Hematopoietic stem cell (HSC) self-renewal in vitro has been reported to result in a diminished proliferative capacity or acquisition of a homing defect that might compromise marrow repopulation. Our group has demonstrated that human HSC expanded ex vivo in the presence of porcine microvascular endothelial cells (PMVEC) retain the capacity to competitively repopulate human bone fragments implanted in severe combined immunodeficiency (SCID) mice. To further test the marrow repopulating capacity of expanded stem cells, our laboratory has established a myeloablative, fractionated total body irradiation conditioning protocol for autologous marrow transplantation in baboons. A control animal, which received no transplant, as well as two animals, which received a suboptimal number of marrow mononuclear cells, died 37, 43, and 59 days postirradiation, respectively. Immunomagnetically selected CD34+ marrow cells from two baboons were placed in PMVEC coculture with exogenous human cytokines. After 10 days of expansion, the grafts represented a 14-fold to 22-fold increase in cell number, a 4-fold to 5-fold expansion of CD34+ cells, a 3-fold to 4-fold increase of colony-forming unit–granulocyte-macrophage (CFU-GM), and a 12-fold to 17-fold increase of cobblestone area-forming cells (CAFC) over input. Both baboons became transfusion independent by day 23 posttransplant and achieved absolute neutrophil count (ANC) >500/μL by day 25 ± 1 and platelets >20,000/μL by day 29 ± 2. This hematopoietic recovery was delayed in comparison to two animals that received either a graft consisting of freshly isolated, unexpanded CD34+ cells or 175 × 106/kg unfractionated marrow mononuclear cells. Analysis of the proliferative status of cells in PMVEC expansion cultures demonstrated that by 10 days, 99.8% of CD34+ cells present in the cultures had undergone cycling, and that the population of cells expressing a CD34+ CD38− phenotype in the cultures was also the result of active cell division. These data indicate that isolated bone marrow CD34+ cells may undergo cell division during ex vivo expansion in the presence of endothelial cells to provide a graft capable of rescuing a myeloablated autologous host.
THE SAFETY AND efficacy of ex vivo–expanded human hematopoietic cell populations is already being evaluated in the clinical setting.1-3 Currently, such protocols are focused on the in vitro culture of large numbers of mobilized peripheral blood CD34+ cells. Stem cell number has been demonstrated to determine the marrow repopulating potential of a graft.4-6 Ex vivo hematopoietic stem cell (HSC) expansion is particularly important for cord blood transplantation. The potential use of umbilical cord blood HSC as a source of grafts is limited by the relatively low numbers of cells obtained, especially for an adult recipient.7,8 The multitude of retroviral gene transfer strategies currently being designed is also limited by the need for the target HSC population to undergo cell division for integration of the transgene to occur.9 Such cycling must occur in the absence of differentiation for durable expression by cells derived from modified stem cells to be possible. Several studies have shown, however, that HSC lose their proliferative capacity after multiple rounds of division, or that in vitro exposure to cytokines results in a defect in homing or engraftment ability.10-15 Hematopoietic cells comprising a graft must possess both the ability to home to the marrow as well as retain a high proliferative capacity to reconstitute hematopoiesis. The ideal ex vivo stem cell expansion system would thus induce HSC to replicate in vitro without a loss of self-renewal capacity or ability to home to the marrow.
