Abstract
The long-term culture (LTC) system has been useful for analyzing mechanisms by which stromal cells regulate the proliferative activity of primitive normal, but not chronic myeloid leukemia (CML), hematopoietic progenitor cells. In previous studies, we identified two endogenous inhibitors in this system. One is transforming growth factor-β (TGF-β), which is equally active on primitive normal and CML progenitors. The other we now show to be monocyte chemoattractant protein-1 (MCP-1). Thus, MCP-1, when added to LTC, blocked the activation of primitive normal progenitors but did not arrest the cycling of primitive CML progenitors. Moreover, the endogenous inhibitory activity of LTC stromal layers could be overcome by the addition of neutralizing antibodies to MCP-1, but not to macrophage inflammatory protein-1α (MIP-1α). However, neither of these antibodies antagonized the inhibitory activity of NAc-Ser-Asp-Lys-Pro (AcSDKP) on primitive normal but not CML progenitor cycling in this system. Moreover, none of six other -C-C- or -C-X-C- chemokines, previously shown to inhibit primitive normal human CFC proliferation in semisolid assays, were found to act as negative regulators when added to normal LTC. These results provide further support for the concept that primitive CML progenitor cell proliferation is deregulated when these cells are exposed to limiting concentrations of multiple inhibitors, only some of which have differential actions on normal and Ph+/BCR-ABL+ cells.
MAINTENANCE OF AN appropriate output of mature blood cells in vivo relies on an effective interplay between hematopoietic progenitors at different stages of differentiation and multiple positively and negatively acting cytokines. In this context, positively acting cytokines are those that stimulate a given cell type to enter S-phase as well as those that sustain their viability. Conversely, negatively acting cytokines refer to those that inhibit the cell-cycle progression of the same cells and/or that induce apoptosis. Previous studies have shown that, in the microenvironment of normal adult marrow tissue, hematopoietic progenitors at different stages of differentiation are maintained in different turnover states,1-3 although this situation is subject to alteration by developmental4 as well as genetic2 or physiologic perturbations.5-7 In addition, we now know from extensive in vitro studies that progenitors at all stages of differentiation react to many stimulators and inhibitors, although the particular set to which a given progenitor is responsive and the type of response elicited varies with progenitor type. In vitro studies have also shown that stromal cells, either spontaneously or after activation, produce many of the cytokines that can regulate hematopoietic progenitor proliferative activity.8-12However, because of the complexity of their ranges of effects, it has been difficult to establish which cytokines are the key regulators of specific progenitor populations in vivo.
In this respect, the long-term marrow culture (LTC) system has served as an interesting experimental model because it appears to embody many of the features of hematopoietic cell regulation in vivo. In LTC, primitive hematopoietic cells become associated with and are maintained and regulated by a layer of adherent cells which consists primarily of fibroblasts and macrophages.13,14 In unperturbed LTC, the primitive (high proliferative potential) but lineage-restricted colony-forming cells (CFC) contained within the adherent layer become quiescent, albeit reversibly, whereas the more mature (low proliferative potential) CFC also present proliferate continuously.15 Thus, the different cycling behavior of these progenitor populations in LTC mimics that seen in vivo.15 When hematopoietic cells from patients with chronic myeloid leukemia (CML) are cocultured with stromal cells under the same conditions, the primitive neoplastic progenitors do not become arrested in Go,16 an abnormal behavior they also display in vivo.2 Such findings suggest that the LTC model would be useful for identifying physiologically relevant inhibitors of primitive normal hematopoietic progenitor proliferation.
