Abstract
Fas, a cell surface receptor, can induce apoptosis after cross-linking with its ligand. We report that Fas antigen is constitutively expressed in medullary epithelial cells of the human thymus. Expression is decreased in cultured thymic epithelial cells (TEC), similarly to HLA-DR antigen. TEC are resistant to anti-Fas–induced apoptosis after 4 days of primary culture, and this resistance is reversed by concomitant addition of cycloheximide. Cycloheximide also downregulated the expression of Fas-associated phosphatase-1, which has been found to inhibit Fas-induced apoptosis. This phosphatase could be involved in the resistance to Fas-induced apoptosis observed on day 4 of TEC culture. When TEC were subcultured after 10 to 13 days of primary culture, exposure to interleukin-1-β, tumor necrosis factor-, and interferon-γ, alone or together, reinduced Fas mRNA and protein expression. In coculture with activated thymocytes, TEC also upregulated Fas protein expression. Cytokine-activated TEC became sensitive to apoptosis induced by an agonistic anti-Fas antibody. This apoptosis was inhibited by Z-VAD-fmk but not by Z-DEVD-fmk and DEVDase activity was slightly increased in Fas-stimulated TEC, suggesting that DEVDase activity is not sufficient to induce TEC apoptosis. Taken together, these data show that the Fas receptor is expressed in medullary epithelial cells of the human thymus and is able to induce apoptosis.
Fas (APO-1/CD95) IS A CELL surface receptor expressed in a variety of tissues; on cross-linking, Fas can induce apoptosis in vitro and in vivo.1 The Fas receptor shows homology with several members of the tumor necrosis factor receptor (TNFR) family, including CD40 and P55-TNFR. Its major function appears to be the induction of apoptosis in cells expressing it. Fas expression in several cell types is upregulated by the cytokines interferon-γ (IFN-γ), interleukin-1β (IL-1β), and TNF-α.2-4 The role of the Fas/FasL system is well characterized in lymphocytes in two mechanisms: activation-induced cell death of peripheral lymphocytes5-7 and T-cell cytotoxicity.8
Cross-linking of Fas receptors activates an array of cysteine proteases (caspases) in a cascade-like fashion. Upon Fas ligand binding, Fas recruits FADD/MORT1 and RIP via the interaction of their death domains.9 Caspase-8 (FLICE/MACH) acts very early in Fas-induced apoptosis and potentially acts as a direct link between the Fas signaling complex and ICE-like proteases,10,11 each of the latter playing a distinct role in Fas-induced apoptosis.12 A tyrosine phosphatase, Fas-associated phosphatase-1 (FAP-1), acts as a negative switch in the Fas pathway and interacts with the carboxyl terminus of the Fas receptor.13
The thymus plays a central role in T-cell differentiation and T-cell repertoire selection. Clonal deletion of immature thymocytes is an important mechanism ensuring self-tolerance. Some thymocytes generated in the thymus survive to become mature T cells, but most are deleted by apoptosis through thymic selection.14,15 T-cell maturation in the thymus is driven by interactions between developing thymocytes and thymic stromal cells.16 Thymic epithelial cells (TEC) are a major cellular component of the thymic stroma and play a key role in T-cell commitment.17
Fas is widely expressed in mouse thymocytes,18,19 which can be induced to undergo apoptosis by an agonistic anti-Fas antibody, both in vitro and in vivo.18,20-22 The involvement of Fas in thymic negative selection is controversial.23-27
A subpopulation strongly expressing Fas antigen represents 1% to 4% of total thymocytes in humans.28-30 We recently described two distinct pathways (an antigen-dependent pathway and a cytokine-dependent pathway) that can upregulate Fas expression in human thymocytes31; cytokine-activated thymocytes display a higher susceptibility to Fas-induced apoptosis in vitro than anti-CD3–activated thymocytes.
Fas is also expressed in nonlymphoid tissues, notably in various epithelial cells.32 The expression and function of Fas antigen in nonlymphoid cells of the human thymus has not yet been documented. We therefore studied Fas expression in TEC both in situ by means of immunochemistry and in vitro by using cultured cells.
MATERIALS AND METHODS
Thymic tissue and TEC culture.
Normal thymus fragments were obtained from infants aged from 5 days to 2 years undergoing heart surgery at Marie-Lannelongue Hospital (Le Plessis-Robinson, France). For some experiments, fragments of thymic tissue were flash-frozen in liquid nitrogen and stored at −80°C.
Primary TEC cultures were established as previously described.33 Briefly, small fragments of human thymic tissue were washed in RPMI medium and transferred to 75-cm2culture dishes. The culture medium, RPMI 1640 supplemented with 20% horse serum (Life Technologies, Cergy-Pontoise, France), 0.2% Ultroser (Life Technologies), 2 mmol/L L-glutamine, 100 IU/mL penicillin, and 100 μg/mL streptomycin was replaced twice a week. After 10 to 13 days of primary culture, the confluent monolayers were washed with phosphate-buffered saline (PBS) and treated with 0.075% trypsin (Life Technologies) and 0.16% EDTA for 5 minutes at 37°C. In some experiments, TEC were collected after 2, 4, 7, or 10 days of primary culture. The epithelial nature of the cells was checked by immunocytochemical analysis of cytocentrifuged cells, using the antikeratin monoclonal antibody CK-1 (Dako, Trappes, France). Our culture conditions select medullary epithelial cells, because cells collected after 10 to 13 days of culture strongly express antikeratin CK-1, a marker of the medullary epithelial network. The proportion of keratin-positive cells was consistently greater than 95%.
After 10 to 13 days of primary culture, TEC were subcultured (5 × 105 cells/well) in 24-well Primaria plates (Polylabo, Paris, France) and incubated for 24 hours to allow them to adhere. After two washes with Hank’s Balanced Salt Solution (HBSS), the medium was replaced with RPMI supplemented with 5% horse serum and the following cytokines: 1 ng/mL recombinant human IL-1β (Sigma Chemical Co, Saint Quentin Fallavier, France), 10 ng/mL recombinant TNF-α (Genzyme, Cergy Saint Christophe, France), and 500 U/mL recombinant human IFN-γ (Genzyme), separately or together. TEC were treated with trypsin-EDTA after 24, 48, or 72 hours. After three washes, the cells were used for immunofluorescence studies.
Immunofluorescence studies.
TEC were labeled fluorescein isothiocyanate (FITC)-conjugated anti–HLA-DR (Immunotech, Marseille, France) or anti-Fas antibodies (Dako). TEC were first incubated with anti-Fas (anti-CD95) monoclonal antibody (clone UB2; Immunotech) for 30 minutes at 4°C, then washed twice in Hank’s solution (HBSS) supplemented with 5% fetal serum calf, stained with biotin-coupled goat antimouse IgG antibody (Immunotech), washed twice, and incubated with Quantum Red-conjugated streptavidin (Sigma).
Cell labeling was analyzed on a FACScan flow cytometer (Becton Dickinson, Grenoble, France) using Cell Quest software. A gate was set on intact cells by using forward- and side-scatter analysis; 104 cells were analyzed in the gate. The proportion of cells expressing HLA-DR among total cells or the mean fluorescence intensity (MFI) of Fas staining was measured.
TEC/thymocytes cocultures.
After 10 to 13 days of primary culture, TEC were subcultured in 24-well plates (0.5 × 106 cells/well). Heterologous thymocytes were mechanically isolated by gently scraping fresh thymic tissue, filtering the cells through sterile gauze, and washing them with HBSS. After two washes of adherent TEC, 5 × 106thymocytes (in 1 mL RPMI containing 5% horse serum) were added per well. When indicated, 5 ng/mL phorbol 12-myristate 13-acetate (PMA; Sigma) and 500 ng/mL ionomycin (Boehringer Mannheim, Meylan, France) were added to the coculture. After a 3-day coculture period, wells were washed three times and TEC were collected as previously described. After staining with anti-Fas antibody, a gate was set on TEC by using forward- and side-scatter analysis during the acquisition of data on the FACScan flow cytometer.