In the absence of an appropriate human model, the baboon has proven useful as a tool for the preclinical evaluation of HSC transplant protocols.16-19 Several monoclonal antibodies, which recognize the human CD34 epitope, also cross-react with the analogous glycoprotein present on primate hematopoietic cells.16-20CD34+ cells isolated from baboon bone marrow and mobilized peripheral blood have been demonstrated to rescue lethally irradiated animals and to restore lymphohematopoiesis.16,17,19 The in vivo mobilization of baboon HSC and in vitro support of baboon hematopoietic cells can be performed using readily available recombinant human cytokines.18 20
Our group has recently reported the rapid in vitro expansion of adult human marrow cells that retain a phenotype consistent with HSC when cultured in the presence of a porcine microvascular endothelial cell (PMVEC) line.21,22 These expanded HSC are capable of engrafting and competitively repopulating human bone fragments implanted in severe combined immunodeficiency (SCID) mice with lymphoid, myeloid, and CD34+ progeny.21Our goal was not to expand progenitor cell number, but rather to expand the number of marrow repopulating cells by self-renewal and assess the function of the expanded graft. In this report, we tested the ability of the expansion product of autologous, selected CD34+cells to engraft lethally irradiated baboons after 10 days of ex vivo expansion culture in the presence of PMVEC and human cytokines. These cocultures promote the in vitro cycling of HSC that remain capable of reconstituting hematopoiesis in the host.
MATERIALS AND METHODS
Animals.
Healthy juvenile baboons (Papio anubis) of both sexes and weighing 8.5 to 11 kg were used. The animals were housed under conditions approved by the Association for the Assessment and Accreditation of Laboratory Animal Care. The studies were performed under protocols approved by the Animal Care Committee of the University of Illinois at Chicago. Two weeks before transplant, the animals were fitted with jackets and placed on a tether system; 1 week later, central venous catheters were placed in the jugular and femoral veins. Beginning 4 days before transplant, the animals received eight fractions of 125 cGy total body irradiation (TBI) from a linear accelerator administered twice daily for a total of 1,000 cGy. After completion of TBI (day 0), the animals were infused with a graft composed of either mononuclear marrow cells, CD34+ marrow cells, or the total cellular expansion product derived from marrow CD34+ cells. The animals were administered 175 to 280 mL whole irradiated blood transfusions from ABO compatible donors when platelet counts fell below 20,000/μL or upon clinical evidence of bleeding. Complete blood counts (CBC) were performed at 24- to 72-hour intervals until the animals reached transfusion independence and catheter removal; thereafter, CBCs were performed at weekly intervals by blood collection under ketamine hydrochloride (HCL) (10 mg/kg) sedation. On reaching a neutropenic state defined as absolute neutrophil count (ANC) <500/μL, prophylactic antibiotics, antivirals, and antifungals (ceftazidine 1,500 mg/d, gentamicin 100 mg/d, fluconazole 60 mg/d, acyclovir 100 mg/d, vancomycin 400 mg/d) were administered via continuous infusion until ANC >500/μL was achieved.
Collection, selection, and cryopreservation of CD34+ marrow cells.
At least 6 weeks before transplantation, bone marrow aspirates were obtained from the humeri and iliac crests of juvenile baboons after ketamine and xylazine (1 mg/kg) anesthesia. Three sets of aspirates per animal were obtained at intervals of at least 2 weeks to minimize stress and the effects of loss of blood volume. The heparinized marrow was diluted 1:15 in phosphate-buffered saline (PBS) and the mononuclear cell fraction obtained by centrifugation over 60% Percoll (Pharmacia LKB, Uppsala, Sweden) at 500g for 30 minutes. The monoclonal antibody (MoAb) K6.1 (gift of the Naval Medical Research Institute, Bethesda, MD), a murine IgG2a, which recognizes the analogous baboon CD34 epitope, was used for the selection of the CD34+ fraction of marrow cells.20 22 The mononuclear cells were suspended in PBS containing 0.2% bovine serum albumin (Sigma Chemical Co, St Louis, MO) and human immune globulin (Bayer Corp, Elkhart, IN) and stained first with biotin-conjugated K6.1 (20 μg/mL), washed, and labeled with Miltenyi streptavidin-conjugated iron microbeads (Miltenyi Biotech, Auburn, CA) and selected by passage through a magnetic column according to the manufacturer’s instructions. Purity of the positively and negatively selected cells was determined flow cytometrically by counterstaining with streptavidin-phycoerythrin (PE) (Southern Biotechnology, Birmingham, AL). Purity of the CD34+ fractions was 93% to 98%. The selection procedures were more than 99% efficient in enrichment of the progenitor cells in the K6.1+ fraction as determined by methylcellulose colony-forming cell assay and cobblestone area-forming cells (CAFC) assays of the K6.1-positive and -negative cell fractions (data not shown). The K6.1-selected or unselected mononuclear marrow cells were cryopreserved at 2 × 107 cells/mL in 50% Iscove’s modified Dulbecco’s medium (IMDM), 40% fetal bovine serum (FBS), and 10% dimethyl sulfoxide (DMSO).
