Hematopoietic progenitor kinase-1 (HPK1), which is expressed predominantly in hematopoietic cells, was identified as a mammalian Ste20 homologue that, upon transfection, leads to activation of JNK/SAPK in nonhematopoietic cells. The JNK/SAPK pathway is activated by various environmental stresses and proinflammatory and hematopoietic cytokines. Upstream activators of HPK1 currently remain elusive, and its precise role in hematopoiesis has yet to be defined. We therefore examined the possible involvement of HPK1 in erythropoietin (Epo) and environmental stress-induced JNK/SAPK activation in the Epo-dependent FD-EPO cells and Epo-responsive SKT6 cells. We found that Epo, but not environmental stresses, induced rapid and transient activation of HPK1, whereas both induced activation of JNK/SAPK. A screen for HPK1 binding proteins identified the hematopoietic cell-specific protein 1 (HS1) as a potential HPK1 interaction partner. We found HPK1 constitutively associated with HS1 and that HS1 was tyrosine-phosphorylated in response to cellular stresses as well as Epo stimulation. Furthermore, antisense oligonucleotides to HPK1 suppressed Epo-dependent cell growth and Epo-induced erythroid differentiation. We therefore conclude that Epo induces activation of both HPK1 and HS1, whereas cellular stresses activate only HS1, and that the HPK1-JNK/SAPK pathway is involved in Epo-induced growth and differentiation signals.
MITOGEN-ACTIVATED protein (MAP) kinases form a large family of serine-threonine protein kinases conserved through evolution.1,2 In mammalian cells, four distinct MAP kinase cascades have been identified: extracellular signal-regulated kinases (ERK),3,4 c-Jun amino-terminal kinases (JNK) or stress-activated protein kinases (SAPK),5,6 p38 MAP kinase (p38) or cytokine suppressive anti-inflammatory drug binding protein,7,8 and Erk5/BMK.9,10 The classical MAP kinases (ERK1 and ERK2) are activated by a variety of cell growth and differentiation stimuli2,11,12 and play a critical role in mitogenic signaling.13 The p38 and JNK/SAPK cascades are primarily activated by various environmental stresses (osmotic shock, UV radiation, heat shock, x-ray radiation, hydrogen peroxide, and protein synthesis inhibitors) and by proinflammatory cytokines such as tumor necrosis factor-α and interleukin-1 (IL-1).5-8,14-21 Cellular stresses and proinflammatory cytokines induce apoptotic cell death21; thus, it has been suggested that JNK/SAPK and p38 have a function in apoptotic signals.22,23 Various hematopoietic cytokines, interleukins, and colony-stimulating factors regulating hematopoietic cell growth, survival, and differentiation were also found to activate JNK/SAPK and p38.24-28 Furthermore, we recently found that JNK/SAPK and p38 play a crucial role in erythropoietin (Epo)-induced erythroid differentiation as well as Epo-dependent cell growth.29 Crawley et al30 reported that p38 acts in IL-2– and IL-7–driven T-cell proliferation. Very recently it was reported that p38 plays a critical role in neuronal differentiation of PC12 cells.31 Thus, JNK/SAPK and p38 appear to act not only in apoptotic signaling, but also in mitogenic and differentiation processes.
Hematopoietic progenitor kinase-1 (HPK1) is a member of a family of mammalian Ste20-related kinases.32,33 Initially identified by subtractive hybridization in hematopoietic progenitor cells and subsequently shown to be predominantly expressed in hematopoietic cells,32 HPK1 bears extensive homology to the Ste20 homologues GC-kinase,34 KHS,35 and GLK.36 Transfection studies in nonhematopoietic cells identified HPK1 as a specific activator of the JNK/SAPK pathway.32,33 Within the kinase cascade leading to its activation JNK/SAPK is immediately preceded by the upstream kinases MKK4/SEK137 and MKK7/SEK2,38-40 which themselves can be phosphorylated and activated by different kinases including MEKK1,41 MLK2/3,42,43 or TPL-2.44 All members of the GC-kinase family of Ste20 homologues are capable of activating the JNK/SAPK cascade in transfection models. While kinase activity has been demonstrated to be a prerequisite for that function, the exact mechanism of activation of subsequent pathway elements remains elusive. At all levels of the JNK/SAPK kinase cascade either multiple kinases or different homologues of a given kinase have been shown to be active. While the existence of multiple input elements enhances the possible levels of regulation of MAPK pathways and contributes elements of developmental and tissue specificity, it also complicates their analysis. The specificity and precise biological function of the various upstream kinases involved in JNK/SAPK activation in hematopoiesis remains currently unclear. Several pathways leading to JNK/SAPK activation by HPK1 have been postulated, involving the three different MAPK kinase kinases, MLK3,32MEKK133 and TAK1.45 However, no direct involvement of HPK1 in the activation of the JNK/SAPK cascade in hematopoietic cells has been demonstrated to date. Furthermore, it has not been examined whether environmental stresses can activate HPK1, and natural upstream agonists causing HPK1 activation have yet to be identified. The identification of upstream agonists and interacting proteins is therefore an important step towards a better understanding of the biology of HPK1 and related Ste20 homologous kinases.
