Abstract
Hematopoietic stem cells (HSC) are cells with self-renewing multilineage differentiation potential. Although engraftment in xenogeneic recipients can be used to measure human HSC, these assays do not allow assessment of individual progenitors. We developed an in vitro assay that allows the identification of a single human bone marrow progenitor closely related to HSC, which we termed “Myeloid-Lymphoid Initiating Cell,” or ML-IC, because it is capable of generating multiple secondary progenitors that can reinitiate long-term myeloid and lymphoid hematopoiesis in vitro. The assay is done in contact with murine AFT024 fetal liver stromal cells and with Flt3-Ligand, stem cell factor, and interleukin-7. In this assay, 0.2% to 1.7% of Lin−/34+/DRdim cells could generate 1 to 3 long-term culture initiating cells (LTC-IC) as well as 1 to 4 NK-IC after 4 to 6 weeks. In addition, this assay measures contribution of net-progenitor conservation and net-progenitor proliferation over time, providing insight in the fate of individual LTC-IC and NK-IC. This assay will prove useful to enumerate the number of very primitive human progenitors with multilineage differentiation potential, as well as to evaluate future ex vivo culture conditions.
HEMATOPOIETIC STEM cells (HSC) are defined as cells capable of self renewal as well as multilineage differentiation. In mice, the phenotype and function of HSC have been characterized using competitive in vivo repopulation assays.1-4 Because such repopulation assays cannot be performed in humans, surrogate in vivo or in vitro assays are used to evaluate human HSC. Transplantation of human progenitors in xenogeneic transplant recipients, such as severe combined immunodeficient (SCID) mice,5-7 beige-nude-SCID (BNX)-mice,8,9 nonobese diabetic (NOD)-SCID-mice,10-13 or fetal sheep,14 15allows detection of engrafting human cells. In vivo production of myeloid, natural killer (NK), T-lymphoid, and B-lymphoid blood elements is seen for several months to years after transplantation. Through transplantation of limiting numbers of CD34+subpopulations, these xenotransplant models provide a quantitative assay for engrafting cells.
A number of in vitro assays have been described that assess primitive human progenitors. These include long-term culture initiating cell (LTC-IC) assays,16 cobblestone area forming cell (CAFC) assays,17,18 and extended (E)-LTC-IC assays.19These assays enumerate primitive progenitors that can eventually generate myeloid cells, but not cells with multilineage differentiation or self-renewal potential. Several groups have developed cultures that allow differentiation of single human CD34+Lin− cells into cells with myeloid, NK, B-lymphoid, dendritic, and/or T-lymphoid phenotype showing that a single cell can differentiate in vitro into multiple lineages.20-22 Because of its multilineage differentiation capacities, this cell is thought to be more immature than the LTC-IC.
In the work described here, we wanted to develop an assay that would allow us to enumerate even more primitive cells that, besides having multilineage differentiation potential, also can generate secondary primitive progenitors that again have multilineage differentiation potential. A number of observations prompted us to use a stroma-based culture system to accomplish this. Long-term ex vivo maintenance of murine HSC also requires stroma.23 It has also been shown that noncommitted human progenitors require stromal cell interactions that cannot be replaced by soluble factors to commit to the B-lymphoid lineage.24-27 Our group has recently shown that human CD34+/HLA-DR−/Lin− bone marrow progenitors require stromal contact to differentiate into functional NK cells.28
We used the murine fetal liver cell line, AFT024, known to support murine repopulating HSC for up to 7 weeks ex vivo,23 as well as human primitive myeloid and lymphoid progenitors.29 30 We show that a culture system based on this AFT024 feeder, supplemented with the early acting cytokines Flt3-Ligand (Flt3-L), stem cell factor (SCF), and interleukin-7 (IL-7), can enumerate very primitive human progenitors capable of generating multiple secondary progenitors that have the ability of reinitiating long-term multilineage hematopoiesis. We termed these cells Myeloid-Lymphoid Initiating Cells or ML-IC. This single cell assay also provides insight in the mechanisms underlying ex vivo expansion of primitive myeloid and lymphoid progenitors.
