haemophilus influenzae can enhance early recovery of specific antibody.24
Transfer of humoral immunity suggests that differentiated antigen-selected B cells in the graft are a significant source of posttransplantation B cells. The ontogeny data presented in the preceding section indicated that stem cells differentiating into Ig expressing B cells represent a significant part of the posttransplantation B-cell population. In addition, findings from B-cell–purged transplants indicate that both recipient and donor stem cells contribute to the posttransplantation B-cell population.15
B-Cell Function
Deficiencies in humoral responsiveness in HSC recipients is attributed to both decreased T-cell help and to intrinsic B-cell defects.25-27 Serum Ig levels remain low during the first 3 months after ABMT during the same period in which the numbers of circulating B cells is reduced11,28 and B-cell proliferative response to the T-cell–independent antigenStaphylococcus aureus Cowan strain I (SAC) blunted.12 While IgM production in response to pokeweed mitogen and SAC normalize at 3 months, IgG production is suppressed for 12 to 24 months in most patients.12 This delay in Ig production parallels the pattern seen in B-cell ontogeny, and may reflect the failure of posttransplant B cells to receive or respond to T-cell factors involved in isotype switching.
In patients receiving B-cell–purged BM for B-cell hematologic malignancies, responsiveness to the normally proliferative effects of cross-linking anti-Ig antibody is significantly lower than that of normal controls at 3 and 6 months.15 As in engraftment of unpurged marrow, recovery of in vivo B-cell function demonstrates a selective defect with normal serum levels of IgM returning at 6 months, IgG at 12 months, and IgA after 2 years, reflecting a recapitulation of normal B-cell development.15
T CELLS
BM-derived hematopoietic stem cells in the normal process of differentiation home to the thymus, the major site of T-cell differentiation. However, the thymus is not the only site of T-cell development. T-cell differentiation also occurs through extrathymic pathways in the gut mucosa, in the liver,29 and, at least in the case of murine T-cell development, in the BM as well.30 The contribution of these extrathymic sites may play a role in posttransplant immune reconstitution.
Reconstitution of T-Cell Subsets
Surface markers have proven to be critically useful in characterizing T lymphocytes and their functional subsets. Nevertheless, however useful in our understanding of lymphoid ontogeny, phenotypic subtyping identified to date doubtlessly represents only a part of the overall T-cell functional repertoire. Evidence for the as-yet limited nature of our T-cell characterization is the fact that phenotypic shifts in posttransplantation T cells do not necessary result in immune dysfunction. This must be borne in mind when discussing T-cell phenotypes and their correlation to immune reconstitution after stem cell transplantation.
T-Cell Subsets After ABMT
After ABMT, the relative number of CD3+ cells is significantly decreased compared with those of normal controls during the first month postgrafting, returning to normal levels within 3 months.31 In addition, a decrease in the relative and absolute numbers of CD4+ cells in the peripheral blood is commonly seen and can persist for a year or more.31-33 In contrast, the relative and absolute number of CD8+ cells reconstitutes fairly rapidly resulting in an inverted CD4/CD8 ratio in the months following autologous transplantation.31-33
The observation of an inverse correlation between the size of the thymus and the level of the CD4+ cells in the peripheral blood after high-dose chemotherapy supports the notion that helper T-cell development depends on residual thymic function.34In addition, thymic epithelium has been shown to play a more important role in helper T-cell development than in differentiation of the suppressor T cell.35 The inverted CD4/CD8 ratio seen after transplantation is consistent with such a schema. Thymic involution starts as early as 1 year of age, continues at a rate of approximately 3% per year until middle age and thereafter decreases to less than 1% per year.36 The fact that CD8+ T-cell reconstitution appears not to be significantly impaired by age-related involution of the thymus suggests that thymic-independent pathways primarily contribute to its regeneration.37 A predominance of CD28− and CD57+ cells among the CD8+ subset has been observed following chemotherapy that may persist up to 9 months.37 The absence of CD28 expression is similar to that seen in extrathymically derived CD8+ cells.38
Functional subsets of CD4+ and CD8+ T-cell populations have been studied after ABMT and can be distinguished on the basis of the expression of CD45 isoforms (CD45RA, CD45RO). CD45RO+ T cells respond in vitro to recall antigens and correspond to “memory cells” while CD45RA+ T cells correspond to naı̈ve cells recently issued from the thymus. During the first 3 months following ABMT, the number of CD45RA+ lymphocytes is decreased, but returns to normal levels by 1 year posttransplantation.31 39 The CD4+CD45RA+ subset is profoundly reduced and may take up to 2 years to recover. In contrast, the CD8+CD45RA+ population normalizes within the first month after ABMT.
T-Cell Subsets After ABSCT
Because peripheral blood stem cells (PBSC) contain a larger proportion of more differentiated progenitor cells as well as terminally differentiated effector cells than BM, one might suppose that the kinetics of T-cell recovery may be accelerated following ABSCT as compared with ABMT. The fraction of cells designated PBSC in fact does contain large numbers of T cells. CD3+ cells may represent more than 20% of peripheral cells collected after granulocyte colony-stimulating factor (G-CSF) mobilization yielding substantially more T cells (up to 1 log greater) than found in BM.40 41
Immediately after transplantation of mobilized PBSC, total CD3+ cell levels return to normal, CD4+ cell levels remain below normal, and the number of CD8+ cells increases resulting (depending on the study) in either severely42,43 or slightly44-47 reduced CD4/CD8 ratios. Overall, when CD4/CD8 and CD45RA/CD45RO ratios are examined, recovery of T-cell subsets appears more promptly after ABSCT than after ABMT.43,47 48 As noted however, here as in all similar analyses, precise correlation of T-subset distribution to overall T-cell function is uncertain.
T-Cell Repertoire (TCR)
Shifts in T-cell subsets as defined by TCR V gene expression have been described early after both allo-BMT and ABMT.49-53 It is not clear whether particular T-cell subsets as defined by V gene usage are derived from T-cell precursors (either donor or recipient) or from expansion of donor-derived mature T cells. A significant proportion of patients shows increased usage of TCR γ/δ during the early period post-BMT. Within the TCR γ/δ subpopulation, there is a preferential expression of the Vγ9 and Vδ2 genes as is seen during early fetal life.54 This suggests that recapitulation of T-cell ontogeny may occur early following BMT, analogous to that described for B-cell regeneration.50 51 This early postengraftment predominance of Vγ9+Vδ2+ cells in the periphery may subsequently be further increased by antigen-driven expansion of the newly generated γδ cells. An alternative possibility exists that mature γδ T cells are already present in the BM graft, are expanded after contact with antigen, and do not represent an ontologic recapitulation.