Hematopoietic cell-specific protein 1 (HS1) is one of the major substrates of the Src family protein tyrosine kinases (Lyn, Blk, Fyn, or Lck)46-48 and Syk/Zap-70 kinases.46,49,50HS1 is highly tyrosine-phosphorylated during B-cell antigen receptor (BCR)-mediated signaling,46,47 and its phosphorylation is synergistically enhanced by the Src family and Syk/Zap-70 kinases associated with BCR.46,49,50 Phosphorylation of HS1 was also reported to be induced by IL-5 treatment of B cells51and FcεRI cross-linking of mast cells.52 Studies using HS1-deficient mice53 or HS1-deficient mutant WEHI-231 cell lines54 indicated that HS1 may function not only in B-cell proliferation, but also in apoptotic events after BCR cross-linking. It was recently found that tyrosine phosphorylation of HS1 is actually required for BCR-induced apoptosis.46 However, the precise role of HS1 in cell growth and apoptosis has not been determined.
We examined a possible involvement of HPK1 in Epo receptor-mediated and environmental stress-induced JNK/SAPK activation in FD-EPO cells, which grow in response to Epo,25 and in SKT6 cells, which can be differentiated to hemoglobinized cells in response to Epo.55 We found that Epo, but not environmental stresses, induced rapid and transient activation of HPK1, whereas both Epo and environmental stresses induced activation of JNK/SAPK. A screen for potential HPK1 binding proteins identified HS1, which we found constitutively associated with HPK1 in hematopoietic cells. HS1 is tyrosine-phosphorylated in response to both Epo stimulation and environmental stresses. The HPK1-JNK/SAPK pathway and the HS1-mediated signaling pathway might share common regulatory elements. We discuss here possible functions of the HPK1-JNK/SAPK and HPK1-HS1 pathways in Epo receptor-mediated and cellular stress-induced signal transduction.
MATERIALS AND METHODS
Reagents.
A polyclonal rabbit antiserum against a HPK1 peptide (#5),32 rabbit antiserum against mouse GST-HS1 (amino acid residues 57 to 233),53 and a mouse monoclonal antibody (2D20) specific to human HS1 (amino acid residues 306 to 320)46 were described previously. Antibodies against mouse JNK1 were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). Antiphosphotyrosine antibody, 4G10, was purchased from Upstate Biotechnology (Lake Placid, NY). Human Epo (2.6 × 105U/mg) was a gift from Kirin Brewery (Tokyo, Japan). GST-c-Jun was prepared as reported.25 Phosphothioester oligonucleotides (S-oligos) were prepared and purified by BEX (Tokyo, Japan).
Cell culture.
Epo-dependent FD-EPO cells25 were maintained in RPMI-1640 medium supplemented with 10% fetal calf serum and 1 U/mL of human Epo. Epo-responsive mouse erythroleukemia SKT6 cells have been described55 and were maintained in Ham F-12 medium supplemented with 10% fetal calf serum. SKT6 cell proliferation is independent of Epo and is neither enhanced nor impaired by the presence of Epo. SKT6 cells were induced to differentiate by the addition of 0.5 U/mL of recombinant human Epo, followed by incubation for 4.5 days, and hemoglobin-positive cells were stained by 0.05% 2,7-diaminofluorene (DAF).29
In vitro HPK1 kinase assay.
FD-EPO cells were starved in RPMI 1640 medium containing 0.4% fetal calf serum, 0.125 mg/mL of transferrin, and 0.01% bovine serum albumin without Epo for 12 hours and were restimulated with or without 1 U/mL of Epo for up to 60 minutes. SKT6 cells were stimulated with Epo without starvation. To test for osmo-stress and heat-shock responses, FD-EPO cells or SKT6 cells were incubated in the medium containing 0.35 mol/L NaCl for 30 minutes or incubated at 42°C for 1 hour. Cells were lysed in a lysis buffer (50 mmol/L Tris, pH 7.5, 0.5% Nonidet P-40, 150 mmol/L NaCl, 100 mmol/L sodium fluoride, 10 mmol/L sodium pyrophosphate, 1 mmol/L EDTA, 2 mmol/L Pefabloc, 10 ng/mL leupeptin, and 10 ng/mL aprotinin). Insoluble material was then removed by centrifugation and the precleared cell lysate was incubated with anti-HPK1 (#5)-specific antibody at 4°C for 2 hours. Immunocomplexes were bound to protein A-Sepharose at 4°C for 1 hour, washed 5 times with TNE (140 mmol/L NaCl, 50 mmol/L Tris-HCl, pH 8.0, 5 mmol/L EDTA) containing 1% Nonidet P-40, twice with TNE, and once with KB (50 mmol/L Tris-HCl, pH 7.5, 8 mmol/L MgCl2, 2 mmol/L MnCl2, 1 mmol/L dithiothreitol [DTT]). Kinase reactions were performed in 30 μL of KB at 30°C for 20 minutes in the presence of 10 μCi of [γ-32P]ATP. The reactions were terminated by mixing with Laemmli sample buffer and boiling. The samples were resolved by 7.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and autoradiographed.
Colt-λ cDNA library screen.