MATERIALS AND METHODS
Cell Source and Preparation
Bone marrow was aspirated from the posterior iliac crest from healthy volunteer donors after obtaining informed consent using guidelines approved by the Committee on the Use of Human Subjects at the University of Minnesota (Minneapolis, MN). Mononuclear cells (MNC) were obtained by Ficoll-Hypaque (Sigma-Diagnostics, St Louis, MO) centrifugation. CD34+ cells were selected with a biotinylated monoclonal anti-CD34 antibody (clone, 12.8 CePrate system; CellPro Inc, Bothell, WA) on an immunoaffinity column (CellPro).
Fluorescence-Activated Cell Sorting (FACS)
CD34+ enriched cells were incubated with anti-CD34-Biotin (CellPro), Streptavidin-SA670 (GIBCO-BRL, Grand Island, NY), anti-HLA-DR-phycoerythrin (PE) (Becton Dickinson, Mountain View, CA), and a lineage cocktail of fluorescein isothiocyanate (FITC)–conjugated antibodies against CD2, CD3, CD4, CD5, CD7, CD8, CD10, CD14, CD15, CD16, CD19 (Becton-Dickinson). Individual Lin−/34+/DRdim cells were sorted into 96-well plates (Costar, Cambridge, MA) containing irradiated AFT024 feeders using the automatic cell deposition unit (ACDU) on a FACS Star-Plus flow cytometry system equipped with a Consort 32 computer (Becton Dickinson). To ensure that only a single cell was deposited, the ACDU was set up in a low event “through-put” (200 cells/s). An oscilloscope was used to adjust phasing of the single droplet sort pulse to the drop drive frequency. Accuracy of the single-cell deposition was determined by sorting single cells in 96-well plates without stroma and scoring wells visually for the number of cells deposited: 83% of the wells contained a single cell, 17% of the wells did not contain a cell, and no well contained more than one cell.
Stromal Feeder
The murine fetal liver cell line, AFT024, was maintained at 33°C in Dulbecco’s modified Eagle’s medium (DMEM; GIBCO-BRL) supplemented with 20% fetal calf serum (FCS; Hyclone Laboratories, Logan, UT), 50 μmol/L 2-Mercaptoethanol (2-ME; Bio-Rad, Hercules, CA). AFT024 cells were subcultured in 24- or 96-well plates (Costar), precoated with 0.1% gelatin (Specialty Media, Lavalette, NJ), and grown to confluence. Confluent plates were irradiated at 2,000 rad and were maintained at 37°C.
Culture Media
Flt3-L/SCF/IL-7 expansion medium.
RPMI 1640 (GIBCO-BRL), 20% FCS, 25 μmol/L 2-ME, 1,000 U/mL penicillin, 100 U/mL streptomycin (GIBCO-BRL), Flt3-L (10 ng/mL; Amgen, Thousand Oaks, CA), SCF (10 ng/mL; Immunex, Seattle, WA), and IL-7 (20 ng/mL; R&D Systems, Minneapolis, MN).
IL-3/MIP-1α expansion medium.
Iscove’s modified Dulbecco’s medium (IMDM), 12.5% FCS, 12.5% horse serum (Stem Cell Technologies, Vancouver, Canada), 2 mmol/L L-Glutamine (GIBCO-BRL), 1,000 U/mL penicillin, 100 U/mL streptomycin, 10−6 mol/L hydrocortisone, IL-3 (5 ng/mL), and macrophage inflammatory protein (MIP)-1α (100 ng/mL; R&D Systems).
Lymphoid differentiation medium.
DMEM and Ham’s F12-medium (GIBCO-BRL) in a 2:1 (vol/vol) mix containing 20% heat inactivated human AB serum (North American Biologicals, Miami, FL), ascorbic acid (20 mg/mL; GIBCO-BRL), selenium selenite (50 μmol; GIBCO-BRL), 2-ME (25 μmol), and ethanolamine (50 μmol; GIBCO-BRL). Cytokines added on day 0: IL-2 (1,000 U/mL; Amgen), IL-3 (5 ng/mL), IL-7 (20 ng/mL), SCF (20 ng/mL), and Flt3-L (10 ng/mL). At weekly intervals, half media change was done using 10% instead of 20% human AB serum. Cytokines added at week 1 and later: IL-2 only (1,000 U/mL).