Transforming growth factor-β (TGF-β) was the first endogenously produced inhibitor shown to be active in the LTC system.17However, primitive CML cells were found to be normally responsive to TGF-β and, if exposed to higher concentrations of TGF-β than are normally present in LTC, their cycling, like that of their primitive normal counterparts, can also be reversibly arrested.18 To reconcile these apparently paradoxical findings, we postulated the existence of a cooperative mechanism responsible for the inhibition of primitive normal, but not CML, progenitor cycling in the LTC system. This mechanism would involve the combined activity of limiting concentrations of TGF-β and a second inhibitor to which primitive CML cells are specifically unresponsive. In subsequent studies, we obtained evidence that the second inhibitor might be a chemokine, because its activity could be neutralized by the addition to LTC of excess macrophage inflammatory protein-1β (MIP-1β).19Subsequent studies showed that MIP-1α, another member of the chemokine -C-C- family with known abilities to inhibit primitive hematopoietic cell proliferation in suspension cultures,20could also block the activation of primitive normal, but not CML, CFC in the LTC system.19 In addition, exogenously added MIP-1β was found to antagonize the ability of NAc-Ser-Asp-Lys-Pro (AcSDKP) to inhibit the proliferation of primitive normal, but not CML, progenitors in the LTC system.21 Furthermore, MIP-1α was shown to be constitutively produced in these cultures.22These findings suggested that MIP-1α might be the endogenously produced chemokine that cooperates with TGF-β to block the cell-cycle progression of primitive normal progenitors in LTC. When neutralizing anti–MIP-1α antibodies eventually became available, it was possible to test this prediction directly. As described in this report, the results of such experiments led to the discovery that monocyte chemoattractant protein-1 (MCP-1), and not MIP-1α, is one of at least two endogenously produced chemokines responsible for the selective inhibitory effects observed on primitive normal progenitors in the LTC system.
MATERIALS AND METHODS
Reagents.
Recombinant human MIP-1α (Genetics Institute, Cambridge, MA) was kept in proprietary buffer designed to minimize aggregation at −20°C. Purified AcSDKP, obtained from the Microsequencing Centre of the University of Victoria (Victoria, Canada), was stored in concentrated form and then diluted just before addition to LTC as previously described.21 Recombinant human regulated-on-activation-normal-T-cell-expressed-and-secreted (RANTES), MCP-1, MCP-2, MCP-3, TGF-β1, interleukin-8 (IL-8), neutralizing anti–MIP-1α and anti–MCP-1 antibodies, and appropriate control Ig preparations were purchased from R & D Systems (Minneapolis, MN). Human platelet factor-4 (PF-4) was purchased from Sigma (St Louis, MO) and biologically active recombinant human interferon inducible protein-10 (IP-10) was prepared as previously described.23High specificity activity 3H-thymidine (25 Ci/mmol) was obtained from Amersham (Oakville, Ontario, Canada). Human erythropoietin (Epo) and granulocyte colony-stimulating factor (G-CSF) were provided by StemCell Technologies (Vancouver, BC, Canada), human Steel factor (SF) by Amgen (Thousand Oaks, CA), human IL-3 and human granulocyte-macrophage CSF (GM-CSF) by Novartis (Basel, Switzerland), and human IL-6 by Cangene (Mississauga, Ontario, Canada).
Cells.
Normal human bone marrow aspirate cells were obtained with informed consent from individuals donating marrow for allogeneic transplants or, alternatively, they were obtained from cadaveric sources (North West Tissue Center, Seattle, WA). The cells were centrifuged on Ficoll-Paque (Pharmacia Biotech, Baie d-Urfe, Quebec, Canada) to isolate the light-density (≤1.077 g/cm3) fraction and the cells were then washed twice before use.
Cells from four patients with chronic phase, Ph chromosome-positive (Ph+) CML were obtained with informed consent as part of routine diagnostic or follow-up procedures.
LTC.
LTC were initiated in 35-mm tissue culture dishes by seeding 8 × 106 normal human light-density bone marrow cells or 2 × 106 light-density CML peripheral blood cells in 2.5 mL of human LTC medium (Myelocult; StemCell Technologies) supplemented just before use with 10−6 mol/L hydrocortisone sodium hemisuccinate (Sigma) onto pre-established, semi-confluent irradiated feeder layers of normal marrow LTC adherent cells as previously described.19 LTC were then incubated at 33°C, without further perturbation, for 10 to 12 days before use in progenitor cycling studies as described.
