Abstract
The effects of thrombopoietin (TPO) and/or stem cell factor (SCF) on the development of human mast cells from CD34+ bone marrow (BM) cells were investigated using a serum-deprived liquid culture system. Mast cells were identified by measurement of intracellular histamine content, immunocytochemical staining, and flow cytometric analysis. Whereas SCF alone generated only a small number of tryptase+ cells, the addition of TPO to the culture containing SCF resulted in an apparent production of mast cells from 3 weeks until at least 15 weeks. Some of the cells reacted with an antichymase monoclonal antibody as well. Based on the effects of growth factor(s) on a later phase of the mast cell growth, TPO may stimulate an early stage of mast cell development in combination with SCF, whereas subsequent growth seems to be supported by SCF alone. Single-cell culture studies indicated that the CD34+CD38−c-kit+ cells and CD34+CD38+c-kit+ cells were responsible for the SCF + TPO–dependent mast cell production. Two-step culture assays clearly showed that mast cells originated from multilineage colony-forming cells that had potential to differentiate into neutrophil/mast cell lineages, neutrophil/macrophage/mast cell lineages, or neutrophil/macrophage/mast cell/erythroid lineages. These results suggest that TPO plays an important role in the development of human mast cells from CD34+ BM cells in concert with SCF, and provide direct evidence of the differentiation into the mast cell lineage of human multipotential BM-derived progenitors.
HUMAN MAST CELLS are distributed over the whole body in tissues including skin, lungs, gut, and nasal mucosa, and are classified into two phenotypically distinct subpopulations on the basis of their protease expression: tryptase+chymase− mast cells and tryptase+chymase+ mast cells.1 It is well-known that human mast cells play important roles in both allergic disorders and inflammatory reactions.
In the murine system, it has been elucidated that mast cells originate from hematopoietic stem cells in vivo2 or multipotential hematopoietic progenitors in vitro.3 Mast cell precursors depart from bone marrow (BM) and migrate into connective or mucous tissues, where they differentiate into the mature form. The stem cell factor (SCF)–c-kit receptor signal transduction pathway is essential for the development of murine mast cells, because both W and steel (SI) mice, which have mutations in the locus of c-kit receptor and SCF, respectively, are deficient in mast cells.4 In addition to SCF, interleukin (IL)-3, IL-4, IL-9, and IL-10 promote the proliferation and differentiation of mast cells.5-8 In the human system, SCF has been shown to act as a major growth and differentiation factor for mast cell development from cord blood (CB) mononuclear cells (MNCs),9 BM cells,10,11 and fetal liver cells.12 However, there are several differences between the human and murine mast cell development systems. First, it was shown that the addition of IL-6 or IL-11 to cultures containing SCF enhances the growth of mast cells from CD34+ CB cells.13 Second, neither IL-3 nor IL-4 induces the differentiation of human mast cells.14-16Although the hypothesis that monocytic precursors or basophils are candidates for mast cell precursors was previously proposed,17,18 Agis et al19 clearly showed that human mast cells are derived from CD34+ cells. However, the clonal origin of human mast cells from multilineage BM-derived progenitor cells has not been established because of the limitation of assays available.
Thrombopoietin (TPO) was cloned by several groups as a potent stimulator in megakaryocytopoiesis.20-24 Recent studies showed that TPO can act on the growth of hematopoietic progenitors (especially multipotential progenitors) in combination with other cytokines, including SCF, IL-3, and flt3 ligand (FL).25 26In the present study, we examined, using CD34+ BM cells, whether TPO can stimulate the SCF-dependent proliferation and differentiation of human mast cell progenitors/precursors in a serum-deprived culture system.
MATERIALS AND METHODS
Factors and antibodies.
Human recombinant TPO, SCF, IL-3, granulocyte-macrophage colony-stimulating factor (GM-CSF), and erythropoietin (EPO) were provided by Kirin Brewery Co Ltd (Takasaki, Japan). Human recombinant FL was purchased from PeproTech EC Inc (Rocky Hill, NJ). Human recombinant granulocyte colony-stimulating factor (G-CSF) was a gift from Chugai Pharmaceutical Co (Tokyo, Japan). Human recombinant IL-6 was kindly provided by Ajinomoto Co (Kawasaki, Japan). Human recombinant IL-11 was purchased from R & D Systems (Minneapolis, MN).
For the flow cytometric analysis and cell sorting, monoclonal antibodies (MoAbs) for CD34 (8G-12, fluorescein isothiocyanate [FITC]; phycoerythrin [PE]), CD38 (HB7, allophycocyanin [APC]), and c-kit (104D2, PE) were purchased from Becton Dickinson Immunocytometry Systems (Mountain View, CA). The MoAbs for CD15 (80H5, FITC) and CD41 (SZ.22, Biotin) were from Immunotech S.A. (Marseilles, France). A MoAb against human c-mpl domain 1 (M1) was obtained from Genzyme Co (Cambridge, MA). In our preliminary experiments, the Western blot analysis showed that M1 MoAb recognized the c-mpl with the molecular weight of 82 kD in a platelet lysate.
For immunocytochemical staining, purified MoAbs for human tryptase (MAB1222) and chymase (3D5) were purchased from Chemicon International Inc (Temecula, CA) and from Biogenesis Inc (Sandown, NH), respectively. MoAbs for human myeloperoxidase (MPO, CLB-MPO-1), CD2 (T11), and CD41 (SZ.22) were obtained from Immunotech S.A. MoAbs for CD19 (HD37) and glycophorin A (GPA, JC159) were from Dako (Glostrup, Denmark).
Isolation of CD34+ cells from bone marrow MNCs.
BM cells were aspirated in heparinized plastic syringes from healthy volunteers after informed consent was obtained. BM MNCs were separated by density centrifugation over Ficoll-Paque (Pharmacia, Piscataway, NJ), washed twice, and suspended in Ca2+- and Mg2+-free phosphate-buffered saline (PBS) containing 1 mmol/L EDTA 2-Na and 2.5% fetal bovine serum (Hyclone, Logan, UT). The cells (2 × 106) were incubated with 20 μL of FITC-conjugated anti-CD34 MoAb for 30 minutes at 4°C. As negative controls, the cells were stained with FITC-conjugated mouse IgG1 (Dako). After two washes, CD34+ cells were sorted by a FACStarplus flow cytometer (Becton Dickinson), as described previously.27
Serum-deprived suspension culture.
