Abstract
Essential thrombocythemia (ET) and idiopathic myelofibrosis (PMF) are two myeloproliferative diseases characterized by a marked megakaryocytic (MK) involvement. The pathogenesis of these two diseases is unknown. Recently it has been shown that overexpression of Mpl-ligand (Mpl-L) in mice induces thrombocytosis and myelofibrosis. In this study, we investigated whether Mpl-L was responsible for the pathogenesis of ET and PMF. Using in vitro cultures of blood or marrow CD34+ cells, we investigated whether MK growth was abnormal in these two diseases. Spontaneous MK growth involving only a fraction (20%) of the MK progenitors, as compared with growth in the presence of pegylated recombinant human megakaryocyte growth and development factor (PEG-rhuMGDF), was found in both diseases (21ET and 14PMF) using serum-free semisolid and liquid cultures, including cultures at one cell per well. We first searched for ac-mpl mutation/deletion by sequencing the entire coding region of the gene by polymerase chain reaction (PCR) in nine ET patients and five PMF patients, but no mutation was found. We subsequently investigated whether an autocrine stimulation by Mpl-L could explain the autonomous MK growth. Addition of different preparations of soluble Mpl receptor (sMpl) containing a Fc domain of IgG1 (sMpl-Fc) markedly inhibited MK spontaneous growth in both ET and PMF patients. This effect was specific for sMpl because a control soluble receptor (s4-1BB-Fc) had no inhibitory effect and an sMpl devoid of the Fc fragment had the same inhibitory efficacy as the sMpl-Fc. This inhibition was reversed by addition of PEG-rhuMGDF or a combination of cytokines. The sMpl-Fc markedly altered the entry into cell cycle of the CD34+ cells and increased the apoptosis that occurs in most patient CD34+ cells in the absence of exogenous cytokine, suggesting an autocrine stimulation. In contrast, a neutralizing antibody against Mpl-L did not alter the spontaneous MK growth, whereas it totally abolished the effects of 10 ng/mL PEG-rhuMGDF on patient or normal CD34+ cells. Mpl-L transcripts were detected at a very low level in the patient CD34+cells and MK and only when a highly sensitive fluorescent PCR technique was used. By quantitative reverse-transcription (RT)-PCR, the number of Mpl-L transcripts per actin transcripts was lower than detected in human Mpl-L–dependent cell lines, suggesting that this synthesis of Mpl-L was not biologically significant. In favor of this hypothesis, the Mpl-L protein was not detected in culture supernatants using either an enzyme-linked immunosorbent assay (ELISA) or a biological (Ba/F3huc-mpl) assay, except in one PMF patient. Investigation of Mpl-L signaling showed an absence of constitutive activation of STATs in spontaneously growing patient MKs. Addition of PEG-rhuMGDF to these MKs activated STATs 3 and 5. This result further suggests that spontaneous growth is neither related to a stimulation by Mpl-L nor to ac-mpl mutation. In conclusion, our results show that Mpl-L or Mpl are not directly implicated in the abnormal proliferation of MK cells from ET and PMF. The mechanisms by which the sMpl mediates a growth inhibition will require further experiments.
ABNORMAL MEGAKARYOCYTE (MK) proliferation is often associated with myeloproliferative diseases, which include chronic myelogenous leukemia (CML), polycythemia vera (PV), essential thrombocythemia (ET), and idiopathic myelofibrosis (PMF). In these two last diseases, alterations of megakaryocytopoiesis are the predominant features. ET is defined by a megakaryocytic (MK) marrow hyperplasia and persistent thrombocytosis, whereas PMF is a more typical myeloproliferative disease characterized by a myelofibrosis initially associated with a marked myeloid proliferation. In PMF, there is an important hyperplasia of morphologically abnormal MK that may be responsible for the marrow myelofibrosis by a local release of fibroblast growth mediators.1-5
With the exception of CML, the molecular mechanisms responsible for the clonal proliferation in these disorders are presently unknown. In PV, the biological hallmark of the disease is an erythropoietin (Epo)-independent colony growth that shows that the control of erythropoiesis is abnormal.6-8 Such endogenous erythroid colony formation has been also described in a minority of patients with ET and PMF.9 In these two diseases, the growth characteristics of MK progenitors have been extensively studied.10-19 A spontaneous MK growth (in the absence of exogenous cytokines) was observed in a majority of patients.10,14-18,20,21-23 However, cultures were mainly performed in the presence of serum or with unpurified cell populations, and it cannot be excluded that the abnormal MK growth could be related to a paracrine stimulation by cytokines secreted in culture or present in the serum,22 although spontaneous MK growth could not be blocked by antibodies against interleukin-3 (IL-3), granulocyte-macrophage colony-stimulating factor (GM-CSF), and IL-6.24 More recently, it has been reported that antisense oligonucleotides against c-mpl, the receptor for thrombopoietin (TPO), markedly inhibited spontaneous MK growth in ET,25suggesting that the Mpl-Ligand (Mpl-L)/Mpl loop was involved in the pathogenesis of this disease.
Mpl-L, also called TPO or megakaryocyte growth and development factor (MGDF), is a cytokine that regulates platelet production.26-30 Mpl-L has similarities with Epo. First, at the structural level, Mpl-L has a 153–amino acid domain that shares 50% similarity with Epo.26,27,29 Second, Mpl-L and Epo are hormonal factors mainly synthesized in adults by the kidney and the liver.26,27,29 However, the two cytokines differ markedly by three properties: (1) Mpl-L has a much broader biological activity than Epo; it acts not only on the early and late stages of megakaryocytopoiesis,28,31,32 but also on primitive hematopoietic cells33-37; (2) Mpl-L is synthesized by many cell types including marrow stromal cells28,38; and (3) Mpl-L plasma levels are directly dependent on the platelet/MK mass and Mpl-L synthesis does not appear to be transcriptionally regulated.39,40 It has been shown that platelets specifically bind Mpl-L with high affinity, internalize, and degrade the protein.41-43
Overexpression in mice of Mpl-L using retroviral or adenoviral gene transfer leads to a MK hyperplasia and a thrombocytosis associated with myelofibrosis.44-48 The disease induced by marked Mpl-L overexpression has characteristics similar to human PMF.46Moreover, a truncated form of c-mpl is an oncogene that leads to an acute myeloproliferative disease associated with autonomous growth of all hematopoietic progenitors.49 In this study, we have analyzed the growth characteristics of MK progenitors from a large series of 21 patients with ET and 14 patients with PMF. Our data show that truly autonomous MK development occurs in both diseases, which do not result from a mutation/deletion in the c-mpl gene. Nevertheless, we observed that different preparations of the soluble murine Mpl receptor could efficiently and significantly interfere with spontaneous MK growth, although we could not detect an autocrine stimulation by Mpl-L.
MATERIALS AND METHODS
Patients
All ET patients had more than 650 × 109/L platelets without any evidence for secondary thrombocytosis.
All PMF patients exhibited splenomegaly, circulating immature cells of the granulocytic lineage, tear drop–shaped red blood cells, and fibrosis on bone marrow biopsy.
Clinical data are summarized in Table 1.
Blood and Bone Marrow Cells
Heparinized bone marrow (BM) samples were obtained from 21 patients with a typical ET after informed consent. Heparinized blood samples (20 to 50 mL) were obtained from 14 patients with a PMF after informed consent. Mononuclear cells were separated on a Ficoll gradient (Lymphoprep; Nycomed Pharma, Oslo, Norway). Low density cells (<1.077 g/cm3) were recovered, washed, and used directly for culture or isolation of CD34+ cells.
As controls, samples from normal BM and leukapheresis samples from patients with lymphoma or solid tumors were used.
Reagents for Cytometry
Directly conjugated R-phycoerythrin (PE)-HPCA2 (anti-CD34) monoclonal antibody (MoAb; Becton Dickinson, Mountain View, CA) was used for cell sorting. Fluorescein isothiocyanate (FITC)-TAB (anti-CD41b; Tab provided by Dr R. McEver, Oklahoma Medical Research Foundation, Oklahoma City, OK) and R-PE CD41 (Pharmingen, San Diego, CA) were used for analysis of MKs by flow cytometry. Control FITC- and PE-conjugated IgG1 MoAbs were obtained from Becton Dickinson. Unconjugated MoAb Y2/51 (anti-CD61) was a generous gift from Dr D. Mason (Oxford, UK). FITC-Annexin-V (Immunotech, Marseille, France), 7 actinomycin D (7 AAD; Sigma, St Louis, MO), propidium iodide (PI; Sigma), R-PE CD41 (Pharmingen), and allophycocyanin (APC)-conjugated anti-CD34 MoAb (Becton Dickinson) were used for cell cycle and apoptosis analysis.