Ex vivo expansion cultures.
PMVEC were maintained and used for stem cell expansion cultures as previously described in detail.21 22 Briefly, cryopreserved baboon K6.1+ cells were thawed and placed onto previously established PMVEC monolayers in 162-cm2 flasks (Costar Corp, Cambridge, MA) at 4 × 105 cells/mL in IMDM containing 7% FBS and recombinant human stem cell factor (SCF) at 100 ng/mL and interleukin-3 (IL-3), IL-6, and granulocyte-macrophage colony-stimulating factor (GM-CSF) at 10 ng/mL (gift of Amgen, Inc, Thousand Oaks, CA). At 3 to 4 days of culture, the cocultures were dispersed by gentle agitation and half of the volumes distributed to additional confluent PMVEC monolayers in 162-cm2 flasks and fed by replacement of fresh medium and cytokines to the original volume in each flask. This procedure was repeated at 3- to 4-day intervals to maintain a nonadherent cell density of <2 × 106/mL. Mononuclear marrow cells from an unrelated animal were also immunomagnetically depleted of K6.1+ cells and the K6.1− fraction plated in 35-mm tissue culture dishes (Costar) at 106 cells/well in 2 mL IMDM containing 10% FBS and 2 × 10−5mol/L 6α-methylprednisolone (Sigma). Cultures were fed weekly by removal of all nonadherent cells and complete medium replacement weekly for 4 weeks, by which time they were confluent with a population of predominantly fibroblasts including adipocytes and macrophages.
Hematopoietic progenitor and CAFC.
Colony-forming units-granulocyte/macrophage (CFU-GM), burst-forming units-erythroid (BFU-E), and colony-forming units-mixed lineage (CFU-Mix) were assayed in methylcellulose culture as previously described.21 Briefly, 5 × 103to 3 × 104 cells were plated in replicates of 1 mL IMDM containing 1.1% methylcellulose, 30% FBS, 5 × 10−5 mol/L 2-mercaptoethanol, 100 ng SCF, 10 ng IL-3, 10 ng GM-CSF, and 5 U human recombinant erythropoietin (gift of Amgen, Inc) in 35-mm tissue culture dishes (Costar). After 13 to 15 days of incubation at 37°C in a 100% humidified atmosphere of 5% CO2 in air, the colonies were scored with an inverted microscope using standard criteria for their identification. CAFC give rise to uniform, multipotential aggregates of more than 50 uniformly sized, refractile cells when cocultured for at least 5 weeks with murine stromal fibroblasts in the presence of human cytokines. Marrow mononuclear cells, CD34+, or expanded cells were placed in limiting dilution of 100 to 0.78 cells/well in 96-well plates onto confluent, irradiated (7,000 cGy) monolayers of the murine stromal fibroblast line M2-10B4. Each well contained 200 μL of a 50:50 mixture of IMDM and RPMI with 10% FBS, 50 ng/mL SCF, 50 ng/mL leukemia inhibitory factor (LIF), and 5 ng/mL IL-3, IL-6, and GM-CSF, which has been shown in our laboratory to optimize the development of CAFC. The cultures were fed weekly by replacement of one half of the culture volume with fresh medium containing the above cytokines at two times final concentration. After 5 weeks of culture at 37°C in a 100% humidified atmosphere of 5% CO2 in air, the CAFC-derived colonies were scored with an inverted microscope using standard criteria for their identification.23 CAFC frequency was computed using minimization of χ by regression to the cell number at which 37% of wells show negative CAFC growth, with 95% statistical precision.