Single Cell Cultures
To determine the LTC-IC frequency on day 0, cells were sorted individually in 96-well plates (“day 0 plates”), maintained either in AFT024/Flt3-L/SCF/IL-7 or AFT024/IL-3/MIP-1α conditions for 5 weeks, and then overlaid with clonogenic methylcellulose medium (Methylcellulose [Fisher, Chicago, IL] in a final concentration of 1.12% containing IMDM, supplemented with 30% FCS, 3 IU/mL erythropoietin [Amgen], and supernatant of the bladder carcinoma cell line 5637 [7.5%]) and scored for secondary colony-forming cell (CFC) after an additional 2 weeks. To determine the NK-culture-initiating cell (IC) frequency on day 0, single cell cultures were maintained with lymphoid differentiation medium for 5 to 6 weeks. Wells were scored visually for presence of mature progeny. To demonstrate presence of NK cells, wells were harvested and cells were stained with anti-CD56-PE and anti-CD3-FITC (Becton Dickinson) and analyzed by FACS to determine the presence of CD56+ NK cells. We also tested secondary CD56+NK cells for their capability to kill specifically K562 targets as previously described.31
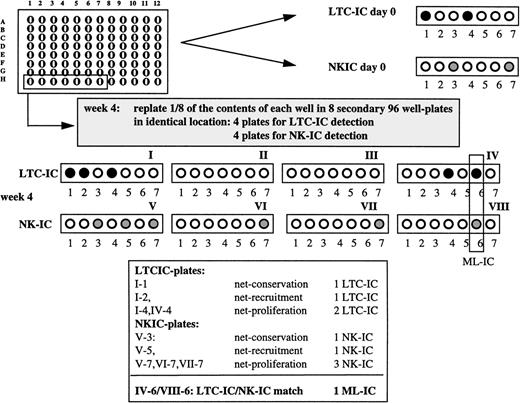
To assess ML-IC, cells were sorted individually in 96-well plates (“expansion cultures”) and maintained in either AFT024/Flt3-L/SCF/II-7 or AFT024/IL-3/MIP-1α expansion conditions for 4 weeks. The content of a single well was then harvested with trypsin and divided equally over 8 secondary 96-well plates containing irradiated AFT024 feeders in such a manner that one eighth of each single cell progeny was deposited in the identical location in the 8 secondary plates. Four of the 8 secondary plates were maintained in either AFT024/Flt3-L/SCF/IL-7 or AFT024/IL-3/MIP-1α expansion conditions for an additional 5 weeks and assessed for LTC-IC as described above. The other 4 secondary plates were maintained for 6 to 7 weeks with lymphoid differentiation medium and assessed for the presence of NK cells by FACS. LTC-IC and NK-IC expansion was determined as described in Table 1.
In other experiments, individually sorted Lin−/34+/DRdim cells were cultured in Flt3-L/SCF/IL-7 expansion medium on AFT024 feeders. After 3 weeks, the primary plates were harvested by trypsinization, and the contents of each well were divided equally into 4 secondary 96-well plates in such a manner that one fourth of the progeny of each single cell was deposited in the identical location in the 4 secondary plates. Secondary cultures were maintained for an additional 3 weeks in Flt3-L/SCF/IL-7 expansion medium. After 6 weeks, secondary plates were harvested by trypsinization, and the contents of each well were divided equally into 4 tertiary 96-well plates in such a manner that one fourth of the content of each secondary well was deposited in the identical location of the 4 tertiary plates. Two of the tertiary plates (8 for each primary plate) were assessed for LTC-IC, and two of the tertiary plates (8 for each primary plate) were assessed for NK-IC as described above.
Definitions
Overall progenitor expansion is equal to recovery of >100% LTC-IC/NK-IC in all week 4 or 6 progeny plates compared with the day 0 plates. Progenitor proliferation is equal to a single cell that gives rise to two or more LTC-IC/NK-IC, ie, can reinitiate hematopoiesis in at least 2 secondary plates. Progenitor conservation is equal to a single cell that persists over time with or without proliferation, ie, can reinitiate hematopoiesis in at least one secondary LTC-IC/NK-IC culture. Progenitor recruitment is equal to a single cell that reads out as an LTC-IC/NK-IC after 4 to 6 weeks, but not on day 0.
Statistics
Results of experimental points from different experiments were reported as the mean ± standard error of the mean (SEM). Significance levels were determined by either paired or nonpaired two-sided Student’st-test analysis as indicated.