3H-thymidine suicide procedure.
LTC adherent cells were detached using trypsin24 after removal of the nonadherent cells, and then washed twice and resuspended in Iscove's medium without fetal calf serum (FCS; StemCell) at a concentration of 106 cells/mL. One-milliliter aliquots were then incubated at 37°C in an environment of 5% CO2 for 1 hour after which high-specific 3H-thymidine (20 μCi) was added to one of the two aliquots. Both tubes were then returned to the incubator for 20 minutes. The reaction was arrested by washing the cells twice in Iscove's medium containing 2% FCS and 400 μg/mL of cold thymidine before finally plating suitable aliquots of the cells in methylcellulose-containing medium (Methocult H4330; StemCell) supplemented with 3 U/mL of human Epo, 50 ng/mL of SF, and 20 ng/mL each of IL-3, IL-6, G-CSF, and GM-CSF as previously described.25 Erythroid and granulocyte-macrophage colonies of different sizes were scored after 2 to 3 weeks of growth at 37°C and subdivided as large (>8 clusters of erythroblasts or >1,000 cells) or small (3 to 8 clusters of erythroblasts or 20 to 1,000 cells) to allow the cycling status of primitive and mature erythroid (BFU-E) and granulopoietic (CFU-GM) progenitors to be separately evaluated.15,16 The proportion of each type of progenitor that was in S-phase at the time of the LTC harvest is indicated by the “percent kill” value. This value was calculated from the reduction in colony yields due to the brief in vitro exposure of the cells to 3H-thymidine just before plating.15For all of the LTC treatments evaluated here, there was no evidence of any significant effect of the prior treatment of the LTC with cytokines or antibodies on the number of any type of CFC present when the LTC were subsequently assessed (as seen by comparing the CFC numbers in control v treated LTC). Hence, only percent kill values are shown for the different CFC categories assessed. The Student'st-test was used to assess the significance of the different responses measured.
RESULTS
Addition of anti–MIP-1α antibodies to preactivated LTC of normal human marrow cells does not influence the proliferation of the primitive hematopoietic progenitors present in the adherent layer.
In an initial series of experiments, we used neutralizing anti–MIP-1α antibodies to determine whether endogenously-produced MIP-1α could be shown to contribute to the cell-cycle arrest of primitive normal CFC that occurs in unperturbed LTC. For this, LTC were initiated with normal human marrow cells as described in Materials and Methods and then given a first medium change 10 to 12 days later. Another 3 days after that, 200 μg/mL of anti–MIP-1α antibodies or 300 ng/mL of MIP-1β (as a positive control), or 200 μg/mL of a control Ig preparation (as a negative control) were added. As shown previously, the added MIP-1β blocked the return of the primitive CFC to a quiescent state 4 days later (Table1). In contrast, the added anti–MIP-1α antibodies had no effect on the cycling behavior of the primitive CFC in these experiments. Even 3 successive daily additions of the anti–MIP-1α antibodies failed to mimic the effect of a single addition of MIP-1β (data not shown).
To validate the effectiveness of the anti–MIP-1α antibodies, their ability to neutralize the inhibitory action of exogenously added MIP-1α on the activation of normal progenitor cycling in the LTC system was assessed. Therefore, a series of replicate normal LTC was initiated, and then 10 days later a half medium change was performed to activate the primitive CFC in the adherent layer. At the same time, 100 ng/mL of MIP-1α with or without 100 μg/mL of the same anti–MIP-1α antibody preparation used for the experiments shown in Table 1 (or control Ig) were also added. Two or 3 days later, the cycling status of the primitive CFC in the adherent layer of these LTC was assessed. As shown in Table 2, 100 μg/mL of these anti–MIP-1α antibodies were sufficient to specifically and completely overcome the inhibitory activity of 100 ng/mL of simultaneously added MIP-1α. Because this concentration of MIP-1α is much higher than what has been detected in the LTC system, it seemed likely that there might be another endogenously produced chemokine involved.