Proliferation analysis using PKH26 dye.
To ascertain the proliferative status of the CD34+CD38− cells in PMVEC coculture, three baboon marrow specimens were immunomagnetically selected for CD34+ cells as described above. The CD34+ cells were next labeled with the lipophilic membrane dye PKH-26 (Sigma), which fluoresces in the PE channel, according to the manufacturer’s instructions. The dye is stably integrated into the cell membrane and the molecules are equally distributed between daughter cells during cellular division; the fluorescent intensity of each successive generation is thus half that of the preceding one.21 The CD34+ marrow cells, uniformly labeled with PKH-26, were placed into duplicate 35-mm tissue culture dishes (Costar) containing preestablished monolayers of either allogeneic baboon marrow stroma derived from the K6.1− fraction of a fourth immunomagnetically selected animal or PMVEC. At initiation and at days 7, 10, and 14 of allogeneic stromal or PMVEC coculture, the cells were harvested and the PKH-26 fluorescence of the CD34+ CD38−cells compared with that of the cells used to initiate the cocultures. CD38+ cells were stained with mIgG1 produced by hybridoma OKT-10 (ATCC, Manassas, VA), which has been demonstrated to cross-react with the baboon24 as well as rhesus25 CD38 epitope, and labeled with fluorescein isothiocyanate (FITC)-conjugated anti-mIgG1 (Southern Biotechnology). Harvested cells were stained with K6.1-biotin and OKT-10 supernatant followed by streptavidin-allophycocyanin (APC) and anti-mIgG1–FITC after two high-volume washes to remove any residual serum. The cells were analyzed on a Becton Dickinson FACS Vantage flow cytometer equipped with argon and helium/neon lasers (Becton Dickinson, San Jose, CA). Nonviable cells were excluded by detection of propidium iodide uptake. The flow cytometer was calibrated with reference microbeads (Sigma), as well as FITC and APC Calibrite reference beads (Becton Dickinson) and the photomultiplier tube voltage and compensation settings saved for the subsequent analyses. Controls consisted of cells stained with irrelevant mIgG1 followed by anti-mIgG1–FITC (Southern Biotechnology) and irrelevant IgG-biotin (Coulter Immunology, Hialeah, FL) and streptavidin-APC (Becton Dickinson). Greater than or equal to 3 × 104cells were analyzed per culture at each time point.