A Colt (cloning of ligand targets) library screen was performed as published by Sparks et al.56 Briefly, a 15-day mouse embryo cDNA library in λEXlox (Novagen, Madison, WI) was screened following the manufacturer’s protocol at approximately 50,000 plaques/15-cm dish. Six to 8 hours after plating, plaque size was determined and isopropylβ-D-thiogalactopyranoside–soaked filters, which had previously been air-dried, were applied. After overnight incubation at 37°C, the filters were removed and washed in phosphate-buffered saline (PBS) containing 0.1% Triton X-100 until no bacterial debris was left. Filters were then blocked in PBS containing 2.5% bovine serum albumin (BSA) for 30 minutes at ambient temperature. A biotin-labeled peptide (APFENIPPPLPPKPKFR) comprising the second proline-rich/SH3-ligand motif of the HPK1 central portion was synthesized and purified by high-performance liquid chromatography (HPLC). To generate multivalent complexes, 60 pmol of peptide was incubated in 100 μL PBS with 2 μg streptavidin-coupled alkaline phosphatase (Bio-Rad, Hercules, CA). Excess biotin binding sites were blocked by addition of 125 μg biotin and 5 minutes of incubation. The whole reaction mixture was then dissolved in 3 mL PBS containing 2.5% BSA and added to a preblocked filter. After incubation for 1 hour at room temperature, the filters were washed three times with PBS containing 0.1% Triton X-100 and then developed by the addition of 10 mL buffer (100 mmol/L Tris-HCl, pH 9.5, 100 mmol/L NaCl, 50 mmol/L MgCl2) containing 44 μL nitroblue tetrazolium chloride (75 mg/mL 70% dimethylformamide) and 33 μL 5-bromo-4-chloro-3-indoyl-phosphate-p-toluidine salt (50 mg/mL in dimethylformamide). At optimal signal strength, the chromogenic buffer was removed and the reaction was stopped by the addition of 50 mmol/L HCl. Positive plaques were then collected, phage-eluted, and rescreened until homogeneous preparations were obtained.
Immunoprecipitation and immunoblotting.
FD-EPO cells were starved in RPMI 1640 medium containing 0.4% fetal calf serum, 0.125 mg/mL transferrin, and 0.01% BSA without Epo for 12 hours and restimulated with or without 1 U/mL Epo for up to 3 hours. SKT6 cells were stimulated with Epo without starvation. In some cases, the cells were incubated in the medium containing 0.35 mol/L NaCl for 30 minutes or incubated at 42°C for 1 hour. The stimulated and unstimulated cells were immediately lysed in a lysis buffer (50 mmol/L Tris-HCl, pH 7.5, 0.5% Nonidet P-40, 150 mmol/L NaCl, 100 mmol/L sodium fluoride, 10 mmol/L sodium pyrophosphate, 1 mmol/L EDTA, 2 mmol/L Pefabloc, 10 ng/mL leupeptin, and 10 ng/mL aprotinin). Insoluble material was then removed by centrifugation and the cell lysate was incubated with anti–HS1-specific rabbit antibody at 4°C for 2 hours. The immunocomplexes were then bound to protein A-Sepharose at 4°C for 1 hour. The beads were washed 5 times with lysis buffer containing 0.1 % Nonidet P-40 before being boiled in Laemmli sample buffer. Samples were fractionated in 7.5% SDS-PAGE and electrotransferred to ECL membrane (Amersham, Little Chalfont, UK). The membrane was blocked in 5% BSA in TBS-T (20 mmol/L Tris-HCl, pH 7.5, 150 mmol/L NaCl, and 0.5% Tween 20) and incubated with anti-HPK1 antibody (#5), anti-HS1 antibody (2D20), or antiphosphotyrosine antibody (4G10) for 2 hours. After washing 3 times with TBS-T, the membrane was incubated with antimouse or antirabbit IgG-conjugated horseradish peroxidase antibody, and the antibody complexes were visualized by an ECL system (Amersham).
Effect of S-oligonucleotides.
Various concentrations of antisense, sense, or scrambled S-oligonucleotides derived from the HPK1 mRNA sequence were mixed in the culture medium before the addition of 0.5 U/mL Epo. The S-oligonucleotides in the medium were replenished after 24 hours. The antisense HPK1 S-oligonucleotide used was 5′-AGGGTCCACGACGTCCATCC-3′. As a control, the corresponding sense S-oligonucleotides and scrambled S-oligonucleotides were used.
Cell proliferation of FD-EPO cells was measured by a colorimetric assay using 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT; Sigma, St Louis, MO), originally developed by Mosmann.57 Exponentially growing cells (exactly 3 × 104) were plated on microtiter plates in 100 μL of culture medium in the presence or absence of Epo and various concentrations of antisense, sense, or scrambled S-oligonucleotides. After incubation at 37°C for 3 days, 10 μL of 5 mg/mL MTT in PBS was added to each well and incubated at 37°C for an additional 4 hours; the colorimetric reaction was stopped by the addition of 100 μL of 0.04 mol/L HCl in isopropanol and thorough mixing. Optical densities were measured using a microplate reader with a test wavelength of 570 nm and a reference wavelength of 630 nm.