Immunohistochemical analysis of thymic sections.
Acetone-fixed frozen sections 6-μm thick were incubated for 30 minutes at room temperature with polyclonal rabbit antikeratin antibody (Dako) and monoclonal anti-Fas antibody (clone UB2). They were then washed three times in PBS and incubated with rhodamine-coupled goat antirabbit antibody (Immunotech) and FITC-conjugated antimouse antibody (Silenus Laboratories, Hawthorn, Australia) for 30 minutes. After three washes in PBS, the sections were mounted in PBS/glycerol. Control sections were incubated with the fluorescent conjugates only.
Anti-Fas antibody assay and measurement of FITC-conjugated annexin-V binding.
After two washes with HBSS, subcultured TEC were treated with various amounts of anti-Fas IgM antibody (clone CH-11; Upstate Biotechnology Inc, Lake Placid, NY) or the same concentrations of irrelevant IgM antibody (Dako). When indicated, 10 μg/mL cycloheximide (Sigma) was added to the culture. After 24 hours of exposure to IgM or anti-Fas CH-11 antibody, the cells were washed twice with PBS and harvested by trypsin-EDTA treatment; living cells were counted using the Trypan blue exclusion method. In some experiments cells were washed in PBS and fixed for 5 minutes in ethanol containing 5% acetic acid. Cells were then stained with Toluidine blue for 30 seconds and rinsed with water.
Apoptosis was analyzed by quantifying phosphatidylserine residues exposed on the external cell membrane. Indeed, one of the plasma membrane alterations occurring in the early stages of apoptosis is the externalization of phosphatidylserine at the cell surface; it triggers specific recognition and removal by phagocytes.34 Annexin-V is a calcium-dependent phospholipid binding protein with high affinity for phosphatidylserine and is used for the detection of apoptotic cells.35 One microliter of human recombinant FITC-conjugated annexin-V (Boehringer Mannheim) and 2 μg/mL propidium iodide were added to 100 μL of cell suspension in binding buffer (10 mmol/L HEPES/NaOH, pH 7.4, 140 mmol/L NaCl, 5 mmol/L Ca Cl2). After 15 minutes of incubation in the dark, dual-color analysis was performed on a FacsScan flow cytometer. Cells incorporating propidium iodide, ie, dead cells, were excluded from the analysis.
Caspase inhibitory peptides and DEVDase assay.
In some experiments, TEC were preincubated with 20 μmol/L Z-Val-Ala-Asp-fluoromethylketone (Z-VAD-fmk) or Z-Asp-Glu-Val-Asp-fluoromethylketone (Z-DEVD-fmk; Enzyme Systems Products, Dublin, CA) for 2 hours before adding IgM or anti-Fas CH-11 antibody. Both Z-VAD-fmk and Z-DEVD-fmk peptides were cell-permeant; Z-VAD-fmk is a cystein protease inhibitor or broad specificity, whereas Z-DEVD-fmk inhibits more specifically caspases from the caspase-3 family, also called DEVDases (DEVD-cleaving caspases).
After 7 hours of incubation with IgM or CH-11, TEC were harvested, washed three times in PBS, and lysed in a buffer containing 10 mmol/L HEPES, pH 7.4, 2 mmol/L EDTA, 0.1% CHAPS, 5 mmol/L dithiothreitol (DTT), and a cocktail of protease inhibitors (17 μg/mL phenylmethyl sulfonyl fluoride [PMSF], 10 μg/mL leupeptin, 10 μg/mL aprotinin, and 10 μg/mL pepstatin). Samples were centrifuged at 100,000g at 4°C to obtain cytosolic proteins. Proteolytic reactions were performed in a buffer containing 20 mmol/L HEPES, pH 7.4, 10% glycerol, and 2 mmol/L DTT. Fifty micrograms of proteins and 100 μmol/L N-acetyl-Asp-Glu-Val-Asp-pNA (DEVD-pNA) were added and the mixture was incubated during 3 hours at 37°C. The formation of p-nitroanilide was measured at 405 nm using a Uvikon 930 spectrophotometer (Kontron Instruments, Saint Quentin en Yvelines, France). In the same experiment, we used an internal control of DEVDase activity, ie, K562 target cells killed by lymphokine-activated killer cells, as previously described.36
RNA preparation and reverse transcriptase-polymerase chain reaction (RT-PCR).
Total RNA was extracted using the RNAplus kit (Bioprobe, Paris, France), then purified with 0.5 vol of 7.5 mol/L ammonium acetate and 2.5 vol of 100% ethanol, and centrifuged at 15,000 rpm for 30 minutes at 4°C. The pellet was washed in 75% ethanol, dried under a vacuum, and stored at −80°C after dissolution in water. The total RNA concentration was determined by measuring absorbance at 260 nm with a Gene Quant spectrophotometer (Pharmacia, Cambridge, UK). The purity of the RNA preparation was checked by measuring the 260 nm/280 nm ratio.
The oligonucleotide primers used for RT-PCR were from Genset (Paris, France) and the sequences were as follows: Fas primers, forward 5′-GACAAAGCCCATTTTTCTTCC-3′ and reverse 5′-ATTTATTGCCACTGTTTCAGG-3′; FAP-1 primers, forward 5′-GAATACGAGTGTCAGACATGG-3′ and reverse 5′-AGGTCTGCAGAGAAGCAAGAATAC-3′; β-actin primers, forward 5′-GGGTCAGAAGGATTCCTATG-3′ and reverse 5′-GGTCTCAAACATGATCTGGG-3′. A 50-μL reverse transcription reaction mixture containing 1 μg of total RNA, 5 μL of 10× RT buffer (Eurobio, Les Ulis, France), 1.5 mmol/L each dNTP (Eurobio), 40 U of RNasin (Promega, Charbonnières, France), 1 μmol/L reverse primer, and 4 U of avian myeloblastosis virus (AMV) reverse transcriptase (Eurobio) was incubated at 42°C for 60 minutes. PCR was performed in a total volume of 100 μL containing 10 μL of RT reaction mixture, 10 μL of PCR buffer (Eurobio), 1.5 mmol/L MgCl2, 0.5 μmol/L of each primer, 0.2 mmol/L of each dNTP, and 2.5 U of EurobioTaq II polymerase (Eurobio). The mixture was overlaid with mineral oil and amplified in a PHC3 thermal cycler (Techne, Cambridge, UK) as follows: denaturation at 94°C for 1 minute; annealing at 53°C (Fas), 60°C (FAP-1), or 58°C (β-actin) for 1 minute; and extension at 72°C for 2 minutes. The final elongation step lasted 10 minutes at 72°C. PCR products were analyzed on 1.5% agarose gel containing ethidium bromide.
Western blotting.
In some experiments, TEC were collected and solubilized in a lysis buffer containing 150 mmol/L NaCl, 50 mmol/L Tris, pH 8.0, 5 mmol/L EDTA, 1% Triton X-100, 0.02% NaN3, 1 mmol/L PMSF (Sigma), and 0.15 U/mL aprotinin (Sigma) for 20 minutes at 4°C. Insoluble material was removed by centrifugation at 4°C for 10 minutes. After boiling, the samples (20 μg total protein) were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel (7.5%) and proteins were transferred to polyvinylidene difluoride (PVDF) membranes. A second SDS-PAGE gel was colored with Coomassie blue to check that similar amounts of protein were loaded into the gel. Blots were saturated in PBS containing 0.1% Tween-20 and 5% dry nonfat milk and incubated for 4 hours at 4°C in PBS containing 0.1% Tween-20, 0.1% dry nonfat milk, and 0.2 μg/mL polyclonal goat anti–FAP-1 antibody (Santa Cruz Biotechnology, Santa Cruz, CA). Bound antibody was detected by using peroxidase-conjugated antigoat Ig (Santa Cruz Biotechnology). Immunoreactivity was determined using the ECL chemiluminescence reaction (Amersham France S.A., Les Ulis, France).