RESULTS
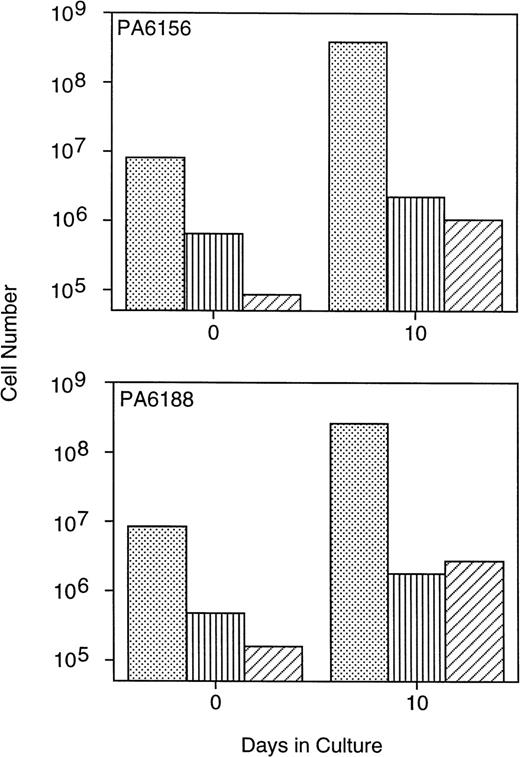
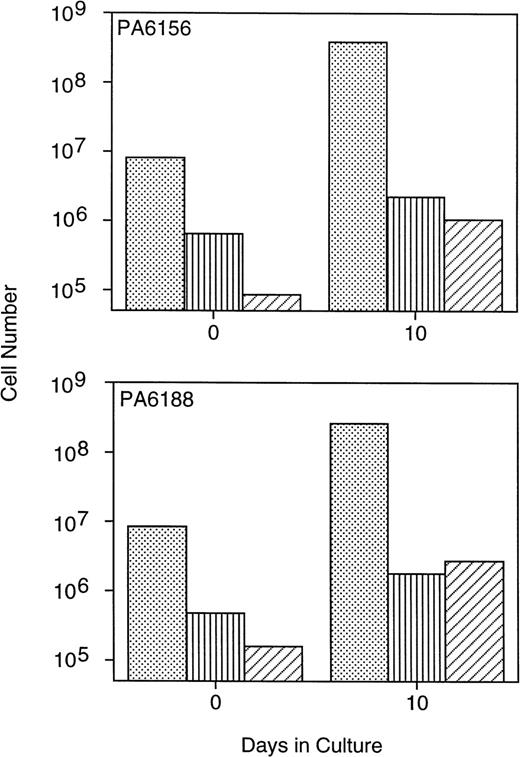
Multiple bone marrow aspirates obtained from three baboons were enriched for CD34+ cells using the MoAb K6.1 coupled with immunomagnetic column selection and immediately cryopreserved. Ten days before transplant, 8.1 × 106/kg (PA6156) and 8.4 × 106/kg (PA6188) CD34+ marrow cells were thawed and placed into PMVEC cocultures, and expanded ex vivo for 10 days in the presence of exogenous human SCF, IL-3, IL-6, and GM-CSF. This resulted in a fourfold to fivefold increase in the total number of CD34+ cells and a threefold to fourfold increase in the number of assayable CFU-GM after 10 days of PMVEC coculture (Fig 1). Erythroid burst-forming cells were also expanded in number by 9-fold to 17-fold (data not shown). Primitive CAFC were expanded in number by greater than one log by day 10 of PMVEC coculture (Fig 1).
Expansion of baboon CD34+ cells and progenitor cells in PMVEC coculture. Bone marrow CD34+cells from two baboons were immunomagnetically selected using the MoAb K6.1 and used to inoculate 10-day expansion cultures in coculture with the porcine microvascular endothelial cell line PMVEC in the presence of exogenous human SCF, IL-3, IL-6, and GM-CSF. The total number of CD34+ cells (dots), CFU-GM (vertical lines), and CAFC (diagonal lines) were determined flow cytometrically, by methylcellulose culture, and by limiting-dilution assay on murine stromal fibroblasts, respectively, at inoculation (day 0) and at harvest (day 10) immediately before infusion into the animals.
Expansion of baboon CD34+ cells and progenitor cells in PMVEC coculture. Bone marrow CD34+cells from two baboons were immunomagnetically selected using the MoAb K6.1 and used to inoculate 10-day expansion cultures in coculture with the porcine microvascular endothelial cell line PMVEC in the presence of exogenous human SCF, IL-3, IL-6, and GM-CSF. The total number of CD34+ cells (dots), CFU-GM (vertical lines), and CAFC (diagonal lines) were determined flow cytometrically, by methylcellulose culture, and by limiting-dilution assay on murine stromal fibroblasts, respectively, at inoculation (day 0) and at harvest (day 10) immediately before infusion into the animals.