SKT6 cells treated with S-ologonucleotides were cultured in the presence of 0.5 U/mL Epo for 4.5 days, and hemoglobin-positive cells were stained by DAF.
RESULTS
Epo but not osmotic shock or heat shock activates HPK1.
To identify upstream regulators of HPK1, we tested for a possible activation of HPK1 in response to Epo stimulation in the Epo-dependent FD-EPO cells and Epo-responsive SKT6 cells. Using an HPK1-specific polyclonal antipeptide rabbit serum (αHPK1 #5), HPK1 was immunoprecipitated from FD-EPO or SKT6 cells before and at various times after Epo stimulation. The activity of HPK1 kinase was then determined by its ability to autophosphorylate in vitro in the presence of [γ-32P]ATP and Mg2+. A distinct increase in HPK1 in vitro autophosphorylation in response to Epo was observed in both FD-EPO cells (Fig 1A) and SKT6 cells (Fig 1B). In both cell lines, autophosphorylation levels rapidly increased as early as 3 minutes after Epo stimulation (lane 2) and reached their maximum about 5 minutes after stimulation (lane 3). Within the next 30 minutes, HPK1 kinase activity decreased, and 1 hour after stimulation only basal level activity was detectable (lanes 4 through 6). Half of the HPK1 immunoprecipitates used for kinase assays were probed with anti-HPK1 antibody to confirm that there were equal amounts of HPK1 in these samples (Fig 1A and B, bottom). These results indicate that HPK1 is rapidly and transiently activated in FD-EPO cells as well as in SKT6 cells after Epo-receptor engagement.
Epo but not osmotic shock or heat shock lead to activation of HPK1 kinase activity. (A) In vitro HPK1 autophosphorylation in Epo-dependent FD-EPO cells (A) and in Epo-responsive SKT6 cells (B). HPK1 was immunoprecipitated using a HPK1-specific antibody after Epo stimulation at the indicated time points (lanes 1 through 6) or after 0.35 mol/L NaCl treatment for 30 minutes (lane 7) or heat shock at 42°C for 30 minutes (lane 8). In vitro kinase assays were performed in the presence of [γ-32P]ATP. The arrow indicates the autophosphorylated HPK1, and the band below is nonspecific.
Epo but not osmotic shock or heat shock lead to activation of HPK1 kinase activity. (A) In vitro HPK1 autophosphorylation in Epo-dependent FD-EPO cells (A) and in Epo-responsive SKT6 cells (B). HPK1 was immunoprecipitated using a HPK1-specific antibody after Epo stimulation at the indicated time points (lanes 1 through 6) or after 0.35 mol/L NaCl treatment for 30 minutes (lane 7) or heat shock at 42°C for 30 minutes (lane 8). In vitro kinase assays were performed in the presence of [γ-32P]ATP. The arrow indicates the autophosphorylated HPK1, and the band below is nonspecific.
Various environmental stresses are known to induce activation of the JNK/SAPK and p38 kinase pathways.5-8,14-21 We therefore investigated a possible activation of HPK1 in response to osmotic shock and heat shock in FD-EPO and SKT6 cells. However, HPK1 kinase activity was neither affected by exposure of FD-EPO or SKT6 cells to 0.35 mol/L NaCl for 30 minutes (lane 7) nor by a heat shock at 42°C for 30 minutes (lane 8). In contrast, JNK1 was fully activated under the same conditions as described previously.25 Thus, neither in FD-EPO cells nor in SKT6 cells is HPK1 activity regulated by environmental stresses. HPK1 is therefore not likely to mediate JNK/SAPK activation in response to stressful stimuli.
In parallel to HPK1 activation in response to Epo, a JNK/SAPK in vitro kinase assay showed that JNK1 was clearly activated by both Epo stimulation and environmental stresses, as reported previously.25 The JNK1 activation profile in response to Epo stimulation was somewhat delayed compared with that of HPK1. Maximal HPK1 activity was observed after 5 minutes (Fig 1A and B), whereas JNK1 activity peaked only 15 minutes after Epo stimulation. These results suggest that Epo-receptor engagement but not environmental stress induces activation of HPK1, which may in turn lead to activation of JNK/SAPK in hematopoietic FD-EPO and SKT6 cells, as previously described for transfected nonhematopoietic cells.32 33
Identification of HPK1 interacting proteins.
To further define the Epo-dependent HPK1 signaling pathway in FD-EPO cells, we attempted to identify proteins that physically interact with HPK1. HPK1 consists of an N-terminally located kinase domain, followed by a central region harboring 4 proline-rich motifs and a long presumably regulatory C-terminal tail. Three of the 4 proline-rich regions have previously been shown to be capable to act as SH3 domain ligands.58 As recently demonstrated by Sparks et al,56 peptides modeled after such SH3 ligands can be used to identify their target SH3 domains with relatively limited cross-reactivity. Of the 4 proline-rich regions in HPK1 (P1 through P4), we selected proline-rich region P2 as a bait for our Colt (cloning of ligand targets) screen. In previous experiments, P2 had displayed the strongest SH3 domain affinity.58
Screening of a 14-day mouse embryonic λ cDNA library led to the isolation of 12 cDNAs, all of which encoded SH3 domain containing proteins (Table 1). Two pairs of isolates were derived from a shared progenitor-phage each, so that the screen yielded in total 10 independent isolates. For each cDNA, we identified a corresponding entry in the data sequence base. Table 1 summarizes the identities of all 12 cDNAs. Somewhat surprisingly, drebrin, a neuronal actin binding protein, was most highly represented within the HPK1 interacting proteins. When we aligned the amino acid sequences of the different SH3-domains of the identified candidate proteins, we noticed an unusually high level of homology between the drebrin, cortactin, and HS1 SH3 domains (Fig 2).
