Abstract
Methotrexate (MTX) is not cytotoxic to patient-derived acute lymphoblastic leukemia (ALL) cells in total-cell-kill assays, such as the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, putatively due to the rescue effects of hypoxanthine and thymidine released from dying cells. This was mimicked by a diminished methotrexate (MTX) cytotoxicity for the cell lines HL60 and U937 in the presence of hypoxanthine, thymidine, or lysed ALL cells. However, enzymatic depletion or inhibition of nucleoside membrane transport did not result in MTX dose-dependent cytotoxicity in patient samples. Alternatively, a thymidylate synthase inhibition assay (TSIA), based on inhibition of the TS-catalyzed conversion of 3H-dUMP to dTMP and 3H2O, correlated with the MTT assay for antifolate sensitivity in four human leukemia cell lines with different modes of MTX resistance. For 86 ALL patient samples, TSI50 values after 21 hours exposure to MTX were not different between T- and c/preB-ALL (P = .46). After 3 hours incubation with MTX followed by an 18-hour drug-free period, T-ALL samples were 3.4-fold more resistant to MTX compared with c/preB-ALL samples (P = .001) reflecting the clinical differences in MTX sensitivity. TSI50 values correlated with MTX accumulation (r = −.58, P < .001). In conclusion, the TSIA, but not the MTT assay, can measure dose-response curves for MTX in patient-derived ALL cells and showed relative MTX resistance in T-ALL compared with c/preB-ALL.
ACUTE LYMPHOBLASTIC leukemia (ALL) is the most frequently occurring type of cancer in children. Today, more than 95% of the children with newly diagnosed ALL will reach complete remission by combination chemotherapy. However, the leukemia will relapse in one third of the patients.1 This treatment failure may be explained by unfavorable clinical pharmacokinetics,2 regrowth potential of the residual leukemic cells, and by cellular drug resistance.3 Testing of in vitro drug resistance, eg, by using the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, has provided good correlations with clinical outcome.4-8 Unfortunately, the cytotoxicity of methotrexate (MTX), a cornerstone in the treatment of ALL, cannot be evaluated on primary patient-derived leukemic cells by the MTT assay9-12or by other 4-day culture assays, such as the differential staining cytotoxicity (DiSC) assay.13 A test system, which allows the determination of MTX resistance of primary ALL cells, might provide additional prognostic information, thereby facilitating the identification of low- and high-risk patients. In addition, an in vitro assay may be used to determine the relation between clinical and cell biological features and MTX resistance.
In contrast to the nonproliferating patient-derived ALL cells, MTX-induced growth inhibition can easily be observed in cell lines using the MTT assay.14 The mechanism of action of MTX is through the inhibition of folate requiring enzymes in the pyrimidine and purine de novo synthesis pathways, ultimately resulting in an inhibition of the synthesis of DNA, RNA, and protein.15However, cells can be rescued from this cytotoxic action by salvage pathways in which, eg, hypoxanthine (Hx) is converted to inosine monophosphate (IMP) and subsequently to adenosine monophosphate (AMP) and guanosine monophosphate (GMP). Inhibition of the de novo synthesis of thymidylate catalyzed by thymidylate synthase (TS) can be bypassed by the consumption of exogenous thymidine (TdR), which will be converted by thymidine kinases to dTMP. Hx and TdR have been reported to be released by spontaneously dying lymphocytes16 due to degradation of DNA and RNA. Because on average 35% of ALL cells derived from patients spontaneously die during 4 days of culture,10 thereby releasing Hx and TdR, this may explain the absence of a dose-response curve of MTX for ALL cells. Additional evidence for this hypothesis is provided by a report in which enzymatic depletion of Hx and TdR resulted in MTX cytotoxicity on GKTL cells, a leukemia xenograft, which is unable to grow ex vivo, similar to ALL cells.17 This approach, however, was not applied to samples directly derived from patients, and so far it is unknown whether total-cell-kill assays can be adapted for the screening of cells derived from pediatric ALL patients for MTX sensitivity.
In the present study, we investigated several adaptations of the MTT assay for the screening of ALL cells from childhood leukemia patients for MTX sensitivity or resistance, but no dose-dependent cytotoxicity of MTX was observed. Alternatively, we showed that an in situ thymidylate synthase inhibition assay (TSIA)18,19 could be used to determine relative sensitivities of cell lines to MTX, obtaining similar data as for the MTT assay. Therefore, we optimized this indirect MTX resistance assay for the screening of patient-derived leukemic cells. The results show that the TSIA provides dose-response curves in patient-derived ALL cells with large interpatient variation and that this assay can detect differential MTX resistance in T- versus c/preB-ALL after short-term exposure. This is in accordance with the clinically observed relative chemoresistance of T-ALL compared with c/preB-ALL cells.20
MATERIALS AND METHODS
Patient specimens.
Bone marrow and peripheral blood samples were obtained from newly diagnosed pediatric ALL patients; infants (<12 months old) and patients with proB- (CD10− precursor B-lineage) and mature B-ALL were excluded. Mononuclear cells were isolated by centrifugation (500g, 25 minutes) with Ficoll Isopaque, as described previously.9 After isolation, cells were washed twice in RPMI containing 1% fetal calf serum (FCS) with 10-minute periods of centrifugation at 300g and suspended at 2 × 106 cells/mL in culture medium (RPMI 1640 containing 20% heat-inactivated FCS, 100 IU/mL penicillin, 100 μg/mL streptomycin, 0.125 μg/mL fungizone, 200 μg/mL gentamycin, 2 mmol/L L-glutamine, 5 μg/mL insulin, 5 μg/mL transferrin, and 5 ng/mL sodium selenite). Remaining cells were cryopreserved in RPMI containing 20% FCS and 10% dimethyl sulfoxide (DMSO) in liquid nitrogen.
Cell lines.