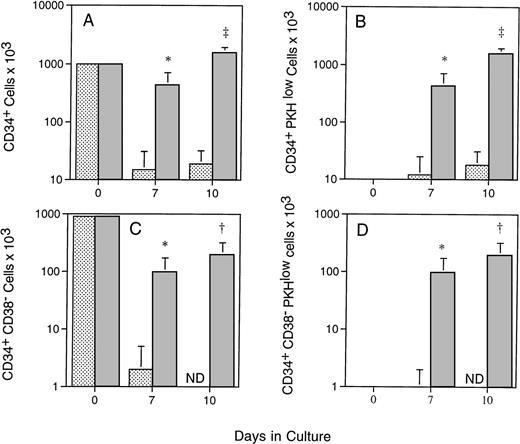
To better understand the kinetics of primitive hematopoietic cell expansion in the PMVEC cocultures, CD34+ cells were selected from marrow specimens obtained from three baboons, labeled with PKH-26, and inoculated onto either preestablished allogeneic marrow stroma derived from the CD34− marrow cell fraction of a fourth animal, or PMVEC monolayers. These cultures were also supplemented with exogenous human SCF, IL-3, IL-6, and GM-CSF, and the proliferation of CD34+ CD38− cells in the cultures monitored using MoAbs K6.1 and OKT-10 (Fig 2). By day 10, the total number of CD34+ cells in the PMVEC cocultures had increased to about 1.5 times the number used to inoculate the cultures, while CD34+ cell numbers in the allogeneic stromal cell cultures had declined to only 15% of input by day 7 (Fig 2A). By 7 days in culture, 99.2% ± 0.3% of the CD34+ cells present in the PMVEC cocultures had undergone multiple rounds of cell division versus 84.7% ± 13.8% of the diminished number in the allogeneic baboon stromal cell cultures; at day 10, the percentages of PKH-26low were 99.8 ± 0.1 and 95.5 ± 4.5, respectively. These differences were reflected in the absolute number of CD34+ PKH-26low cells present in the cultures at 7 and 10 days, showing that the presence of CD34+ cells in the cultures was due to proliferation rather than survival of quiescent cells (Fig 2B). In contrast, the number of cells in both culture systems expressing a primitive CD34+CD38− phenotype decreased. CD34+CD38− cells declined to an undetectable number in the marrow stromal cell cultures (Fig 2C and Fig 3), whereas significantly greater numbers of CD34+ CD38− cells were maintained in the PMVEC cocultures after an initial decline (Fig 2C). PKH-26 labeling confirmed that the presence of CD34+CD38− cells in the PMVEC cocultures was also due to proliferation of cells in this phenotypic compartment, where 99.25% ± 0.85% of the CD34+ CD38− cells present in the cultures had become PKHlow by 10 days. This contrasted with the proliferating CD34+ cells in the allogeneic stromal cell cultures, which progressively gained CD38 expression (Fig 2D and Fig 3).
Proliferation of CD34+ CD38−cells in allogeneic stromal and PMVEC coculture. Immunomagnetically selected CD34+ baboon marrow cells were labeled with the lipophilic dye PKH-26, which stably integrates into the cell membrane and is distributed equally to daughter cells at division, and placed onto preestablished allogeneic baboon marrow stromal cell (light bars) or PMVEC monolayers (dark bars) in the presence of exogenous human SCF, IL-3, IL-6, and GM-CSF. After 7 and 10 days of culture, CD34 and CD38 expression of the expanded cells was determined using the MoAbs K6.1 and OKT-10, respectively. Proliferative status of the cells was determined by PKH fluorescence as described. The total numbers of CD34+ cells (A), CD34+ cells, which have undergone division in vitro (B), CD34+CD38− cells (C), and CD34+CD38− cells, which have undergone division (D) in the cultures are shown. The data represent the mean ± standard deviation (SD) of experiments performed on three separate animals. ND, not detectable above 0.005% of events. *P < .05 (Student’st-test); †P < .02; and ‡P < .001.