Sequence alignment of 5 potential HPK1 interacting proteins. Residues identical between the individual proteins and murine HS1 are shaded. Characteristic residues found in a large number of SH3 domains are indicated by hollow letters.
Sequence alignment of 5 potential HPK1 interacting proteins. Residues identical between the individual proteins and murine HS1 are shaded. Characteristic residues found in a large number of SH3 domains are indicated by hollow letters.
HS1 is constitutively associated with HPK1 and is tyrosine-phosphorylated in response to Epo and to osmotic shock.
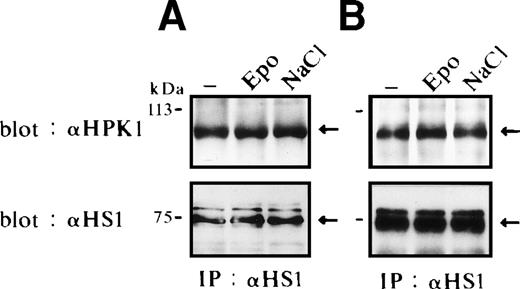
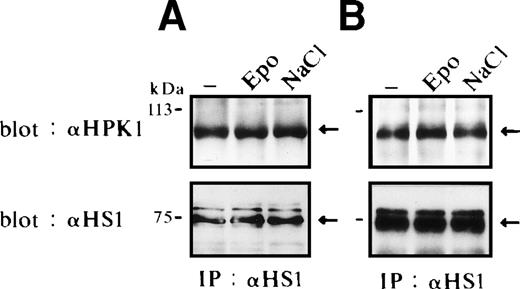
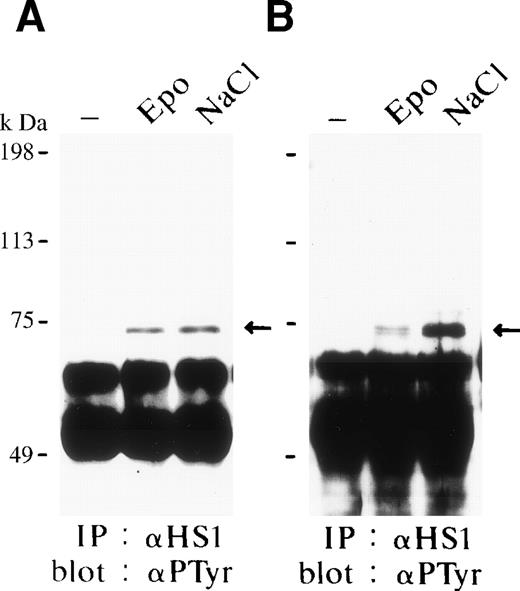
HS1 has been described to be expressed in hematopoietic cells, drebrin is specifically expressed in neurons, and cortactin is not expressed in hematopoietic cells except megakaryocytes/platelets.59-61We found HS1 but not cortactin or drebrin expressed in FD-EPO cells and SKT6 cells (data not shown). We therefore tested for a possible interaction between HS1 and HPK1 in FD-EPO cells (Fig 3A) and SKT6 cells (Fig 3B) before and after Epo stimulation as well as in cells that had been exposed to osmotic shock. HS1 was immunoprecipitated with an anti–HS1-specific rabbit antibody, and the immunocomplexes were separated by SDS-PAGE and probed with anti-HPK1 antiserum #5. HPK1 was found to be constitutively associated with HS1, independent of stimulation of the Epo receptor (Fig 3A and B, upper panels, lanes 1 and 2) in both cell lines. Association levels were also insensitive to osmotic shock (Fig 3A and B, upper panels, lanes 1 and 3) in both cell lines. Similar result was obtained after exposure of cells to heat shock (data not shown). Equal amounts of immunoprecipitated HS1 were demonstrated by immunoblotting (Fig 3A and B, lower panels). We failed to detect HS1 in anti-HPK1 immunoprecipitates, most likely due to interference of the anti-HPK1 serum #5 with HS1 binding. Anti-HPK1 serum #5 is directed against a peptide derived from the proline-rich central part of HPK1. Our results suggest that HPK1 constitutively interacts with HS1 and thereby forms a possible link between the JNK/SAPK and HS1 signaling cascades.
HS1 constitutively associates with HPK1. Binding of HPK1 and HS1 in FD-EPO cells (A) or in SKT6 cells (B) before (lane 1) and after (lane 2) Epo stimulation or osmotic shock (lane 3). Anti-HS1 immunoprecipitates were probed with anti-HPK1 antibody (upper panels) or anti-HS1 antibody (lower panels). Arrows indicate HS1.