Statistical analysis.
Differences between groups were identified by using the Mann-Whitney or Wilcoxon test (Instat software). A difference was considered statistically significant if the P value was less than .05.
RESULTS
Fas antigen is expressed in situ in human thymus.
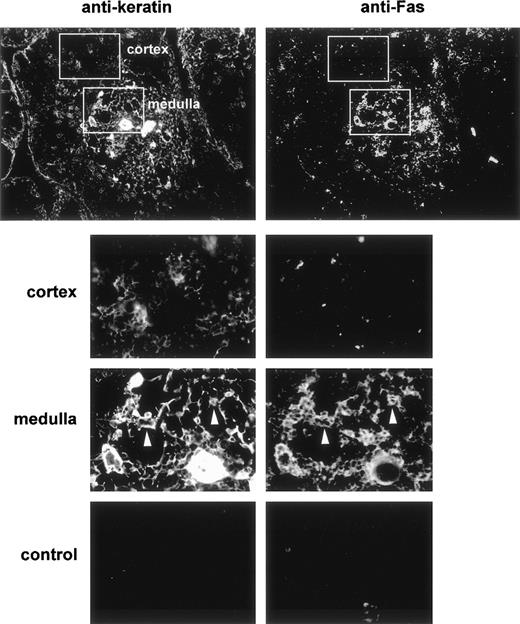
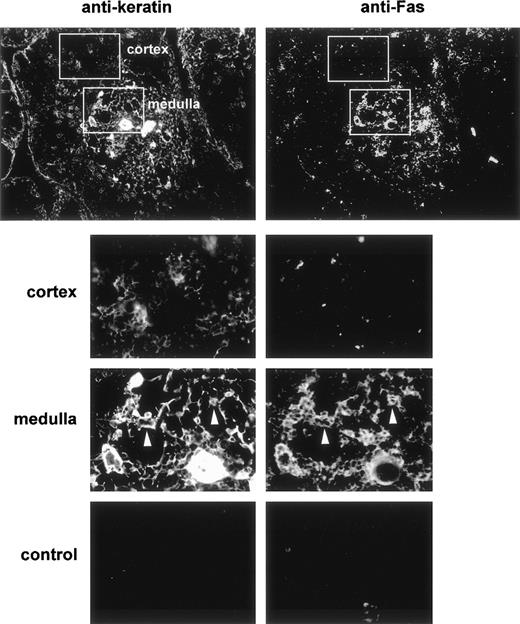
Fas expression was analyzed on cryosections of human thymuses in double-staining experiments with antikeratin to visualize the epithelial network. We used a polyclonal antikeratin antibody that stains thymic epithelial cells more strongly in the medulla than in the cortex. Fas was mainly expressed in the medulla (Fig 1). Double-staining showed that most cells expressing keratin were also Fas-positive, whereas cortical epithelial cells were clearly Fas-negative. Thus, a subset of TEC express Fas antigen in the human thymus. A subset of human thymocyte (<3% of total thymocytes) with a strong expression of Fas was previously described using cytofluorimetry.28-30 We could rarely distinguish Fas expression in some medullary thymocytes; thus, Fas expression in the human thymus is mostly epithelial.
Double immunofluorescence distribution of Fas and keratin in the human thymus. Frozen thymus sections were fixed in acetone and then double-stained with antikeratin (left) and anti-Fas (right) antibodies. After three washes they were stained with rhodamine-coupled antirabbit and FITC-coupled antimouse antibodies. The top plates (×10) show that Fas expression is mainly observed in the medullary area of the human thymus. Control sections (×20) were incubated with the fluorescent conjugates only. The lower plates are enlargements of the framed areas in the upper plates. The white arrows indicate medullary epithelial cells expressing keratin and Fas antigen.
Double immunofluorescence distribution of Fas and keratin in the human thymus. Frozen thymus sections were fixed in acetone and then double-stained with antikeratin (left) and anti-Fas (right) antibodies. After three washes they were stained with rhodamine-coupled antirabbit and FITC-coupled antimouse antibodies. The top plates (×10) show that Fas expression is mainly observed in the medullary area of the human thymus. Control sections (×20) were incubated with the fluorescent conjugates only. The lower plates are enlargements of the framed areas in the upper plates. The white arrows indicate medullary epithelial cells expressing keratin and Fas antigen.
Fas antigen expression falls in cultured TEC.
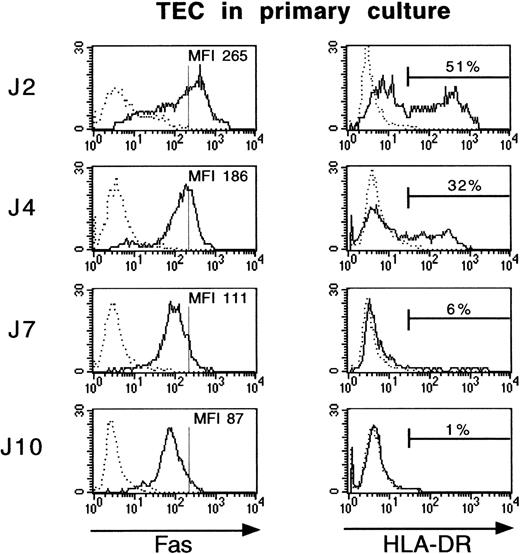
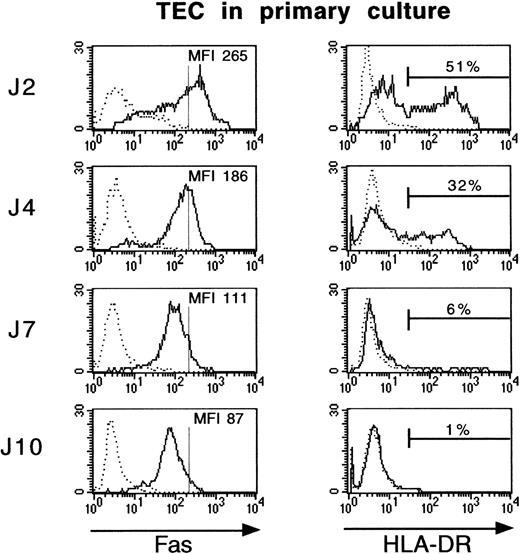
We examined Fas and HLA-DR expression by immunofluorescence in TEC after 2, 4, 7, or 10 days of culture. Only about 1% of cultured TEC expressed HLA-DR after 10 days in primary culture, whereas 51% of TEC were HLA-DR–positive cells after 2 days (Fig 2). Fas antigen expression followed the same pattern and decreased threefold during primary culture, between day 2 and day 10.
Fas and HLA-DR expression is lost during culture of TEC. Primary cultures of human TEC were established as described in Materials and Methods. TEC were collected after 2, 4, 7, or 10 days of primary culture by trypsin treatment and then labeled with FITC-conjugated anti–HLA-DR or anti-Fas antibodies. TEC were first incubated with anti-Fas monoclonal antibody for 30 minutes at 4°C, then washed in HBSS supplemented with 5% fetal serum calf, stained with biotin-coupled goat antimouse IgG antibody, washed, and incubated with streptavidin/Quantum Red conjugate. Cell labeling was analyzed on a FACScan flow cytometer. Vertical bars on Fas histograms indicate the Fas MFI level in TEC on day 2. Fas antigen expression fell during culture, similarly to HLA-DR antigen expression.