Origin of Posttransplant T Cells and the Role of Thymic and Extrathymic Pathways
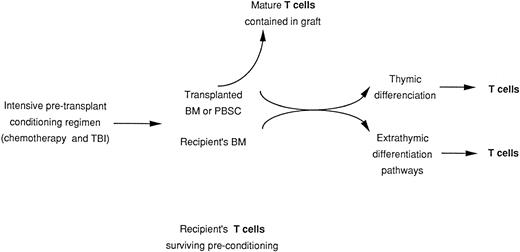
Examination of TCR leads to the question of T-cell origin. After ABMT or ABSCT, T cells may reconstitute from at least four different sources: (1) rare recipient T cells that survived the conditioning regimen; these cells might be seeded in the BM, lymph nodes, or spleen; (2) T cells present in the graft; (3) hematopoietic stem cell progenitors of the graft that differentiate in the recipient; and (4) residual recipient stem cells (Fig 1). Studies of TCR alluded to in the preceding section suggest that the quantity of T cells present in the graft might influence the rapidity and the quality of T-cell recovery. However, it remains unclear how much passively transferred T lymphocytes will contribute to sustained cellular immunity.
Origin of mature T cells in transplant recipients. Derivation from transplanted stem cells and mature T cells contained in the graft and derivation from surviving host T cells.
Origin of mature T cells in transplant recipients. Derivation from transplanted stem cells and mature T cells contained in the graft and derivation from surviving host T cells.
In autologous grafting where the source of cells as either transplanted or nontransplanted cannot be readily identified, data from gene marking studies may help to trace the origin of T cells after transplantation. Unfortunately, because gene transfer into BM cells is neither very efficient nor very specific, gene transfer studies may not yet not give a precise quantitative determination of the role of transplanted BM in T-cell recovery. However that may be, following transplantation of autologous BM that had been transduced with a retroviral vector containing a neomycin resistance gene, T cells carrying the gene could be detected by polymerase chain reaction (PCR) as early as 1 month and remain detectable for at least 18 months.55 Nevertheless, although the protocol of retroviral infection in the study cited was intended to transfer the marker gene into stem cells only, one cannot exclude that T cells from the graft had been transduced as well.
Data from allo-BMT are helpful in distinguishing the contribution of grafted BM and T cells from that of postchemotherapy residual stem cells or T cells. In most conditioning regimens used for allo-BMT, recipient hematopoiesis is ablated. However, T cells can survive conditioning regimens.56 Using PCR amplification of minisatellite DNA regions, Roux et al57 were able to determine the recipient or donor origin of T cells after allo-BMT. Their data suggest that in recipients of T-cell–depleted (TCD) BM, within the first year following transplantation, the T-cell compartment has a mixed origin with T cells derived from both transferred donor cells and surviving host T cells. In contrast, when a patient was grafted with an unmanipulated BM, few or no recipient T-cell clones are detected.
Another source of information is the transfer of antigen-specific cellular immunity from the donor to the recipient. Reports indicate that cellular immunity against varicella zoster virus and tuberculin-purified protein derivative can be effectively transferred from immune marrow donor to recipient.58 59 These findings transposed to autologous transplantation suggest that T cells in the graft can expand.
To study the relative contributions of peripheral lymphoid populations and BM-derived precursors to T-cell regeneration after BMT and to understand the role of the thymic function in the immune recovery,60 Mackall et al61 performed a series of elegant experiments where they studied lethally irradiated thymus-bearing and thymectomized mice that had received congenic lymph node cells as a source of peripheral T-cell progenitors and syngeneic T-cell–depleted BM. They showed that in hosts lacking thymic function, the predominant reconstituting T cells were derived from peripheral T-cell progenitors contained in the transplant which expanded in the thymectomized recipients. In the presence of a functional thymus, the expansion of peripheral T-cell progenitors is downregulated. Furthermore, in thymectomized hosts, the majority of T lymphocytes did not express the high-molecular-weight (HMW) CD45 isoform which indicated normal maturation, while in thymus-bearing animals there was an increase in the level of naive type cells. There is evidence that thymic-independent T-cell regeneration occurs primarily via expansion of peripheral T cells and is Ag driven because significant expansion of CD4+ and CD8+transgenic/TCR-bearing cells appears only in the presence of Ag specific for the TCR.62 In humans, where thymic regenerative capacity is compromised because of age- and chemotherapy-related changes, it appears that a thymic-independent pathway must predominate.60
The capacity of the thymic and extrathymic pathways to produce CD4+ cells differed in thymectomized and thymus bearing murine hosts.61 Lower numbers of CD4+ cells were derived from TCD BM transplanted into thymectomized recipients than when transplanted in thymus-bearing mice, while the percentage of CD8+ cells did not differ significantly between the two types of recipients. Although the BM is an inefficient source of CD4+ cells in the absence of a functional thymus, the peripheral lymphoid progenitor pathway appears capable of compensating, resulting in adequate numbers of CD4+ cells in transplanted thymectomized mice.
T-Cell Function
T-cell competence after stem cell transplantation can be gauged at three distinct functional levels: cell proliferation, cytokine production, and lytic capacity.
T-Cell Proliferation
Using limiting dilution analysis, the frequency of mitogen-responsive T cells in peripheral blood, including the frequency of cytokine-secreting helper T cells, interleukin-2 (IL-2) responding T cells, and cytotoxic T cells, was found to be low after ABMT.63
Cayeux et al64 reported that isolated T cells at an early post-ABMT stage (<2 months) had defective responses to normally proliferation-inducing MoAbs such as anti-CD3 or anti-CD2 even in the presence of IL-2 affecting both CD4+ and CD8+subsets. In response to anti-CD3 and IL-2, PB mononuclear cells (PBMC) from ABMT recipients proliferate but to a lesser degree than in normal individuals.65 Sugita et al66 found similar results: anti-CD3 alone or in combination with anti-CD2, anti-CD26, or anti-CD29 could not induce T-cell proliferation within 4 months after ABMT. However, T-cell proliferation induced by anti-CD3 + anti-CD2 and by anti-CD3 + anti-CD26 reaches almost normal levels by 1 year. Just as in normal T cells, coactivation with anti-CD28 MoAb can enhance the response in some transplant patients, particularly in long-term recipients (above 6 months).67
In a mixed leukocyte reaction (MLR), accessory cells from auto-transplanted recipients were unable to trigger normal levels of proliferation of normal control T cells or to induce them to synthesize IL-2.68 69 In addition, normal allogeneic accessory cells failed to provide the necessary signals to activate transplant-derived T cells or induce them to produce IL-2 and proliferate. This deficiency could be corrected by addition of exogenous IL-2. The fact that recipient T cells do respond to IL-2 indicates that the IL-2 cell receptor is present on their surface and functional. These observations may indicate that during T-cell proliferation cell-to-cell contact does not lead to T-cell activation but can induce IL-2 responsiveness.