Human Cytokines and Cytokine Soluble Receptors
Recombinant human (rhu) IL-3 (a gift from Immunex, Seattle, WA) and rhuIL-6 were both used at a final concentration of 100 U/mL (3 and 5 ng/mL, respectively). Recombinant human stem cell factor (rhuSCF) and PEG-rhuMGDF (gifts from Amgen, Thousand Oaks, CA) were usually used at a final concentration of 50 ng/mL and 10 ng/mL, respectively. In some experiments, different preparations of murine soluble Mpl receptor (sMpl) were added at concentrations from 1 to 50 μg/mL. Two preparations of sMpl were chimeric fusion proteins comprising the extracellular domain of the murine Mpl and the Fc fragment of a human IgG1 (generous gifts from ZymoGenetics and Immunex, Seattle, WA). A third preparation consisted of the extracellular domain of a murine Mpl variant (missing part of the fourth exon due to alternate splicing), which was associated with a Tag (a generous gift from Dr S. Lok, ZymoGenetics).
Cell Lines
Ba/F3 cells (parental) and transfected with human c-mpl cDNA (Ba/F3hu c-mpl) were maintained in alpha-minimum essential medium (MEM) supplemented with 10% fetal calf serum (FCS) and 2.5% WEHI-3 conditioned medium (CM) as a source of murine IL-3. For coculture experiments, Ba/F3 cells were twice washed and deprived of WEHI-3 CM for 4 hours before being plated at a concentration of 1 × 103 cells/mL in the upper layer of a transwell (Costar, Cambridge, MA). In the lower chamber, CD34+ cells were cultured. These cultures were performed in serum-free medium supplemented with 1% FCS. Cell growth was examined with an inverted microscope.
HEL, DAMI, K562, UT7, UT7/huc-mpl, HEPG2, Meg0,1 and MO-7E cell lines were used as controls for quantitative determination of Mpl-L transcripts and studies of the effects of sMpl-Fc on cell cycle and apoptosis.
Isolation of CD34+ Cells
Mononuclear cells were separated using a magnetic cell sorting system (mini MACS; Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) in accordance with the manufacturer’s recommendations. The purity of recovered CD34+ cells was determined by flow cytometry using PE-HPCA2 and was routinely over 80%.
Cell Sorting
Mononuclear marrow cells (1 × 106) were washed and incubated with R-PE-HPCA2 (20 μL) in 100 μL at 4°C for 45 minutes. After one wash, cells were suspended in Iscove’s modified Dulbecco’s medium (IMDM) at a concentration of 5 × 105cells /mL and separated by cell sorting. Cells were sorted on a FACS Vantage flow cytometer (Becton Dickinson) equipped with an automatic cloning unit device (ACDU). A morphologic gate including 80% of the events and all of the CD34+cells was determined on two-parameter histograms (side scatter [SSC] versus forward scatter [FSC]). Positivity for the CD34 antigen was determined using control cells labeled with a R-PE–irrelevant IgG1 MoAb. The CD34high cells were sorted.
Cell Cultures
Liquid culture.
CD34+ cells (from 1 × 104 to 1 × 105 cells per mL) were cultured in IMDM with penicillin/streptomycin/glutamine and 11.5 μmol/L α-thioglycerol (Sigma). In cultures performed in serum-free conditions, IMDM was supplemented with 1.5% bovine serum albumin (BSA; Cohn’s fraction V; Sigma), sonicated lipids, and iron-saturated human transferrin.32 Cultures were performed in three conditions, ie, absence of any cytokine, presence of PEG-rhuMGDF (10 ng/mL), and a combination of three cytokines (25 ng/mL SCF, 100 U/mL IL-3, 100 U/mL IL-6 ). In some experiments a rabbit neutralizing polyclonal anti-human Mpl-L antibody (Amgen) was added at 10 μg/mL.
Semisolid cultures.
Cultures were performed in serum-free fibrin clot in the presence or absence of cytokines. Ingredients for serum-free cultures were similar to those of liquid cultures in which bovine plasma fibrinogen (1 mg/mL; Sigma), 0.01 mol/L ε amino caproic acid, and horse thrombin (6 mU/mL; Stago, Asnières, France) were added. Target cells were either low-density marrow cells (5 × 104 to 1 × 105 cells/mL) or CD34+ cells (1 × 103 cells/mL). Cultures were incubated at 37°C in a fully humidified atmosphere containing 5% CO2 in air and scored after 10 to 12 days. Colonies were quantitated by an indirect immuno-alkaline phosphatase labeling technique using an anti-GPIIIa MoAb (CD61, Y2-51) as previously described.50 Dishes were entirely scanned under an inverted microscope at a 4× or 100× magnification.
Limiting dilution experiments.
For limiting dilution experiments, individual CD34+ were sorted with the ACDU of the cell sorter into 96-well plates.32 Serum-free cultures were performed in the absence or presence of 10 ng/mL PEG-rhuMGDF. Plates were examined at days 7 and 12 after incubation at 37°C in an air atmosphere supplemented with 5% CO2.
Determination of MK Numbers Produced in Culture
Total numbers of MKs were determined by flow cytometry after labeling with FITC-Tab (anti-CD41) or an R-PE anti-CD41 antibody (Pharmingen). After collection and rinsing with phosphate-buffered saline (PBS)/EDTA (3 mmol/L), cultured cells were centrifuged at 350g for 15 minutes and incubated with the antibody for 30 minutes. Cells from each culture condition were distributed in the same volume (400 μL) and rapidly analyzed by flow cytometry. For each sample, the acquisition rate was 2 μL/s for 100 seconds. MKs were defined as brightly positive CD41 cells with scatter properties of nucleated cells. Samples were analyzed with a FACSort flow cytometer (Becton Dickinson).
Quantitative Titration of mRNA by PCR
The quantitative titration of the different mRNAs was performed as previously described by PCR run to saturation.51
RNA and cDNA preparation.
Total RNA was isolated using RNA PLUS (Bioprobe Systems, Montrevil, France), a modification of the acid-guanidinium thiocyanate-phenol-chloroform extraction method of Chomczynski and Sacchi.52 RNA were reverse transcribed with random hexamers (GIBCO-BRL/Life Technologies, Cergy Pontoise, France) using AMV-reverse transcriptase (Promega Laboratories, Madison, WI).
Construction of internal standards.
A Mpl-L plasmid was obtained by cloning theEcoR1-Xba 1 fragment of the Mpl-L cDNA into the EcoR1-Xba 1 site of Bluescript pBS SK+ plasmid. The internal standard for Mpl-L was generated by PCR using the mutational primer A, which created a 5-bp deletion in the sequence of the Mpl-L cDNA. The PCR product was digested withEcoR1-BamH1 and directly cloned into the Mpl-L cDNA Bluescript pBS SK+ plasmid previously cleaved byEcoR1-BamH1. β-Actin was used to normalize the results obtained. The β-actin control was obtained by cloning theXho 1-BamH1 PCR fragment of reverse-transcribed MO7E β-actin mRNA into the Xho 1-BamH1 site of the Bluescript pBS SK+ plasmid. A 6-bp deletion was generated according to the same procedure as above using primer B. The two plasmids were subjected to a spectrophotometric quantitation (260 nm).
Quantitative PCR and run-off reaction.
Two coamplifications were performed for quantitation of Mpl-L (primer C) and β-actin (primer D) transcripts, with serial dilutions ranging from 10 to 105 copies of the internal standard for Mpl-L or β-actin, as previously reported.51For the run-off, a part (2 μL) of the amplified reaction was mixed in a final reaction volume of 10 μL containing 0.1 μmol/L of the dye-labeled oligonucleotide (E, F), 0.15 U Taq polymerase, 200 μmol/L dNTP, in ATGC buffer and subjected to a 2-minute denaturation step at 94°C, followed by 1 minute at 60°C and incubation at 72°C. Samples were electrophoresed on a 6% acrylamide, 8 mol/L urea gel, using an Applied Biosystems (Roissy, France) 373A DNA sequencer. A gel scanner (Genescan, Perkin Elmer Co, Norwalk, CT) was used to detect DNA peaks and to measure their area.
Oligonucleotides.
Primer A: 5′-CAGGAAGATGGCATTGGGATCCTTGTGTGGTCCTGCC-3′; Primer B: 5′-CTCGTCGTCGACAACGGCTCCGGCATGTGCAGCTTCGCG-3′; Primers C: 5′-ACTTTAGCTTGGGAGAATGGAAA-3′; 5′-ACCAGGAATCTTGGCTCTGAAT-3′; Primers D: 5′-TCGTCGACAACGGCTCCGGCATGTGC-3′; 5′-GCCAGCCAGGTCCAGACGCAGGATGG-3′; Primer E: 5′-(6-FAM)CTTTCCTCGGAGCAGGTGTT-3′; Primer F: 5′-(HEX)GAGGATGCCTCTCTTGCTCTG-3′.