RESULTS
Measurement of Multilineage Initiating Cells
To determine if a single cell can give rise to secondary progenitors with both LTC-IC and NK-IC characteristics, we cultured single Lin−/34+/DRdim cells from 4 individual donors (176 to 264 cells per donor) in Flt3-L/SCF/IL-7 expansion medium on AFT024 feeders. After 4 weeks, one eighth of the contents of each individual well was replated in the identical location of 8 secondary AFT024-containing 96-well plates. Four of the 8 secondary plates were maintained for 5 weeks in Flt3-L/SCF/IL-7 expansion medium and then overlaid with clonogenic methylcellulose medium to assess presence of LTC-IC in single-cell progeny. To assess NK-IC, the other 4 plates were maintained under lymphoid differentiation conditions for 6 to 7 weeks (Fig1). Detection of ≥1 LTC-IC and ≥1 NK-IC in the identical location in secondary plates was required to consider the initially plated Lin−/34+/DRdim cell an ML-IC.
Single cell cultures. Lin−/34+/DRdim cells were sorted into 88 wells of 96-well plates with AFT024 stromal feeders using the FACS Star Plus ACDU system. Cells were cultured as indicated with weekly medium exchange. After 4 weeks, the content of each well was collected by trypsinization and divided equally over 8 secondary 96-well plates with pre-established AFT024 feeders in such a manner that one eighth of the content was deposited in the identical location in the 8 secondary plates, 4 for LTC-IC detection and 4 for NK-IC detection. To detect LTC-IC/NK-IC at day 0, single cells were cultured in 96-well plates containing AFT024 feeders for 5 weeks, and plates were overlaid with clonogenic methylcellulose. To detect NK-IC at day 0, single cells were cultured in separate 96-well plates containing AFT024 feeders as described in Materials and Methods. An ML-IC is defined as a single Lin−/34+/DRdim cell with multilineage generative capacity, ie, this cell can generate at least one LTC-IC and one NK-IC.
Single cell cultures. Lin−/34+/DRdim cells were sorted into 88 wells of 96-well plates with AFT024 stromal feeders using the FACS Star Plus ACDU system. Cells were cultured as indicated with weekly medium exchange. After 4 weeks, the content of each well was collected by trypsinization and divided equally over 8 secondary 96-well plates with pre-established AFT024 feeders in such a manner that one eighth of the content was deposited in the identical location in the 8 secondary plates, 4 for LTC-IC detection and 4 for NK-IC detection. To detect LTC-IC/NK-IC at day 0, single cells were cultured in 96-well plates containing AFT024 feeders for 5 weeks, and plates were overlaid with clonogenic methylcellulose. To detect NK-IC at day 0, single cells were cultured in separate 96-well plates containing AFT024 feeders as described in Materials and Methods. An ML-IC is defined as a single Lin−/34+/DRdim cell with multilineage generative capacity, ie, this cell can generate at least one LTC-IC and one NK-IC.
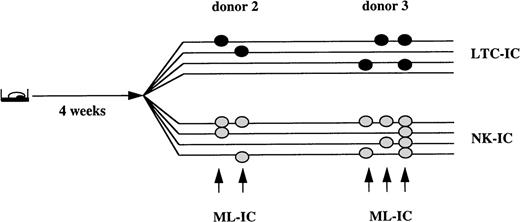
In 2 out of 4 donors, we found that 0.8% and 1.7% of Lin−/34+/DRdim cells could generate both secondary LTC-IC and NK-IC after the initial 4-week expansion culture, or were ML-IC. Each initially sorted ML-IC gave rise to 1 or 2 LTC-IC, as well as 2 to 4 NK-IC. In the other 2 donors, such cells were not detected (Fig 2). In additional experiments, we extended the initial culture in Flt3-L/SCF/IL-7 expansion medium to 6 weeks (data not shown). We detected ML-IC in 3 of 4 donors. The frequency of ML-IC ranged between 0.2% and 0.7%, and each ML-IC generated 1 to 3 LTC-IC and 1 NK-IC.