HL60, a human promyelocytic leukemia cell line, and U937, derived from a patient with monoblastic leukemia, and four human T-lymphoblastic leukemia cell lines CCRF-CEM (the parental CEM/S and three MTX-resistant sublines with either defective MTX transport, increased dihydrofolate reductase [DHFR], or defective MTX-polyglutamylation)21 were grown as suspension cultures in RPMI medium 1640 supplemented with 10% heat-inactivated FCS and 1 mmol/L L-glutamine. Cultures were maintained in exponential growth at 37°C in a humified atmosphere of 95% air/5% carbon dioxide. In the experimental set up, HL60 cells were suspended at 0.2 × 106 cells/mL, U937 and CEM cells at 0.1 × 106 cells/mL for the MTT assay and at 2 × 106 cells/mL for the TSIA.
Chemicals.
MTX was obtained as a gift from Pharmachemie (Haarlem, The Netherlands). Fluoro-MTX (F-MTX), a nonglutamylatable MTX analogue,22 was kindly provided by Dr J.J. McGuire (Grace Cancer Center, Buffalo, NY). AG337, edatrexate, GW1843U89, trimetrexate, and ZD1694 were provided by Agouron Pharmaceuticals (La Jolla, CA), Ciba-Geigy (Basel, Switzerland), Glaxo Wellcome (Research Triangle Park, NC), Werner-Lambert/Parke Davis (Ann Arbor, MI), and Zeneca (Maccles Field, UK), respectively. FCS, penicillin, streptomycin, fungizone, gentamycin, and L-glutamine were obtained from Flow Laboratories (Irvine, UK), bovine serum albumin from Organon Technika (Oss, The Netherlands), and Ficoll Isopaque (Lymphoprep 1.077 g/mL) was provided by Nyegaard (Oslo, Norway). Insulin, transferrin, sodium selenite, and MTT were purchased from Sigma (Zwijndrecht, The Netherlands), as well as thymidine, hypoxanthine, thymidine phosphorylase (TP), and xanthine oxidase (XO). 2-(aminocarbonyl)-N-(4-amino-2,6-dichlorophenyl)-4-[5,5-bis-(4-fluorophenyl-l0-phenyl]-1-piperazineacetamide (R75231) was kindly provided by Dr H. van Belle (Jansen Pharmaceuticals, Beerse, Belgium). [5-3H]-2′-deoxyuridine (22 Ci/mmol) and [5-3H]-2′-deoxycytidine (25 Ci/mmol) were purchased from Moravek Biochemicals (Brea, CA).
MTT and DiSC assay in blast cells from patients.
Round-bottomed 96-well microculture plates (Costar, Badhoevedorp, The Netherlands) were filled with 20 μL of different MTX concentrations and stored at −20°C. Aliquots of 80 μL of the cell suspension (2 × 106 cells/mL) were added to each well as described previously.9 Four wells contained medium without drugs or cells for blanking the plate reader and eight wells contained cells and no drug for measuring control cell viability. Twelve concentrations of MTX ranging from 51.2 pg/mL to 2,500 μg/mL (0.11 nmol/L to 5.5 mmol/L) were tested in duplicate. Plates were incubated in a humified incubator in 5% CO2 for 4 days at 37°C.
On day 4, 10 μL of MTT solution (final concentration, 0.45 mg/mL) was added and after shaking until the cell pellet was resuspended, the plate was incubated at 37°C for 6 hours. Formazan crystals were dissolved with 100 μL of 0.04 N HCl-isopropyl alcohol (acid isopropanol). The optical density (OD) of the wells was measured using an EL312 microplate spectrophotometer (Biotek Instruments Inc, Winooski, VT) at 540 and 562 nm. The OD is linearly related to cell number.23 The OD of wells containing MTX, but no cells, was subtracted from the OD of corresponding wells containing cells and MTX. Leukemic cell survival (LCS) was calculated by the equation: LCS = (ODday 4 treated wells/ODday 4 control wells) × 100%. In simultaneous experiments, the medium was preincubated for 15 minutes with TP (final concentration, 0.15 IU/mL) and XO (final concentration, 0.02 IU/mL) at 37°C to deplete TdR and Hx. Nucleoside transport was inhibited by the addition of R75231 (final concentration, 1.65 μmol/L). The DiSC assay was performed as described elsewhere.24
MTT and DiSC assay in cell lines.
Individual wells of a 96-well microculture plate were filled with 50 μL cell suspension, 25 μL medium, and 25 μL MTX (final concentrations ranging from 10−11 to 10−5 mol/L). Plates were incubated in a humified incubator in 5% CO2 for 3 days at 37°C. Subsequently, 10 μL of MTT solution (final concentration, 0.45 mg/mL) was added and incubated for 3 hours at 37°C. Formazan crystals were dissolved with 100 μL of 0.04 N HCl-isopropyl alcohol (acid isopropanol). The concentration resulting in 50% inhibition of the cell growth (IC50) was determined by assigning 100% to the mean value for the OD of the control wells at day 3 after subtraction of the mean blank value. To distinguish between cell growth inhibition and cell kill, the OD after 3 days was corrected for the mean OD observed for the control wells at the day of drug addition.25 In some experiments, Hx, TdR, or lysed ALL cells were added to the medium and IC50 values were determined relative to control cells also incubated in the presence of these additives. Lysed leukemic cells (final concentration, 1 × 106/mL) were obtained by snap-freezing and thawing three times.
Measurements of Hx and TdR.
After 4 days of culture, microculture plates were kept at −20°C, at which temperature Hx and TdR are stable,26 until analyzed. To determine the concentration of Hx and TdR, 7 μL of 80% trichloroacidic acid (TCA) was added to 70 μL medium and put on ice for 30 minutes. After 2 minutes of centrifugation at 12,000g, the supernatant was neutralized by the addition of 140 μL of tri-octylamine/1,1,2-tri-chloro-tri-fluoro-ethane (1/4; vol/vol). This mixture was centrifuged for 1 minute at 12,000g. The separation of the nucleosides casu quo bases in the supernatant was performed on a reverse-phase microsphere C18 column (Chrompack; 100 × 4.6 mm, 3 μm) at a flow rate of 1 mL/min. Eluents were made of 60 mmol/L KH2PO4 in 5 mmol/L tetrabutylammonium hydrogensulphate (TBA), at pH 6 (solvent A) and 50% acetonitril (solvent B). A gradient (2 minutes 2% B, followed by linear increase of 2% to 40% B over 10 minutes) separated the peaks, which were identified by their absorbance ratios and retention times by comparison with standards. Column output was monitored at 254 and 280 nm.