DNA Sequencing
Genomic DNA was isolated from peripheral blood cells (leukocytes and mononuclear cells) or bone marrow cells using the proteinase K/sodium dodecyl sulfate (SDS) treatment and phenol/chloroform extraction.53 Each of the 12 exons of the c-mplgene was amplified and sequenced using the PCR technique with the oligonucleotide primers described in Table 2.
All of the oligonucleotide primers were chosen in the flanking intronic sequences. PCR was performed using 2.5 μg DNA in a 100-μL mixture containing 1.5 U Taq polymerase (ATGC; Noisy le Grand, France), 200 μmol/L dNTP, 5% formamide, and 2 μmol/L of each of the two primers, in ATGC buffer. Dideoxy-terminator sequencing reactions were performed with fluorescent dye-labeled terminator and 3.2 pmol of the 5′ sense or 3′antisense primer previously used according to the manufacturer’s protocols (Applied Biosystems). Samples were purified by Sephadex G-50 columns (Quick Spin Columns, Boehringer Mannheim, Meylan, France) and stored at −20°C until ready for sequencing on a DNA Sequencer (Model 373 A; Applied Biosystems). All computed sequence analyses were performed using the SeqEd software (Applied Biosystems).
PCR with a fluorescent dye.
RT-PCR for Mpl-L transcript detection was performed in a 25-μL mixture containing 2.5 μL reverse transcribed mRNA, 0.3 U Taq polymerase (ATGC), 200 μmol/L dNTP, and 2 μmol/L of each of the two primers, in ATGC buffer. The two primers used were 5′-TCCTGCTTGTGACCTCCGAGT-3′ for the sense and 5′-(HEX)CCAGAATGTCCTGTGCCTTGG-3′ for the antisense. PCR reaction (1 μL) was mixed with a 20-mmol/L EDTA, 5% formamide solution and electrophoresed using an Applied Biosystem 373A DNA sequencer. A scanner (Genescan, Perkin Elmer) was used to detect DNA peaks.
STAT Activation
CD34+ cells were maintained in culture for 10 days in the presence or absence of the indicated cytokine combination (PEG-rhuMGDF and SCF or IL-3, IL-6 and SCF) and CD41+ cells were purified,washed in PBS, and incubated for 15 minutes in the presence or absence of PEG-rhuMGDF (30 to 100 ng/mL) or interferon-γ (IFN-γ; 1,000 U/mL; Boerhinger Mannheim) at 37°C. Incubation was stopped by a brief centrifugation and washes in PBS medium. Nuclear extracts were prepared and incubated (1 to 3 μg proteins) with 10 to 15 fmol of a32P-labeled oligonucleotide as previously described.54 Protein concentration was evaluated using the BCA protein assay kit (Pierce, Rockford, IL). The oligonucleotide probes (m67 SIE, 5′-CATTTCCCGTAAATC-3′; IRF1-GAS, 5′-GATCCATTTCCCCGAAATGA-3′; β-casein-GAS, 5′AGATTCTAGGAATTCAAATC-3′) were end-labeled using T4 polynucleotide kinase to a specific activity of 3,000 cpm/fmol.54
Mpl-L Enzyme-Linked Immunosorbent Assay (ELISA)
The ELISA for the detection of human Mpl-L was the Human TPO Quantikine kit (R&D system, Minneapolis, MN), which was used according to the manufacturer’s instructions. The ELISA sensitivity limit was 25 pg/mL of Mpl-L.
Cell Cycle and Apoptosis Analysis by Flow Cytometry
Cells were washed and labeled overnight in citrate buffer containing 25 μg/mL PI, 50 μg/mL RNase (Merck, Darmstadt, Germany) and 0.1% Nonidet P40 (Sigma) in the dark at 4°C. For determination of apoptosis, cells were stained with anti-CD34 and anti-CD41 MoAbs conjugated to APC and R-PE, respectively, then washed and stained with annexin V-FITC and 7 AAD according to the manufacturer’s instruction. Cell cycle and labeling were analyzed by flow cytometry using a FACSort (Becton Dickinson) equipped with laser and a photodiode tuned at 488 nm and 560 nm, respectively.
RESULTS
CD34+ Cells From Patients
Twenty-one patients with a typical ET were studied. Most samples (20 out of 21) were obtained by means of BM aspiration. After separation on ficoll gradient, the number of mononuclear cells varied from 0.8 × 106 to 30 × 106 per volume of 1 to 3 mL of BM aspiration. CD34+ cells were purified either by the immunomagnetic bead technique or by flow cytometry. On the average, 1% of the ET BM cells were CD34+cells (0.01% to 1.6%). Three blood samples from ET patients contained an average of 0.6% CD34+ cells (0.2% to 1.3%) in the mononuclear cell fraction, and CD34+ cells were purified. However, because insufficient numbers of CD34+ cells were collected, studies were only performed with BM samples. In contrast, in patients with PMF, the entire study was performed with blood samples. In 14 blood samples (20 to 50 mL), the average CD34+ cell recovery was 2.3% (0.2% to 8%) of the initial number of mononuclear cells, and more than 3 × 106 CD34+cells per 10 mL of blood were obtained in most samples. A 15th sample was obtained from a cytapheresis sample and contained 0.12% CD34+ cells. This patient (m6) developed a blastic transformation.
Presence of Autonomous MK Growth in ET and PMF Patients
In a first set of experiments, low-density (d = 1.077) bone marrow cells (5 × 104/mL) from 20 ET patients were cultured in serum-free fibrin clot. All samples exhibited spontaneous MK growth, which represented 34.5% of the MK colony number obtained after PEG-rhuMGDF stimulation (Table 3). In the majority of cases, spontaneous colonies were composed of a low MK number (from 3 to 6). Spontaneous erythroid colony formation was also observed in only 8 of 20 ET samples tested.
To diminish the cellular interactions and the possible role of cytokine secretion by accessory cells, subsequent experiments were performed with purified CD34+ cells cultured either in semisolid or liquid media. CD34+ cells from 6 ET and 1 PMF patients were cultured in serum-free fibrin clots (Table 4). In all cases, autonomous MK growth was observed. The mean percentage of spontaneous MK colonies in comparison to the number of MK colonies obtained with PEG-rhuMGDF was 20%. The increase in MK colony formation induced by PEG-rhuMGDF was statistically significant (P < .025 for nonpurified BM and P < .0005 for purified CD34+ cells). In liquid cultures and for all patients (10 patients with ET, 3 patients with PMF), MK could be obtained from CD34+ cells without addition of any exogenous cytokine (Table 5). MKs could be easily identified under inverted microscopy by their morphology as big, round, and opalescent cells. They were precisely quantitated by flow cytometry as cells expressing high level of CD41. CD41+cells represented from 50% to 90% of the cells obtained after 10 days of culture. Autonomous growth was even more significant in PMF patients than in ET patients with an average number of 3 MK (from 2 to 4) per plated CD34+cell. PEG-rhuMGDF increased the MK number by threefold (8.5 MK per plated CD34+cell). Platelet shedding MKs were observed in the absence of any exogenous cytokine in ET and PMF patients, showing that a complete cytoplasmic maturation occurred in these unstimulated cultures. The mean ploidy of spontaneously growing MK was higher than in PEG-rhuMGDF–stimulated cultures (≥8N MK: 43.7% v 22.4%, respectively). In controls (bone marrow or cytapheresis), no spontaneous MK growth from CD34+ cells was observed in semisolid or liquid cultures.
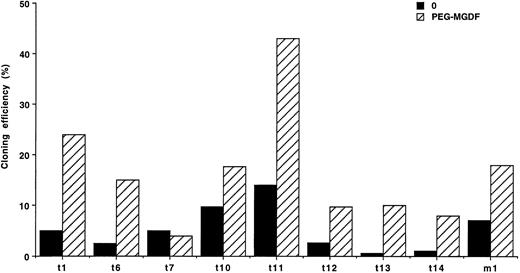
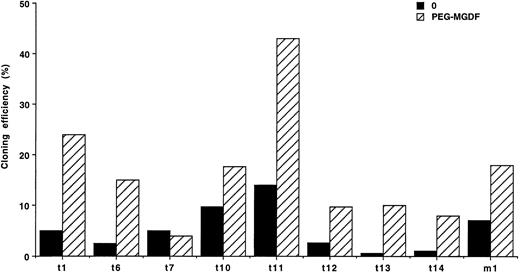
These results show that only a fraction of MK progenitors grew in the absence of exogenous cytokine. Because a paracrine stimulation due to accessory cells present in some CD34+ subsets could occur, limiting dilution liquid cultures were performed at one cell per well in 8 cases of ET and 1 case of PMF. Spontaneous MK growth was observed in all of these patients, with an average cloning efficiency of 5.6% for ET (from 0.6% to 14%) and 7% for the PMF patient (Fig 1). Clones were composed of 1 to 19 MKs, with a majority of 1 to 3 MKs per clone. PEG-rhuMGDF increased the cloning efficiency on average of fivefold with clones ranging from 1 to 44 MKs (data not shown).