Serum-deprived liquid cultures were carried out in 24-well culture plates (#3047; Becton Dickinson), using the technique described previously.28 29 Five to 10 × 103CD34+ BM cells were cultured in each well containing 2 mL of α-medium (Flow Laboratories Inc, Rockville, MD) supplemented with 1% deionized bovine serum albumin (Sigma Chemical Co, St Louis, MO), 600 μg/mL fully iron-saturated human transferrin (approximately 98% pure; Sigma), 16 μg/mL soybean lecithin (Sigma), and 9.6 μg/mL cholesterol (Nakalai Tesque Inc, Kyoto, Japan) in the presence of 10 ng/mL TPO, 10 ng/mL SCF, 10 ng/mL GM-CSF, 50 ng/mL FL, 50 ng/mL IL-6, 50 ng/mL IL-11, or 10 ng/mL G-CSF, alone or in combination. The plates were incubated at 37°C in a humidified atmosphere flushed with a mixture of 5% CO2, 5% O2, and 90% N2. Half of the cell-free supernatant was replaced with fresh medium containing growth factor(s) every 5 to 7 days. The number of viable cells was determined by a trypan-blue exclusion test using hemocytometers, and the cells were processed for the cytochemical and immunologic stainings and flow cytometric analysis.
Serum-deprived single-cell culture.
Single-cell sorting was performed by two-step sorting, as described previously.29 BM MNCs (2 × 106) were incubated with 20 μL of FITC-conjugated anti-CD34 MoAb, 5 μL of APC-conjugated anti-CD38 MoAb, and 20 μL of PE-conjugated anti–c-kit MoAb for 30 minutes at 4°C. As negative controls, the cells were stained with FITC-, APC-, and PE-conjugated mouse IgG1 (Becton Dickinson). After two washes, CD34+CD38+c-kit+ cells, CD34+CD38+c-kit− cells, CD34+CD38−c-kit+ cells, and CD34+CD38−c-kit− cells were individually sorted in 5-mL tubes by the FACStarplus flow cytometer. The cells in each group were then resorted into individual wells of a 96-well U-bottomed tissue culture plate (#3077; Becton Dickinson) containing 100 μL of the serum-deprived culture medium supplemented with SCF, TPO, or FL, alone or in combination, using a FACStarplus flow cytometer equipped with an automatic cell deposition unit. Ninety-nine percent of the wells contained a single cell on the first day of culture. The plates were incubated at 37°C in a humidified atmosphere flushed with a mixture of 5% CO2, 5% O2, and 90% N2. After 3 weeks, colonies of more than 20 cells were scored in situ on an inverted microscope, and the constituent cells of colonies were identified on cytocentrifuged preparations stained with May-Grünwald-Giemsa. Megakaryocyte colonies were scored when they had three or more cells.
To assess the differentiative potentials of mast cell–containing colony-forming cells supported by various types of growth factor combinations, we performed a two-step culture assay. Each of the sorted cells was incubated with SCF + TPO, SCF + FL, TPO + FL, or SCF + TPO + FL. When the cell number in each well reached 10 cells or more between day 10 and day 32, the cells were divided into two aliquots. One half of the sample was replated in a well containing SCF+TPO. The other half was recultured in a well containing a cocktail of the growth factors (GFs: 10 ng/mL G-CSF + 10 ng/mL SCF + 10 ng/mL TPO + 50 ng/mL FL + 10 ng/mL GM-CSF + 100 U/mL IL-3 + 2 U/mL EPO). The culture plates were further incubated for 1 to 3 weeks, and the progenies were identified on cytospin preparations stained with May-Grünwald-Giemsa.
Flow cytometric analysis.
For the analysis of surface markers on the cultured cells, the cells were collected in plastic tubes and incubated with appropriately diluted FITC- or PE-MoAbs, as described previously.29 The cells were washed twice, and their surface markers were analyzed with a FACScan flow cytometer (Becton Dickinson) using the Lysis 2 software program (Becton Dickinson). Viable cells were gated according to their forward light scatter characteristics (FSC) and side scatter characteristics (SSC). The proportion of positive cells was determined by comparison with cells stained with FITC- or PE-conjugated mouse isotype matched Ig. To analyze the surface expression of c-mpl on CD34+ BM cells, cultured mast cells, and cultured megakaryocytic cells, the cells were incubated with 20 μL anti–c-mpl MoAb for 30 minutes at 4°C. Isotype MoAb was used as a control. The cells were washed three times and stained with FITC-conjugated goat antimouse immunoglobulin (GAM; Becton Dickinson) for 15 minutes. CD34+ BM cells and cultured cells grown by TPO were washed three times and treated with mouse serum for 15 minutes. Then, the cells were stained with PE-conjugated anti-CD34 MoAb or biotinized anti-CD41 MoAb, followed by Cy-Chrome–labeled streptavidin (PharMingen, San Diego, CA). PE-conjugated and biotinized isotype antibodies were used as controls.
For the identification of mast cells and neutrophils, we carried out a two-color analysis. The cultured cells were stained with 20 μL of PE-conjugated anti–c-kit MoAb and 20 μL of FITC-conjugated anti-CD15 MoAb for 30 minutes at 4°C and then analyzed using the flow cytometer. As negative controls, cells were stained with PE- and FITC-conjugated mouse IgG1.
Cytochemical and immunologic stainings.
Cultured cells were spread on glass slides using a Cytospin II (Shandon Southern, Sewickly, PA) and stained with May-Grünwald-Giemsa. A cytochemical reaction with peroxidase (POX) was performed by the conventional method.
Reactions with mouse MoAbs against human tryptase, chymase, MPO, and CD41 were detected using the alkaline phosphatase–antialkaline phosphatase method (Dako APAAP Kit System; Dako Corp, Carpinteria, CA), as described previously.29 30 The isotype mouse MoAb was used as a control. Briefly, cytocentrifuged samples were fixed with Carnoy’s fluid, washed with PBS, and preincubated with normal rabbit serum to saturate Fc receptors on the cell surface. After being washed with PBS three times, the samples were reacted with mouse MoAb for 30 minutes at room temperature in a humidified chamber. After additional three washes with PBS, the samples were reacted with rabbit antimouse IgG antibody, washed three times, and successively reacted with the calf intestinal alkaline phosphatase–mouse monoclonal antialkaline phosphatase complex. Finally, alkaline phosphatase activity was detected with naphthol AS-MX phosphate, Fast Red TR, and levamisole to inhibit nonspecific alkaline phosphatase activity. The specimens were counterstained with hematoxylin. Three hundred cells were examined.