Fas and HLA-DR expression is lost during culture of TEC. Primary cultures of human TEC were established as described in Materials and Methods. TEC were collected after 2, 4, 7, or 10 days of primary culture by trypsin treatment and then labeled with FITC-conjugated anti–HLA-DR or anti-Fas antibodies. TEC were first incubated with anti-Fas monoclonal antibody for 30 minutes at 4°C, then washed in HBSS supplemented with 5% fetal serum calf, stained with biotin-coupled goat antimouse IgG antibody, washed, and incubated with streptavidin/Quantum Red conjugate. Cell labeling was analyzed on a FACScan flow cytometer. Vertical bars on Fas histograms indicate the Fas MFI level in TEC on day 2. Fas antigen expression fell during culture, similarly to HLA-DR antigen expression.
Fas antigen expression by cultured TEC is upregulated by cytokines.
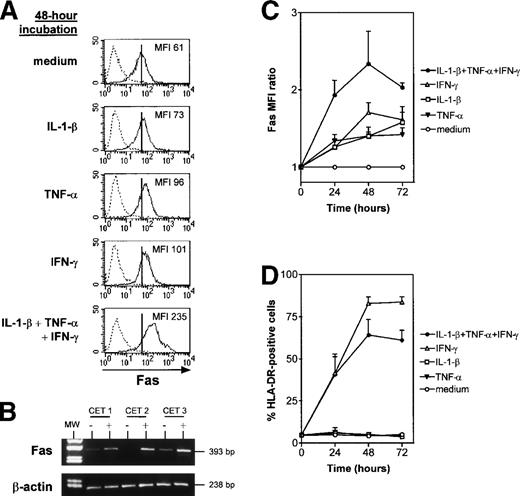
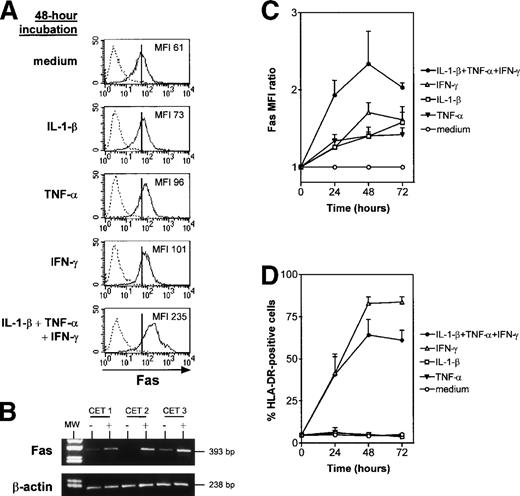
Fas and HLA-DR expression were monitored by means of flow cytometry after cytokine treatment (IL-1-β, TNF-α, and IFN-γ) of TEC subcultures. All three cytokines individually upregulated Fas expression (Fig 3A). The Fas MFI in the presence of one or several cytokines was expressed as a ratio relative to control values. Fas expression by cultured TEC increased more strongly when the three cytokines were added together than when they were added separately (Fig 3A and C): the Fas MFI ratio was 2.4 ± 0.4 after 48 hours in the presence of IL-1-β, TNF-α, and IFN-γ, compared with 1.4 ± 0.05 with IL-1-β, 1.4 ± 0.1 with TNF-α, and 1.7 ± 0.1 with IFN-γ. Like HLA-DR, the effect was maximal after 48 hours of incubation (Fig 3C and D). HLA-DR expression was increased by IFN-γ, as previously described,33 but not by IL-1-β and TNF-α (Fig 3D); the combined effect of the three cytokines was slightly less potent than that of IFN-γ alone.
IL-1-β, TNF-, and IFN-γ, alone and in combination, upregulate Fas expression in cultured TEC. TEC subcultured after 10 to 13 days of primary culture were incubated with the cytokines. At 24, 48, or 72 hours, TEC were collected by trypsin treatment and labeled with anti–HLA-DR or anti-Fas antibody. (A) A representative experiment shows that, after 48 hours of incubation, IL-1-β, TNF-, and IFN-γ, both alone and together, increased Fas MFI. Vertical bars on Fas staining histograms indicate the Fas MFI level in TEC cultured in control conditions, ie, in medium. (B) mRNA was extracted from TEC cultured without (−) or with (+) 1 ng/mL IL-1-β, 10 ng/mL TNF-, and 500 U/mL IFN-γ. Lane 1 corresponds to the molecular weight (MW) marker (pUC18 DNA Marker Hae III digest; Sigma). mRNA was submitted to RT-PCR. In three independent experiments, cytokine-activated TEC showed a strong increase in Fas mRNA levels in comparison to TEC cultured in control conditions, whereas β-actin expression was not modified. (C) The MFI ratio is the ratio between Fas MFI measured in the presence of one or several cytokines and Fas MFI measured in control conditions. MFI ratios are expressed as a function of time. Results are means ± SEM of four independent experiments. (D) The proportion of HLA-DR–positive cells was analyzed in the same experiments.
IL-1-β, TNF-, and IFN-γ, alone and in combination, upregulate Fas expression in cultured TEC. TEC subcultured after 10 to 13 days of primary culture were incubated with the cytokines. At 24, 48, or 72 hours, TEC were collected by trypsin treatment and labeled with anti–HLA-DR or anti-Fas antibody. (A) A representative experiment shows that, after 48 hours of incubation, IL-1-β, TNF-, and IFN-γ, both alone and together, increased Fas MFI. Vertical bars on Fas staining histograms indicate the Fas MFI level in TEC cultured in control conditions, ie, in medium. (B) mRNA was extracted from TEC cultured without (−) or with (+) 1 ng/mL IL-1-β, 10 ng/mL TNF-, and 500 U/mL IFN-γ. Lane 1 corresponds to the molecular weight (MW) marker (pUC18 DNA Marker Hae III digest; Sigma). mRNA was submitted to RT-PCR. In three independent experiments, cytokine-activated TEC showed a strong increase in Fas mRNA levels in comparison to TEC cultured in control conditions, whereas β-actin expression was not modified. (C) The MFI ratio is the ratio between Fas MFI measured in the presence of one or several cytokines and Fas MFI measured in control conditions. MFI ratios are expressed as a function of time. Results are means ± SEM of four independent experiments. (D) The proportion of HLA-DR–positive cells was analyzed in the same experiments.
Moreover, Fas mRNA levels monitored by RT-PCR were low or undetectable in control conditions and were strikingly increased after 24 hours in the presence of the three cytokines, suggesting that Fas upregulation occurred at the transcription level (Fig 3B).
Activated thymocytes induce Fas upregulation in TEC.
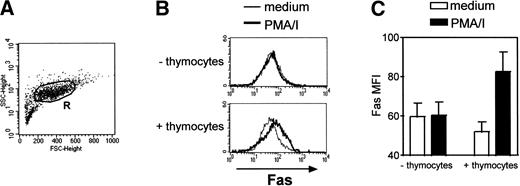
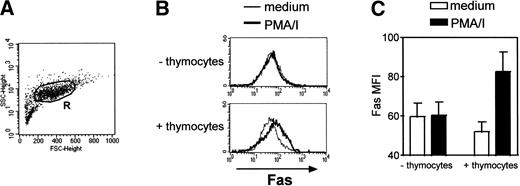
Heterologous thymocytes were cocultured with TEC for a 3-day period. Fas expression was analyzed in TEC only, ie, in cells in the R region (Fig 4A) according to forward-and side-scatter parameters. Thus, thymocytes (that have smaller size) were excluded from the analysis. In the absence of any activation, Fas expression was not modified in TEC (Fig 4). In the absence of thymocytes, the addition of PMA, a phorbol ester that activated protein kinase C, and ionomycin, a calcium ionophore, did not have any effect on Fas expression in TEC. By contrast, when thymocytes activated by these agents were cocultured with TEC, we measured an increase in Fas MFI in TEC: it was 52.0 ± 5.0 in TEC cocultured with thymocytes in medium and 82.7 ± 9.9 in TEC cocultured with activated thymocytes (n = 3). Thus, activated thymocytes are able to upregulate Fas expression in TEC.