In situ TSIA.
Inhibition of TS was determined in whole cells based on a previously described assay18,19 by measuring the TS-catalyzed conversion of 3H-dUMP to dTMP and3H2O. A total of 135 μL cell suspensions (1 × 106 cells/mL; 4 × 106 cells/mL when deoxyuridine was used as a substrate) were incubated at 37°C with either 15 μL RPMI as controls or with 15 μL MTX solution. Blanks in triplicate were included containing 135 μL culture medium and 15 μL RPMI. Two conditions were tested: (1) continuous incubation in which cells were incubated with or without drugs for 21 hours (five final concentrations ranging from 0.0039 μmol/L to 1 μmol/L for MTX; from 0.039 μmol/L to 10 μmol/L for F-MTX ) and (2) short exposure in which the drug was washed away after 3 hours followed by an 18-hour drug-free period (five final MTX concentrations ranging from 0.156 μmol/L to 40 μmol/L), based on pilot experiments reported elsewhere.27 Controls were included in triplicate for both conditions. [5-3H]-2′-deoxycytidine or [5-3H]-2′-deoxyuridine (final concentration, 1 μmol/L, 2.5 Ci/mmol) was added 4 hours after the start of the experiment as precursor for dUMP, the substrate for TS. After a total incubation time of 21 hours, cells were put on ice and 150 μL 35% ice-cold TCA was added together with 750 μL 10% activated charcoal solution (10 g washed charcoal, 0.5 g dextran, and 2.5 g bovine serum albumin [BSA] in 100 mL demineralized water) as described previously for the in vitro TS catalytic activity assay.28 After vortexing, samples were left on ice for 30 minutes and centrifuged (800g, 30 minutes, 4°C); 450 μL of the aqueous phase, containing 3H2O, was transferred to a scintillation vial and counted for radioactivity. After subtraction of the mean blank counts, the data were evaluated by calculation of the concentration of drug needed to inhibit 50% of the control TS activity, assuming a linear dose-response curve between the two flanking concentration points. Data were expressed as TSI50,cont. referring to the continuous exposure condition and as TSI50,short for the short exposure condition. Experiments in which cell lines were assayed for antifolate sensitivity were performed by incubating 2 × 106 cells/mL applying the same time schedule. In this case,3H-deoxyuridine was added as a substrate 1 hour before the end of the experiment (final concentration, 1 μmol/L, 0.7 Ci/mmol).
MTX accumulation and polyglutamylation.
Of samples for which a sufficient number of cells was available, 107 cells were exposed for 24 hours to 1 μmol/L [3H]-MTX (2 Ci/mmol), washed three times in ice-cold phosphate-buffered saline (PBS) and resuspended in 1 mL ice-cold PBS. Total accumulation was determined by counting 90 μL for radioactivity and 10 μL for cell survival. The remaining 900 μL was centrifuged and analyzed for polyglutamate formation by high-performance liquid chromatography (HPLC) as described previously.29
Statistics.
To analyze correlations between parameters obtained with the TSIA and with the MTT assay, the Wilcoxon signed rank sum test was applied. The Spearman’s rank correlation coefficient was calculated for a relation between MTX sensitivity and polyglutamylation parameters. The Mann-Whitney U test was performed to determine differences in MTX sensitivity between T- and c/preB-ALL. All tests were performed by applying SPSS 7.5 for Windows (SPSS Benelux BV, Gorinchem, The Netherlands) software.
RESULTS
Prevention of MTX-induced growth inhibition on leukemic cell lines.
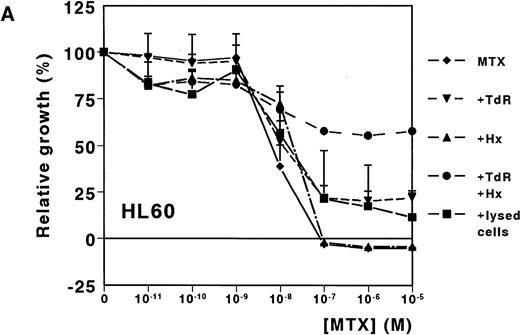
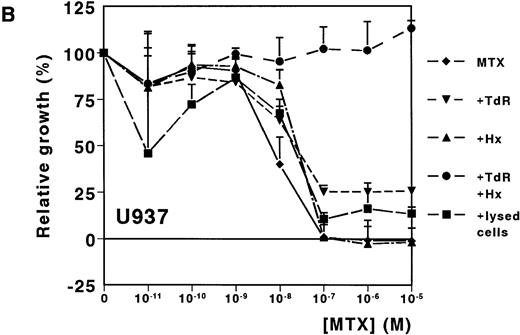
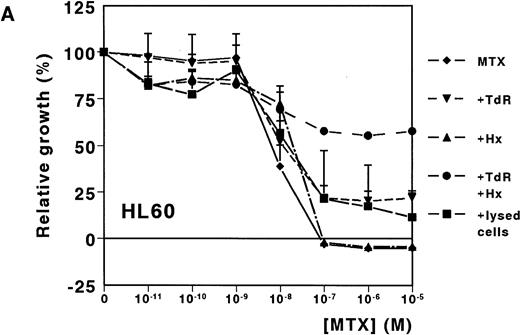
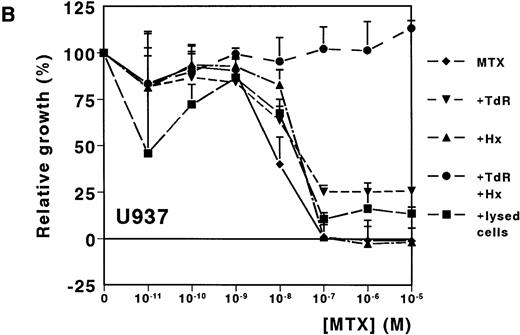
A dose-dependent growth inhibition was induced by MTX for the leukemic cell lines HL60 and U937, as determined with the MTT assay (IC50 values, 8.8 nmol/L ± 1.0 and 11.0 nmol/L ± 4.0, respectively). For both cell lines, cell growth was completely inhibited at MTX concentrations higher than 10−7mol/L (Fig 1A and B). To investigate whether Hx and TdR can protect cells from MTX-induced growth inhibition, these metabolites were added to the medium. Hx (25 μmol/L) did not protect the cells from growth inhibition by high MTX concentrations (>10−7 mol/L), but IC50values increased fourfold and fivefold for HL60 and U937, respectively. TdR (10 μmol/L) by itself increased the IC50 value of MTX by 2.7-fold and 3.8-fold, respectively. In contrast to the addition of Hx, TdR partly protected cells from high MTX concentrations (Fig 1A and B). When Hx and TdR were added simultaneously, complete prevention of the MTX effect was observed for U937 (Fig 1B). Also for HL60 cells, no IC50 could be calculated (IC50 > 10−5 mol/L) (Fig 1A). To test the hypothesis that compounds released by dying patient-derived ALL cells prevented MTX-induced cell kill in the MTT-assay, lysed ALL cells were added to HL60 and U937 cultures. IC50 values increased fourfold for both cell lines (Fig 1A and B).