Histamine assay.
Cultured cells were lysed with 0.5% Nonidet P-40 (Nakalai Tesque Inc), and the content of histamine in the cell lysate was measured by a radioimmunoassay (RIA; Immunotech S.A.). All assays were conducted in triplicate.
Reverse transcription-polymerase chain reaction (RT-PCR).
RT-PCR was performed according to a modification of the procedure described previously.28 CD34+ BM cells, 15-week-old c-kit+ cultured cells grown by SCF+TPO, and day 10–cultured CD41+ cells grown by TPO were sorted by the FACStarplus flow cytometer, as described previously.27 Total RNA was individually isolated from the cells, using Isogen (Wako Pure Chemical Industries, Osaka, Japan). Next, 200 ng of RNA was reverse-transcribed in 200 U SuperScript II (Life Technologies, Gaithersburg, MD), 10−7 mol/L oligo dT primer (Takara Shuzo Co, Ohtsu, Japan), and 10 U RNase inhibitor (Boehringer Mannheim, Mannheim, Germany) in 50 mmol/L Tris-HCl (pH 8.3), 75 mmol/L KCl, and 3 mmol/L MgCl2. The prepared solution was incubated at 37°C for 1 hour. PCR amplification was performed using the cDNA corresponding to 10 ng of total RNA, Taq polymerase (Takara Shuzo Co), and c-kit– or c-mpl–specific primers, according to the manufacturer’s instructions. The primers for amplification were 5′-AAGGACTTGAGGTTTATTCCT-3′ (nt 494-514) and 5′-CTGACGTTCATAATTGAAGTC-3′ (nt 837-817) for c-kit; 5′-CTTGGTGACCGCTCTGCATCT-3′ (nt 1479-1499) and 5′-GAGGATTTCAAGGAGGCTGGG-3′ (nt 1713-1693) for P-form of c-mpl. For c-kit expression, 30 cycles consisting of denaturation at 95°C for 1 minute, annealing at 55°C for 1 minute, and polymerization at 72°C for 1 minute were performed in a GeneAmp PCR System 9600 (Perkin-Elmer Cetus, Norwalk, CT). For c-mpl expression, 35 cycles consisting of denaturation at 95°C for 20 seconds, annealing at 61°C for 20 seconds, and polymerization at 72°C for 1 minute were performed. As a control, we used β-actin cDNA, 5′-CTGGACTTCGAGCAAGAGAT-3′ (nt 702-721), and 5′-TCGTCATACGCCTGCTTGCT-3′ (nt 1132-1113).
Statistical analysis.
All experiments were carried out at least three times and were shown to be reproducible. Values are expressed as mean ± standard deviation (SD). One-way analysis of variance, followed by post hoc contrasts with Bonferroni limitation, was employed for four independent groups.
RESULTS
Combination of SCF and TPO stimulates the growth of mast cells by CD34+ BM cells in long-term serum-deprived liquid cultures.
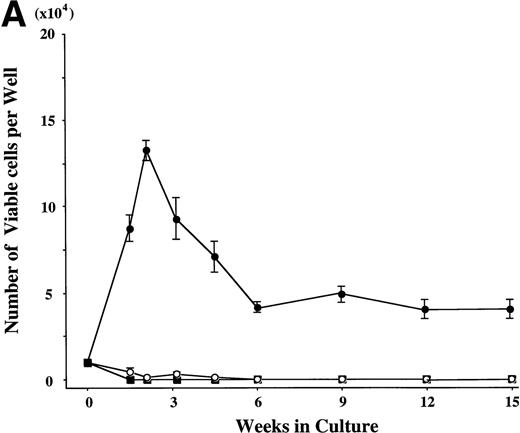
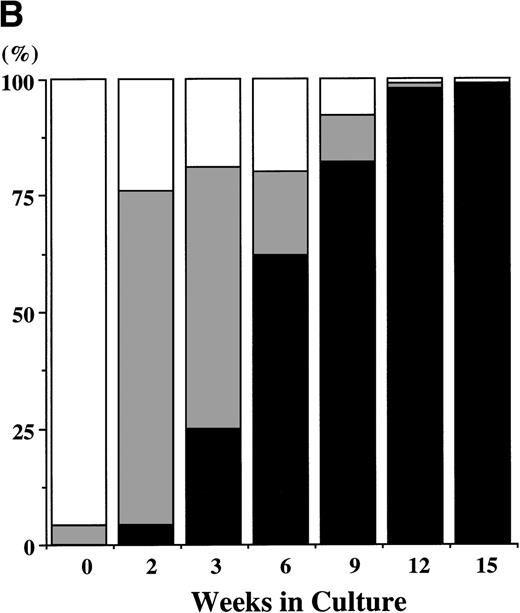
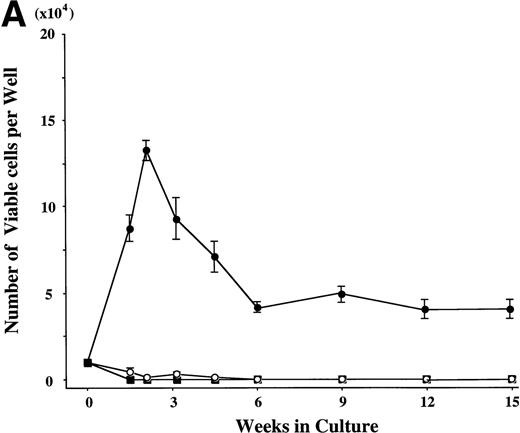
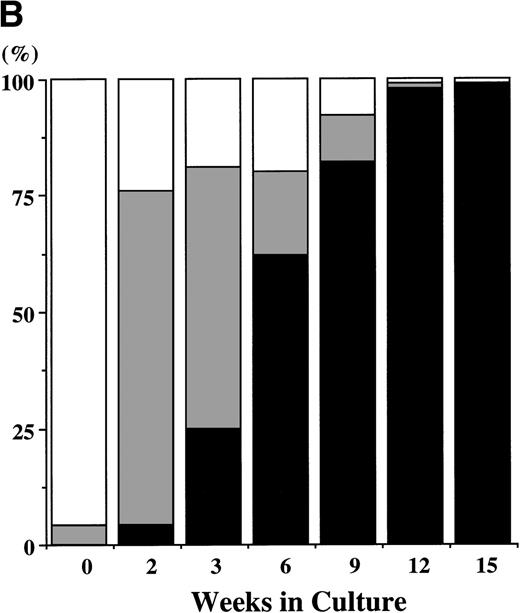
CD34+ BM cells were cultured at 10,000 cells per well containing serum-deprived liquid culture medium supplemented with 10 ng/mL of TPO and/or 10 ng/mL of SCF. The results are shown in Fig1A. In the absence of the growth factors, almost all of the cells degenerated within 2 weeks. The addition of SCF alone supported the growth of approximately 1,000 to 2,000 viable cells at 2 to 3 weeks. Although portions of the cells were megakaryocytes, neutrophils, and mast cells, the remaining cells were blastic and degenerated without terminal differentiation within 3 weeks. Even 100 ng/mL of SCF failed to increase the cell production (data not shown). Under stimulation with TPO, a small number of megakaryocytes (approximately 200 cells) were generated between day 7 and day 10, but there were no viable cells beyond 2 weeks. In contrast, a combination of SCF and TPO caused marked cell production. The total viable cell number increased to approximately 13 times the input quantity after 2 weeks. It decreased after 3 weeks, but remained unchanged from 6 to 15 weeks. Under this culture condition, a low percentage of megakaryocytes was seen until 2 weeks. After 3 weeks of culture, a large part of the cultured cells became positive for either POX or tryptase (a mast cell–specific protease), as shown in Fig 1B. The POX+cells were of the neutrophilic lineage, in the light of the reaction with anti-MPO MoAb. The frequency of tryptase+ cells was 25% at 3 weeks of culture and reached levels higher than 95% after 12 weeks. Consequently, the absolute numbers of cultured mast cells grown by SCF + TPO were relatively constant between 3 weeks and 15 weeks. Some tryptase+ cells showed nuclear lobulation. The frequency of the chymase+ cells was 5% ± 2% (mean ± SD) at 3 weeks and 31% ± 3% at 15 weeks of culture in three independent experiments. In addition, the 9-week-old cultured cells contained a significant amount of histamine (3 to 8 ng per 5 × 104 cells). The percentage of GPA-, CD2-, or CD19-positive cells was less than 1% at 2, 3, 6, and 12 weeks of the culture.