Fas upregulation is induced in TEC cocultured with activated thymocytes. TEC subcultured after 10 to 13 days of primary culture were cocultured with heterologous thymocytes in the presence or in the absence of 5 ng/mL PMA and 500 ng/mL ionomycin (PMA/I). After 3 days, TEC were collected. (A) Fas expression was analyzed in TEC, ie, in cells gated in the R region. (B) A representative experiment shows that, without thymocytes, PMA and ionomycin did not induce any effect on Fas expression in TEC. When cocultured with thymocytes, Fas expression is upregulated in TEC when PMA and ionomycin were added. (C) Data are the mean ± SEM from three independent experiments.
Fas upregulation is induced in TEC cocultured with activated thymocytes. TEC subcultured after 10 to 13 days of primary culture were cocultured with heterologous thymocytes in the presence or in the absence of 5 ng/mL PMA and 500 ng/mL ionomycin (PMA/I). After 3 days, TEC were collected. (A) Fas expression was analyzed in TEC, ie, in cells gated in the R region. (B) A representative experiment shows that, without thymocytes, PMA and ionomycin did not induce any effect on Fas expression in TEC. When cocultured with thymocytes, Fas expression is upregulated in TEC when PMA and ionomycin were added. (C) Data are the mean ± SEM from three independent experiments.
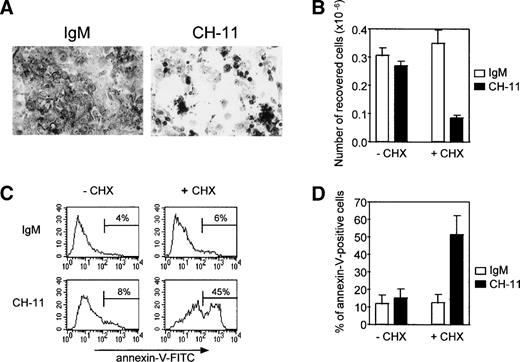
Susceptibility of TEC to cell death induced by an agonistic anti-Fas antibody on day 4 of culture.
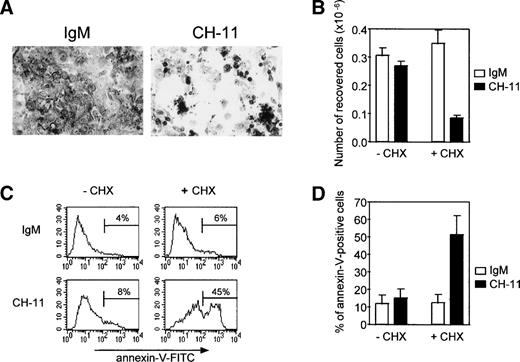
Because Fas antigen expression decreased gradually during culture, we examined whether the remaining Fas antigen was functional and whether TEC in primary culture were sensitive to an agonistic anti-Fas antibody. TEC on day 4 were treated with 0.5 μg/mL anti-Fas IgM antibody (clone CH-11) or with 0.5 μg/mL mouse IgM. Susceptibility to Fas-induced apoptosis was assessed by (1) the number of viable cells recovered and (2) the proportion of annexin-V–positive cells (Fig 5). Although Fas antigen expression was still strong on day 4 of primary culture, TEC were not sensitive to Fas-induced apoptosis at this time. This resistance to Fas-induced apoptosis was reversed by concomitant addition of 10 μg/mL cycloheximide, an inhibitor of protein synthesis. In these conditions, cell density was reduced (Fig 5A), cell recovery was about 70% of that in the presence of IgM and cycloheximide or in the absence of cycloheximide (Fig 5B), and the proportion of annexin-V–positive cells was significantly increased (P < .05, n = 4; Fig 5C and D). TEC on day 4 of culture were also resistant to 0.5 μg/mL recombinant soluble Fas ligand and this resistance was similarly reversed by concomitant addition of cycloheximide (not shown).
Resistance to Fas-induced apoptosis of human TEC on day 4 of primary culture can be raised by concomitant addition of cycloheximide. TEC were collected on day 3 of primary culture and subcultured in 24-well plates. After a 24-hour period to allow cells to adhere, 0.5 μg/mL agonistic anti-Fas IgM antibody (clone CH-11) or 0.5 μg/mL mouse IgM antibody was added in the presence or absence of 10 μg/mL cycloheximide. (A) After cell fixation in ethanol containing 5% acetic acid, cells were stained with Toluidine blue and photographed. In the absence of cycloheximide, no change in cell density was observed (data not shown). When cycloheximide was added, cell density was clearly reduced in the presence of agonistic anti-Fas antibody (clone CH-11) in comparison to cells treated with IgM. (B) Living cells recovered from 0.5 × 106 cells subcultured in 24 wells on day 3 were counted after the culture by using the Trypan blue exclusion method. In the absence of cycloheximide, the number of cells collected was not significantly modified by anti-Fas. By contrast, when cycloheximide was added, the number of cells recovered was clearly reduced in the presence of anti-Fas. Data are the means ± SEM of three independent experiments. (C) Apoptosis was analyzed by quantifying phosphatidylserine residues exposed on the external cell membrane. Annexin-V binding was performed as previously described. A representative analysis shows that TEC undergo anti-Fas (CH-11)–induced apoptosis in the presence of cycloheximide, because the proportion of annexin-V–positive cells is increased relative to IgM treatment, whereas TEC were resistant in the absence of cycloheximide. (D) Data are the means ± SEM of four independent experiments.
Resistance to Fas-induced apoptosis of human TEC on day 4 of primary culture can be raised by concomitant addition of cycloheximide. TEC were collected on day 3 of primary culture and subcultured in 24-well plates. After a 24-hour period to allow cells to adhere, 0.5 μg/mL agonistic anti-Fas IgM antibody (clone CH-11) or 0.5 μg/mL mouse IgM antibody was added in the presence or absence of 10 μg/mL cycloheximide. (A) After cell fixation in ethanol containing 5% acetic acid, cells were stained with Toluidine blue and photographed. In the absence of cycloheximide, no change in cell density was observed (data not shown). When cycloheximide was added, cell density was clearly reduced in the presence of agonistic anti-Fas antibody (clone CH-11) in comparison to cells treated with IgM. (B) Living cells recovered from 0.5 × 106 cells subcultured in 24 wells on day 3 were counted after the culture by using the Trypan blue exclusion method. In the absence of cycloheximide, the number of cells collected was not significantly modified by anti-Fas. By contrast, when cycloheximide was added, the number of cells recovered was clearly reduced in the presence of anti-Fas. Data are the means ± SEM of three independent experiments. (C) Apoptosis was analyzed by quantifying phosphatidylserine residues exposed on the external cell membrane. Annexin-V binding was performed as previously described. A representative analysis shows that TEC undergo anti-Fas (CH-11)–induced apoptosis in the presence of cycloheximide, because the proportion of annexin-V–positive cells is increased relative to IgM treatment, whereas TEC were resistant in the absence of cycloheximide. (D) Data are the means ± SEM of four independent experiments.