0.8% to 1.7% of Lin−/34+/DRdim cells are myeloid-lymphoid initiating cells. Progeny from single Lin−/34+/DRdim cells cultured on AFT024 in expansion medium with Flt3-L, SCF, and IL-7 were replated after 4 weeks in 8 secondary plates. Four secondary plates were maintained in expansion medium with Flt3-L, SCF, and IL-7 to enumerate LTC-IC. The remaining 4 secondary plates were maintained in lymphoid differentiation medium to detect NK-IC. Lin−/34+/DRdim cells that generated ≥1 LTC-IC (black circles) and ≥1 NK-IC (gray circles) were identified as ML-IC.
0.8% to 1.7% of Lin−/34+/DRdim cells are myeloid-lymphoid initiating cells. Progeny from single Lin−/34+/DRdim cells cultured on AFT024 in expansion medium with Flt3-L, SCF, and IL-7 were replated after 4 weeks in 8 secondary plates. Four secondary plates were maintained in expansion medium with Flt3-L, SCF, and IL-7 to enumerate LTC-IC. The remaining 4 secondary plates were maintained in lymphoid differentiation medium to detect NK-IC. Lin−/34+/DRdim cells that generated ≥1 LTC-IC (black circles) and ≥1 NK-IC (gray circles) were identified as ML-IC.
Net-Conservation and Net-Proliferation Contribute to LTC-IC and NK-IC Expansion in Flt3-L/SCF/IL-7 Expansion Medium Cultures
We hypothesized that this single cell assay with LTC-IC and NK-IC readout assays would also provide information on the fate of a single LTC-IC or NK-IC progenitor over time; specifically, if an LTC-IC or NK-IC persists over time or if there is net conservation, and if a single cell can give rise to two or more LTC-IC or NK-IC (net-proliferation) during the primary culture step.
Single Lin−/34+/DRdim cells were cultured in Flt3-L/SCF/IL-7 expansion medium for 4 weeks, and progeny were evaluated for LTC-IC and NK-IC, as described above. The absolute number of Lin−/34+/DRdim cells capable of initiating long-term cultures on day 0 was 6.6% ± 0.9%. Of the initially plated Lin−/34+/DRdim cells, 9.3% ± 1.2% were capable of initiating and sustaining hematopoiesis in one or more secondary stromal cultures. Thus, culture for 4 weeks in Flt3-L/SCF/IL-7 expansion medium on AFT024 feeders supported net conservation of LTC-IC. Cultured progeny of 47.5% ± 7% of single Lin−/34+/DRdim LTC-IC were able to initiate hematopoiesis in 2, 3, or 4 secondary long-term cultures and had therefore proliferated. The overall LTC-IC frequency after 4 weeks, or all LTC-IC present in the 4 secondary cultures, was 15.9% ± 1.8%, or 2.5 ± 0.2–fold higher than that measured in day 0 Lin−/34+/DRdim cells (Table 1). In three additional experiments, all 8 secondary plates were evaluated for the presence of secondary LTC-IC. The maximal number of secondary LTC-IC generated from one individual cell was 6.
To examine the fate of single NK-IC, progeny of the primary Flt3-L/SCF/IL-7 expansion medium cultures were replated in contact with AFT024 feeders with lymphoid differentiation medium for 6 to 7 weeks. The NK-IC frequency in freshly sorted Lin−/34+/DRdim cells measured by culturing for 6 to 7 weeks in lymphoid differentiation medium was 0.12% ± 0.02%. In 3 of 4 experiments, progeny of 3.9% ± 1.7% of the initial Lin−/34+/DRdim cells cultured for 4 weeks in expansion medium were capable of generating functional NK cells in one or more secondary lymphoid differentiation cultures indicating net conservation of NK-IC (Table2). 46% ± 26% of single-sorted Lin−/34+/DRdim cells that generated NK-IC after 4 weeks could initiate 2 to 4 secondary NK long-term cultures, indicating proliferation. Thus, a total of 33 NK-IC (7.5% ± 3.7%) was present in all 4 secondary cultures initiated with progeny of 440 Lin−/34+/DRdim cells, which is 37.3 ± 19–fold higher than the day 0 frequency of NK-IC.