Combination of SCF and TPO stimulates mast cell production by CD34+ BM cells in serum-deprived liquid culture. (A) CD34+ BM cells (1 × 104) were plated per well containing serum-deprived liquid culture medium supplemented with 10 ng/mL of SCF and/or 10 ng/mL of TPO. Half of the cell-free supernatant was replaced with fresh medium containing the growth factor(s) every 5 to 7 days. The numbers of viable cells were serially counted. This experiment was performed three times. Values are expressed as the mean ± SD of three experiments. (□), no growth factors; (○), SCF; (▪), TPO; (•), SCF + TPO. (B) Time course of the relative frequency of tryptase-positive cells and peroxidase-positive cells grown by SCF + TPO. The cultured cells grown by SCF + TPO were stained with a MoAb against tryptase or with POX. (▪), tryptase-positive cells; (▩), POX-positive cells; (□), others.
Combination of SCF and TPO stimulates mast cell production by CD34+ BM cells in serum-deprived liquid culture. (A) CD34+ BM cells (1 × 104) were plated per well containing serum-deprived liquid culture medium supplemented with 10 ng/mL of SCF and/or 10 ng/mL of TPO. Half of the cell-free supernatant was replaced with fresh medium containing the growth factor(s) every 5 to 7 days. The numbers of viable cells were serially counted. This experiment was performed three times. Values are expressed as the mean ± SD of three experiments. (□), no growth factors; (○), SCF; (▪), TPO; (•), SCF + TPO. (B) Time course of the relative frequency of tryptase-positive cells and peroxidase-positive cells grown by SCF + TPO. The cultured cells grown by SCF + TPO were stained with a MoAb against tryptase or with POX. (▪), tryptase-positive cells; (▩), POX-positive cells; (□), others.
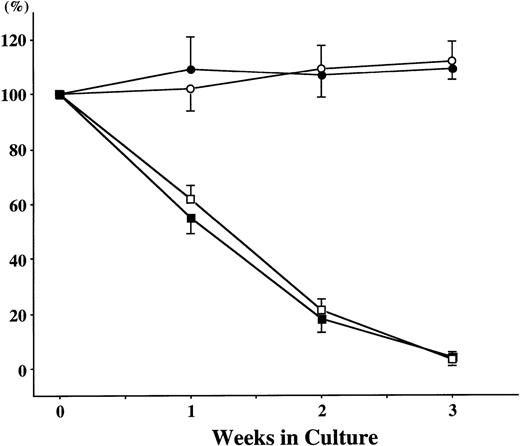
We examined whether TPO exerted the effects on the later phase of mast cell growth. Fifteen-week-old cultured cells (more than 95% of the cells were positive for tryptase) generated by SCF + TPO from CD34+ BM cells were harvested and replated with SCF and/or TPO. The results are shown in Fig 2. Whereas the total viable cell number was maintained in the culture containing SCF alone for 3 weeks, no effects were observed under stimulation with TPO alone. Moreover, the addition of TPO to the culture containing SCF did not influence the total viable cell number.
Effects of SCF and/or TPO on the later phase of mast cell growth. Twenty thousand 15-week-old cultured cells, a large number of which reacted with antitryptase MoAb, were incubated in culture wells containing 10 ng/mL of SCF and/or 10 ng/mL of TPO for 3 weeks. The numbers of viable cells were counted weekly. The values are expressed as a percentage of the cell number at the beginning of the culture. The results shown are from one representative experiment of three. Similar results were obtained in the other two experiments. (□), no growth factors, (○), SCF; (▪), TPO, (•), SCF + TPO.
Effects of SCF and/or TPO on the later phase of mast cell growth. Twenty thousand 15-week-old cultured cells, a large number of which reacted with antitryptase MoAb, were incubated in culture wells containing 10 ng/mL of SCF and/or 10 ng/mL of TPO for 3 weeks. The numbers of viable cells were counted weekly. The values are expressed as a percentage of the cell number at the beginning of the culture. The results shown are from one representative experiment of three. Similar results were obtained in the other two experiments. (□), no growth factors, (○), SCF; (▪), TPO, (•), SCF + TPO.