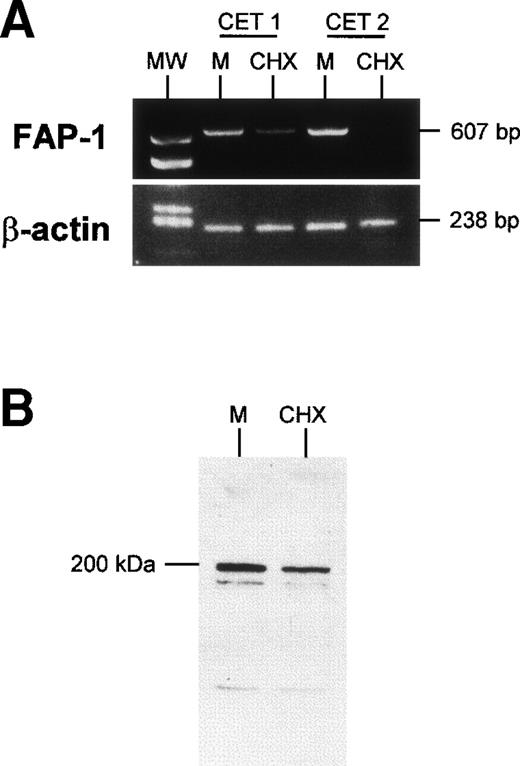
FAP-1 regulation in TEC.
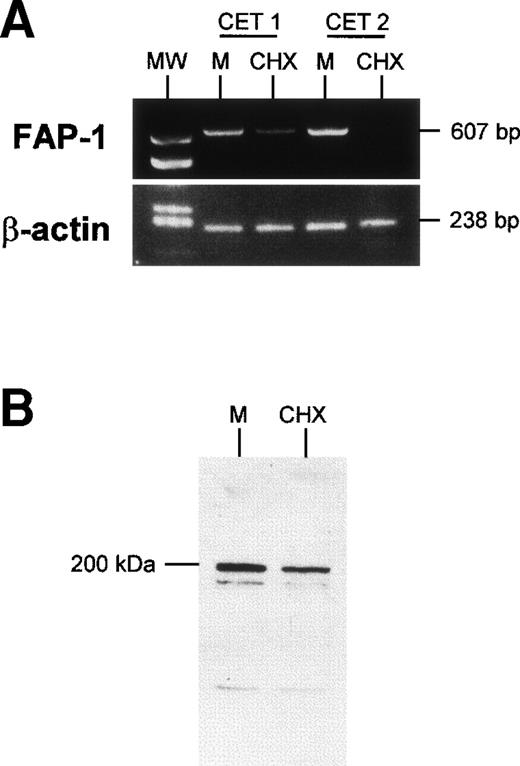
Because expression of FAP-1, a Fas-associated phophatase, protects cells from Fas-induced apoptosis,13 we wondered whether FAP-1 expression was altered by cycloheximide treatment. TEC collected on day 3 of primary culture and left to adhere for 24 hours were further cultured for 24 hours in the presence or absence of 10 μg/mL cycloheximide. They were then harvested and FAP-1 mRNA was examined. Cycloheximide strongly reduced FAP-1 mRNA levels, whereas it did not affect β-actin mRNA levels (Fig 6). Fas mRNA levels were not significantly affected by the cycloheximide treatment (data not shown). In similar experiments, TEC protein extracts (20 μg protein in each condition) were analyzed in Western blot assay. We observed a major protein band (apparent molecular weight, 200 kD) detected by anti–FAP-1 antibody; it was clearly reduced in the presence of cycloheximide. These results show that FAP-1 downregulation coincides with the acquisition of susceptibility to Fas-induced apoptosis in human TEC.
Downregulation of FAP-1 expression in TEC in the presence of cycloheximide. TEC on day 4 of primary culture were incubated for 24 hours with 10 μg/mL cycloheximide (CHX) or in medium (M). (A) mRNA was extracted and submitted to RT-PCR. In two independent experiments, treatment of TEC with cyloheximide induced a clear decrease in FAP-1 mRNA levels, whereas β-actin expression was not modified. (B) Western blot analysis of FAP-1 expression. TEC were solubilized, and 20 μg total protein in each condition was analyzed on SDS-PAGE 7.5% and transferred to PVDF membranes. The blot was incubated with anti–FAP-1 antibody and then with peroxidase-conjugated antigoat Ig. Immunoreactivity was determined using the ECL chemiluminescence reaction. A major protein band (apparent molecular weight, 200 kD) was decreased in the presence of cycloheximide.
Downregulation of FAP-1 expression in TEC in the presence of cycloheximide. TEC on day 4 of primary culture were incubated for 24 hours with 10 μg/mL cycloheximide (CHX) or in medium (M). (A) mRNA was extracted and submitted to RT-PCR. In two independent experiments, treatment of TEC with cyloheximide induced a clear decrease in FAP-1 mRNA levels, whereas β-actin expression was not modified. (B) Western blot analysis of FAP-1 expression. TEC were solubilized, and 20 μg total protein in each condition was analyzed on SDS-PAGE 7.5% and transferred to PVDF membranes. The blot was incubated with anti–FAP-1 antibody and then with peroxidase-conjugated antigoat Ig. Immunoreactivity was determined using the ECL chemiluminescence reaction. A major protein band (apparent molecular weight, 200 kD) was decreased in the presence of cycloheximide.
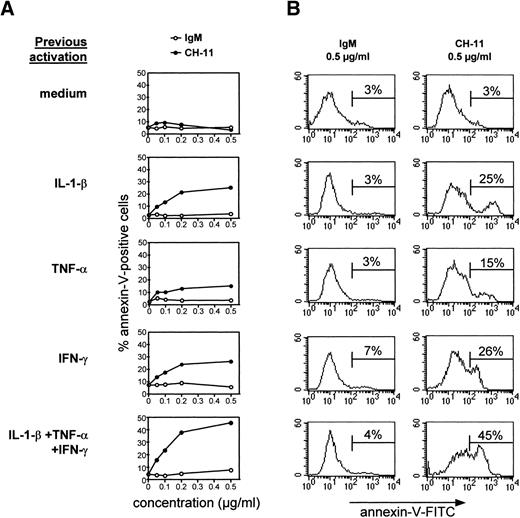
An agonistic anti-Fas antibody induces apoptosis of cytokine-activated TEC.
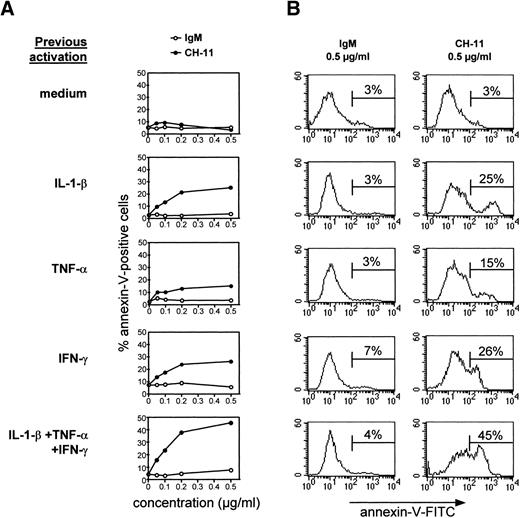
To determine if human TEC activated by cytokines and reexpressing Fas antigen were susceptible to Fas-induced apoptosis, subcultured TEC were incubated for 48 hours with IL-1-β, TNF-α, and IFN-γ, alone or in combination, and then treated with various concentrations of IgM or anti-Fas antibody (clone CH-11). TEC were collected by trypsin treatment after 24 hours and annexin-V binding was examined. TEC cultured in the absence of cytokines were not susceptible to apoptosis induced by agonistic anti-Fas antibody (Fig7) or recombinant soluble Fas ligand (not shown); this resistance was not reversed by concomitant addition of cycloheximide (not shown). By contrast, TEC previously treated with IL-1-β, TNF-α, and IFN-γ, especially in combination, underwent apoptosis. Thus, the level of anti-Fas–induced apoptosis was thus related to the level of Fas expression. Apoptosis was maximal with 0.5 μg/mL agonistic anti-Fas antibody.