T-cell proliferation is also impaired after autologous PBSC, but recovery appears faster than that observed after ABMT.47The depressed immune function might be partly related to the suppressive effects caused by high numbers of monocytes present in growth factor–mobilized PBSC.70 71 Although hematopoietic engraftment after infusion of positively selected CD34+cells appears similar to that observed after unselected PBSCT, no study has compared the immune recovery after infusion of selected CD34+ cells and after PBSC or BM.
Cytolytic Function
Cytomegalovirus (CMV)-specific HLA-restricted CD8+cytotoxic T cells (CTL) have been shown in the majority of patients within the first 3 months after ABMT or APBSCT and are associated with protection from CMV infection.72 In contrast, specific CTL response against Epstein-Barr virus (EBV) are impaired during the first 2 months after autologous stem cell transplantation.73Further investigation of specific CTL activity are required in developing of adjuvant vaccines in patients undergoing transplantation.
Lymphokine-activated killer (LAK)-like activity, that is cells incubated with IL-2 capable of killing NK-resistant target cells, is mediated primarily by CD16+CD3− cells and to a lesser degree by CD16−CD3+ cells and appears 4 to 6 weeks after ABMT.74 LAK activity is not observed after conventional chemotherapy not followed by transplantation, but IL-2–responsive LAK precursor cells are rapidly reconstituted after ABMT or ABSCT.75-77 Development of IL-2–mediated cytotoxic activity may prove to be functionally important for eradication of residual malignant cells in vivo. Consistent with such a role is the finding that the cytotoxic activity of PBMC after ABMT against NK-sensitive and NK-resistant target in response to anti-CD3 and IL-2 is actually greater than that of normal controls.67 IL-7 also induces significant LAK activity in ABMT recipients to levels comparable to those obtained with IL-2, suggesting that IL-7 may have an immunotherapeutic role alone or in conjunction with IL-2.78 The potential therapeutic use of cytokines in inducing cytotoxic activity is discussed under NK Cells.
Cytokine Production and T-Cell Responsiveness to Cytokines
In vitro–stimulated PBMC from recipients of ABMT have significant defects in the production of a number of T-cell–derived cytokines important in immune homeostasis, particularly IL-2, in the early posttransplant period.64 79
The origin of the observed decreased cytokine production (notably IL-2) and of the blunted T-cell proliferation can be ascribed to several causes: subnormal levels of cytokine receptor expression, abnormal accessory function, production of suppressive cytokines, or an intrinsic T-cell defect. Insufficient IL-2 receptor cannot account for the decreased proliferation since stimulation of posttransplant T cells has been shown to induce the expression of the α chain of the IL-2 receptor.66,80,81 Normal accessory cells do not restore normal production of IL-2 and, vice versa, patients’ accessory cells fail to activate normal T cells and induce IL-2 synthesis.68 However, the addition of exogenous IL-2 can at least partially compensate the abnormal proliferative response. Possibly signal transduction required for T-cell–non T-cell interactions are in some way dysfunctional. These may entail the interactions between CD40-CD40L, between CD4-MHC II, or between B7-CD28.
NK CELLS
NK cells are defined as large granular lymphocytes capable of mediating major histocompatability complex (MHC)-unrestricted cytolytic reactions against tumor or virally infected cells and not expressing receptors for antigen (ie, neither surface Ig or TCR). In addition, NK cells characteristically have a CD3−CD16+CD56+ phenotype. NK cells are among the first cells to recover after transplantation: in contrast to B- and T-cell function, NK activity following both ABMT and ABSCT reaches normal level within 1 month.46,48,80 82-84
IL-12, a 70-kD heterodimeric cytokine with pleiotropic activities produced by antigen-presenting cells (APC), stimulates the proliferation and cytotoxic activity of NK cells and enhances generation of cytotoxic T cells.85 In addition, it induces transcription and secretion of interferon-γ (IFN-γ) either directly or in synergy with other inducers and promotes commitment of naive T cells to the TH1 pathway resulting in the production of TH1 cytokines including IL-2.85As a result of these effects, IL-12 appears to have strong antitumor and antimetastatic effects as shown in a murine model,86,87with evidence suggesting a similar role in human malignancy.88 SAC stimulated PBMC derived from autologous hematopoietic transplants show no decrease in the production of IL-12 as compared with control PBMC.89 However, this seemingly normal in vitro production of IL-12 does not exclude the possibility that in vivo IL-12 production remains abnormal. T cells play a role in priming APC to produce IL-1290 and posttransplantation T cells may be unable to carry out this function. Transplantation-derived PBMC do not appear to have defective responsiveness to exogeneously provided IL-12 as evidenced by their degree of in vitro proliferation and IL-12–mediated IL-2 production.89 In addition, significant increases in NK and LAK activity are observed with IL-12 alone or in combination with IL-2.91
The sum of these findings suggests that the recovery of NK cells seen after ABMT and ABSCT is prompt both in quantity and in functional quality. Maturation of NK cells can occur in the absence of a functional thymus in mice and humans.92 93 This may account for the prompt NK recovery. In the early posttransplant period, when specific immunity is still recovering, NK cells may provide an important defense against infections or tumor relapse. Expansion of NK cells with growth factors immediately following transplantation might therefore serve to increase host defenses. Such an immunotherapeutic strategy is discussed below. However, despite our ability to accelerate or potentiate cytotoxic cells, there is no direct evidence showing a correlation between NK activity after transplantation and tumor relapse.