Limiting dilution experiments. Cloning efficiency of CD34+ cells at limiting dilution in serum-free liquid cultures (1 cell/well), without growth factor (0) (▪) or with PEG-rhuMGDF (10 ng/mL) (▨). In 8 ET patients (t) and 1 PMF patient (m). Results are derived from 288 wells.
Limiting dilution experiments. Cloning efficiency of CD34+ cells at limiting dilution in serum-free liquid cultures (1 cell/well), without growth factor (0) (▪) or with PEG-rhuMGDF (10 ng/mL) (▨). In 8 ET patients (t) and 1 PMF patient (m). Results are derived from 288 wells.
Together, these experiments show that a true autonomous MK growth occurs in ET and PMF patients. We next investigated whether this abnormal growth was due to a mutation in the c-mpl gene.
Absence of Mutation in the Coding Region of the c-mpl Gene in ET and PMF Patients
All of the 12 exons, including the flanking intronic sequences ofc-mpl gene, were individually sequenced by the PCR technique in 9 ET patients and 5 PMF patients. In comparison to the published normal sequence, one allelic base change was found in two brothers. This base change located in the fourth exon was silent and did not modify the amino acid sequence of the protein. This mutation corresponds to the same recently reported polymorphism.55
This result shows that the autonomous MK proliferation in ET and PMF is not related to a c-mpl mutation located in the coding sequence. We next explored the hypothesis of an autocrine stimulation by Mpl-L in these two diseases.
Search for Autocrine Stimulation by Mpl-L in ET and PMF
In a first series of experiments, we used murine sMpl to block the spontaneous MK growth.
Blocking of spontaneous MK growth in ET and PMF by sMpl.
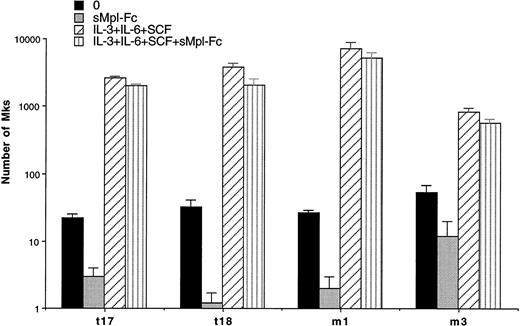
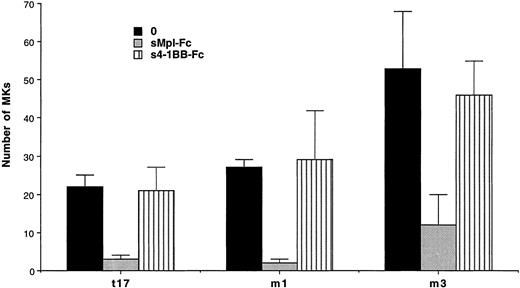
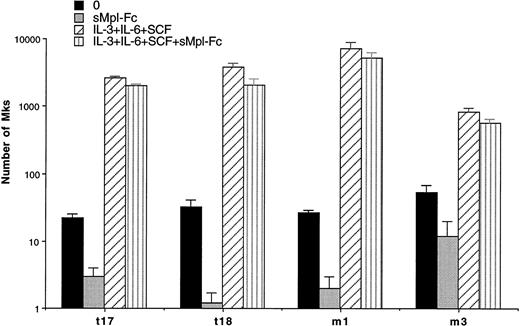
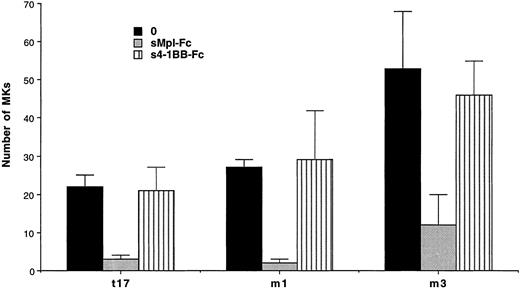
Experiments were performed in semisolid and liquid media cultures. In semisolid medium, cultures were performed with CD34+ cells from 3 ET and 1 PMF patients at a concentration of 103cells per mL (Table 4). A partial and significant inhibition (from 66% to 98%, P < .01) of the spontaneous growth was elicited by the chimeric sMpl (sMpl-Fc, 5 μg/mL) in all these cases. Addition of 10 ng/mL PEG-rhuMGDF did not induce a complete reversion of the inhibitory effect mediated by the sMpl-Fc (P < .05). A larger number of experiments (9 ET and 3 PMF patients) were performed in liquid cultures with CD34+ cells (10 to 50 × 103 per mL; Table 5). Quantitation of MKs was performed using either an inverted microscope when a low number of CD34+cells was available (Table 5A) or by flow cytometry after labeling with an R-PE or FITC anti-CD41 antibody (Table 5B). MK spontaneous growth was significantly inhibited by the sMpl-Fc (40% to 100% inhibition, P < .02). A dose-response curve showed that 5 μg/mL of sMpl-Fc had the maximum inhibitory effect (data not shown). This effect was nearly completely reversed by 10 ng/mL PEG-rhuMGDF (no significant difference with PEG-rhuMGDF cultures,P > .05). In contrast, sMpl-Fc had no effect on ET or PMF CD34+ cells stimulated by a combination of IL-3, IL-6, and SCF (Fig 2). When tested on normal CD34+ cells, 5 μg/mL of sMpl-Fc inhibited the effect of 1 ng/mL of PEG-rhuMGDF (77% inhibition). This effect was nearly totaly reversed by concentration of PEG-rhuMGDF exceeding 20 ng/mL (on the average, a 15% residual inhibition that was not statistically significant). A similar and nonsignificant inhibition was also found by 5 μg/mL of sMpl-Fc on the MK growth stimulated by a combination of SCF, IL-3, and IL-6. To confirm the specificity of the effect of the sMpl-Fc, two other preparations of sMpl (sMpl-Fc and sMpl-Tag) were tested. Both inhibited spontaneous MK growth from CD34+cells in the two cases of PMF tested (data not shown). In contrast, a control irrelevant soluble receptor (s4-1BB-Fc; 5 μg/mL) had no inhibitory effect (Fig 3).
Inhibition of MK spontaneous growth by the sMpl-Fc. Number of MKs obtained after 10 days of serum-free liquid culture without growth factor (0) (▪), with the sMpl-Fc at 5 μg/mL (▩), with a combination of rhuIL-3, rhuIL-6, and rhuSCF (IL-3+IL-6+SCF) (▨), or with the three cytokine combination and the sMpl-Fc (IL-3+IL-6+SCF+sMpl-Fc) (▥). CD34+cells were seeded at 2 × 104 cells/mL in 100 μL in triplicate, and MKs were quantitated by morphological analysis.
Inhibition of MK spontaneous growth by the sMpl-Fc. Number of MKs obtained after 10 days of serum-free liquid culture without growth factor (0) (▪), with the sMpl-Fc at 5 μg/mL (▩), with a combination of rhuIL-3, rhuIL-6, and rhuSCF (IL-3+IL-6+SCF) (▨), or with the three cytokine combination and the sMpl-Fc (IL-3+IL-6+SCF+sMpl-Fc) (▥). CD34+cells were seeded at 2 × 104 cells/mL in 100 μL in triplicate, and MKs were quantitated by morphological analysis.
Absence of inhibition by a control soluble receptor. Number of MKs obtained after 10 days of serum-free liquid culture without growth factor (0) (▪), with the sMpl-Fc at 5 μg/mL (▩), or with the control soluble receptor 4-1BB-Fc at 5 μg/mL (s4-1BB-Fc) (▥). CD34+ cells were seeded at 2 × 104cells/mL in 100 μL in triplicate, and MKs were quantitated by morphological analysis.
Absence of inhibition by a control soluble receptor. Number of MKs obtained after 10 days of serum-free liquid culture without growth factor (0) (▪), with the sMpl-Fc at 5 μg/mL (▩), or with the control soluble receptor 4-1BB-Fc at 5 μg/mL (s4-1BB-Fc) (▥). CD34+ cells were seeded at 2 × 104cells/mL in 100 μL in triplicate, and MKs were quantitated by morphological analysis.