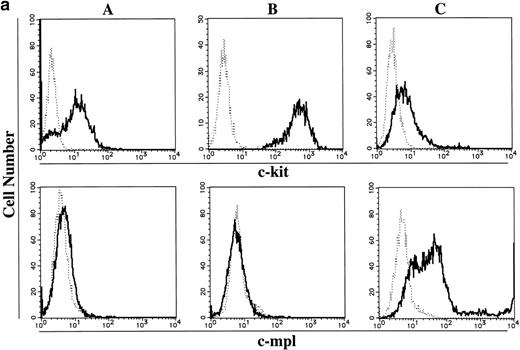
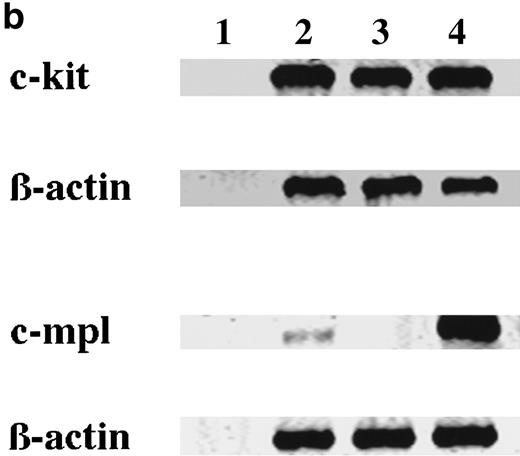
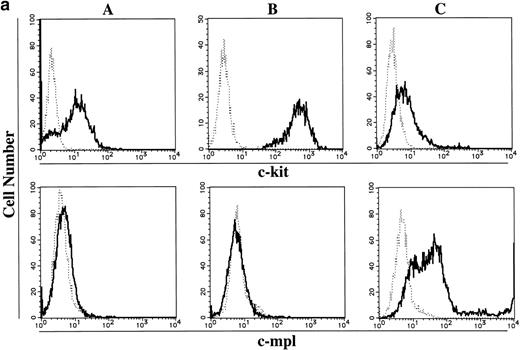
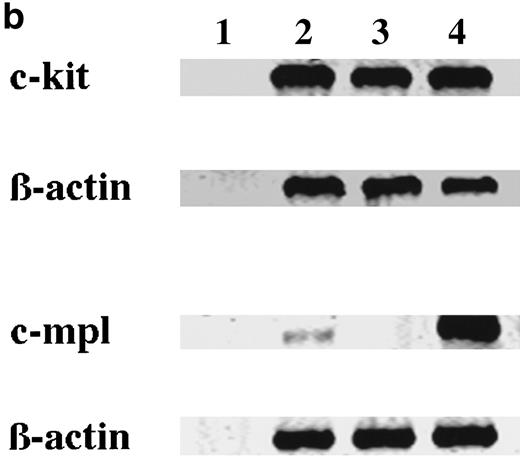
To elucidate whether the ability of 15-week-old cultured cells to maintain the absolute number under stimulation with SCF, but not TPO, was based on the receptor level, the expressions of c-kit and c-mpl in CD34+ BM cells and cultured mast cells grown in SCF + TPO were examined using flow cytometric analysis and RT-PCR analysis. Day 10 megakaryocytic cells generated by TPO alone from CD34+CB cells28 were used as positive controls. The results are presented in Fig 3. In the flow cytometric analysis, CD34+ BM cells and 15-week-old cultured cells grown in SCF + TPO were positive for c-kit antigen. Cultured CD41+ cells grown in TPO reacted with both the anti–c-kit MoAb and the anti–c-mpl MoAb. On the other hand, the c-mpl expression was not detectable on the surface of CD34+ BM cells and cultured cells grown in SCF + TPO. Next, we performed RT-PCR analysis. Thirty- and 35-cycle RT-PCR were used for expression of c-kit and c-mpl, respectively. The molecular sizes of RT-PCR products obtained with the primers were compatible with the expected molecular size: 344 bp for c-kit and 232 bp for c-mpl. The expression of c-kit and c-mpl was observed in CD34+ BM cells. On the other hand, cultured c-kit+ cells grown by SCF + TPO expressed c-kit mRNA, but not c-mpl mRNA.
Expression of c-kit and c-mpl in CD34+ BM cells and cultured mast cells grown by SCF + TPO. The expression of c-kit and c-mpl in CD34+ BM cells and 15-week-old cultured cells grown by SCF + TPO were examined using (a) flow cytometric analysis and (b) RT-PCR analysis. Day 10 megakaryocytic cells generated by TPO alone from CD34+ cord blood cells were used as positive controls. (a) Flow cytometric analysis: (A), CD34+ BM cells; (B), 15-week-old cultured cells grown by SCF + TPO; (C), Day 10 cultured CD41+ cells grown by TPO. (—), labeled with PE-conjugated anti–c-kit MoAb or anti–c-mpl MoAb followed by FITC-conjugated GAM. (---), labeled with PE-conjugated mouse IgG1 or control mouse IgG1 followed by FITC-conjugated GAM. The abscissa shows the fluorescence intensity of each surface marker, and the ordinate shows the number of cells. (b) RT-PCR analysis: lane 1, no RNA; lane 2, CD34+ BM cells; lane 3, cultured c-kit+ cells grown by SCF + TPO; lane 4, cultured CD41+ cells grown by TPO.
Expression of c-kit and c-mpl in CD34+ BM cells and cultured mast cells grown by SCF + TPO. The expression of c-kit and c-mpl in CD34+ BM cells and 15-week-old cultured cells grown by SCF + TPO were examined using (a) flow cytometric analysis and (b) RT-PCR analysis. Day 10 megakaryocytic cells generated by TPO alone from CD34+ cord blood cells were used as positive controls. (a) Flow cytometric analysis: (A), CD34+ BM cells; (B), 15-week-old cultured cells grown by SCF + TPO; (C), Day 10 cultured CD41+ cells grown by TPO. (—), labeled with PE-conjugated anti–c-kit MoAb or anti–c-mpl MoAb followed by FITC-conjugated GAM. (---), labeled with PE-conjugated mouse IgG1 or control mouse IgG1 followed by FITC-conjugated GAM. The abscissa shows the fluorescence intensity of each surface marker, and the ordinate shows the number of cells. (b) RT-PCR analysis: lane 1, no RNA; lane 2, CD34+ BM cells; lane 3, cultured c-kit+ cells grown by SCF + TPO; lane 4, cultured CD41+ cells grown by TPO.