Lack of Progenitor Proliferation Is Responsible for Lack of LTC-IC and NK-IC Expansion in IL-3/MIP-1α Expansion Medium Cultures
To confirm that this assay will be helpful in assessing the contribution of net proliferation and net conservation of progenitors to overall progenitor expansion, we cultured single-sorted Lin−/34+/DRdim cells from 5 donors in contact with AFT024 feeders under conditions known to maintain LTC-IC, namely IL-3/MIP-1α expansion medium (unpublished observations). We hypothesized that our assay should show that lack of expansion is caused by either poor conservation or poor proliferation of LTC-IC and NK-IC. After culture of single Lin−/34+/DRdim cells for 4 weeks in IL-3/MIP-1α expansion medium, the contents of each well of the primary cultures were replated in 8 secondary AFT024-containing 96-well plates to assess presence of LTC-IC (4 plates) and NK-IC (4 plates). On day 0, 7.7% ± 1.5% of Lin−/34+/DRdim cells had LTC-IC characteristics. After 4 weeks in IL-3/MIP-1α expansion medium, 6.5% ± 1.2% of the initially plated Lin−/34+/DRdim cells could initiate hematopoiesis in one or more secondary cultures, indicating net conservation of LTC-IC. In contrast to the Flt3-L/SCF/IL-7 expansion medium cultures, only 13.9% ± 8.5% of the single Lin−/34+/DRdim gave rise to more than 1 LTC-IC. The overall LTC-IC frequency of 8.6% ± 2.1% at week 4 was only 1.2-fold higher than that measured in freshly sorted Lin−/34+/DRdim cells (Table 3). Thus, lack of long-term expansion is caused by poor proliferation, and not poor net-conservation. Further, only 4 of 528 Lin−/34+/DRdim cells cultured for 4 weeks in IL-3/MIP-1α expansion medium cultures could generate one or more NK-IC in secondary cultures. Finally, no ML-IC could be detected under these conditions.
DISCUSSION
In this report, we describe a novel in vitro assay that can enumerate very primitive human progenitors capable of generating multiple secondary LTC-IC and NK-IC. We term this primitive progenitor a Myeloid-Lymphoid Initiating Cell, or ML-IC. A similar progenitor, termed LTC-ICML, capable of initiating secondary long-term myeloid cultures as well as pre-B cell cultures, has been characterized in murine bone marrow.32 As for the human ML-IC, which is five to ten times less frequent than human LTC-IC, murine LTC-ICML are 15 times less frequent than murine LTC-IC. A number of studies have described assays that allow human progenitors to differentiate into mature myeloid as well as NK and B- and T-lymphoid cells.20-22,33-36 The assay described here can assess a possibly even more primitive human bone marrow cell capable of generating multiple secondary progenitors that can reinitiate both myeloid and lymphoid long-term cultures. We believe that this characteristic places the ML-IC ontogenetically very close to the HSC. We have, however, not proven that ML-IC can self-replicate. Therefore, we have not yet shown that ML-IC are human HSC. This will require retroviral tagging. The frequency of ML-ICs is still 10-fold higher than that of SCID-repopulating cells (SRCs), as defined by Larochelle et al.10 Thus, ML-IC may still contain a population of cells that is less primitive than SRC. Alternatively, differences in frequency may be related to the relative inefficient seeding efficiency of human cells in vivo, leading to underestimation of the absolute number of engrafting human cells. Studies are currently underway to address the relation between SRC and ML-IC.
What are the disadvantages and advantages of the ML-IC assay over the SRC assay? A disadvantage of the ML-IC assay is the cumbersome nature of the technically demanding long-term culture system. A second disadvantage of the ML-IC assay is that “engraftment” per se—a characteristic ascribed to stem cells—cannot be shown. The first advantage of the ML-IC assay is that it can provide information concerning the multipotentiality of a single-sorted CD34 cell. Similar information can be obtained from experiments in which retrovirally tagged human progenitors are transplanted in immunodeficient mice.
Another advantage of the ML-IC assay is that it provides insight into the fate of single LTC-IC and NK-IC cells in ex vivo cultures. We show, for instance, that differences in LTC-IC or NK-IC expansion in different culture systems can be attributed to variable losses in progenitor conservation or progenitor proliferation. These conclusions are in part indirect because progenitor differentiation can occur in the presence or absence of progenitor proliferation. Indeed, an LTC-IC can remain quiescent for the duration of the expansion culture and retain its LTC-IC function. Alternatively, an LTC-IC can die or remain quiescent, but differentiate. Finally, an LTC-IC can proliferate symmetrically or asymmetrically. Each of these outcomes can be investigated using the ML-IC assay.