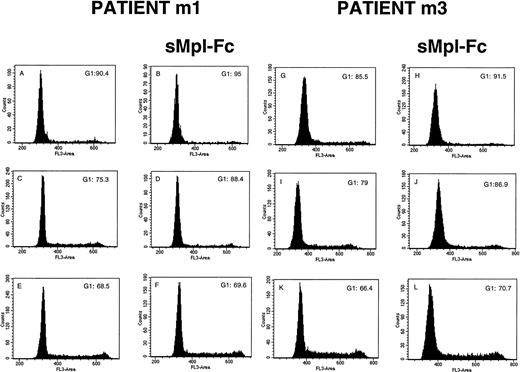
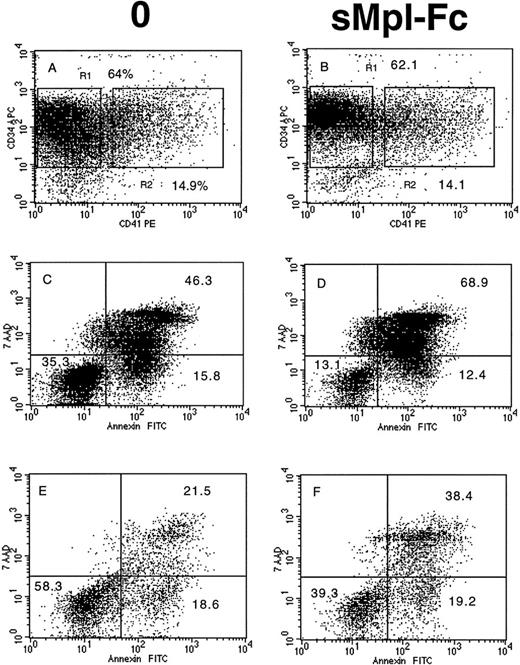
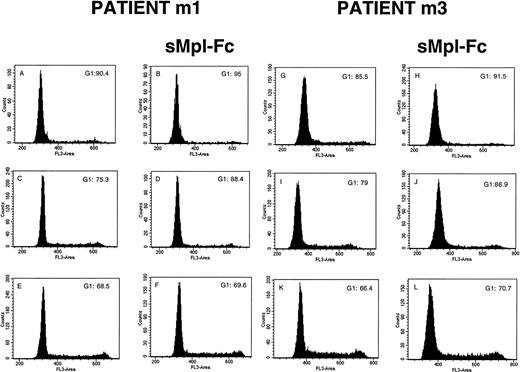
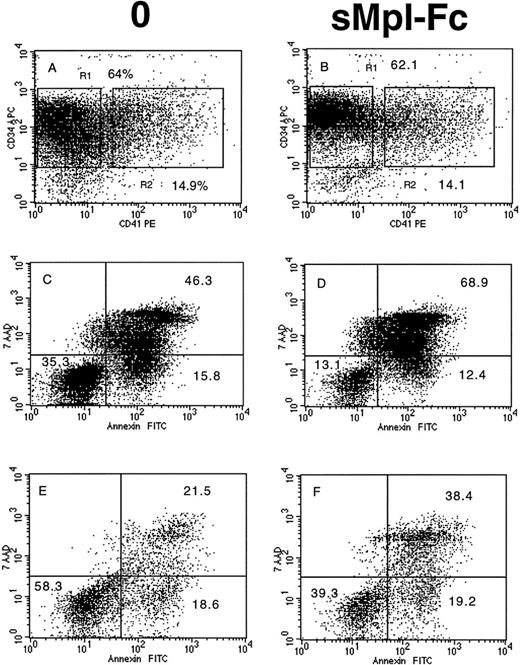
The effects on sMpl-Fc on the cell cycle and apoptosis of patient CD34+ cells were investigated in four cases of PMF. Without growth factor addition, the percentage of CD34+ cells in S, G2/M phases was low (5% to 10%) and was reduced about twofold by addition of the sMpl-Fc (Fig 4A, B, G, and H). In contrast, sMpl-Fc slightly but not significantly reduced the percentage of cells in cycle after stimulation by three cytokines (Fig4E, F, K, and L) or 100 ng/mL PEG-rhuMGDF (data not shown). At 10 ng/mL PEG-rhuMGDF, sMpl-Fc moderately inhibited cycling of the patient cells (Fig 4C, D, I, and J). A significant amount of apoptosis of patient CD34+ CD41− cells was observed without growth factor (Fig 5A and C) at day 4 of culture (mainly late apoptosis). This apoptosis was much less significant in the CD34+CD41+ cell population (Fig 5A and E). Addition of sMpl-Fc accelerated and increased the apoptosis because a higher percentage of cells were in late apoptosis (AnnexinV+ 7AAD+), both in the CD34+ CD41− and CD34+CD41+ cell populations (Fig 5B, D,and F). In contrast, no effect was seen on growth factor–stimulated cells (data not shown). Several cell lines that express Mpl (HEL, UT-7/huc-mpl) or do not express Mpl (K562, UT-7) were studied. sMpl-Fc did not modify the cell cycle of UT-7 or UT-7/huc-mpl stimulated by the GM-CSF or PEG-rhuMGDF (for UT-7/huc-mpl). A slight but insignificant decrease (less than 5%) in cycling cells was observed for K562 and HEL cells. In addition, no induction of apoptosis by the sMpl-Fc was observed in these cell lines. By flow cytometry we could not detect a specific binding of the sMpl-Fc to patient CD34+ cells, normal or patient MKs, or the different cell lines previously studied.
Effects of the soluble receptor on the cell cycle. CD34+ cells from 2 PMF patients (m1 and m3) were cultured without growth factor in the absence (A and G) or the presence of the sMpl-Fc (B and H), with 10 ng/mL PEG-rhuMGDF in the absence (C and I) or the presence of the sMpl-Fc (D and J) or with a combination of 3 cytokines (IL-3, IL-6, and SCF) in the absence (E and K) or the presence of 5 μg/mL sMpl-Fc (F and L). Cell cycle was studied after staining with propidium iodide after 4 days of culture. Two other patients were studied, and similar results were obtained.
Effects of the soluble receptor on the cell cycle. CD34+ cells from 2 PMF patients (m1 and m3) were cultured without growth factor in the absence (A and G) or the presence of the sMpl-Fc (B and H), with 10 ng/mL PEG-rhuMGDF in the absence (C and I) or the presence of the sMpl-Fc (D and J) or with a combination of 3 cytokines (IL-3, IL-6, and SCF) in the absence (E and K) or the presence of 5 μg/mL sMpl-Fc (F and L). Cell cycle was studied after staining with propidium iodide after 4 days of culture. Two other patients were studied, and similar results were obtained.
Effects of the soluble receptor on apoptosis. CD34+ cells from 1 PMF patient (m2) were cultured without growth factor in the absence (A, C, and E) or the presence of the sMpl-Fc (B, D, and F). At day 4 of culture, cells were labeled by an APC-conjugated anti-CD34 MoAb and a R-PE–conjugated anti-CD41a MoAb. They were subsequently stained by Annexin-FITC and 7AAD. CD34+CD41− (R1) and CD34+CD41+ (R2) were gated only on the fluorescence labeling without taking into account their scatter properties. 7AAD and Annexin binding was studied in the gate R1 (C and D) and in the gate R2 (E and F). Early and late apoptosis correspond to the low right quadrant (AnnexinV+7AAD−) and the up right quadrant (AnnexinV+7AAD+), respectively. Similar results were obtained with cells of two other patients.
Effects of the soluble receptor on apoptosis. CD34+ cells from 1 PMF patient (m2) were cultured without growth factor in the absence (A, C, and E) or the presence of the sMpl-Fc (B, D, and F). At day 4 of culture, cells were labeled by an APC-conjugated anti-CD34 MoAb and a R-PE–conjugated anti-CD41a MoAb. They were subsequently stained by Annexin-FITC and 7AAD. CD34+CD41− (R1) and CD34+CD41+ (R2) were gated only on the fluorescence labeling without taking into account their scatter properties. 7AAD and Annexin binding was studied in the gate R1 (C and D) and in the gate R2 (E and F). Early and late apoptosis correspond to the low right quadrant (AnnexinV+7AAD−) and the up right quadrant (AnnexinV+7AAD+), respectively. Similar results were obtained with cells of two other patients.
These results show that sMpl significantly inhibited spontaneous MK growth in ET and PMF and that this effect is likely due to the Mpl domain and not to the Fc part of the chimeric protein. These observations strongly suggested that an autocrine stimulation by Mpl-L was possible in ET and PMF. We tried to confirm this hypothesis first by studying the effect of a neutralizing anti–Mpl-L antibody.
Neutralizing anti–Mpl-L antibody does not inhibit spontaneous MK growth in ET and PMF.