Dose-response curves of MTX in absence or presence of 25 μmol/L Hx, 10 μmol/L TdR, or 1 × 106/mL lysed primary ALL cells for (A) HL60 and (B) U937. The results are expressed as mean values of three experiments ± SD. Protection from the MTX-induced growth inhibition was most obvious when Hx and TdR were added together.
Dose-response curves of MTX in absence or presence of 25 μmol/L Hx, 10 μmol/L TdR, or 1 × 106/mL lysed primary ALL cells for (A) HL60 and (B) U937. The results are expressed as mean values of three experiments ± SD. Protection from the MTX-induced growth inhibition was most obvious when Hx and TdR were added together.
Lack of in vitro MTX cytotoxicity in patient-derived blast cells using the MTT assay.
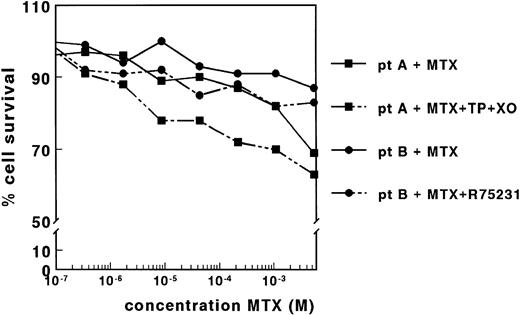
The effect of MTX was determined for blast cells of 83 leukemic patients by the MTT assay. LC50 values could, however, not be determined in 90% of the samples because the leukemic cell survival in the presence of MTX did not decrease below 50% of the control cell survival. This lack of MTX cytotoxicity was observed even at very high MTX concentrations up to 5.5 × 10−3 mol/L, as shown for two patients in Fig 2. By applying the DiSC assay, also no dose-response curves were obtained (data not shown).
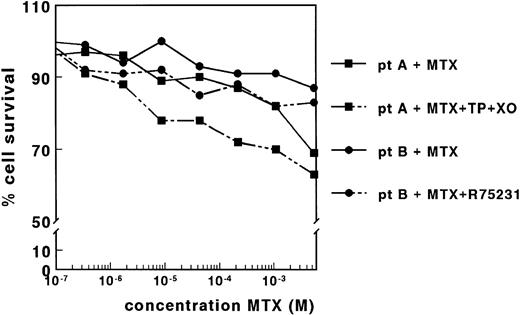
Dose-response curves of MTX for samples derived from two patients with ALL, in the absence and presence of TP (0.15 IU/mL) and XO (0.02 IU/mL) (patient A) or in the absence and presence of R75231 (1.65 μmol/L) (patient B). Both adaptations of the MTT assay did not result in dose-response curves for MTX for 11 patient samples.
Dose-response curves of MTX for samples derived from two patients with ALL, in the absence and presence of TP (0.15 IU/mL) and XO (0.02 IU/mL) (patient A) or in the absence and presence of R75231 (1.65 μmol/L) (patient B). Both adaptations of the MTT assay did not result in dose-response curves for MTX for 11 patient samples.
Prevention of rescue from MTX cytotoxicity.
The lack of MTX cytotoxicity on nondividing patient cells might be associated with the presence of high concentrations of TdR and Hx. Therefore, we determined the concentrations of these compounds in the control wells of four patient samples after 4 days of incubation. The concentration of Hx varied between 36.4 and 58.7 μmol/L, which was not increased relative to the concentrations measured in the corresponding wells without cells (Table 1). The TdR concentrations ranged between <0.1 and 2.1 μmol/L, which is twice the concentration measured in the absence of cells (Table 1). Preincubation of the medium with TP and XO markedly reduced the TdR and Hx concentrations in four samples measured (Table 1). However, this reduction in the concentration of the metabolites did not result in dose-response curves for 11 patient samples, as shown for a representative sample in Fig 2. Cell membrane nucleoside transport was inhibited by coincubation with R75231 to prevent protection of cells by the presence of TdR in the medium. For 12 samples tested, R75231 did not increase the cytotoxicity of MTX, as shown for one sample in Fig 2.
Optimization and validation of the in situ TSIA.