Cytokine-activated TEC are sensitive to anti-Fas–induced apoptosis. TEC subcultured after 10 to 13 days of primary culture were incubated in the presence of 1 ng/mL IL-1-β, 10 ng/mL TNF-, and 500 U/mL IFN-γ, alone or in combination, for 48 hours. After two washes, various concentrations of agonistic anti-Fas antibody or control IgM were added. After 24 hours, cells were collected by trypsin treatment and labeled with annexin-V–FITC and propidium iodide. Dead cells, ie, cells incorporating propidium iodide, were excluded from the analysis. A representative analysis is shown. (A) The proportion of annexin-V–positive cells among total living cells is expressed as a function of the concentration of IgM or anti-Fas. Increasing concentrations of IgM were inactive, whereas Fas-mediated apoptosis was concentration-dependent. (B) The analysis of annexin-V–FITC binding in TEC previously activated by cytokines and incubated with 0.5 μg/mL anti-Fas CH-11 or IgM is presented.
Cytokine-activated TEC are sensitive to anti-Fas–induced apoptosis. TEC subcultured after 10 to 13 days of primary culture were incubated in the presence of 1 ng/mL IL-1-β, 10 ng/mL TNF-, and 500 U/mL IFN-γ, alone or in combination, for 48 hours. After two washes, various concentrations of agonistic anti-Fas antibody or control IgM were added. After 24 hours, cells were collected by trypsin treatment and labeled with annexin-V–FITC and propidium iodide. Dead cells, ie, cells incorporating propidium iodide, were excluded from the analysis. A representative analysis is shown. (A) The proportion of annexin-V–positive cells among total living cells is expressed as a function of the concentration of IgM or anti-Fas. Increasing concentrations of IgM were inactive, whereas Fas-mediated apoptosis was concentration-dependent. (B) The analysis of annexin-V–FITC binding in TEC previously activated by cytokines and incubated with 0.5 μg/mL anti-Fas CH-11 or IgM is presented.
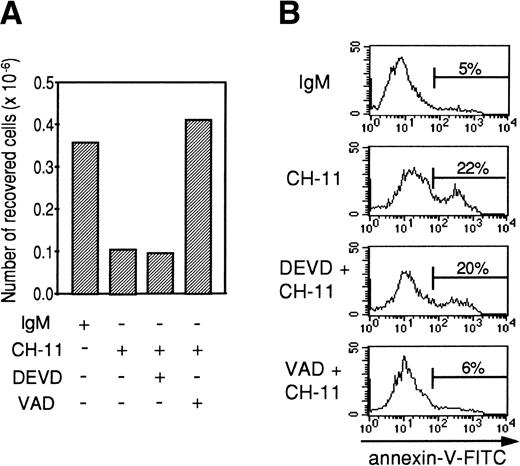
Effect of Z-DEVD-fmk and Z-VAD-fmk peptides on anti-Fas–induced apoptosis in TEC and DEVDase assay in Fas-stimulated TEC.
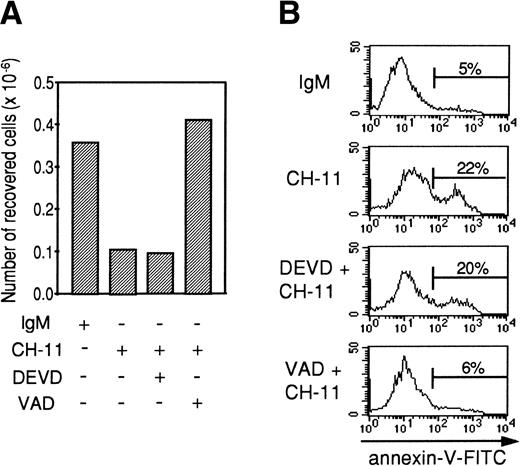
Tripeptide Z-VAD-fmk is a cystein protease inhibitor of broad specificity, whereas Z-DEVD-fmk inhibits more specifically cystein proteases from the caspase-3 family (DEVDases). In preliminary experiments, Z-VAD-fmk and Z-DEVD-fmk alone did not have any effect on TEC apoptosis. To study the effects of these caspase inhibitors on anti-Fas–induced apoptosis, TEC were first cultured for 48 hours with the combination of IL-1-β, TNF-α, and IFN-γ. After two washes, TEC were incubated for 2 hours with 20 μmol/L Z-VAD-fmk or Z-DEVD-fmk, and 0.5 μg/mL agonistic anti-Fas antibody or mouse IgM was then added. After 24 hours, the cells were collected by trypsin treatment. As expected (Fig 8), the agonistic anti-Fas antibody depleted viable cells relative to the control IgM antibody. Z-DEVD-fmk did not modify the number of cells recovered or the proportion of annexin-V–positive cells. By contrast, Z-VAD-fmk restored the number of cells collected and inhibited anti-Fas–induced apoptosis. Using a specific colorimetric substrate (DEVD-pNA), DEVDase activity was measured in cytokine-activated TEC that were cultured during 7 hours in the presence of 0.5 μg/mL IgM or 0.5 μg/mL CH-11. We also evaluated the proportion of apoptotic cells among living cells using annexin-V staining. As shown in Table 1, after 7 hours of incubation with agonistic anti-Fas antibody, TEC already undergo Fas-specific apoptosis, because the proportion of annexin-V–positive cells among living cells was increased in the presence of CH-11. DEVDase activity was slightly increased (2.1 and 2.4 times in 2 independent experiments). In comparison, in a granule-mediated apoptosis in which caspase-3 family was clearly involved,36 DEVDase activity was increased 12.1 and 15.6 times, respectively, in K562 target cells killed by lymphokine-activated killer cells compared with K562 alone. Thus, the slight activation of DEVDase in Fas-stimulated TEC is well correlated to the noninhibition of apoptosis induced by z-DEVD-fmk and is probably not sufficient to induce TEC apoptosis.
Z-VAD-fmk, contrary to Z-DEVD-fmk, inhibits Fas-mediated apoptosis in cytokine-activated TEC. A representative experiment (from 3 independent experiments) is shown. Human TEC were activated for 48 hours with 1 ng/mL IL-1-β, 10 ng/mL TNF-, and 500 U/mL IFN-γ. After two washes, cells were preincubated with 20 μmol/L Z-VAD-fmk or 20 μmol/L Z-DEVD-fmk where indicated, before adding 0.5 μg/mL IgM or 0.5 μg/mL anti-Fas. Twenty-four hours later, cells were harvested and the number of living cells was measured using the Trypan exclusion dye method (A); annexin-V–FITC binding was also measured (B).
Z-VAD-fmk, contrary to Z-DEVD-fmk, inhibits Fas-mediated apoptosis in cytokine-activated TEC. A representative experiment (from 3 independent experiments) is shown. Human TEC were activated for 48 hours with 1 ng/mL IL-1-β, 10 ng/mL TNF-, and 500 U/mL IFN-γ. After two washes, cells were preincubated with 20 μmol/L Z-VAD-fmk or 20 μmol/L Z-DEVD-fmk where indicated, before adding 0.5 μg/mL IgM or 0.5 μg/mL anti-Fas. Twenty-four hours later, cells were harvested and the number of living cells was measured using the Trypan exclusion dye method (A); annexin-V–FITC binding was also measured (B).