Prior studies had shown that, like the addition of MIP-1α, the addition of AcSDKP to normal LTC could inhibit the activation of primitive hematopoietic progenitors contained within the adherent layer. Moreover, this inhibitory effect could be similarly antagonized by the simultaneous addition of MIP-1β.21 Therefore, it was also of interest to determine whether the effects of AcSDKP might be mediated by induced increases in endogenous MIP-1α. To test this possibility, we used the same type of protocol as described for the experiments shown in Table 2. Therefore, another series of replicate normal LTC were initiated and 10 days later at the time of the first half medium change, 300 ng/mL AcSDKP with (or without) 300 ng/mL of MIP-1β, or 100 μg/mL of the anti–MIP-1α antibody, or a control Ig preparation were added. The different effects of these various treatments on the cycling status of the primitive CFC present in the adherent layer 2 or 3 days later are shown in Table 3. The results for all previously studied treatments were reproduced in these experiments. However, the addition of 100 μg/mL of anti–MIP-1α antibodies, unlike the addition of 300 ng/mL of MIP-1β, did not block the ability of concurrently added AcSDKP to prevent the activation of primitive CFC cycling.
Addition to normal LTC of MCP-1, but not PF4, IL-8, IP-10, RANTES, MCP-2, or MCP-3, can prevent the activation of quiescent primitive normal human progenitors.
The preceding experiments suggested that there might be another relevant chemokine produced in LTC that is different from MIP-1α, but able to be antagonized by MIP-1β and be similarly ineffective in suppressing the proliferation of primitive CML cells. To determine first what chemokine(s) might mimic the inhibitory activity of MIP-1α on primitive CFC in the LTC system, a number of chemokines from both the C-C (like MIP-1α) and the C-X-C families were tested, including several shown to inhibit the SF-enhanced growth of human CFC in short-term methylcellulose assays.23 26 The protocol followed involved adding each chemokine to a 10-day-old normal marrow LTC at the same time as the first half-medium change. Table 4 shows that, of the 7 chemokines added, only MCP-1 was able to block the proliferation of the primitive CFC in the adherent layer when these progenitors were assessed after 2 to 3 days of chemokine treatment. Moreover, both primitive (high proliferative potential) erythroid and granulopoietic progenitors were sensitive to the inhibitory effects of MCP-1, whereas the mature (low proliferative potential) granulopoietic progenitors, coexisting in the adherent layer of the same cultures, were not.
Addition of anti–MCP-1 antibodies to previously activated normal LTC prevents the return of the primitive human progenitors to a quiescent state 4 to 5 days later.
We next examined the possibility that MCP-1 might also be an endogenously produced inhibitor in the LTC system. Analysis by enzyme-linked immunosorbent assay (ELISA; R & D Systems) showed the medium harvested from LTC several days after feeding to contain readily detectable levels (0.4 to 2.9 ng/mL) of MCP-1 to be present, raising the likelihood of their availability at higher levels within the adherent layer.27 Accordingly, the same type of experiments described above (Table 1) were repeated. However, in this case, 1.6 μg/mL of a neutralizing MCP-1 antibody preparation (ie, sufficient to neutralize 100 ng/mL of MCP-1), instead of anti–MIP-1α antibodies, were added to LTC that had been given a half-medium change 2 to 3 days previously to activate the primitive CFC population in the adherent layer. The cycling activity of these progenitors was then assessed another 4 days later. As shown in Table 5, the primitive CFC continued to proliferate in the cultures to which the anti–MCP-1 antibodies had been added, in contrast to the control LTC in which this subpopulation of CFC (but not the mature CFC) had, as expected, become quiescent.