HS1 constitutively associates with HPK1. Binding of HPK1 and HS1 in FD-EPO cells (A) or in SKT6 cells (B) before (lane 1) and after (lane 2) Epo stimulation or osmotic shock (lane 3). Anti-HS1 immunoprecipitates were probed with anti-HPK1 antibody (upper panels) or anti-HS1 antibody (lower panels). Arrows indicate HS1.
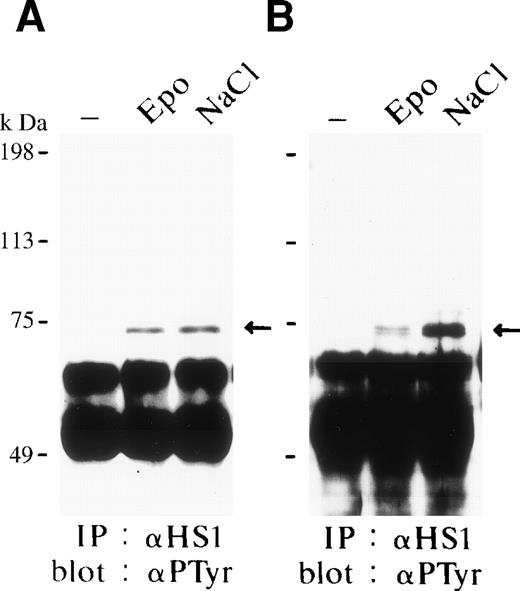
The same filters shown in Fig 3A and B were reprobed with the antiphosphotyrosine-specific antibody 4G10. We found that both Epo and osmotic shock induced tyrosine phosphorylation of HS1 in FD-EPO cells (Fig 4A) and SKT6 cells (Fig 4B). In contrast, we failed to detect tyrosine-phosphorylation of HPK1. These results clearly indicate that HS1 can be tyrosine-phosphorylated in response to Epo stimulation and osmotic shock.
HS1 is tyrosine-phosphorylated in response to Epo and osmotic shock. The same filters shown in Fig 3 were reprobed with the antiphosphotyrosine antibody 4G10. The arrow indicates the tyrosine-phosphorylated HS1.
HS1 is tyrosine-phosphorylated in response to Epo and osmotic shock. The same filters shown in Fig 3 were reprobed with the antiphosphotyrosine antibody 4G10. The arrow indicates the tyrosine-phosphorylated HS1.
HPK1 activation is involved in Epo-dependent cell growth.
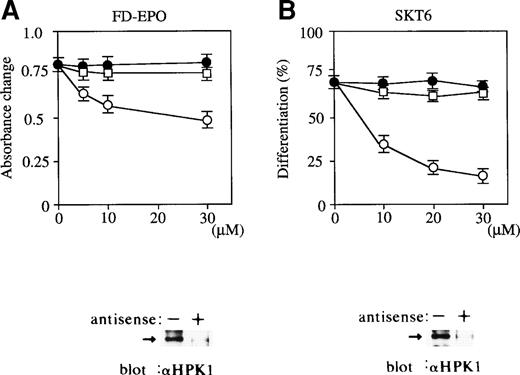
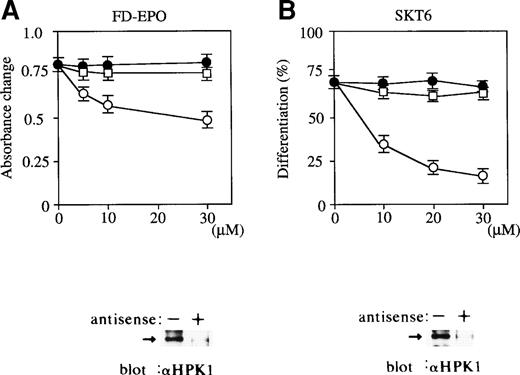
We studied the possible involvement of HPK1 in Epo-dependent FD-EPO cell growth by application of an antisense S-oligonucleotide strategy. In the presence of antisense S-oligonuleotides to HPK1 mRNA, Epo-dependent cell growth was significantly inhibited in a dose-dependent manner (Fig 5A, ○). In contrast, the scrambled S-oligonucleotide (Fig 5A, •) and sense S-oligonucleotide (Fig 5A, □) had little effect. Immunoblot analysis of HPK1 demonstrated a clear reduction in HPK1 expression levels in the presence of antisense S-oligonucleotides (Fig 5A, below). Our results argue in favor of an involvement of Epo-induced HPK1 activation, which in turn contributes to activation of JNK/SAPK pathway in Epo-dependent cell proliferation of FD-EPO cells.
Antisense oligonucleotide-mediated abrogation of HPK1 expression in FD-EPO cells and SKT6 cells leads to reduced Epo-dependent proliferation and inhibition of erythroid differentiation. Various concentrations (0 to 30 μmol/L) of HPK1 antisense S-oligonucleotides (○), HPK1 sense S-oligonucleotides (□), or scrambled S-oligonucleotides (•) were mixed with FD-EPO cells (A) or with SKT6 cells (B) in the presence of Epo. Cellular proliferation was measured using a MTT assay. The hemoglobinized cells were stained with DAF, and the percentage of hemoglobinized cells without oligonucleotides is shown as 100%. Values shown are the means of six experiments.