Comparison of the ability of various two-factor combinations to generate mast cells.
Because Nakahata et al13 described the generation of a sufficient number of mast cells by CB MNCs in the culture with SCF+IL-6 or SCF+IL-11, but not SCF + G-CSF, we compared effects of these two-factor combinations with those of SCF + TPO on the growth of mast cells from CD34+ BM cells. SCF, IL-6, IL-11, and G-CSF were used at 10 ng/mL, 50 ng/mL, 50 ng/mL, and 10 ng/mL, respectively. After 3 weeks, numbers of c-kit+CD15− cells grown by SCF + IL-6 and SCF + IL-11 were one twentieth and one ninth of the value obtained by SCF + TPO, respectively. SCF+G-CSF could yield 12-fold more cells than SCF + TPO did. However, only 0.9% of the cells were c-kit+CD15− cells. Thus, the combination of SCF and TPO was the most favorable stimulator for mast cell growth by CD34+ BM cells among the two-factor combinations tested.
Effects of FL or GM-CSF on the SCF + TPO–dependent mast cell growth.
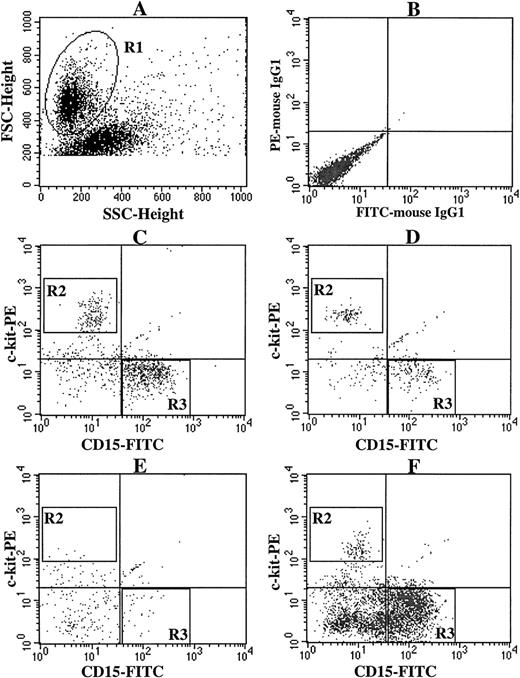
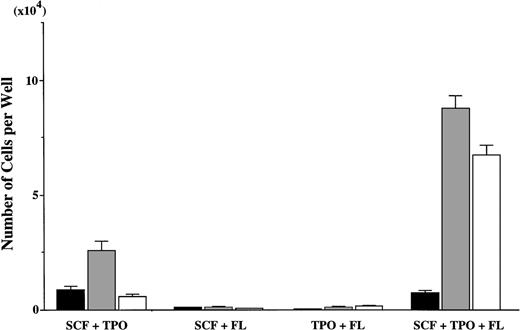
Next, we examined the effects of FL on the SCF + TPO–dependent mast cell production by CD34+ BM cells. Five thousand CD34+ cells were plated in a well containing 10 ng/mL of SCF, 10 ng/mL of TPO, or 50 ng/mL of FL, alone or in combination. The cultured mast cells and myeloid cells were distinguished on the basis of c-kit and CD15 expressions, ie, the c-kit+CD15− cells were identified as mast cells, and c-kit−CD15+ cells were identified as myeloid cells, as shown in Fig 4. Although FL alone had no effects on the cell growth (data not shown), the addition of FL to the culture containing SCF + TPO resulted in a significant increase in the generation of c-kit−CD15+ cells, as compared with the value obtained by SCF + TPO (P < 0.0001; Fig5). However, there was no difference in the SCF + TPO–dependent production of c-kit+CD15− cells in the presence or absence of FL. Neither SCF + FL nor TPO + FL showed any ability to support the growth of sufficient numbers of mast cells and myeloid cells.
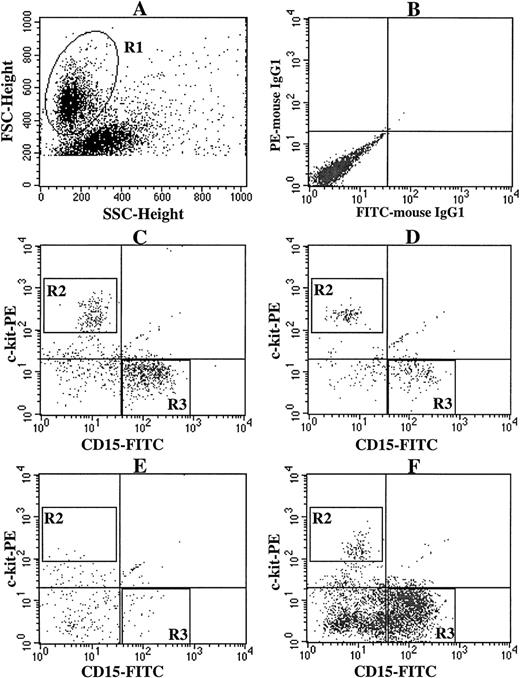
Expression of c-kit and CD15 on cultured cells grown by various combinations of SCF, TPO, and FL. CD34+ BM cells (5 × 103) were plated per well containing various combinations of 10 ng/mL of SCF, 10 ng/mL of TPO, and 50 ng/mL of FL. After the numbers of viable cells were counted at 3 weeks of culture, the cells were stained with PE-conjugated anti–c-kit MoAb and FITC-conjugated anti-CD15 MoAb. (A) The viable cell region (R1) was gated on the basis of FSC and SSC. (B) As negative controls, PE- and FITC-conjugated mouse IgG1 were used. The c-kit+CD15− cells (R2) were identified as mast cells, and the c-kit−CD15+ cells (R3) as myeloid cells. Stimulation with (C) SCF + TPO, (D) SCF + FL, (E) TPO + FL, and (F) SCF + TPO + FL.