To determine whether endogenously produced MCP-1 might also mediate the inhibitory effects obtained by adding AcSDKP to normal LTC, we undertook a further series of experiments. In these, anti–MCP-1 antibodies (1.6 μg/mL) were added simultaneously with AcSDKP to 10-day-old LTC at the time of a first half-medium change and the cycling status of the primitive CFC obtained from the adherent layer was then assessed 2 or 3 days later. The results of these experiments are shown in Table 6. Although the ability of AcSDKP to inhibit the proliferation of primitive progenitors in the LTC system was again seen, this was not affected by the addition of the same preparation and dose of anti–MCP-1 antibodies that had been effective in blocking the spontaneous mechanism of endogenous inhibition analyzed in the experiments shown in Table 5.
Added MCP-1 fails to inhibit the cycling of neoplastic progenitors in the adherent layer of CML LTC.
Previously we showed that primitive CML progenitors, when cocultured with normal LTC adherent layers, proliferate continuously, despite their apparently normal sensitivity to the inhibitory effects of TGF-β.18 A potential explanation for this paradox was suggested by the observation that the proliferation of primitive CML progenitors is not inhibited by their exposure to MIP-1α in the LTC system. Additional studies with MIP-1β suggested that primitive normal CFC cycling in these cultures is downregulated by the cooperative action of limiting concentrations of an endogenously produced chemokine (like MIP-1α) working in concert with limiting concentrations of TGF-β. The experiments described above indicate that MCP-1, and not MIP-1α, is the endogenous chemokine involved. Therefore, to determine if the insensitivity of primitive CML CFC in LTC to MIP-1α would extend to MCP-1, a final series of experiments with CML LTC were undertaken. Preselected cryopreserved samples of cells from CML patients (previously shown to contain exclusively Ph+ progenitors28) were seeded onto normal human marrow-derived LTC adherent layers. Ten days later, at the time of the first half-medium change, different candidate inhibitors were added. Another 2 days later, the cycling status of the CFC in the layer was assessed. As shown in Table 7, addition of MCP-1, at the same concentration found to be effective in normal LTC (100 ng/mL), did not alter the proliferative activity of the primitive CML CFC in these LTC, although in parallel cultures it was again possible to show an arrest of the proliferation of the same type of CML CFC in cultures to which 5 ng/mL of TGF-β was added.
DISCUSSION
Chemokines constitute a large group of basic heparin-binding polypeptides that are identified by their conservation of a primary structure containing up to four cysteines.29,30 The two largest and best characterized subfamilies of chemokines are distinguished by the first two cysteines being adjacent (C-C) or separated by an intervening amino acid (C-X-C). The genes encoding the C-C chemokines are closely clustered on human chromosome 17 and the genes for the C-X-C chemokines are located on chromosome 4. The C-C family includes MIP-1α, MIP-1β, MCP-1, -2, and -3, and RANTES. PF-4, IL-8, and IP-10 are all members of the C-X-C family. Perhaps the best-studied chemokine inhibitor of hematopoiesis is MIP-1α. It was first identified as the active inhibitory component in media obtained from cultures of a macrophage-like cell line31 and subsequently shown to be active on primitive human as well as murine CFC in semisolid cultures.20,26 Later studies showed that MIP-1α also has a selectively inhibitory effect on primitive progenitors present in the adherent layer of LTC established from normal human marrow.19 Nevertheless, knock-out of the MIP-1α gene did not result in any obvious perturbation in vivo of hematopoiesis.32 Further understanding of how chemokines may regulate the cell-cycle progression of primitive hematopoietic cells has been made difficult by the lack of cell line models that reproducibly exhibit this response. In addition, in both in vivo and in vitro studies, a stimulatory (or no) effect of MIP-1α on more mature hematopoietic progenitors has been observed.19,26,31 33