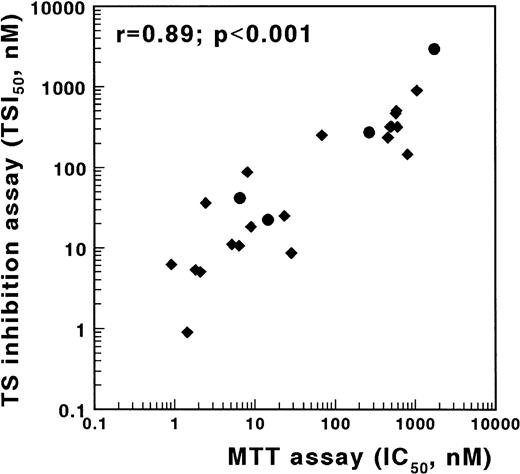
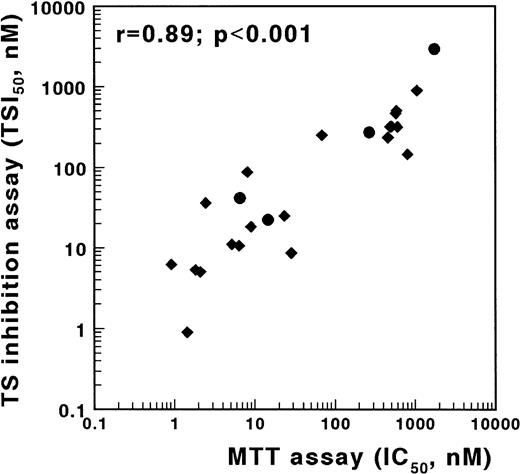
An alternative method to screen cells for antifolate resistance is based on the inhibition of TS in intact cells.18,19 30 To validate the TSIA, we determined antifolate sensitivity obtained with the MTT assay and with the TSIA using the MTX-sensitive T-lymphoblastic leukemia cell line CCRF-CEM and three sublines, which are resistant to MTX due to defective transport, impaired polyglutamylation, or elevated DHFR. In addition to MTX, sensitivity to five other antifolates, mentioned in the Materials and Methods section, was determined. These compounds differ from MTX at the level of transport, polyglutamylation, and/or target enzyme. A strong and significant correlation was found between the IC50 values determined in the MTT assay and both the TSI50,cont. (r = .89, P < .001, Fig 3) and the TSI50,short (r = .66, P = .001) in the TSIA. The Wilcoxon signed rank sum test showed that antifolate sensitivity, as measured with the TSIA after continuous exposure, was not different from antifolate sensitivity as measured with the MTT assay (P = .69).
Correlation between TSIA and antifolate growth inhibition measured by the MTT assay for wild-type CCRF/CEM leukemia cells and three MTX-resistant sublines. TSIA and MTT-based growth inhibition were determined for MTX (•) and five other antifolates (AG337, edatrexate, GW1843U89, trimetrexate, and ZD1694) (⧫). The TSI50 values, measured by the 21-hour incubation TSIA, and the IC50 values after 3 days of culture in the MTT assay were significantly correlated (r = .89, P < .001).
Correlation between TSIA and antifolate growth inhibition measured by the MTT assay for wild-type CCRF/CEM leukemia cells and three MTX-resistant sublines. TSIA and MTT-based growth inhibition were determined for MTX (•) and five other antifolates (AG337, edatrexate, GW1843U89, trimetrexate, and ZD1694) (⧫). The TSI50 values, measured by the 21-hour incubation TSIA, and the IC50 values after 3 days of culture in the MTT assay were significantly correlated (r = .89, P < .001).
Because the original TSIA19 required 2 × 106 cells per drug per time point, we modified the assay by testing different concentrations of drugs instead of different time points. In addition, the substrate was changed from deoxyuridine to deoxycytidine, which is more efficiently converted to dUMP, the direct substrate for TS. The amount of 3H2O formed was increased fourfold when deoxycytidine was used as a substrate instead of deoxyuridine, reducing the amount of cells to 0.1 × 106 cells per drug per concentration. No difference in TSI50 values was observed using these substrates as shown in Fig 4A.
(A) Comparisons of TSI50 (•, TSI50, cont.; ▴, TSI50, short) values obtained by the use of [3H]-dUrd and [3H]-dCyd as substrates in the in situ TSIA. (B) Comparisons of TSI50 values (•, TSIcont.; ▴, TSIshort) obtained in fresh samples and after cryopreservation from 10 patients after 21 hours of continuous MTX incubation and after a 3-hour incubation period followed by an 18-hour drug-free period.
(A) Comparisons of TSI50 (•, TSI50, cont.; ▴, TSI50, short) values obtained by the use of [3H]-dUrd and [3H]-dCyd as substrates in the in situ TSIA. (B) Comparisons of TSI50 values (•, TSIcont.; ▴, TSIshort) obtained in fresh samples and after cryopreservation from 10 patients after 21 hours of continuous MTX incubation and after a 3-hour incubation period followed by an 18-hour drug-free period.
Comparison of fresh and cryopreserved samples from 10 primary acute leukemia patients showed that cryopreservation did not influence MTX-induced TS inhibition during the continuous drug exposure or the short drug exposure condition of the TSIA (Fig 4B). In paired blast samples isolated from peripheral blood and from bone marrow derived from seven patients, no differences in TSI50values were observed (data not shown).
TSIA in T- and c/preB-ALL.
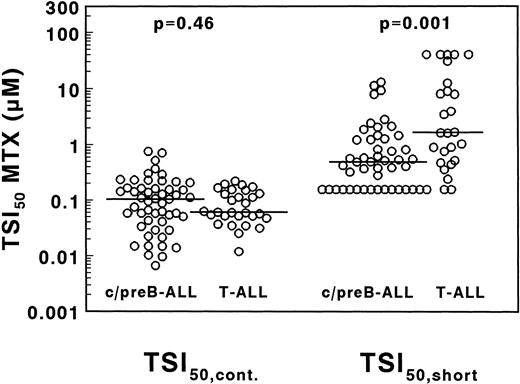
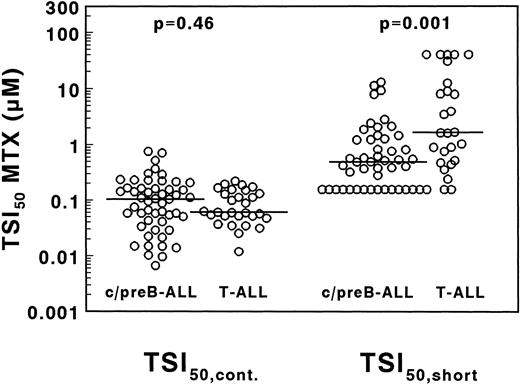
A large interpatient variation in MTX sensitivity was observed both in TSI50,cont., ranging from 0.0067 to 0.76 μmol/L MTX and in TSI50,short, ranging from <0.156 to >40 μmol/L MTX (Fig 5). The median TSI50,cont. was not significantly different between 29 T- and 51 c/preB-ALL samples (0.061 v 0.104 μmol/L MTX;P = .46). The median TSI50, short, however, was 3.4-fold higher in T-ALL compared with c/preB-ALL (1.66 v 0.49 μmol/L; P = .001), but with a large overlap between both groups (Fig 5).