Antisense oligonucleotide-mediated abrogation of HPK1 expression in FD-EPO cells and SKT6 cells leads to reduced Epo-dependent proliferation and inhibition of erythroid differentiation. Various concentrations (0 to 30 μmol/L) of HPK1 antisense S-oligonucleotides (○), HPK1 sense S-oligonucleotides (□), or scrambled S-oligonucleotides (•) were mixed with FD-EPO cells (A) or with SKT6 cells (B) in the presence of Epo. Cellular proliferation was measured using a MTT assay. The hemoglobinized cells were stained with DAF, and the percentage of hemoglobinized cells without oligonucleotides is shown as 100%. Values shown are the means of six experiments.
HPK1 activation is involved in Epo-induced erythroid differentiation.
Similarly, we examined the possible involvement of HPK1 in Epo-induced hemoglobinization of SKT6 cells. In the presence of antisense S-oligonuleotides to HPK1 mRNA, Epo-induced erythroid differentiation was significantly inhibited in a dose-dependent manner (Fig 5B, ○), whereas scrambled S-oligonucleotide (Fig 5B, •) and sense S-oligonucleotide (Fig 5B, □) had little effect. Immunoblot analysis of HPK1 showed a clear reduction in HPK1 expression by antisense S-oligonucleotides (Fig 5B, below). Taken together, these results indicate that the HPK1-JNK/SAPK pathway, at least in part, plays a role in Epo-dependent cell growth and differentiation.
DISCUSSION
Various hematopoietic cytokines, interleukins, and colony-stimulating factors regulating hematopoietic cell growth, survival, and differentiation were recently found to activate the JNK/SAPK, p38, and ERK kinases.24-30 However, the role of these three distinct MAP kinases in hematopoiesis has not yet been determined in detail. We show here that HPK1 is activated by Epo but not by environmental stresses in FD-EPO and SKT6 cells. This finding makes an involvement of HPK1 as an upstream element mediating the Epo-induced activation of the JNK/SAPK cascade likely. Interestingly, in the same cell lines, HPK1 does not seem to be involved in environmental stress-induced JNK/SAPK activation. Potentially, HPK1 could also function as an upstream kinase in other hematopoietic cytokine-induced pathways leading to JNK/SAPK activation.24,25,27 Environmental stresses induce the production of ceramide as a second messenger,62 which in turn stimulates activation of TAK1,63 which may function as a MAPK kinase kinase within the JNK/SAPK cascade. Based on the capacity of dominant negative versions of TAK1 to inhibit HPK1-induced JNK/SAPK activation in transfection experiments, TAK1 was implicated as a HPK1 downstream element. Our results implicate HPK1 in growth and/or differentiation factor-stimulated JNK/SAPK activation rather than stress-induced JNK/SAPK activation. Discovery and identification of the JNK/SAPK interacting protein JIP1 as a mammalian Ste5 analogous scaffolding protein defines a possible HPK1 downstream signaling complex, comprising the MLK-family, MKK7, and JNK/SAPK.64Although the precise mechanism of subsequent activation within the triple kinase cascade MLK-MKK-JNK/SAPK is known to be phosphorylation of regulatory threonine and tyrosine residues in kinase subdomain VIII, it is not clear how Ste20 homologues activate their downstream effectors mechanistically. Given the dependence of JNK/SAPK activation on kinase activity of the respective upstream Ste20 homologue, it is reasonable to assume, however, that kinase activity reflects activity as an upstream pathway activator. We identified here Epo as a natural agonist of HPK1 activation and found that HPK1-JNK/SAPK pathway plays a role in Epo-induced cell growth and differentiation.
The elements that link HPK1 to the engaged Epo receptor remain unknown. We have previously shown that after ectopic expression the adapter molecule Grb2 can link HPK1 to tyrosine kinases like Src family kinases or the EGF receptor.58 To pursue the quest for upstream elements, we screened for additional HPK1 interaction proteins using a peptide derived from the central proline-rich region P2 of HPK1. We found the neuronal actin binding protein drebrin to be highly represented in our screen for HPK1 binding SH3 domains. This result may simply reflect a bias of the screening procedure or a representational bias of the λ cDNA library, because a published screen using similar proline-rich peptides of the class II type and an identical cDNA library yielded identical SH3 domains.56 However, given the expression of HPK1 in mouse embryos and neonates, we cannot exclude the possibility that drebrin may be a valid HPK1 interaction partner at those developmental stages. Nevertheless, we interpret the remarkably high homology between the SH3 domains of drebrin, cortactin, and HS1 as an indication of specificity of our screen. We present evidence here that, at endogenous expression levels, HPK1 is constitutively associated with HS1.