Expression of c-kit and CD15 on cultured cells grown by various combinations of SCF, TPO, and FL. CD34+ BM cells (5 × 103) were plated per well containing various combinations of 10 ng/mL of SCF, 10 ng/mL of TPO, and 50 ng/mL of FL. After the numbers of viable cells were counted at 3 weeks of culture, the cells were stained with PE-conjugated anti–c-kit MoAb and FITC-conjugated anti-CD15 MoAb. (A) The viable cell region (R1) was gated on the basis of FSC and SSC. (B) As negative controls, PE- and FITC-conjugated mouse IgG1 were used. The c-kit+CD15− cells (R2) were identified as mast cells, and the c-kit−CD15+ cells (R3) as myeloid cells. Stimulation with (C) SCF + TPO, (D) SCF + FL, (E) TPO + FL, and (F) SCF + TPO + FL.
Effects of FL on SCF + TPO–dependent mast cell growth. The absolute numbers of c-kit+CD15− cells and c-kit−CD15+ cells were estimated from the results shown in Fig 4. (▪), c-kit+CD15− cells; (▩), c-kit−CD15+ cells; (□), others.
Effects of FL on SCF + TPO–dependent mast cell growth. The absolute numbers of c-kit+CD15− cells and c-kit−CD15+ cells were estimated from the results shown in Fig 4. (▪), c-kit+CD15− cells; (▩), c-kit−CD15+ cells; (□), others.
GM-CSF was reported to suppress the SCF- or SCF + IL-6–dependent generation of mast cells by human fetal liver or CB cells.31 32 Therefore, we examined the effects of GM-CSF on the SCF + TPO–dependent mast cell growth. The addition of 10 ng/mL of GM-CSF to the culture containing SCF + TPO increased the total viable cell number by fourfold to sixfold after 3 weeks of culture, compared with the value obtained by SCF + TPO. However, a large portion of the constituent cells was neutrophils, macrophages, and eosinophils. Less than 2% of the cultured cells were mast cells according to the immunocytochemical staining. Thus, the absolute number of mast cells was substantially decreased by the addition of GM-CSF.
Production of mast cells by CD34+CD38+c-kit+ cells and CD34+CD38−c-kit+ cells under stimulation by SCF plus TPO.
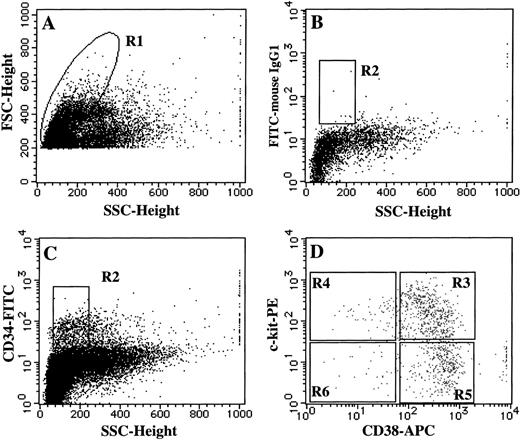
To determine which subpopulations of CD34+ BM cells were responsible for SCF + TPO–dependent mast cell production, we divided CD34+ cells into four subsets on the basis of their CD38 and c-kit expression: CD38+c-kit+, CD38+c-kit−, CD38−c-kit+, and CD38−c-kit− cells, as presented in Fig6. Single-cell sorting was performed in all of the subsets by a FACStarplus flow cytometer, and the sorted individual cells were incubated with SCF, TPO, or FL, alone or in combination, for 3 weeks. The results are presented in Table1. When each of the factors was added alone, only SCF supported the growth of a minimum level of neutrophil/mast cell colonies by the CD34+CD38+c-kit+ cells. Under stimulation with SCF + TPO, one sixth of the CD34+CD38+c-kit+ cells proliferated, and half of them yielded neutrophils and mast cells. The CD34+CD38−c-kit+ cells also responded to the two-factor combination to generate progeny, 40% of which were of the neutrophil/mast cell lineages. Pure mast cell colonies were not seen. In these two subsets, the addition of FL to the culture containing SCF + TPO increased the numbers of neutrophil colonies, but failed to influence the growth of neutrophil/mast cell colonies. SCF+FL and TPO+FL each stimulated the formation of various types of colonies by the CD34+CD38+c-kit+ and CD34+CD38−c-kit+ cells, but had no or a weak ability to support the growth of neutrophil/mast cell colonies. The proliferative response of the CD34+CD38+c-kit− and CD34+CD38−c-kit− cells to all of the types of growth factor combinations was at a low or negligible level.
Single-cell sorting of four subpopulations of CD34+ BM cells. BM MNCs were stained with an FITC-conjugated anti-CD34 MoAb, APC-conjugated anti-CD38 MoAb, and PE-conjugated anti–c-kit MoAb. As negative controls, FITC-, APC-, and PE-conjugated mouse IgG1 were used. (A) The lymphoblastic region (R1) was gated on the basis of FSC and SSC. (B, C). The gate (R2) was set on CD34+ cells. (D) The expression of CD38 and c-kit on CD34+ cells were examined. The single cells in the R3, R4, R5, or R6 region were sorted as CD34+CD38+c-kit+, CD34+CD38−c-kit+, CD34+CD38+c-kit−, or CD34+CD38−c-kit− cells, respectively, using an automatic cell deposition unit equipped with the FACStarplus flow cytometer, as described in Materials and Methods.
Single-cell sorting of four subpopulations of CD34+ BM cells. BM MNCs were stained with an FITC-conjugated anti-CD34 MoAb, APC-conjugated anti-CD38 MoAb, and PE-conjugated anti–c-kit MoAb. As negative controls, FITC-, APC-, and PE-conjugated mouse IgG1 were used. (A) The lymphoblastic region (R1) was gated on the basis of FSC and SSC. (B, C). The gate (R2) was set on CD34+ cells. (D) The expression of CD38 and c-kit on CD34+ cells were examined. The single cells in the R3, R4, R5, or R6 region were sorted as CD34+CD38+c-kit+, CD34+CD38−c-kit+, CD34+CD38+c-kit−, or CD34+CD38−c-kit− cells, respectively, using an automatic cell deposition unit equipped with the FACStarplus flow cytometer, as described in Materials and Methods.
Differentiative potentials of mast cell–producing colony-forming cells supported by SCF plus TPO.