Concentrations of MTX necessary to inhibit 50% of the in situ TS activity in T- and c/preB-ALL, measured after 21 hours continuous drug exposure (TSI50,cont., left panel) or after 3 hours of drug incubation followed by an 18-hour drug-free period (TSI50,short, right panel). Median values are represented as a horizontal line.
Concentrations of MTX necessary to inhibit 50% of the in situ TS activity in T- and c/preB-ALL, measured after 21 hours continuous drug exposure (TSI50,cont., left panel) or after 3 hours of drug incubation followed by an 18-hour drug-free period (TSI50,short, right panel). Median values are represented as a horizontal line.
The role of MTX polyglutamates.
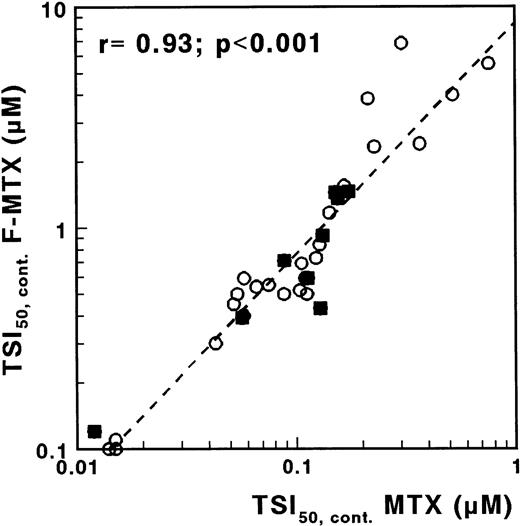
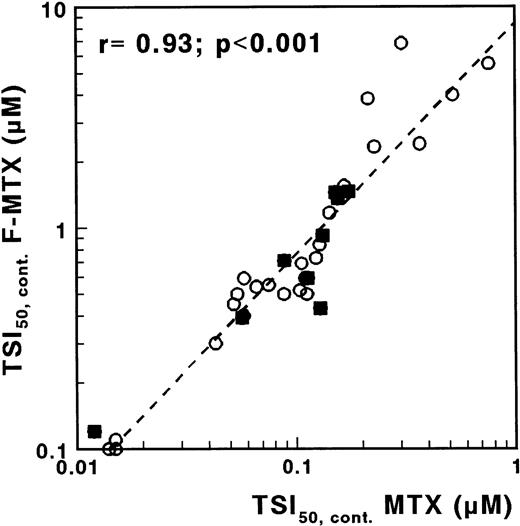
F-MTX is a nonglutamylatable analogue of MTX, with otherwise similar characteristics.22 Therefore, by measuring the sensitivity to both MTX and F-MTX, more insight into the role of MTX polyglutamates would be provided. For F-MTX, no difference was observed for continuous exposure between T- and c/preB-ALL (median TSI50 values, 0.65 and 0.64 μmol/L, respectively; P = .68). The ratio TSI50,cont. F-MTX/TSI50,cont. MTX was median 7 (range, 3 to 21), and the sensitivity for F-MTX was significantly related to the sensitivity for MTX (r = .93; P < .001; Fig 6). After incubation in drug-free medium, TS activity was fully recovered in the majority of the samples incubated with F-MTX, in contrast to the results obtained with MTX.
Correlation between TSI50,cont values for MTX and F-MTX determined with the in situ TS inhibition assay, in which cells were incubated with MTX or with F-MTX for 21 hours. (○), c/preB-ALL; (▪), T-ALL.
Correlation between TSI50,cont values for MTX and F-MTX determined with the in situ TS inhibition assay, in which cells were incubated with MTX or with F-MTX for 21 hours. (○), c/preB-ALL; (▪), T-ALL.
Because the difference in retention of TS inhibition between F-MTX and MTX clearly indicated an important role for polyglutamylation of MTX, we determined MTX accumulation and polyglutamylation for 47 samples. TSI50,cont. and TSI50,short were both significantly correlated with MTX accumulation (Table 2). The ratio TSI50,short/TSI50,cont., excluding the two T- and the 16 B-lineage samples with TSI50,short<0.156 μmol/L, was not correlated with overall MTX accumulation, but rather with the percentage of MTX, which had been converted to the pharmacologically more important MTX-Glu4-6 (Table 2).
DISCUSSION
In this report, we showed that MTX resistance can be studied in primary ALL cells by an in situ TSIA. Conventional total cell kill assays such as the MTT assay9-12 and the DiSC assay13cannot be used for this purpose due to salvage by purines and thymidine, which protect cells from the cytotoxic effects of MTX.26 31-34 A test to determine MTX resistance is particularly important because MTX is an essential drug in the treatment of ALL, and evaluation of MTX resistance in primary ALL samples may improve the prognostic value of in vitro drug resistance testing. In addition, research on clinically relevant mechanisms of MTX resistance will be facilitated by the identification of resistant subgroups of patient samples.
In the present report, we showed that Hx, TdR, and lysed ALL cells can protect cell line cells against MTX-induced growth inhibition. Protection by lysed ALL cells can (at least partly) be explained by the release of metabolites such as Hx and TdR.16 In our experiments, the concentrations of metabolites released by 1 × 106 lysed ALL cells/mL were high enough to increase TSI50 values by fourfold. Higher concentrations of Hx (25 μmol/L) and TdR (10 μmol/L), however, were required for complete protection of U937 cells against MTX-induced growth inhibition. Also for HL60 cells, the simultaneous addition of Hx and TdR was more efficient in protecting cells compared with the ALL lysate. In the nonproliferating system of ALL lymphoblasts, however, the concentrations of Hx and TdR released by spontaneously dying patient cells may be sufficient to protect cells from MTX-induced death.