TRANSFER OF T- AND B-CELL–MEDIATED IMMUNITY
The degree of T-cell incompetence after autologous transplantation is difficult to gauge exactly in vitro. The capacity to mount an effective immune response against foreign antigen better reflects the immune status of transplant recipients than do in vitro tests. Information garnered from allogeneic transplantation indicates that with the passive transfer of allogeneic lymphocytes, transplantation recipients show short-term production of antibodies against viral pathogens.20,94 However, vaccination in the months after transplantation does not always result in sustained protection. This pattern of temporarily effective but nonsustainable protection suggests that B lymphocytes derived from the donor, which generate specific antibody in the short term, are transferred together with the transplant, but that antigen-specific T cells necessary for ongoing sustained protection must be regenerated anew since the transfer of T cells functional against specific infections appears less efficient.95-97 The reduced transfer of antigen-specific T lymphocytes may be caused by an already impaired T-cell–mediated immunity before transplant in patients who have received significant prior chemotherapy.
ABMT patients have an overall increased risk of infections, including CMV, influenza, herpes simplex virus, and varicella zoster virus,98-100 although the incidence of infections and severity are lower in ABMT than among allo-BMT recipients.72,95,101 This disparity may be accountable by the possibility that T-cell transfer from an allogeneic donor is further compromised by GVHD prophylaxis. Lymphocyte proliferative responses to herpes simplex virus and CMV in seropositive patients return more rapidly in ABMT recipients than allo-BMT.72
Overall, the transfer of antigen-specific T cells is substantially less efficient than the passive transfer of antibody-producing B lymphocytes transfer. This has implications in designing infection-specific vaccines for administration before and after transplantation.
STRATEGIES TO ENHANCE IMMUNE RESPONSIVENESS AFTER AUTOLOGOUS STEM CELL GRAFTING
The effectiveness of any cancer immunotherapy in limiting or eliminating malignant cells is a function of the target tumor mass. Even when therapeutically bolstered, immune mechanisms can be rendered ineffective by the presence of overwhelming numbers of target malignant cells. Because high-dose chemotherapy followed by ABMT or ABSCT results in (or is presumed to result) in minimal burden of residual malignant disease it provides a potentially ideal setting for immunotherapy.
Administration of Cytokines
Interleukin-2
It has been 15 years since the first demonstration that IL-2 activates and promotes proliferation of murine and human NK cells in vitro resulting in both a greater degree and wider spectrum of lytic activity and initial preclinical studies had shown that systemic IL-2 had significant antitumor effects.102,103This lead to clinical studies using IL-2 regimens with or without adoptive transfer of in vitro activated LAK cells. After the exhilarating results of the initial clinical trials involving high-dose recombinant IL-2 and LAK cells,104 the true response rate of IL-2 with or without the addition of LAK cells was found to be in the range of 15% to 25% for renal cell carcinoma and melanoma.105 Although a variety of tumors of different histological types are sensitive to IL-2/LAK cell therapy, complete eradication of tumor is rare. Nevertheless, because of the measurable tumor regression seen in a significant minority of patients with metastatic malignancies, a logical extension was to perform similar trials of IL-2 after ABMT for hematologic malignancies. The rationale for using IL-2 is based on the following: (1) preclinical data showing that human hematologic malignant cells can be lysed in vitro by IL-2–activated effector cells106-108; (2) IL-2–responsive cells are present early following transplantation74-76; (3) immunotherapy is likely to be more effective when minimal tumor burden is present; and (4) chemotherapy-resistant cells can be lysed by IL-2–activated NK and T cells. IL-2–based trials have included both preclinical and clinical studies.
Preclinical studies.
Mice inoculated with a leukemic cell line were administered intravenous cyclophosphamide and syngeneic BMT 24 hours later. This was followed by either recombinant (r) IL-2 for 5 days starting on day 1, 7, or 21 or by no further therapy.109Mice not treated with IL-2 relapsed and died within 50 days posttransplantation, whereas mice receiving IL-2 had long-term disease-free survivals. The maximal antileukemic effect was observed in mice receiving IL-2 3 weeks after BMT. The delayed maximal effect is consistent with and likely results from the delay needed to first achieve maximal lymphocyte reconstitution that can then respond to IL-2.
Clinical studies.
Trials of continuous-infusion IL-2 administration after ABMT have been performed primarily in hematologic malignancies,83,110-124although several studies of its use following ABMT for solid tumors have also been performed.83,114 125
IL-2 administered after ABMT has significant immunomodulatory effects, including an increase in circulating lymphocytes expressing CD3, CD4, CD8, and the NK-associated markers CD16 and CD56 as well as an increase in circulating NK and LAK activity.83,110,111,114,115,117However, in one trial where IL-2 treatment was begun on the day after transplantation for acute lymphoblastic leukemia (ALL), cells with the NK phenotype and circulating NK activity were not enhanced.119 Nevertheless, the majority of IL-2–treated patients showed strong cytotoxicity against an ALL cell line.
One of the primary drawbacks to the use of high-dose IL-2 is its systemic toxicity. Fever, fatigue, diffuse rash, and varying degrees of a capillary leak syndrome with weight gain and hypotension are frequently observed in most of the studies using high doses of IL-2 (between 9 and 24 × 106/IU/m2/d). The major hematopoietic toxicity of IL-2 after ABMT has been thrombocytopenia. Lymphocytosis, neutrophilia, and eosinophilia are commonly reported as well. These effects may be caused by the induction of secretion of other cytokines by IL-2.126
The conclusion drawn from these clinical trials is that although the administration of IL-2 is complicated by significant side effects in at least 30% of recipients, it remains a viable option for some patients. Most groups administer IL-2 once hematopoietic engrafment has been achieved, generally 2 to 3 months after transplantation while endogenously activated cells and IL-2–responsive immunocompetent precursors cells are present earlier in the posttransplant period. Immune modulation with marked time-dependent increases of NK cells (CD56bright+CD16+CD3−) have been achieved with low doses of IL-2 (2 to 4 × 105IU/m2/d) administered for a period of 3 months without significant toxicity.114,122,127 NK and LAK activities were also found to be enhanced by low doses of IL-2.114
Furthermore, none of the studies including two randomized ones in acute leukemia in adults have shown that the immunomodulatory effects of IL-2 were beneficial in terms of survival.120 124 It is probably unlikely that IL-2 administered alone will be effective, but it may find its place to expand or sustain in vivo NK or cytotoxic T cells injected after transplantation.