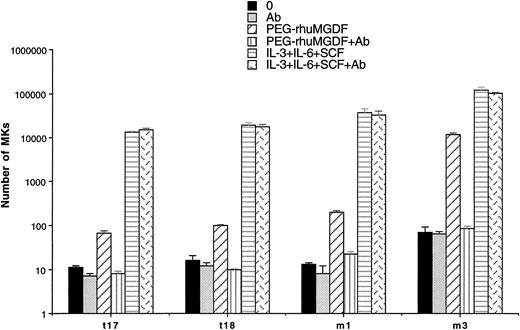
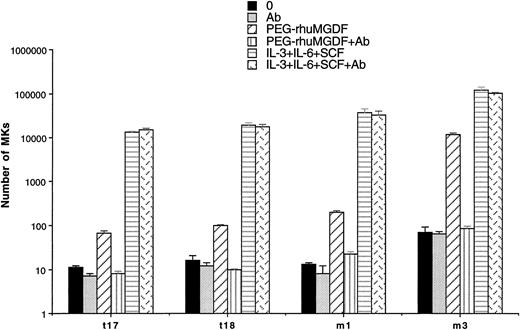
To show a possible autocrine stimulation by Mpl-L in spontaneous MK growth, the effect of a neutralizing anti–Mpl-L antibody was studied in two cases of ET and PMF. The antibody used at 10 μg/mL had no inhibitory activity on the spontaneous growth in liquid cultures performed with 2 to 10 × 103 CD34+cells. In contrast, the antibody totally inhibited the stimulatory effect of 10 ng/mL PEG-rhuMGDF but had no inhibitory effects on MK cultures stimulated by three cytokines (SCF, IL-3, IL-6; Fig 6). This antibody at 10 μg/mL abolished the stimulatory effects of 10 ng/mL PEG-rhuMGDF on the growth of MK progenitors from normal CD34+ bone marrow cells (data not shown).
Effect of an anti–Mpl-L antibody on spontaneous MK growth. Number of MKs obtained after 10 days of serum-free liquid culture without growth factor (0) (▪), with 10 μg/mL anti–Mpl-L antibody (Ab) (▩), with PEG-rhuMGDF (10 ng/mL) (▨), with the combination of PEG-rhuMGDF (10 ng/mL) and the anti–Mpl-L antibody (PEG-rhuMGDF+Ab) (▥), with three cytokines (IL-3, IL-6, and SCF) (▤), or with three cytokines (IL-3, IL-6, and SCF) associated with the anti–Mpl-L antibody (IL-3, IL-6, and SCF+Ab ) (). CD34+ cells were seeded at concentration ranging from from 2 × 104 cells/mL to 1 × 105 cells/mL in 100 μL in triplicate. MKs were quantitated by morphological analysis and results are expressed as the number of MKs per 1 × 103 seeded CD34+ cells.
Effect of an anti–Mpl-L antibody on spontaneous MK growth. Number of MKs obtained after 10 days of serum-free liquid culture without growth factor (0) (▪), with 10 μg/mL anti–Mpl-L antibody (Ab) (▩), with PEG-rhuMGDF (10 ng/mL) (▨), with the combination of PEG-rhuMGDF (10 ng/mL) and the anti–Mpl-L antibody (PEG-rhuMGDF+Ab) (▥), with three cytokines (IL-3, IL-6, and SCF) (▤), or with three cytokines (IL-3, IL-6, and SCF) associated with the anti–Mpl-L antibody (IL-3, IL-6, and SCF+Ab ) (). CD34+ cells were seeded at concentration ranging from from 2 × 104 cells/mL to 1 × 105 cells/mL in 100 μL in triplicate. MKs were quantitated by morphological analysis and results are expressed as the number of MKs per 1 × 103 seeded CD34+ cells.
Thus, markedly divergent results were observed between the anti–Mpl-L neutralizing antibody and the sMpl. However, it could not be totally excluded that the absence of inhibition mediated by the antibody could be due to a decreased affinity for Mpl-L because in an autocrine stimulation, cytokines are synthesized in contact with their receptors.
For these reasons, we investigated whether CD34+cells or cultured MK from ET or PMF patients synthesized Mpl-L.
Studies of Mpl-L Transcripts by RT-PCR in CD34+Cells or CD34+-Derived MK from ET and PMF Patients
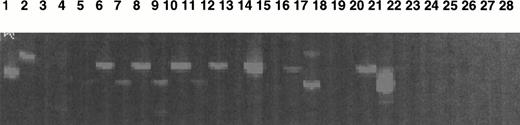
A quantitative RT-PCR was performed using a competitor, and the number of transcripts was quantitated in comparison to the number of actin transcripts. Different cell types (as shown in Fig 7) were used in these experiments.
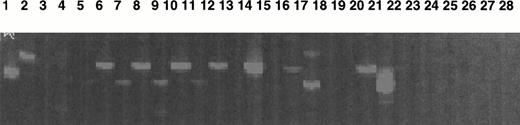
Study of Mpl-L transcripts by a sensitive fluorescent RT-PCR assay. Mpl-L transcripts were amplified from total RNA of different cell types, and the amplification products were run on a sequencing gel. Size of the amplified product is 183 bp. Lane 1, HepG2 diluted 1:10; lane 2, HEL; lane 3, CD34+ cells from peripheral blood cytapheresis; lane 4, cultured MKs from a normal marrow; lane 5, spontaneously growing MKs from t10; lane 6, spontaneously growing MKs from t11; lane 7, spontaneously growing MKs from t12; lane 8, CD34+ from t16; lane 9, spontaneously growing MKs from t16; lane 10, CD34+ from t19; lane 11, spontaneously growing MKs from t19; lane 12, blood monuclear cells from m1; lane 13, CD34+ cells from m1; lane 14, spontaneously growing MKs from m1; lane 15, CD34+ from m2; lane 16, spontaneously growing MKs from m2; lane 17, CD34+ from m3; lane 18, spontaneously growing MKs from m3; lane 19, cultured MK after PEG-rhuMGDF stimulation from m3; lane 20, CD34+cells from m7; lane 21, spontaneously growing MKs from m6; lane 22, cultured MK after PEG-rhuMGDF stimulation from m6; lane 23, spontaneously growing MKs from m8; lane 24, cultured MK after PEG-rhuMGDF stimulation from m8; lane 25, CD34+ cells from m4; lane 26, spontaneously growing MKs from m4; lane 27, H2O control; lane 28, H2O control.
Study of Mpl-L transcripts by a sensitive fluorescent RT-PCR assay. Mpl-L transcripts were amplified from total RNA of different cell types, and the amplification products were run on a sequencing gel. Size of the amplified product is 183 bp. Lane 1, HepG2 diluted 1:10; lane 2, HEL; lane 3, CD34+ cells from peripheral blood cytapheresis; lane 4, cultured MKs from a normal marrow; lane 5, spontaneously growing MKs from t10; lane 6, spontaneously growing MKs from t11; lane 7, spontaneously growing MKs from t12; lane 8, CD34+ from t16; lane 9, spontaneously growing MKs from t16; lane 10, CD34+ from t19; lane 11, spontaneously growing MKs from t19; lane 12, blood monuclear cells from m1; lane 13, CD34+ cells from m1; lane 14, spontaneously growing MKs from m1; lane 15, CD34+ from m2; lane 16, spontaneously growing MKs from m2; lane 17, CD34+ from m3; lane 18, spontaneously growing MKs from m3; lane 19, cultured MK after PEG-rhuMGDF stimulation from m3; lane 20, CD34+cells from m7; lane 21, spontaneously growing MKs from m6; lane 22, cultured MK after PEG-rhuMGDF stimulation from m6; lane 23, spontaneously growing MKs from m8; lane 24, cultured MK after PEG-rhuMGDF stimulation from m8; lane 25, CD34+ cells from m4; lane 26, spontaneously growing MKs from m4; lane 27, H2O control; lane 28, H2O control.
Table 6 illustrates the results obtained by quantitative PCR. Fetal liver and HEP-G2 cells were strongly positive, whereas the factor-dependent UT7/huc-mpl and MO-7E cell lines were weakly positive. However, in all the patient samples (CD34+cells, MK obtained from unstimulated cultures or PEG-rhuMGDF–stimulated cells), no Mpl-L transcripts could be detected by this quantitative RT-PCR method. In addition, no transcripts were detected in normal adult or cord blood CD34+cells or MKs.
In contrast, when a highly sensitive fluorescent RT-PCR technique was used, all the patient samples were weakly positive with about the same level of Mpl-L transcripts as in HEL cells. This cell line expressed less than 0.5 Mpl-L transcript per 104 actin transcripts as measured by quantitative RT-PCR (Fig 7). Cells from patient m6 with PMF in transformation expressed more Mpl-L transcripts than contain those of the other patients.
These data show that ET or PMF cells exhibit extremely low level of Mpl-L transcripts. To understand whether this low number of Mpl-L transcripts had a biological significance, we searched for the Mpl-L protein in the supernatant of ET and PMF cultured cells.