To assess the differentiative potentials of mast cell–producing colony-forming cells supported by various types of growth factor combinations, we performed a two-step culture assay. Each of the sorted cells was initially incubated with SCF + TPO, SCF + FL, TPO + FL, or SCF + TPO + FL. When the cell number in each well reached more than 10 cells between day 10 and day 32, the cells were divided into two aliquots. One half of the sample was replated in a well containing SCF + TPO for the expression of neutrophil and mast cell lineages. The other half was recultured in a well containing G-CSF, SCF, TPO, FL, GM-CSF, IL-3, and EPO (GFs) for the expression of neutrophil, macrophage, megakaryocyte, and erythroid lineages. We defined the sum total of cell lineages expressed under stimulation with SCF + TPO or GFs as the full potentials of hematopoietic progenitors. The results are presented in Table 2. In the CD34+CD38+c-kit+ subset, all of the SCF + TPO–responsive progenitors yielded progenies including mast cells. Half of them had a differentiative capacity restricted to neutrophil/mast cell lineages, and the remaining half could differentiate into neutrophil/macrophage/mast cell lineages. In the CD34+CD38−c-kit+ subset, approximately 80% of the SCF + TPO–responsive progenitors had the ability to differentiate into the neutrophil/macrophage/mast cell pathways, and one yielded erythroid cells as well. With regard to mast cell–containing colony-forming cells supported by SCF + TPO+FL, neutrophil/macrophage/mast cell progenitors and neutrophil/macrophage/mast cell/erythroid progenitors were the major types of progenitors in the CD34+CD38+c-kit+ subpopulation and CD34+CD38−c-kit+ subpopulation, respectively. Megakaryocytes were not detected in any of the wells.
DISCUSSION
Activation via c-kit is essential for murine mast cell development, because SCF- or c-kit–deficient mice lack mast cells. As in mice, human SCF by itself can induce human mast cell development from hematopoietic progenitors in serum-containing culture condition.9,11,12,19 However, in our serum-deprived culture of CD34+ BM cells, SCF seldom generated mast cells, and TPO was required for the substantial growth. The identification of mast cells was performed by measurements of intracellular histamine, immunocytochemical staining, and flow cytometric analysis. One possible explanation for the poor mast cell growth obtained from CD34+ BM cells stimulated by SCF alone may be an inferior proliferative potential of SCF-responsive mast cell–containing colony-forming cells under our culture condition. Fetal bovine serum has been thought to be a potential endogenous source of hematopoietic factors.33,34 Coupled with the evidence that both adult peripheral blood and CB contain significant levels of TPO,35 36 BM mast cell progenitors may be dependent on the synergistic factor(s) present in the serum. Nevertheless, the SCF–c-kit signaling system seems to be important for the generation of mast cells from human BM progenitors, based on the finding that the progenitors responding to SCF + TPO were exclusively restricted to those expressing c-kit receptors.
FL has been shown to play important roles in the proliferation of primitive hematopoietic progenitors. The present results showed that the addition of FL to the culture containing SCF + TPO augmented the colony growth by CD34+CD38−c-kit+cells. However, the comparative analysis of the two-factor combinations showed that the mast cell growth stimulated with SCF+FL was at much lower levels, compared with that stimulated with SCF + TPO. These results are consistent with the finding reported by Hjertson et al37 that FL failed to increase the SCF-dependent mast cell growth by human CB MNCs. Taken together, these observations suggest that the FL does not always stimulate the growth of mast cells with the aid of SCF and that TPO is a crucial factor for SCF-dependent mast cell growth.
The combination of SCF with TPO was a requisite for the significant growth of mast cell–containing colonies from CD34+ BM cells. As shown in Fig 3, both c-kit and c-mpl were expressed in CD34+ BM cells, consistent with the previous reports.27,29,38 39 However, the cultured mast cells grown by SCF + TPO were positive for c-kit, but not c-mpl. Additionally, 15-week-old cultured cells grown by SCF + TPO, a large portion of which reacted with antitryptase MoAb, maintained the absolute number under stimulation with SCF for 3 weeks, but deteriorated in the presence of TPO. Moreover, the addition of TPO to the culture containing SCF did not influence the total viable cell number. Thus, TPO may stimulate an early stage of the mast cell development in concert with SCF, and subsequent growth seems to be supported by SCF alone.
Kirshenbaum et al10 showed that human mast cells are derived from CD34+ BM cells in vitro. Födinger et al40 showed that the donor-derived mast cells were detectable 198 days after allogeneic bone marrow transplantation. These observations suggest that human mast cells are generated by hematopoietic progenitor cells. Our two-step single-cell culture study clearly showed that mast cells were originated from multilineage colony-forming cells that had potential to differentiate into neutrophil/mast cell lineages, neutrophil/macrophage/mast cell lineages, or neutrophil/macrophage/mast cell/erythroid lineages. A large part of these multipotential progenitors belonged to the CD34+CD38−c-kit+ cells and CD34+CD38+c-kit+ cells. To our knowledge, the results presented here are the first evidence of the clonal origin of mast cells from primitive BM progenitors in human hematopoiesis. Additionally, the present data support the existence of common precursors for granulocytes and mast cells, as described by Kitamura et al.2 However, a developmental pathway common to mast cell and lymphoid lineages remains to be determined.
The differentiation of human mast cells is inducible under long-term culture conditions with bone marrow stroma cells.41 Human stroma cells can produce cytokines including SCF, TPO, and GM-CSF.42-44 In the present study, we observed that GM-CSF enhanced the proliferation and differentiation of myeloid cells, but inhibited the human mast cell growth from BM hematopoietic progenitors. These results are in agreement with those obtained from cultures with CB cells.31 32 Coupled with the fact that GM-CSF can be further produced by monocytes and macrophages activated with GM-CSF, the generation of monocytes and macrophages may regulate the production of mast cells in the bone marrow environment. This may be related to the low number of mast cells among the BM cells.
In conclusion, we showed that by means of stimulation at the level of hematopoietic progenitors including primitive progenitors, TPO may play important roles in the SCF-dependent development of human mast cells from CD34+ BM cells. Our cultured mast cells may provide a useful model for investigations of the physiological and pathological characteristics of human mast cells.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Kenichi Koike, MD, Department of Pediatrics, Shinshu University School of Medicine, 3-1-1, Asahi, Matsumoto, 390-8621, Japan.