In the present study, we have shown that primitive, but not mature, normal human hematopoietic cells, when contained in the environment of an LTC-adherent layer, can be inhibited from entering S-phase by added MCP-1. In addition, we have shown that antibody-mediated neutralization of endogenous MCP-1 (like antibody-mediated neutralization of TGF-β11) can allow primitive cycling progenitors to continue to proliferate under conditions where they would otherwise become quiescent. Moreover, the inhibitory activity of MCP-1 in the LTC system is not shared by any of several other chemokines except MIP-1α. Thus, at first glance, our results appear to differ from those reported by Broxmeyer et al, who found several of the C-C and C-X-C chemokines tested here to inhibit colony formation by progenitor subsets that are stimulated by SF-containing cytokine combinations in methylcellulose assays.20,23,26,34 Very recently this group also obtained evidence that the effects of these different chemokines may be mediated by different chemokine receptors because only the inhibitory response to MCP-1 was inactivated in the hematopoietic progenitors of CCR2−/− mice.35 A different explanation for our findings may reside in the fact that the inhibitory effectiveness of a given chemokine on a potentially sensitive progenitor can be influenced by the context in which the progenitor is exposed to it.36 37
In the adherent layer of the LTC system, the identity of the endogenously produced factors responsible for activating primitive human CFC into cycle after the addition of fresh medium has not been conclusively established. However, a combined effect of G-CSF, GM-CSF, and IL-6 (perhaps in combination with other as yet unidentified factors), appears a reasonable possibility.38 Time-course studies have shown that the continuous addition of a sufficient concentration of these positively acting factors over a period of 2 to 3 days can stimulate the proliferation of primitive CFC in LTC-adherent layers.17 Other treatments, like the addition of fresh medium or IL-1, that appear to activate primitive CFC indirectly, increase the endogenous production, in LTC, of G-CSF, GM-CSF, and IL-6.11 On the other hand, current evidence indicates that endogenously produced SF does not contribute to the basal level of hematopoiesis seen in LTC.39 Thus, the inability of some chemokines to inhibit primitive normal CFC in LTC-adherent layers may also be explained by the presence of different positive stimulatory cytokine combinations than those used previously in semisolid assays. Whether the effects seen in the LTC are physiologically relevant must await equivalent in vivo studies. Interestingly, MCP-1−/− mice have not been reported to display altered blood cell production,40 suggesting that if the cycling of their primitive progenitors is deregulated, compensatory mechanisms modulate any changes caused.
The presence of receptors for C-C chemokines on a number of primary human hematopoietic cell types has been reported, including both myeloid and lymphoid cells.29,30 However, the results of such studies have not yet clarified the particular receptor(s) responsible for inhibiting the entry into S-phase of primitive normal CFC given their promiscuous expression and binding abilities. In addition, the same receptor may bind multiple chemokines but activate different responses.41,42 One recently described receptor, designated hD6/CCR 9, binds a large number of C-C chemokines including MIP-1α, MIP-1β, MCP-1, -2, -3, -4, and RANTES with varying affinity. It may be a general C-C family receptor expressed on different hematopoietic cells, although its ability to signal has not yet been shown.43 Competitive binding studies have indicated the existence of at least two other high-affinity receptors for MCP-1 (CCR-244,45 and CCR1046). However, none of the C-C chemokine binding patterns of these receptors match the activity profile described here for primitive normal CFC cycling inhibition in LTC. Thus, none of the cellular chemokine receptors reported to date appear to explain the singular inhibitory effects of MCP-1 and MIP-1α on primitive normal CFC proliferation and the ability of their activities to be antagonized by MIP-1β.
The ability of one chemokine to cause a decreased response to a second chemokine is a noted feature of chemokine action, and a two-step model of chemokine receptor activation has been proposed to explain this behavior. According to this two-step model, a chemokine may bind with high affinity to the amino-terminal extracellular domain of a receptor which, only if followed by a second interaction with one or more of the extracellular loops of the same receptor, then initiates transduction of an intracellular signal.47 Such a model has been proposed to explain, in part, why different ligands might not necessarily elicit the same outcomes by virtue of their different abilities to bind to one site on a given receptor with different consequences for the second site.