Interleukin-7
As noted, T-cell precursors leaving the BM enter the thymus for terminal differentiation. Data from cytokine-deficient mice (knockout mice) suggest that with the exception of IL-7, cytokines can be removed without significantly affecting intra-thymic development.128,129 Mice lacking the IL-7 gene or a functional IL-7 receptor gene have severe impairment of early lymphocyte expansion.128 129 The severe lymphopenia seen in IL-7–deficient mice is associated with normal distribution of T-cell subsets and response to mitogens, suggesting that IL-7 acts on the expansion and proliferation of T cells rather than on their differentiation and function. These multiple effects of IL-7 on T-cell development have led to preclinical studies in murine BMT.
Preclinical studies.
In a murine model of BMT, IL-7 administration accelerates both T- and B-cell reconstitution by up to 2 to 4 weeks130 and both CD4+ and CD8+ cell counts are found to be expanded. Bolotin et al131 reported that in a syngeneic murine BMT model, injections of IL-7 from day 5 to 18 induced an increase in the cellularity of the thymus by 4 weeks, while the proliferation of early thymic precursor cells was increased nearly eightfold. In contrast, in mice not receiving IL-7, normal numbers of thymocytes appeared only by week 8. Furthermore, in the IL-7–treated mice, distribution of thymic subpopulations approximated those of normal untransplanted mice. Abdul-Hai et al132 reported similar findings of accelerated repopulation of the thymus with IL-7 injected for 10 days immediately after murine transplantation. IL-7 also increased RAG-1 (recombination activator gene) expression in the thymus. Taken together, these data suggest that IL-7 may find a therapeutic role in accelerating T-cell reconstitution after autologous transplantation.
Incubation of HSC With Cytokines and Growth Factors
Preclinical Studies
Incubation of murine BM with IL-2 results in the generation of killer cells with non–MHC-restricted cytotoxicity against tumor cells which appears superior to the cytotoxicity of spleen LAK cells.133-135 Transplantation of IL-2–activated BM (ABM) immediately followed by administration of systemic IL-2 reduces the dissemination of established melanoma and sarcoma in mice more effectively than transplantation with untreated BM with or without systemic IL-2 alone.133,134 This suggests that priming with IL-2 before ABMT induces an antitumor effect capable of eradicating residual malignant disease. When IL-2 therapy is delayed for 1 or 2 weeks after transplantation with ABM, there is a progressive decrease in the cure rate,136 suggesting that ABM cells cannot maintain a prolonged cytotoxicity in vivo. The hematopoietic regenerative capacity of IL-2–activated BM was preserved despite a reduction in viable cell number.
As in the murine studies, in vitro incubation of human BM with IL-2 leads to the generation of cells with cytotoxicity against tumor cell lines.137-141 In vitro activation of BM cells with IL-2 significantly enhances cytotoxicity against chemotherapy-resistant leukemic cells, suggesting that this approach might be useful to eliminate drug-resistant minimal residual disease.142Significantly, PBSC cultured in IL-2 for 24 hours retain adequate potential for hematopoietic reconstitution.143
NK cells present potentially ideal instruments of immunotherapy. Large scale ex vivo culture of PBSC enriched in monocytes and NK precursors with IL-2 should yield sufficiently high numbers of activated NK cells for clinical use.144 145 It remains to be seen whether optimal regimens of ex vivo and in vivo IL-2 will result in enhanced cytotoxicity against postchemotherapy/transplantation residual malignancy.
Similar studies with granulocyte-macrophage colony-stimulating factor (GM-CSF) indicate that as with IL-2, ex vivo activation of BM with GM-CSF induces cytotoxicity against tumor cells.146 In the case of GM-CSF, however, this is due to the proliferative effect of GM-CSF on macrophages rather than on T cells. When combined with a tumor-specific antibody, GM-CSF induces specific antibody-dependent cytotoxicity (ADCC) against tumor cells.146
Clinical Studies
Only limited studies of hematopoietic stem cells exposed to IL-2 have been reported so far and these included small numbers of patients with leukemia, breast cancer, and non-Hodgkin’s lymphoma.142 147-149 In the reported studies, BM was maintained with IL-2 for a variety of schedules ranging from 24 hours to 10 days. IL-2–activated BM successfully engrafts patients who had previously received myeloablative chemotherapy. In most studies, additional systemic IL-2 was administered intravenously after transplantation with the expectation that IL-2 would maintain the NK activity. Although in vitro cytotoxicity of IL-2–activated BM against NK-sensitive cell lines was demonstrated, no studies showed any effect against autologous tumor cells. From the preliminary data, it is not yet possible to conclude whether this approach will be successful.
Induction of an Autoaggression Syndrome With Cyclosporine A (CsA)
Preclinical Studies
CsA is a potent immunosuppressive agent that has been used for more than 15 years to prevent GVHD in patients receiving allogeneic BMT. Paradoxically, however, the administration of CsA after syngeneic BMT in rats can lead to the development of an autoimmune phenomenon that is clinically and histologically similar to allogeneic GVHD with the appearance of CD4+ and CD8+ effector cells recognizing MHC class II antigens, including self.150,151Lethally irradiated rats reconstituted with syngeneic BM and treated with CsA for 40 days develop a T-cell–dependent autoimmune syndrome 14 to 28 days after discontinuation of CsA treatment characterized by erythroderma and dermatitis.150 The mechanism for induction of this autoimmune phenomenon remains unclear. It has been suggested that CsA induces modifications in the thymus, including medullary involution, loss of Hassal’s corpuscles, and decreased expression of MHC II antigens in the medulla,152 changes which interfere with intrathymic differentiation of T cells.150,151,153CsA also appears to enhance the development of autoreactive T lymphocytes by blocking their deletion in the thymus.154 The role of the intact thymus in autoaggression is indicated by the finding that syngeneic GVHD cannot be induced in thymectomized animals.153 However, the inhibition of clonal deletion in the thymus and the development of autoreactive T cells in the periphery is insufficient by itself to induce the autoimmune phenomenon. The ablation of the lympho-hematopoietic system with the preparative regimens for transplantation (irradiation or cytotoxic chemotherapy) is also apparently necessary to eliminate peripheral regulatory mechanisms155 156 because infusion of spleen cells from rats with autoreactive disease into normal rats does not transfer the clinical syndrome. In addition, CsA treatment of untransplanted rats does not induce an autoimmune syndrome.