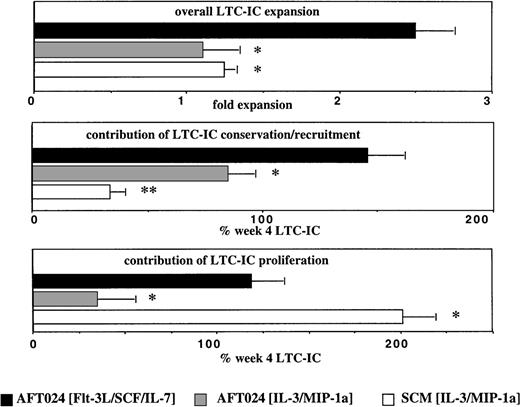
In Fig 3 we included data previously described by our group,37 using stroma-conditioned medium supplemented with IL-3 + MIP-1α (SCM/IL-3/MIP-1α). By comparing the expansion systems, we show that presence of AFT024 feeders in the culture is important for net conservation of LTC-IC (and NK-IC). Net conservation of 80% to 130% of LTC-IC and >100% of NK-IC is observed in AFT024-based cultures, and this irrespective of the expansion medium, whereas <30% of LTC-IC is conserved in SCM + IL-3 + MIP-1α (Fig 3). Thus, factor(s) produced by AFT024 may be responsible for the net conservation of LTC-IC. Moore et al have shown that AFT024 feeders support the in vitro maintenance of competitive repopulating murine stem cells for at least 7 weeks.23 It is thought that expression of one or more novel factors in AFT024 cells is responsible for the improved maintenance of repopulating murine stem cells and possibly for the improved conservation of LTC-IC. One such factor may be the delta-like (dlk) protein/preadipocyte factor-1 (pref-1), which is produced by AFT024 cells, but not by nonsupportive feeders.38 When dlk/pref-1 is introduced in nonsupportive feeders, ex vivo maintenance of murine repopulating stem cells can in part be restored.38 Dlk/pref-1 is related to the family of Notch ligands.39-41 Expression of dlk/pref-1 is downregulated in embryonic tissues during differentiation, and its overexpression prevents terminal differentiation, similar to what is seen with Notch-ligand/Notch-receptor interactions.39-41However, dlk/pref-1 does not contain the delta/serrate/lag-2 (DSL)-domain, thought to be required for the binding of Notch ligands to their receptors.40 Alternatively, AFT024 feeders may produce other novel factors responsible for the improved conservation of primitive progenitors.42 43
Contribution of proliferation and conservation to overall LTC-IC expansion. Single cell assays were performed as described in Methods and Fig 2 legend. In addition, we used data from a study previously published by our group in which LTC-IC were assessed in a single cell assay based on bone marrow (BM) stroma–conditioned media supplemented with IL-3 and MIP-1 in the absence of a stromal feeder.37 Significantly greater LTC-IC expansion is seen in AFT024/Flt3-L/SCF/IL-7 cultures than in AFT024/IL-3/MIP-1 cultures or SCM/AFT024/IL-3/MIP-1 cultures. This is a result of both increased proliferation and conservation when compared with AFT024/IL-3/MIP-1 cultures. The equivalent LTC-IC expansion seen in AFT024/IL-3/MIP-1 and SCM/IL-3/MIP-1 cultures is caused by different mechanisms: extensive self-renewal (80.5% ± 7.0%) of only 33.9% ± 6.0% conserved LTC-IC in SCM/IL-3/MIP-1 cultures and minimal self-renewal (13.9% ± 8.6%) of 84.8% ± 11.5% conserved LTC-IC in AFT024/IL-3/MIP-1 cultures. *P < 0.05; **P < 0.01.