Because about 35% of the untreated ALL cells die spontaneously during the 4 days of culture in the MTT assay,10 the subsequent release of Hx and TdR into the medium partly explains the observed lack of MTX cytotoxicity in primary ALL cells obtained from patients. After 4 days of culture, we detected an increase in TdR concentration only and not in Hx concentration in wells containing cells versus wells without cells. This, however, might be a reflection of the consumption of Hx by the remaining cells to survive the 4 days of culture. The hypothesis that dying cells protect remaining cells from MTX cytotoxicity is supported by several observations. The dilution of plated ALL cells to less than 200 cells/well did result in a dose-dependent effect of MTX, whereas in experiments with an initial concentration of 10,000 cells/well, cells were protected from MTX cytotoxicity.16 In another study with a MTX nonresponsive xenograft, it was shown that incubation with XO and TP to degrade Hx and TdR, respectively, did result in MTX-induced growth inhibition.17
We adapted the MTT assay conditions to block potential rescue effects by purine/pyrimidine salvage pathways. XO and TP indeed decreased the concentrations of Hx and TdR in the medium, but still no dose-response curves were observed for MTX on leukemic cell samples from 11 ALL patients in the MTT assay. This result could imply that residual levels of TdR and Hx were still sufficient to prevent the remaining patient cells from MTX cytotoxicity. Moreover, other purines have been described to rescue cells from MTX cytotoxicity such as guanosine and adenosine.33 These metabolites are also degraded by XO, but only after conversion to Hx. On the present chromatograms, these metabolites could not be evaluated. Another explanation may be found in an increase in extracellular folate pools due to the spontaneously dying cells. However, these folates would prevent MTX toxicity by competition for transport and polyglutamylation suggesting that higher concentrations of MTX should overcome this rescue.
Coincubation of R75231, described to inhibit nucleoside transport of Ehrlich ascites tumor cells,35 did not increase MTX cytotoxicity against ALL cells from patients. However, R75231 will only inhibit the transport of nucleosides, while bases such as Hx are still capable of entering the cell. In view of our cell line experiments and data provided by others,26 it is clear that Hx alone cannot fully rescue the remaining cells from MTX cytotoxicity. As already described, replacing FCS by dialyzed FCS to remove folates, nucleosides, and bases from the medium did not improve MTX cytotoxicity, but decreased leukemic cell survival in general.23 Altogether, it seems that different mechanisms of protection could be involved in patient-derived samples compared with proliferating cell lines.
Because no adaptations of the MTT assay resulted in dose-dependent MTX cytotoxicity curves for primary ALL cells, we investigated an alternative assay, described by others to detect antifolate sensitivity.18,36,37 In four cell lines and testing six antifolates, a highly significant correlation between the 18-hour TSIA and 3-day MTT assay suggested that the TSIA is a suitable antifolate sensitivity test for cell lines. For patient samples, replacing the substrate deoxyuridine by deoxycytidine reduced the number of cells needed, as tritium is released faster from deoxycytidine than from deoxyuridine.19 This could be associated with the fact that conversion of deoxyuridine to dUMP is catalyzed by the cell cycle-dependent thymidine kinase I, which consequently has a low activity in resting cells.38 Deoxycytidine is activated by deoxycytidine kinase, which has a higher activity in leukemic cells and is not cell cycle-dependent.38 The monophosphate dCMP is efficiently deaminated to dUMP, the active substrate for TS. This modification, together with an increase in incubation times, resulted in an in situ MTX sensitivity assay, which can routinely be performed on cryopreserved and on fresh ALL patient cells and requires similar low numbers of cells as the conventional total cell-kill assays.
Using the TSIA, samples from 86 children with ALL, both c/preB-and T-ALL, were screened for MTX resistance. Both subtypes are treated with MTX, but the outcome of T-ALL is worse in conventional therapy regimens compared with c/preB-ALL as reviewed by Uckun et al.20 This may be partly explained by resistance to MTX, as T-ALL displays a less efficient MTX polyglutamylation compared with c/preB-ALL.39,40 Although the TSIA might not always equate MTX-induced cytotoxicity, the relative in vitro MTX resistance for T- versus c/preB-ALL is confirmed by the TSIA measurements. When cells were allowed to efflux MTX during an 18-hour drug-free period after 3 hours of incubation, T-ALL samples were 3.4-fold more resistant to MTX compared with c/preB-ALL cells (P = .001). This is in accordance with the reported low accumulation of long chain polyglutamates in T-ALL, which are preferentially retained inside the cell.41
With the continuous 21-hour exposure condition, no difference in MTX sensitivity between T- and c/preB-ALL could be detected. This suggests that differences in polyglutamylation can be overcome during continuous MTX exposure, which has also been described for cell lines.29,42 43 This is supported by the experiments with F-MTX, as the TSI50,cont. values for MTX were strongly correlated with the TSI50,cont. values obtained for the nonglutamylatable F-MTX.
Patient-derived leukemia samples, when continuously exposed to MTX for 21 hours, displayed TSI50 values varying almost 100-fold. High TSI50,cont. values might reflect MTX resistance, as reviewed by Bertino44 due to (1) a defective transport leading to a lower intracellular concentration of MTX, (2) a mutation in DHFR, the main target enzyme, resulting in a low-affinity for MTX, or (3) a higher level of DHFR. The relative chemoresistance in T-ALL may also be related to the more frequent prevalence of subclones with elevated levels of DHFR in T-ALL compared with B-lineage ALL samples.45 In addition, a human T-cell leukemia cell line was reported to contain twofold higher DHFR protein and mRNA levels compared with a human B-lineage leukemia cell line.46 The TSIA, however, could not detect differences in MTX sensitivity between T- and c/preB-ALL samples in the continuous 21-hour exposure condition. This might be related to elevations in DHFR in subclones occurring in a range of 10% to 90% of the cells; an overall activity assay would not detect these differences. Moreover, the 21 hours of incubation in the TSIA may overcome small differences in DHFR content. In addition, several other factors involved in MTX cytotoxicity might obscure the influence of only one parameter when measured by the end point TSIA.
The overall accumulation of MTX, as measured after 24 hours, is one parameter of MTX resistance that is correlated with the MTX sensitivity as measured with the TSIA. The comparison of a short (3 hours) incubation followed by a drug-free period (18 hours) and a continuous (21 hours) exposure condition may help to investigate intracellular retention of MTX, possibly providing information on MTX polyglutamylation defects. This hypothesis is supported by the significant correlation found between the ratio of TSI50,short/TSI50,cont. and the percentage MTX present as MTX-Glu4-6.