Search for Mpl-L in Patient Serum and the Supernatant of CD34+-Derived MK From ET and PMF
We first used an ELISA that has a sensitivity of 25 pg/mL. The mean serum Mpl-L level was slightly lower in ET patients (n = 44) in comparison to that found in a series of 28 normal subjects. In contrast, it was higher in the 8 PMF patients that had reduced platelet numbers as compared to ET patients (platelet number = 210 × 109v 650 × 109, respectively; Table 7).
The supernatant obtained after 10 days of culture of patient CD34+cells performed at a high cell concentration (1 × 105 CD34+cells/mL) was analyzed by ELISA. Of 14 samples, only 1 from a PMF patient (patient m2) showed a significant positivity (182 pg/mL). Because the sensitivity of the ELISA assay was 25 pg/mL, these results did not totally exclude the possibility that low-level Mpl-L production occured in patient cells.
Therefore, we set up a more sensitive biological test to assay for Mpl-L synthesis. In these cultures, patient CD34+ cells were cultured in the lower chamber of a transwell, whereas Ba/F3huc-mpl cells were cultured in the upper chamber. Ba/F3huc-mpl is a murine factor-dependent cell line that responds to human Mpl-L. The survival of Ba/F3hu c-mpl cells was determined after 7 to 10 days of culture by the Trypan blue exclusion test. The sensitivity limit of this assay was 10 pg/mL of Mpl-L. CD34+cells from 2 ET and 4 PMF samples were cultured in the presence of 1% FCS, a condition optimal for the growth of Ba/F3huc-mpl cells. In none of the cases did the Ba/F3huc-mpl cells survive. Patient CD34+cells did not inhibit the growth of Ba/F3huc-mpl cells stimulated by murine IL-3, showing that Mpl-L secretion was not masked by the release of an inhibitor.
We subsequently performed similar experiments in which Ba/F3 huc-mpl cells were replaced by normal CD34+cells in the upper chamber. In control experiments, patient CD34+cells were omitted from the lower chamber. The presence of MKs was investigated by flow cytometry. In a first experiment, normal CD34+cells were used at a very high cell concentration (1 × 105 cells/mL) in the chamber. These normal CD34+cells survived both in the cocultures with patient CD34+cells and in control cultures without differences between the two types of cultures. In subsequent experiments, normal CD34+cells were seeded at a lower cell concentration (2 × 104 cells/mL) and did not survive in the control cultures. When patient CD34+cells were added in the lower chamber, no effects were observed on the survival or on the induction of MK differentiation of normal CD34+cells placed in the upper chamber. In contrast, when PEG-rhuMGDF (1 to 10 ng/mL) was added, CD34+cells from normal donors showed normal MK differentiation, showing that the transwell cocultures did not alter their proliferation and differentiation capabilities.
Finally, in one experiment, CD34+cells from one patient (m3) were cultured both in the upper and lower chambers of the transwell or only in the upper chamber. Cells in the upper chamber were plated at a low cell concentration (5 × 103cells/mL), whereas 5 × 104 cells were seeded in the lower chamber. No difference in the number of MKs was observed in these cultures whether cells were present or absent in the lower chamber.
Taken together, these results indicate that patient CD34+cells do not secrete detectable levels of biologically active Mpl-L or of other cytokine(s) that would lead to MK differentiation from normal CD34+ cells.
The Autonomous Growth of Patient MKs Does Not Correlate With a Detectable Activation of STAT Factors
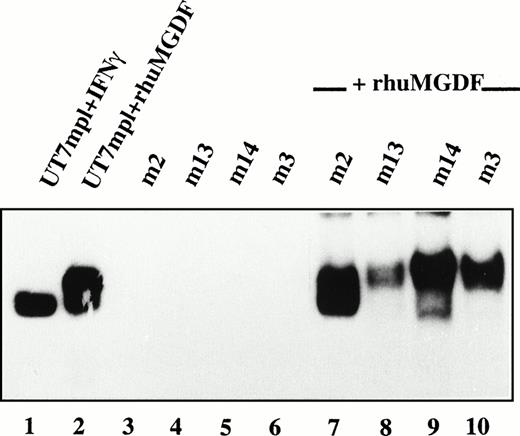
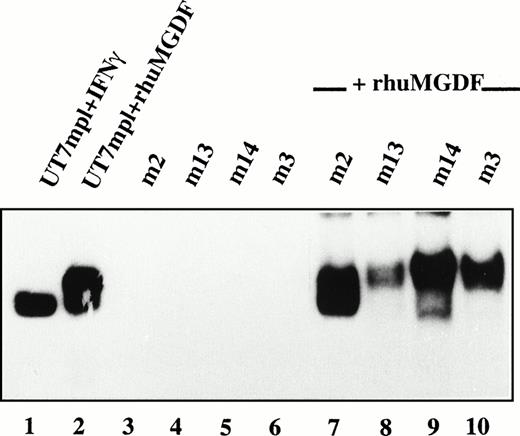
We next investigated Mpl-L signaling in spontaneously growing patient MKs. Nuclear extracts from patient MKs were prepared and tested for the presence of STAT factors by electrophoretic mobility shift assays (EMSA). Using three different oligonucleotides as probes to detect the activation of various STATs, namely m67 SIE, IRF1-GAS, and β-casein-GAS, we were not able to observe any activation of STAT factors in patient MKs (Fig 8, lanes 3 through 6, and data not shown). Under the same conditions, we detected a clear activation of STATs in the Mpl-L responsive UT7/huc-mplcell line following the addition of PEG-rhuMGDF (lane 2) or IFN-γ (lane 1). If, however, patient MKs were incubated for 15 minutes in the presence of PEG-rhuMGDF or IFN-γ, an important activation of STATs was observed (Fig 8, lanes 7 through 10, and data not shown), indicating that patient MKs were responsive to Mpl-L and able to activate STAT factors. When these EMSA were done in the presence of antibodies directed against various STATs (supershift assays), we observed that STAT3 and STAT5 were activated following PEG-rhuMGDF addition in all the patient MKs tested (not shown). Therefore, autonomous growth of patient MKs does not correlate with a constitutive activation of STATs.
Absence of constitutive activation of STATs. CD34+ cells from 4 PMF patients were cultured without growth factor. After 10 days of culture, CD41+ cells were collected and incubated without (lanes 3 through 6) or with (lanes 7 through 10) PEG-rhuMGDF (30 ng/mL) for 15 minutes. Nuclear extracts were prepared and the presence of activated STATs tested by EMSA using IRF1-GAS as a probe. In parallel, as internal controls Mpl-L responsive UT7/huc-mpl cells were incubated in the presence of IFN-γ (lane 1) or PEG-rhuMGDF (lane 2) for the same time, and nuclear extracts were prepared and tested for the presence of activated STATs.
Absence of constitutive activation of STATs. CD34+ cells from 4 PMF patients were cultured without growth factor. After 10 days of culture, CD41+ cells were collected and incubated without (lanes 3 through 6) or with (lanes 7 through 10) PEG-rhuMGDF (30 ng/mL) for 15 minutes. Nuclear extracts were prepared and the presence of activated STATs tested by EMSA using IRF1-GAS as a probe. In parallel, as internal controls Mpl-L responsive UT7/huc-mpl cells were incubated in the presence of IFN-γ (lane 1) or PEG-rhuMGDF (lane 2) for the same time, and nuclear extracts were prepared and tested for the presence of activated STATs.