Contribution of proliferation and conservation to overall LTC-IC expansion. Single cell assays were performed as described in Methods and Fig 2 legend. In addition, we used data from a study previously published by our group in which LTC-IC were assessed in a single cell assay based on bone marrow (BM) stroma–conditioned media supplemented with IL-3 and MIP-1 in the absence of a stromal feeder.37 Significantly greater LTC-IC expansion is seen in AFT024/Flt3-L/SCF/IL-7 cultures than in AFT024/IL-3/MIP-1 cultures or SCM/AFT024/IL-3/MIP-1 cultures. This is a result of both increased proliferation and conservation when compared with AFT024/IL-3/MIP-1 cultures. The equivalent LTC-IC expansion seen in AFT024/IL-3/MIP-1 and SCM/IL-3/MIP-1 cultures is caused by different mechanisms: extensive self-renewal (80.5% ± 7.0%) of only 33.9% ± 6.0% conserved LTC-IC in SCM/IL-3/MIP-1 cultures and minimal self-renewal (13.9% ± 8.6%) of 84.8% ± 11.5% conserved LTC-IC in AFT024/IL-3/MIP-1 cultures. *P < 0.05; **P < 0.01.
These same studies also show that more than 80% of LTC-IC proliferated in SCM/IL-3/MIP-1α cultures, but less than 20% in AFT024/IL-3/MIP-1α cultures (Fig 3). This could be a result of contact-mediated proliferation inhibition of progenitors cultured in contact with AFT024 cells because we have previously shown that contact between progenitors and stromal components, such as fibronectin, inhibits proliferation.44 45 We cannot exclude that some factors present in human marrow–conditioned medium, but not in long-term bone marrow culture (LTBMC) medium used in AFT024/IL-3/MIP-1α, may contribute to the increased proliferation in SCM/IL-3/MIP-1α conditions. Of interest, approximately 50% of the conserved LTC-IC and NK-IC proliferated in AFT024/Flt3-L/SCF/IL-7, the only culture condition allowing significant expansion of LTC-IC and NK-IC. Thus, conservation as well as proliferation is necessary for an effective expansion of primitive progenitors.
In comparing the different culture conditions (AFT024/Flt3-L/SCF/IL-7 and AFT024/IL-3/MIP-1α), we used different culture conditions in the readout system to assess the number of LTC-IC that was present. One could argue that differences in the perceived LTC-IC proliferation and conservation are secondary to the differences in the readout phase, but not the expansion culture phase of the assay. We have, however, previously shown that that assessment of LTC-IC maintenance is similar when measured on AFT024 feeders with or without IL-3/MIP-1α.30 We show here that the number of LTC-IC measured in single cell assays on AFT024 feeders is equivalent when done in Flt3-L/SCF/IL-7 medium (6.6% ± 0.9% LTC-IC frequency) or in IL-3/MIP-1α medium (7.8% ± 1.5% LTC-IC frequency). Therefore, we believe that differences seen between AFT024/Flt3-L/SCF/IL-7 and AFT024/IL-3/MIP-1α expansion cultures are not a result of differences in our ability to assess LTC-IC in the readout phase of the assay.
In conclusion, we developed an assay that provides indirect insight in the fate of single primitive LTC-IC and NK-IC. Although the information on progenitor proliferation and conservation is in part indirect, insight in the role of net-conservation and net-proliferation of progenitors in a given culture system can be used to guide the investigator in the design of improved expansion systems that are adjusted to increase progenitor conservation and/or proliferation. In addition, this assay allows enumeration of a very primitive progenitor capable of generating multiple secondary LTC-IC and NK-IC, which we termed an ML-IC. This cell is ontogenetically closely related to the HSC. The ML-IC assay can show that a single cell can generate multiple progenitors with multilineage potential. Studies are ongoing to determine what the relationship is between ML-IC and SRC.
ACKNOWLEDGMENT
The authors thank Brad Anderson, Kelly Asleson, Valery McCullar, and Kirk VanOverbeke for their excellent technical help.
Supported in part by National Institutes of Health (NIH) Grants No. RO1-HL-49930, RO1-HL-48738, RO1-HL-58739-01, and PO1-CA-65493. Supported by the University of Minnesota Hospitals and Clinics. M.P. was supported by a Grant from the Deutscher Akademischer Austauschdienst. C.M.V. is a Scholar of the Leukemia Society of America.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to C.M. Verfaillie, MD, Professor of Medicine, Division of Hematology, Oncology and Transplantation, Director, Stem Cell Biology Program, Box 806 UMHC, 420 Delaware St SE, Minneapolis, MN 55455; e-mail: verfa001@maroon.tc.umn.edu.