The ability of added anti–MCP-1 and not anti–MIP-1α antibodies to prevent the cell-cycle arrest of primitive normal CFC in unperturbed LTC identifies MCP-1 as the specific endogenously produced chemokine regulating this process (Fig 1). On the other hand, neither MCP-1 nor MIP-1α appears to mediate the inhibitory effect elicited by adding AcSDKP to LTC. However, the fact that the effect of AcSDKP is antagonized by MIP-1β suggests that another, as yet unidentified, chemokine may be produced in the LTC system and contribute to the regulation of primitive cell turnover under certain circumstances. A number of new C-C chemokines have recently been described, including several that showed effects on hematopoietic cells,48-50 and it will clearly be of interest to investigate their potential activities in LTC of normal and CML cells.
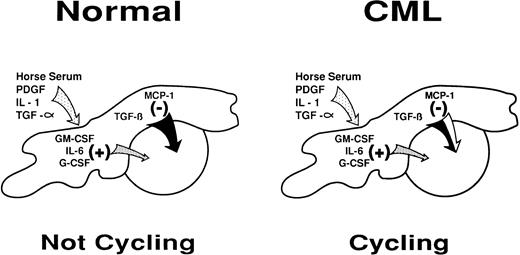
Diagrammatic representation of the intercellular molecular mechanisms by which stromal cells may regulate primitive normal hematopoietic progenitors through a shifting balance in limiting concentrations of multiple positive and negative factors. A partial defect in the inhibitory arm of this mechanism may explain its failure to control the increased proliferative activity of primitive CML progenitors in the LTC system and in vivo.
Diagrammatic representation of the intercellular molecular mechanisms by which stromal cells may regulate primitive normal hematopoietic progenitors through a shifting balance in limiting concentrations of multiple positive and negative factors. A partial defect in the inhibitory arm of this mechanism may explain its failure to control the increased proliferative activity of primitive CML progenitors in the LTC system and in vivo.
Primitive CML cells, unlike their normal counterparts, are actively proliferating in vivo.2 This suggests that one of the consequences of the expression of the BCR-ABL fusion gene in CML cells is the acquisition of signaling properties that allow these cells to ignore (or overcome) the intracellular events stimulated by interactions with chemokines that normally inhibit primitive hematopoietic cell proliferation (Fig 1). A defect in the sensitivity of CML cells to the negative effects of both MCP-1 and MIP-1α, two closely related chemokines, provides further support for this view. However, whether this occurs at the level of chemokine receptor expression or later downstream of chemokine receptor activation is still not clear. Nevertheless, because primitive CML cells do remain sensitive to the inhibitory effects of TGF-β, any effect of BCR-ABL must be proximal to the point of convergence of TGF-β and MCP-1 (or MIP-1α ) in inhibiting primitive progenitors from entering S-phase.
ACKNOWLEDGMENT
The authors thank Dianne Reid for expert technical assistance; Bernadine Fox for preparing the manuscript; and Amgen, Cangene, Genetics Institute, Novartis, and StemCell for their generous gifts of cytokines and culture reagents.
Supported by grants from the National Cancer Institute of Canada (NCIC) with funds from the Terry Fox Run (to A.C.E.), from Novartis (to C.J.E.), and from Glaxo-Wellcome (to A.H.S.). C.J.E. is a Terry Fox Cancer Research Scientist of the NCIC.
Address reprint requests to A.C. Eaves, MD, PhD, Terry Fox Laboratory, 601 W 10th Ave, Vancouver, BC, Canada, V5Z 1L3; e-mail:allen@terryfox.ubc.ca.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked "advertisement" is accordance with 18 U.S.C. section 1734 solely to indicate this fact.