DISCUSSION
ET and PMF are two chronic myeloproliferative diseases characterized by predominant MK involvement, although both diseases are clonal malignant disorders originating from a pluripotent stem cell.3,56,57Numerous studies have shown that spontaneous MK colony formation is frequently observed in ET.14,15,17,20,22 However, a recent study indicates that this growth defect was not observed with purified CD34+cells,58 suggesting that the spontaneous growth could be related to a paracrine cytokine stimulation. In contrast to patients with ET, only a few patients with PMF have been previously studied, and it was reported that their MK progenitors also grew spontaneously.10,24,25 In the present study, we have investigated a large series of 21 patients with ET and 14 patients with PMF. We confirm that the spontaneous growth of MKs is one of the biological hallmarks of these two diseases and is really due to autonomous MK differentiation because it is still observed at limiting dilution and in serum-free conditions. However, in both diseases MK progenitor cell growth remains partially dependent on growth factors because the exogenous addition of PEG-rhuMGDF increases the cloning efficiency by threefold to fourfold, confirming previous observations.15,18 These results are quite similar to those obtained for erythroid progenitors in PV, where spontaneous erythroid colony formation is constantly observed.6-8,11 Furthermore, Epo addition also increases the cloning efficiency of erythroid progenitors in PV. This augmentation is not exclusively due to the recruitment of erythroid progenitors belonging to normal clones,59 therefore showing that the abnormal erythroid progenitors have different Epo sensitivity.60 Similarly in ET and PMF patients, we observed not only autonomous MK growth, but also hypersensitivity to SCF and PEG-rhuMGDF in cell culture studies (data not shown), suggesting that the majority of MK progenitors belong to the malignant clone. More surprisingly, when CD34+cells from PMF patients were cultured in liquid medium, more than 90% of the cells that grew in the absence of cytokines belonged to the MK lineage. In contrast, a combination of IL-3, IL-6, and SCF not only induced a megakaryocytic growth but also a marked myeloid proliferation. This result further suggests that the abnormal MK proliferation plays a crucial role in the pathogenesis of PMF.1,4 61
Because partially autonomous MK growth occurs in PMF and ET, we investigated whether a missense mutation or a deletion in the c-mplgene could explain this abnormal proliferation. In experimental models, either truncation or missense mutations in the extracellular domain of the receptor induce spontaneous growth of normal hematopoietic progenitors or factor-dependent cell lines.49,62,63 Evidence was provided that substitution of amino acids by cysteine residues in critical regions of the extracellular domain induced spontaneous homodimerization of the Mpl receptor.62 Because other mutations could also involve the cytoplasmic domain of the receptor, as demonstrated for the Epo-R in some cases of inherited erythrocytosis in man,64-66 we sequenced all of the c-mpl exons as well as the exon/intron junctions. We were unable to detect any missense mutation in 9 patients with typical ET and 5 patients with PMF. This is not due to lack of sensitivity of our PCR technique because, in two brothers with ET, a polymorphism in the c-mpl gene was detected. One limitation of this method concerns the precise contribution of the malignant clone in the marrow myeloid lineage or in blood granulocytes, which is not yet well-established because ET is not usually associated with a cytogenetic marker. However, recent studies using X-linked markers have shown that the great majority of marrow cells and mature blood granulocytes belong to the malignant clone.57,67 Thus, ifc-mpl missense mutations were responsible for the pathogenesis of ET, it would certainly involve at least one allele. As the malignant clone largely predominates, nearly 50% of the c-mpl genes sequenced from marrow cells or granulocytes would carry the mutation. This is the situation that was observed for the polymorphism detected by our PCR technique in two brothers. Therefore, we conclude that a point mutation in the coding region of c-mpl is not involved in the pathogenesis of ET and PMF. This result is in agreement with a recent report from Kiladjian et al,55 which did not find any mutation of the c-mpl gene in 14 ET patients but detected the same polymorphism.
Two studies have shown that the number of Mpl receptors per platelet is greatly diminished in ET inducing a defect in the uptake of plasma Mpl-L.68,69 In another report, similar findings were observed in PV and PMF but not in ET.70 This mechanism may lead to a regulation of the platelet level at a higher plateau than in normal subjects and may explain high platelet counts in ET and PMF but not the autonomous MK progenitor growth and the clonal origin of the diseases. Therefore, a molecular defect directly acting on cellular proliferation might be present in these two diseases.
It has been recently reported that a donor splicing site mutation in the Mpl-L gene causes hereditary thrombocythemia by inducing an overproduction of Mpl-L by liver cells.71 It seems unlikely that a similar mutation is responsible for sporadic ET because (1) the disease is monoclonal, (2) a normal or slightly elevated Mpl-L serum concentration is found, and (3) spontaneous MK growth is observed in all patients excluding an abnormal synthesis of Mpl-L by liver cells. In contrast, an autocrine stimulation could explain spontaneous MK growth. Thus, we investigated whether an autocrine loop was involved in the pathogenesis of ET and PMF. We found somewhat contradictory results. On the one hand, a chimeric soluble receptor (sMpl-Fc) markedly inhibited spontaneous MK growth. Several criteria used to demonstrate that this effect was specific were met : (1) an irrelevant control receptor (4-1BB-Fc) had no inhibitory effect; (2) two different preparations of sMpl, including one devoid of the Fc fragment, induced inhibition; (3) this inhibition was partially reversed by PEG-rhuMGDF; (4) sMpl did not inhibit the stimulatory effect of a combination of three cytokines; and (5) sMpl had no effects on the growth of factor-dependent or -independent cell lines. On the other hand, Mpl-L transcripts could not be detected by a quantitative RT-PCR technique. Using a more sensitive RT-PCR technique, transcripts were detected but at an even lower level than in the UT7/huc-mpl or MO7E cells, which are both entirely dependent on growth factors, including Mpl-L, for their survival and their growth. An ELISA assay (sensitivity 25 pg/mL) and a coculture biological assay (sensitivity 10 pg/mL) were unable to detect Mpl-L in the supernatant of the cultured patient cells. In agreement with these results, a neutralizing anti–Mpl-L antibody did not inhibit spontaneous MK growth in ET or PMF. Furthermore, in contrast to CML cells and v-mpl expressing cells,54 72 no constitutive activation of STAT5 was found in spontaneously growing MKs from PMF patients. Short-term addition of PEG-rhuMGDF to these MKs induced significant activation of STATs 3 and 5, showing that STATs can be activated by Mpl-L in patient MKs.
Together, these results suggest that there is no autocrine loop of stimulation by Mpl-L in ET and PMF and that sMpl had an inhibitory effect independent of its Mpl-L binding capacity. This inhibition by the sMpl is not specific of malignant cells because we also observed a marked inhibition of the growth of normal MKs cultured in the presence of Mpl-L knock-out stromal cells and without addition of growth factor.73 In this culture technique, no Mpl-L was present and this further suggests that the inhibition by sMpl is independant of its ability to bind Mpl-L. In contrast, sMpl-Fc inhibited the MK growth from normal CD34+ cells stimulated by PEG-rhuMGDF. This effect was reversed by high concentration of PEG-rhuMGDF with a weak residual inhibition (about 15%). Two different mechanisms could explain a specific effect of the sMpl on proliferation: (1) the binding of a cytokine other than Mpl-L and (2) a direct effect of sMpl on the cells by binding to their membrane. Although not totally eliminated, the first hypothesis seems unlikely. Indeed, no stimulatory effect of ET or PMF cell supernatants on the growth of normal or PMF CD34+ cells could be detected. Similarly, we have no direct evidence for the second hypothesis because, by fluorescence labeling, we were unable to show sMpl-Fc binding to patient or normal cells or to different cell lines. However, we cannot exclude that sMpl binds to the extracellular part of another cytokine receptor and modifies its function. Recently, it has been shown that sMpl could stimulate the growth of primitive murine hematopoietic cells in synergy with some cytokines (SCF, FLT3-L).74 It was also speculated that this effect was mediated through a direct interaction with the cell membrane. A direct effect of sMpl on cell differentiation, independently of its Mpl-L binding capacity, could also explain some divergent results concerning the role of Mpl-L on platelet production.73 75-77 Further experiments will be required to show the precise mechanism by which sMpl modifies the growth of normal and malignant cells and to completely exclude that a part of its effect is toxic.
In conclusion, we have shown that autonomous MK growth is present in ET and in PMF. This abnormal proliferation is neither due to a missense activation in c-mpl nor to an autocrine stimulation by Mpl-L, although sMpl inhibits spontaneous MK growth in both diseases. Overall, this suggests that the primary defect in ET and PMF may affect a molecule involved in signal transduction. In CML, the chimeric molecule bcr-abl is able to interact with several cytokine pathways of signal transduction, and recent experiments suggest that a phosphatase may be involved in the growth factor hypersensitivity of PV cells.78 Further investigations are needed to determine if myeloproliferative diseases are due to similar molecular defects.
ACKNOWLEDGMENT
We are grateful to J.-L. Nichol (Amgen, Thousand Oaks, CA) and C. Caillot (Amgen, Neuilly, France) for providing the rhu-SCF, PEG-rhuMGDF, and the poyclonal antibody against Mpl-L; to D. Foster and Z. Lok (ZymoGenetics, Seattle, WA) for soluble murine Mpl receptors (sMpl-Fc and sMpl-Tag); and to D. Cosman (Immunex, Seattle, WA) for rhu-IL-3, murine Mpl (sMpl-Fc), and 4-1BB-Fc soluble-receptors. We are grateful to P. Bourquelot and O. Hermine (Hôpital Necker, Paris, France) for providing patient blood. We thank B. Forget for improving the English manuscript.
Supported by the Institut National de la Santé et de la Recherche médicale (INSERM), the Direction de la Recherche Clinique-Assistance Publique, the Institut Gustave Roussy, grants from the Association de la Recherche contre le Cancer (ARC), Ligue de Paris contre le Cancer, and a Fellowship from Fondation pour la Recherche Médicale to A.L.T.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Najet Debili, PhD, INSERM U 362, PR1, Institut Gustave Roussy, 39 rue Camille Desmoulins, 94805, Villejuif, France; e-mail: denali@igr.fr.