The effector mechanisms of the CsA-induced autoimmune syndrome remain unclear. Because CsA causes a marked decrease in the expression of MHC class II antigens in the thymic medulla,157 developing T cells in the thymus may fail to recognize these MHC determinants as self. Therefore, MHC class II determinants might then turn out to be the principal targets of autoreactivity. The T-cell receptor repertoire of effector T lymphocytes appears restricted, suggesting that only a limited number of class II MHC antigenic determinants may be recognized, and administration of anti–MHC class II antibodies can delay or prevent autoreactivity.158 Recently, the peptide termed CLIP derived from the MHC class II invariant chain which protects the MHC molecules from nonspecific binding of peptides was found to be the target of autoreactive T cells.159
The autoreactive cells generated by CsA treatment after ABMT have been found to have antitumor effect in vitro.160 Unfortunately, the cytotoxic capacity of these cells is directed against tumor cells expressing the MHC II antigen, limiting the practical application of the therapy. Moreover, CsA induces significant autoimmunity in some, but not all, strains of rats and mice.161
In an attempt to widen the potential use of CsA, Charak et al,162 using a murine strain that does not develop autoimmune syndrome after CsA therapy, have shown that mice inoculated with non–Ia-bearing tumors (B16 melanoma and C1498 leukemic cells) which received both IL-2 and CsA after BMT had a better survival than mice receiving either IL-2 or CsA alone. However, no mice were cured. The rationale for linking IL-2 and CsA was based on the finding that CsA-generated T cells are highly responsive to IL-2 in vitro.163 The effectiveness of this two-step mechanism is borne out by the finding that the antitumor effect generated in mice receiving IL-2 and CsA could be transferred into secondary tumor-bearing recipients.164
Clinical Studies
CsA can induce an autoimmune syndrome in patients with lymphoma, acute myeloid leukemia, or breast cancer receiving ABMT.164-170This syndrome is mainly confined to the skin (erythematous maculopapular rash) without clinical evidence of visceral involvement. In one report, the presence of cytotoxic T cells recognizing the patient’s own pretransplant lymphocytes or tumor cell lines that expressed MHC class II determinants could be shown.164 No analysis of cytotoxicity against autologous fresh tumor cells was reported. The reason why clinical signs of autoaggression are located in the skin remains unknown. This could be related to the presence of Langerhans cells in the dermis or a high expression of MHC II in the skin.
Because hematologic malignancies and some solid tumors express MHC class II determinants, they too can presumably serve as the target of a CsA-induced cytotoxic effect. Encouraging results have been reported in a nonrandomized study for the treatment of 40 relapsed or refractory intermediate grade non-Hodgkin’s lymphoma treated with ABMT, CsA, and IFN-α in an attempt to further upregulate the expression of HLA antigens. Thirteen percent of the patients were found to relapse after a median follow-up of 24 months.170 This clinical outcome compares favorably with the trial of ABMT alone in patients with relapsing non-Hodgkin’s lymphoma. However, carefully planned trials comparing APSCT alone versus APBSCT with CsA are still lacking. CsA with or without IFN-γ has been administered after high-dose chemotherapy and ABMT in women with metastatic breast carcinoma, and showed that the combined therapy had an acceptable level of toxicity.166 167 However, no clinical benefits could be shown.
In none of the clinical studies was HLA expression analyzed on primary neoplastic tissue of patients entering the study nor was in vitro evidence of an antitumor effects shown. Overall, it remains to be seen whether there is consistent clinical benefit to the concept of inducing an autoaggressive GVHD-like syndrome in autologous marrow recipients.
Adoptive Transfer of Ex Vivo–Expanded MHC Nonrestricted Effector Cells
As described above, BM or PBSC can be incubated in vitro with cytokines to induce the development of cytotoxic cells. A number of protocols have been devised to select effector cells with cytotoxic activity from PBMC.
Activated NK Cells: Clinical Studies
Initial enthusiasm for the therapeutic use of LAK came from its use in preclinical and clinical studies against lymphoma and leukemia.171,172 In treating minimal residual malignant disease, LAK cells might be expected to be most effective if they are active against chemotherapy-resistant tumor cells which may have survived pretransplantation high-dose chemotherapy regimens. In in vitro studies, LAK cytotoxicity has been demonstrated against tumor cells surviving therapeutic concentrations of chemotherapeutic agents.173 This in vitro data led to pilot clinical trials combining systemic administration of IL-2 followed by apheresis to generate LAK cells after ABMT or ABSCT in lymphoma patients either in relapse or resistant to primary chemotherapy and in acute leukemia with poor prognostic indicators.115 117
CD3−CD56dim peripheral blood cells make up a specific subset of NK cells. These cells are obtained from the post–IL-2 leukapheresis product by adherence to plastic in the presence of IL-2. Such activated NK cells can be expanded in culture for 2 to 3 weeks with IL-2 in the presence of irradiated allogeneic concanavalin A (conA)-preactivated mononuclear cells. The injection of activated NK cells along with IL-2 to support the antitumor activity of NK cells has been used immediately posttransplantation in patients with lymphoma and no major toxicity has been observed with this combination therapy.174
Such manipulations including those involving apheresis after IL-2, expansion of NK cells, injection of IL-2 to support in vivo activity of NK cells entail many practical difficulties and morbidities limiting their applications to selected patients. In addition, these initial phase I trials have been performed on too small a number of patients to allow any conclusions regarding any potential benefit of these approaches.
Cytokine-Induced Killer Cells
A somewhat different therapeutically useful cytotoxic cell can be obtained by in vitro exposure of PBMC to combinations of IFN-γ, IL-2, and anti-CD3 monoclonal antibody. The resultant effector cell termed cytokine-induced killer cells (CIK) bears a CD3+CD56+ (but CD16−) phenotype and demonstrates non-MHC restricted cytotoxicity.175,176 CIK cells have been found to be substantially more cytotoxic in culture than LAK cells against cellular targets and, like LAK cells, they are effective against chemotherapy-resistant cell lines.177 Although ex vivo generation of CIK cells is IL-2 dependent, in vivo use of CIK cells has the advantage over LAK cells of not requiring additional systemic administration of potentially toxic IL-2 to augment their antitumor activity. In direct comparison, CIK cells have been shown to result in greater regression of disseminated human lymphoma in severe combined immunodeficient (SCID) mice than LAK cells.178,179 While a significant minority of CIK-treated lymphoma-bearing SCID mice had long-term survival, none of the LAK-treated mice survived.178 Because of apparent efficient cytotoxicity and limited systemic effects, expansion of CIK cells may find a place in protocols of autologous transplantation.