It has been reported that HS1 is one of the major substrates of the Src family kinases.46-48 One of the Src family members, Lyn, has been reported to physically associate with the Epo receptor and to phosphorylate Stat5 as well as the Epo receptor65; in addition, Lyn is essential for erythroid differentiation.66Thus, we examined the possible scenario that Lyn physically interacts with the Epo receptor and phosphorylates HS1 in response to Epo receptor engagement. However, at endogenous expression levels, we failed to detect the interaction between Lyn and HS1 independent of Epo stimulation or osmotic shock, although Lyn was constitutively tyrosine-phosphorylated (data not shown). Thus, the tyrosine kinase that phosphorylates HS1 after Epo stimulation and osmotic shock remains to be identified. Furthermore, two phosphorylated HS1 bands were detected in Fig 4B. This might indicate that there exists a posttranslationally modified or an alternatively spliced form of HS1 or a very closedly related HS1 gene product in SKT6 cells. This point also has to be clarified.
We demonstrate here that both Epo and environmental stresses induce tyrosine-phosphorylation of HS1. Although the functional significance of tyrosine phosphorylation of HS1 in Epo-induced signaling remains to be understood, HS1 is likely to be involved in erythroid proliferation and differentiation rather than apoptosis. In contrast, in osmotic shock-induced signaling, HS1 phosphorylation may play an important role in signaling cascades leading to apoptotic cell death as observed in BCR-induced apoptosis.
ACKNOWLEDGMENT
The authors thank Dr Tony Pawson for his interest and continuous support and Noriko Takahashi and Junko Iita for technical assistance.
Y.N. and F.K. contributed equally to this work.
Supported in part by a Special Grant for Promotion of Research from The Institute of Physical and Chemical Research (RIKEN) and by grants from the Ministry of Education, Science and Culture of Japan, the Science and Technology Agency of Japan, the Uehara Memorial Foundation, and the Suzuken Memorial Foundation.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Kazuo Todokoro, PhD, Tsukuba Life Science Center, The Institute of Physical and Chemical Research (RIKEN), 3-1, Koyadai, Tsukuba, Ibaraki 305-0074, Japan; e-mail:todokoro@rtc.riken.go.jp.

![Fig. 1. Epo but not osmotic shock or heat shock lead to activation of HPK1 kinase activity. (A) In vitro HPK1 autophosphorylation in Epo-dependent FD-EPO cells (A) and in Epo-responsive SKT6 cells (B). HPK1 was immunoprecipitated using a HPK1-specific antibody after Epo stimulation at the indicated time points (lanes 1 through 6) or after 0.35 mol/L NaCl treatment for 30 minutes (lane 7) or heat shock at 42°C for 30 minutes (lane 8). In vitro kinase assays were performed in the presence of [γ-32P]ATP. The arrow indicates the autophosphorylated HPK1, and the band below is nonspecific.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/10/10.1182_blood.v93.10.3347.410k06_3347_3354/5/m_blod41006001w.jpeg?Expires=1767881675&Signature=AQSvSTBMW-Bq77OBOnWaghH1VC6ORotj5jsNc38wKb6nRCBYKM4um6J8j54ZLz8rzXh9sYKAfpnUXzoNKTJGYJA5BSq37q7yJsh~M31GFOCP6u~9WHTRYwcxeQDHb8kl06TN1Rn3Zu~L8GA9qgjm7UMGNlwaXZEV5Us8jsinO8R44MyKBQUBj7-d1sMl7-iC8GxgTPTxhg3cF4K8euIWZhugZrkt6x~OTzizfKkY-IHu7o8aXSV96JJAxIuYtDxoNlqJgV483u7e5fLo~-OfiTSE3g7ZCk5FKmZTC~ks8dp3ju1BNj57oP8RDnEitZZ9OwRX5RdM8-c09AuhvUOq-A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


![Fig. 1. Epo but not osmotic shock or heat shock lead to activation of HPK1 kinase activity. (A) In vitro HPK1 autophosphorylation in Epo-dependent FD-EPO cells (A) and in Epo-responsive SKT6 cells (B). HPK1 was immunoprecipitated using a HPK1-specific antibody after Epo stimulation at the indicated time points (lanes 1 through 6) or after 0.35 mol/L NaCl treatment for 30 minutes (lane 7) or heat shock at 42°C for 30 minutes (lane 8). In vitro kinase assays were performed in the presence of [γ-32P]ATP. The arrow indicates the autophosphorylated HPK1, and the band below is nonspecific.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/10/10.1182_blood.v93.10.3347.410k06_3347_3354/5/m_blod41006001w.jpeg?Expires=1767956576&Signature=RAmHRrmixLwhNgXXB~B1H2mfT4-Qc01L0x~-TSaxdYxB2qDWVjpUzBMIrabmZtIu1WeL4AXuXsU1npabJW7kccPrkBFCgq-nnxFNy6Dc9P~kz98d34NRLp7O6qfjrxB2PiOLzL35nJ5hpTEQDx~9pN1iDoyD1zyx78EgECiSQFMTJLv7l7LEWQhVTv15h-1vyl6ytY2Cf4esr-~U0Z1v4uOJtHuGuWUMBZTi4KOfRdAeHKGmwwuLtno1QpXWEbnScSC2LfF3gBWFgSnAsB0U9J5yCI3KL18A-LreALCg5U9dfePlM0rhCifO0Jm~HKJV4KT2HMEE6Rm9YZhSt5hNNA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)