In conclusion, the total-cell-kill MTT assay could not be adapted to evaluate MTX cytotoxicity on patient-derived ALL samples. However, the indirect TSIA based on MTX-induced TS inhibition proved to be informative with respect to the extent of MTX sensitivity and resistance. Using this assay, T-ALL samples were more MTX-resistant compared with B-lineage ALL samples after a 3-hour MTX exposure, followed by an 18-hour drug-free period; on long-term MTX exposure, both phenotypes were equally sensitive.
Supported by Grant No. VU 94-679 from the Dutch Cancer Society.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Marianne G. Rots, Department of Pediatric Hematology/Oncology, University Hospital Vrije Universiteit, PO Box 7057, 1007 MB Amsterdam, The Netherlands; e-mail:marianne.rots@azvu.nl.

![Fig. 4. (A) Comparisons of TSI50 (•, TSI50, cont.; ▴, TSI50, short) values obtained by the use of [3H]-dUrd and [3H]-dCyd as substrates in the in situ TSIA. (B) Comparisons of TSI50 values (•, TSIcont.; ▴, TSIshort) obtained in fresh samples and after cryopreservation from 10 patients after 21 hours of continuous MTX incubation and after a 3-hour incubation period followed by an 18-hour drug-free period.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/3/10.1182_blood.v93.3.1067/5/m_blod40301004ax.jpeg?Expires=1768318551&Signature=0FuGl2yXjHTaVYor--R9CtLyP~fGfnkR6cyqFGg1XXiDNDll9RJfLDTdBmI8Eva4QB~oqWKHZ6YKGisjoON0exG~YVvGdWLt56SkZq68ziIq5Z~IzdAt7jtc9pvWgMcHyy-pOv3HkJvYvlZJBCpWX75TYmARceNnlJZB6VanjkV2xr1RawDAGtxAC0iKIi-3nB5jOPd5QIexOSCQIMUw8tArL84Vw14UpnbZNf~xp6pq4WLf7L4z2PKat23ktp1duYiDchSJb~Hx3BiEr0oKdPhwYLOMlV8y-ixMxLzYJi01Bsl8HeoewJqMimExgoQTWh2vrXjSDVkYw2QoelS4SQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. (A) Comparisons of TSI50 (•, TSI50, cont.; ▴, TSI50, short) values obtained by the use of [3H]-dUrd and [3H]-dCyd as substrates in the in situ TSIA. (B) Comparisons of TSI50 values (•, TSIcont.; ▴, TSIshort) obtained in fresh samples and after cryopreservation from 10 patients after 21 hours of continuous MTX incubation and after a 3-hour incubation period followed by an 18-hour drug-free period.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/3/10.1182_blood.v93.3.1067/5/m_blod40301004bx.jpeg?Expires=1768318551&Signature=KinDcUp0L5jU7ftvULgpdI~xfnTTiO-9fXMqlJoD5oJXiPN-al9v-7kWUec1kp94Gh3tXV5K0vijqynkRvy5KItlVBWZGEhsCzqgTFTTUjn9Be47iuDfIJtD2Jf1m7OAUH~EcVjcfgtmtF9IktSnKEcuvgKXbU413kh4nYCjECl73~WJrfbpPup-oCxHTUR76OBhz0AbHQXqZWLvgx2o-0Con~IdOtWfRbTNtyPJmB54Uzcz7oyEJn7GBWYpgqBOsj8-kWaTaThKYLsLG~7FDAkpcrHWizWCL439e0aiilWBBzqldOEzQI7Ugc98m0bhukrGNkwvr7znJLAzakRvCQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

![Fig. 4. (A) Comparisons of TSI50 (•, TSI50, cont.; ▴, TSI50, short) values obtained by the use of [3H]-dUrd and [3H]-dCyd as substrates in the in situ TSIA. (B) Comparisons of TSI50 values (•, TSIcont.; ▴, TSIshort) obtained in fresh samples and after cryopreservation from 10 patients after 21 hours of continuous MTX incubation and after a 3-hour incubation period followed by an 18-hour drug-free period.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/3/10.1182_blood.v93.3.1067/5/m_blod40301004ax.jpeg?Expires=1768380802&Signature=qNrqMVADkk6ocfyE7gKK6h41NZoOXUWfZVS4U78mjcx~beGW6pJLHorwJaDpaUiLJApActDNjhCRr3y2rf1UWsezX2sTy5BQ2RGhA0ud65C7VLAqmNFpPZ8SuHdFmCo6avIkQmYYNglvWgyPvDQMywSBIYglvy9KwAp4TIIwy4PFpWcnCSrunVdKv4Tfz9HHA570CDT2Wry1-W3gKSkhLpGcu5awKZX-GlDTZ~21GpJ4Fa3~hPg0nk6oaxNZ4N-hA1zeqO-iN8N1s-MZZ5Y-RTuryX7V~-p0VkWq2deV~lG13Kq0LtkqAocd4EJtySaP5BEKViy4D7-WxLqM55RwXw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Fig. 4. (A) Comparisons of TSI50 (•, TSI50, cont.; ▴, TSI50, short) values obtained by the use of [3H]-dUrd and [3H]-dCyd as substrates in the in situ TSIA. (B) Comparisons of TSI50 values (•, TSIcont.; ▴, TSIshort) obtained in fresh samples and after cryopreservation from 10 patients after 21 hours of continuous MTX incubation and after a 3-hour incubation period followed by an 18-hour drug-free period.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/93/3/10.1182_blood.v93.3.1067/5/m_blod40301004bx.jpeg?Expires=1768380802&Signature=2xs-0NNZ5nbplElKy7bpNy8aCkHNE1DAusQPbGjdJDZtgM5dsKiVnopGdrfU6epFHgCtiErUByNID~XfUS3MY4REu5~muyZBYH6OCGgWNKLMUfNFNZp5m303KHTG~HSL4cSa29uD1Pdak1bWN4NmQ2RxAQAdCdEqdITgaFoudF-ewDEIRm586x0K5JqgOZueLBueh3uRtptvMITc5z99o6j4FRJ67CL-1KkErb0cjuP7HloTymHhvYL8K-0XAt85OkKA8re-1zprUAXZ4~wl1srdOwLPP-CAKpeluO0XZLiAPp1AiBV880menIZnqt~kiroTWiOgnkAllh6SNqucMw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)