Cytotoxic Cell Lines
Human cell lines with potent MHC nonrestricted cytotoxicity activity against tumor cells have been reported. A cell line termed TALL-104 bearing the characteristic phenotype of cytotoxic cells has been derived from a human acute T-lymphoblastic leukemia and maintained in continuous culture in the presence of IL-2. TALL-104 cells show cytotoxicity exclusively against tumors across species without deleterious effects on normal tissues.179-181 After lethal irradiation, the cells are no longer leukemogenic when injected into SCID mice, but retain their killer function. In murine models bearing human tumors, administration of the human cytotoxic T-cell line had effective antitumor effects when given at early stage of disease.181
Additional NK cell lines have been established.182 183Their capacity to lyse cells appears restricted however to certain tumor types, thereby limiting their potential use. Because cell lines can be expanded continuously in culture, they constitute an unlimited source of effector cells. They may ultimately find a therapeutic niche after transplantation when minimal tumor disease is present and when poorly reactive immunity permits the injection of unmatched allogeneic cells.
Adoptive Transfer of Tumor-Specific MHC-Restricted Effector Cells
Adoptive cellular immunotherapy can be described as the transfer of target-specific effector cells to treat malignant disease. Such an approach entails the isolation and expansion of effector CD4+ and CD8+ T cells with specific reactivity for tumor cells from the host or other donor. In addition, adoptive immunotherapy requires that such cells survive in vivo for a sufficient amount of time to eradicate the tumor.184 This approach could be combined with injection of autologous stem cells.
It is currently feasible to grow T cells to large numbers in vitro by stimulating with antigen and by the addition of cytokines such as IL-2.185 As a result, T-cell therapy has been evaluated as a means to restore protective immunity against CMV following allo-BMT. CD8+ cytotoxic T-cell clones specific for CMV isolated from the blood of BM donors and grown in vitro have been infused to the recipients of allogeneic BM transplants to prevent CMV pneumonia.186 Adoptive transfer of antigen-sensitized T cells may in theory be evaluated in the treatment of malignant diseases. However, adoptive transfer of specific immunity is only possible when specific target tumor antigen(s) are identified and antitumor antigen-specific T cells are expanded. For most tumors currently treatable by high-dose chemotherapy and autologous transplantation, tumor-specific antigens are to date not known.
Ideal tumor antigens should be expressed exclusively or at least preferentially by tumors cells. In the emerging concept of tumor-specific cytotoxicity the context of tumor antigen presentation is no less important than the antigen itself. Once processed, the tumor-related antigen is presented on tumor cells as peptides bound to the HLA molecules. For clinical use then, this HLA restriction must also be identified. The antigen must further be shown to induce T-cell cytolytic responses in vitro and in vivo against tumor cells.
Ig expressed by B-cell malignancies are unique in that they can be distinguished from those expressed by normal B cells. The idiotype resulting from the combination of the variable regions of Ig heavy and light chain therefore represents the best example of a tumor-specific antigen. A number of hematologic malignancies are associated with mutations or translocations that result in expression of cellular oncogenic or chimeric proteins that play a role in malignant transformations and are present only on malignant cells (Table 1). These antigens presented in MHC molecules might induce a T-cell response which may potentially be expanded for clinical use. Prospective candidates for eliciting such a response are two leukemic-associated neoantigens. Chronic myelogenous leukemia (CML) and acute promyelogenous leukemia (APL) are characterized by recurrent translocations leading to the formation of fusion proteins, p210 bcr-abl and promyelocytic leukemia/retinoic acid receptor-α (PML/RAR) in CML and APL, respectively. In the case of p210, peptides have been derived from the fusion sequences and have been analyzed for consensus anchor residues for binding to a given HLA molecules. However, if a proliferation response can be obtained, a cytolytic response has mainly been performed using as target cell lines or normal PBMC bearing appropriate HLA type and loaded with peptides.207-210 Few data are available using fresh leukemic cells as target of cytolytic T cells. In addition, difficulties in obtaining peptide-specific CTL from PBMC of patients with CML in chronic phase suggest that these T cells may frequently be unresponsive to bcr-abl peptides.211 The demonstration of the presence of the processed peptides on the surface of fresh CML cells needs to be confirmed to validate such an approach. Therefore, search for new tumor antigens in addition to the Ig idiotype are needed.
An identical need for tumor-specific cell-surface antigens exists in the immunotherapy of solid tumors. Tumor-specific antigens and CTL-specific responses to these antigens have been most successfully shown for melanoma.212 Unfortunately, antigens uniquely or preferentially expressed by solid tumors currently treatable by high-dose chemotherapy and autologous stem cell support have not been so extensively described as those for melanoma and are now the object of active investigation.212 Solid-tumor–associated antigens might be oncogenic proteins that are mutated and overexpressed as described for breast carcinoma, mutated tumor suppressor proteins, and oncofetal protein (breast carcinoma), or tissue-specific differentiation antigens such as mucin (breast and ovarian carcinoma)213-216 (Table 1). It remains to be determined exactly whether these molecules may prove to induce an immune response and be biologically active antigens for T-cell therapy. In addition, the frequency of expression of putative tumor-specific antigens on tumor cells and the selection of specific epitopes for targeting T cells will have to be clarified.
Having tumor-specific antigens/peptides in hand does not necessary mean that effective T-cell therapy will follow. A composite picture of tumor antigenicity is still incomplete, but to induce a therapeutically useful T-cell response a number of considerations come into play: (1) the frequency of precursor of CTLs capable of recognizing the HLA-restricted peptides may be very low; (2) the relevant antigenic peptide may be present heterogeneically or not be displayed at the surface of tumor cells because they cannot adequately process the antigen or because a different set of peptides is expressed by tumor cells than the peptides predicted; (3) patients must bear the appropriate HLA for which the epitopes have been identified; (4) many tumors are known to have lost completely or partially their HLA molecules; (5) the concentration of peptides needs to be adjusted to avoid selective expansion of low-avidity CTL which are not tumor-reactive217; (6) finally, tumor may fail to attract and activate tumor-specific T cells at the tumor site.218Therefore, adoptive transfer of effector cells will likely have to be worked out on an antigen-by-antigen basis.