DISCUSSION
We report that Fas antigen is constitutively expressed by medullary epithelial cells. Fas expression is lost by cultured TEC but can be restored by cytokines. TEC were sensitive to anti-Fas–induced apoptosis in the presence of cycloheximide on day 4 of culture (when Fas expression was still high) and when subcultured on day 10 to 13 if they had been activated by cytokines. These results point to functional expression of Fas antigen by human medullary TEC.
Fas and Fas ligand expression in the human thymus.
Fas antigen expression has been examined in mouse and human thymic lymphoid cells. Most mouse thymocytes are Fas-positive,18,19 whereas only a minor subset strongly express Fas in the human thymus.28-30 Fas expression in the epithelial compartment also seems to differ between mice and humans. Indeed, Fas expression was not found in the epithelial network of mouse thymus. French et al37 characterized the expression of Fas and its ligand in the mouse thymus by using in situ hybridization and immunohistochemistry. They detected Fas expression in thymocytes and strong Fas ligand expression in thymic epithelial cells and dendritic cells. We found weak expression of Fas ligand mRNA in human thymus by means of RT-PCR, but no protein expression was found in situ (not shown).These results suggest that the Fas/Fas ligand system is differently expressed in the mouse and human thymus and could have different functions.
We found that Fas antigen expression decreased during primary human TEC culture, reaching minimal levels after 10 days. This effect was unlikely to be due to in vitro selection of cortical epithelial cells, which do not express the Fas receptor, because epithelial cells collected after 2, 4, 7, or 10 days of primary culture were strongly labeled by an antikeratin antibody (clone CK1) that mainly stains the medullary epithelial network (not shown). Because Fas was detected in situ, its expression could be downregulated in the absence of the thymic microenvironment, as previously shown for HLA-DR in these cells.33 This is supported by the moderate upregulation of Fas expression in TEC cocultured for 3 days with activated human thymocytes. Fas expression in vivo could be maintained by contact with thymocytes. Fas is mostly expressed in the medulla, where most mature thymocytes are found. Mature human CD3high thymocytes (both double- and single-positive CD4+/CD8+ cells) can secrete consistent amounts of cytokines38 and play a key role in the differentiation of medullary TEC.39 Thus, medullary mature thymocytes might interact with medullary TEC, resulting in the maintenance of Fas expression.
Role of Fas in epithelial cells of the human thymus.
Most nonlymphoid tissues constitutively coexpressing Fas and its ligand in adult mice40 and humans41 are characterized by apoptotic cell turnover, possibly regulated by the Fas system. Fas is directly involved in the regression of the vaginal epithelium after ovariectomy and during the estrous cycle in mice.42Moreover, Fas ligation induces apoptosis in various epithelia in vitro, including ovarian surface epithelial cells,43 thyroid epithelial cells,4,44 and colon epithelial cells.45 Our findings show that Fas can also induce apoptosis of thymic epithelial cells in vitro.
Regarding the physiologic role of Fas in the thymus epithelial compartment, Fas could regulate the turnover of TEC, especially when thymus involutes after childhood. Although no substantial expression of Fas ligand protein is detected in the human thymus, activated rat thymocytes46 and activated human thymocytes and TEC (not shown) can express Fas ligand. Both Fas and its ligand can be produced by TEC in vitro, but we did not observe cell death in those cells in the absence of agonistic anti-Fas antibody. Further studies are needed to determine if Fas ligand produced by activated TEC is also secreted into the extracellular medium, because membrane-associated and secreted Fas ligand seem to have different capacities to induce apoptosis,47 and soluble Fas ligand can block Fas-induced cell death.48
Intracellular partners of the Fas receptor in the induction of cell death.
On day 4 of primary culture, when they still expressed significant amounts of Fas antigen, TEC were resistant to Fas-induced apoptosis, and cycloheximide reversed this resistance. Metabolic inhibitors such as actinomycin D and cycloheximide can sensitize some cells to anti-Fas–mediated apoptosis in both mice18 and humans,44,49 suggesting that a labile protein might inhibit Fas-mediated cell death. We found that cycloheximide downregulated FAP-1, a phosphatase able to inhibit Fas-mediated apoptosis. Similarly, Mori et al49 showed that actinomycin D induced sensitivity to Fas-induced apoptosis and downregulated FAP-1 mRNA in Kaposi’s sarcoma cells. FAP-1 may thus be involved in TEC resistance to Fas-induced apoptosis on day 4 of primary culture. Other molecules could be involved. Intracellular glutathione, levels of which are reduced by cycloheximide, can mediate Fas resistance in human T lymphocytes.50 Fournel et al51 showed that human T cells required IL-2 to acquire susceptibility to Fas-mediated apoptosis, and the investigators suggested that IL-2 may decrease FAP-1 expression. TEC subcultured with or without cytokines contained significant levels of FAP-1 mRNA (not shown), and cytokine-activated TEC were sensitive to Fas-mediated apoptosis, showing that FAP-1 expression was not sufficient to induce resistance to Fas-mediated cell death in cytokine-activated cells. Thus, activation by cytokines can overcome the resistance to Fas-induced cell death induced by FAP-1. In immature20 and mature52 T lymphocytes, additional signals were shown to interfere with the Fas pathway. We show here that Fas-induced apoptosis of cultured TEC requires (1) Fas expression and (2) another signal that can be provided by the removal of a labile protein able to inhibit the Fas signal (eg, the phosphatase FAP-1) or by cell activation by cytokines. In lymphoid and nonlymphoid cells, additional signals that interfere with the Fas pathway (other than the one produced by FAP-1) need to be clarified.
Caspase-8 plays a pivotal role in Fas-induced apoptosis10,11 and links the Fas signaling complex and other ICE-like caspases. Caspase-3 is a major cysteine protease activated after Fas triggering.53 However, in caspase-3 knock-out mice,54 Fas-induced apoptosis and poly(ADP-ribose) polymerase (PARP) cleavage were not impaired. We found that Fas-induced apoptosis of cytokine-activated TEC was inhibited by Z-VAD-fmk, a cysteine protease inhibitor, but not by Z-DEVD-fmk, a specific inhibitor of caspases from the caspase-3 family or DEVDases. Moreover, DEVDase activity is slightly increased in Fas-stimulated TEC, compared with a granule-mediated apoptosis in which DEVDase is implicated and compared with Fas-stimulated cells whose apoptosis is clearly related to DEVDase activity as Jurkat cells (in which DEVDase activity is 26 times increased in the presence of agonistic anti-Fas antibody55). This suggests that Fas-induced apoptosis of TEC is mediated by cysteine proteases but DEVDase activity is probably not sufficient to induce Fas-mediated apoptosis in TEC.
In conclusion, human medullary TEC express Fas in situ and are able to undergo Fas-induced apoptosis. This original system of Fas-mediated cell death in nonlymphoid cells could serve as a model to examine the intracellular signals conferring susceptibility to Fas-mediated apoptosis or to its execution.
ACKNOWLEDGMENT
The authors are grateful to Dr E. Dulmet and S. Planté (Service d’Anatomo-Pathologie, Hôpital Marie-Lannelongue, Le Plessis-Robinson, France) for technical advice in the histological experiments, to Dr J. Bréard (INSERM U461) for the generous gift of Z-DEVD-fmk and Z-VAD-fmk and for helpful discussions, and to N. Riché (INSERM U461) for technical assistance.
Supported by grants from Association Française contre les Myopathies (AFM), Centre National de la Recherche Scientifique (CNRS), and Caisse Nationale d’Assurance Maladie des Travailleurs Salariés (CNAMTS). N.M. received a postdoctoral grant from FRM (Fondation pour la Recherche Médicale).
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Nathalie Moulian, PhD, Laboratoire d’Immunologie Cellulaire et Moléculaire, CNRS UPRESA, Hôpital Marie-Lannelongue, 133 avenue de la Résistance, 92350 Le Plessis Robinson, France.