Autologous Antigen-Pulsed Dendritic Cells
Dendritic cells (DC) are powerful specialized antigen-presenting cells that can initiate T-cell–mediated immune responses.219 DC cultured (or pulsed) in vitro with proteins process them into peptides and present them as antigens to naive T cells in such a way that the T cells can recognize and respond to the stimulus. The processed antigen is recognized by T cells in an MHC-restricted fashion. DC have been pulsed with tumor-associated proteins and peptides, thereby generating antitumor cytolytic responses in preclinical models220-226as well in a clinical trial with patients with follicular B-cell lymphoma.227 As noted, however, T-cell–defined epitopes presentable by DC have not been identified for most tumors. Because of the extensive diversity of MHC antigens, designing common peptides for T-cell recognition for different individuals with the same disease may prove difficult. For hematological malignancies having recurrent translocations resulting in common fusion proteins or for solid tumors uniformly overexpressing identifiable oncogenes, synthetic peptides have been designed based on the presence of HLA anchor motifs.211 228-230 Some public and private epitopes have been found to be recognized by CTL in the context of MHC class I restricted molecules (Table 2). However, the affinity of a peptide for its corresponding HLA molecules does not necessarily predict its recognition by T cells and its capacity to elicit a cytotoxic response.
To circumvent this problem and provide DC with presentable tumor-associated peptides, methods have been developed to isolate immunogenic peptides from tumor cells using mild acid elution.235 Transfer of DC cells with tumor peptides isolated by this method has been shown to result in suppression of the growth of weakly immunogenic tumors in a murine model.224However, this individualized therapy with unique tumor peptides might be performed only when a large number of tumor cells are available, as it is the case for leukemia.
DC can be generated in large numbers from CD34+hematopoietic progenitors cells using a combination of cytokines including primarily GM-CSF and tumor necrosis factor-α (TNF-α).236-239 Expansions of up to approximately 1.7 × 106 mature DC have been obtainable from as little as 1 mL of normal adult BM using stem cell factor (SCF), GM-CSF, and TNF-α.237 Combining flt-3 ligand to the above three growth factors, it was estimated that 0.6 × 109/kg DC could be generated starting from peripheral progenitor cells of a single leukapheresis.238 Because of this potential of obtaining DC in large numbers in vitro and because of their importance in processing tumor antigens into specific peptides, DC represent an attractive target for transfer of genes encoding tumor-associated antigens. Recent efforts have shown that expression can be achieved by retroviral transduction of CD34+ progenitor cells while they are made to differentiate into DC by growth factors.240-242 Alternatively, DC can be initially obtained and subsequently transduced with adenovirus vectors in which tumor-associated antigen cDNA have been inserted.243-245These strategies will be helpful to circumvent the difficulties in identifying peptides derived from tumor antigens of each individual patient.
As a result of these initial studies, clinical protocols incorporating DC are envisioned which will include expansion of CD34+populations into DC followed by either pulsing the cells with tumor antigens and antigen-derived peptides epitopes, or introducing genes encoding tumor-associated antigens into them for therapeutic reinjection into patients.
Induction of Antitumor Effect by Combining Autologous HSC Transplantation With Infusions of Allogeneic T Cells
A major antineoplastic advantage of allo-BMT is the possibility of inducing a graft-versus-host effect and graft-versus-leukemia has been mainly described. This same antineoplastic effect could be exploited in autologous transplantation. In this approach, patients would receive autologous stem cells purged of tumor contamination for hematopoietic regeneration following myeloablative conditioning regimens together with lymphocytes from an allogeneic donor. The combination of autologous BM in conjunction with allogeneic lymphocytes and IL-2 has been performed in patients with non-Hodgkin’s lymphoma and leukemia.246 However, the number of patients involved was too low to draw any conclusion regarding its efficacy. This technique may further be rendered even more specific if the allogeneic donors could be previously vaccinated with tumor antigens in an attempt to induce an antitumor response in the recipients. Conceivably, tumor antigens might needed to be altered because tumor antigens may be “self” protein and fail to induce an immune response in healthy individuals. The limitations of this procedure will likely be the difficulty in obtaining an adequate histocompatible donor and control the development of GVHD. The possibility to confer an inducible suicide phenotype to donor T cells by gene transfer of herpes simplex virus thymidine kinase may provide a means of suppressing the development of severe GVHD.247
SUMMARY AND OUTLOOK
Although the regeneration of the CD4 T-cell compartment is generally delayed because of the absence of normal thymic maturation, cellular immunity regains effective levels of function within the first 3 months after transplantation. The use of PBSC in recent years has further contributed to accelerate immune reconstitution in comparison to BM, although the overall advantage of PBSC in this regard remains unclear. Tumor immunotherapy is defined as attempts in vivo to harness the regenerating posttransplant immune responses and apply them against malignant cells. As reviewed here, these attempts fall into one of two categories: induction of nontumor specific cytotoxicity and activation of tumor-directed cellular immunity. Nonspecific methods, including administration of cytokines or incubation of the graft with cytokines, induction of an autoaggression syndrome with cyclosporine, and expansion of NK cells, have not yet been clearly shown to be effective in reducing relapses and improve survival. Furthermore, none of these therapies are without their own side effects. It is unlikely that major progress will be made in the clinical use of cytokines alone, although they may find a therapeutically useful niche enhancing immune effector cells. As for infusion of non–tumor-specific effector cells, administration of activated NK cells or CIK cells requires large-scale cellular expansion, rendering the procedure as yet technically difficult for routine clinical use.
The second category of immunotherapy, tumor-specific modalities, may prove to be most effective when the amount of postchemotherapy residual disease is reduced to a minimum. However, in devising tumor-specific approaches, numerous obstacles have already been recognized that need to be overcome before even hoping for general clinical use. The identification of tumor-associated antigens (even if not strictly unique antigens) inducing immune responses is a prerequisite to the development of adoptive transfer of either effector immune cells or use of APC to activate cytolytic T cells. In addition, many questions remains to be resolved: How to obtain an adequate number of clonal cytotoxic T cells? What is the optimal way to pulse dendritic cells? What is the fate of the generated antitumor immune effector cells once reinjected to the patients? The next 5 years should clarify to what extent specific—and nonspecific—immunotherapy may be able to fill in the shortcoming of conventional chemotherapy and finally lead to more effective long-term control of tumors.
Address reprint requests to Thierry Guillaume, MD, PhD, Laboratory of Experimental Oncology and Hematology, University of Louvain, 54 Avenue Hippocrate, UCL54.71, 1200 Brussels, Belgium.