Proliferation of CD34+ CD38−cells in allogeneic stromal and PMVEC coculture. Immunomagnetically selected CD34+ baboon marrow cells were labeled with the lipophilic dye PKH-26, which stably integrates into the cell membrane and is distributed equally to daughter cells at division, and placed onto preestablished allogeneic baboon marrow stromal cell (light bars) or PMVEC monolayers (dark bars) in the presence of exogenous human SCF, IL-3, IL-6, and GM-CSF. After 7 and 10 days of culture, CD34 and CD38 expression of the expanded cells was determined using the MoAbs K6.1 and OKT-10, respectively. Proliferative status of the cells was determined by PKH fluorescence as described. The total numbers of CD34+ cells (A), CD34+ cells, which have undergone division in vitro (B), CD34+CD38− cells (C), and CD34+CD38− cells, which have undergone division (D) in the cultures are shown. The data represent the mean ± standard deviation (SD) of experiments performed on three separate animals. ND, not detectable above 0.005% of events. *P < .05 (Student’st-test); †P < .02; and ‡P < .001.
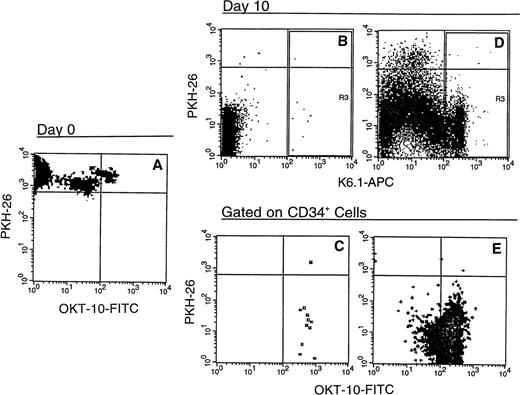
Phenotype of baboon CD34+ bone marrow cells after 10 days in allogeneic stromal and PMVEC coculture. Immunomagnetically selected CD34+ baboon marrow cells (A) were labeled with the lipophilic dye PKH-26 and placed onto preestablished allogeneic baboon marrow stromal cell (B and C) or PMVEC (D and E) monolayers as described in Fig 2. After 10 days of culture in the presence of exogenous human SCF, IL-3, IL-6, and GM-CSF, the cells were harvested and stained with the MoAbs K6.1 and OKT-10, which recognize the baboon CD34 and CD38 antigens, respectively, and secondarily labeled with APC (K6.1) and FITC (OKT-10). After first gating on forward and side scatter and propidium iodide exclusion to consider only live, nucleated cells, events positive for K6.1 (R3, top row) were gated for OKT-10 versus PKH-26 analysis (bottom row). The horizontal cursors define the PKH fluorescence of the freshly isolated and labeled cells; events subsequently below this line have undergone cell division. Machine settings were calibrated using reference microbeads to assure consistency of PKH-26 measurement. The vertical cursors delineate positive K6.1-APC (B and D) and OKT-10-FITC (C and E) fluorescence based on both negative controls consisting of isotype-matched irrelevant antibody secondarily labeled with APC or FITC and positive fluorescence of reference beads; events to the right of these cursors are positive. The data shown represent one of three baboon marrows tested.
Phenotype of baboon CD34+ bone marrow cells after 10 days in allogeneic stromal and PMVEC coculture. Immunomagnetically selected CD34+ baboon marrow cells (A) were labeled with the lipophilic dye PKH-26 and placed onto preestablished allogeneic baboon marrow stromal cell (B and C) or PMVEC (D and E) monolayers as described in Fig 2. After 10 days of culture in the presence of exogenous human SCF, IL-3, IL-6, and GM-CSF, the cells were harvested and stained with the MoAbs K6.1 and OKT-10, which recognize the baboon CD34 and CD38 antigens, respectively, and secondarily labeled with APC (K6.1) and FITC (OKT-10). After first gating on forward and side scatter and propidium iodide exclusion to consider only live, nucleated cells, events positive for K6.1 (R3, top row) were gated for OKT-10 versus PKH-26 analysis (bottom row). The horizontal cursors define the PKH fluorescence of the freshly isolated and labeled cells; events subsequently below this line have undergone cell division. Machine settings were calibrated using reference microbeads to assure consistency of PKH-26 measurement. The vertical cursors delineate positive K6.1-APC (B and D) and OKT-10-FITC (C and E) fluorescence based on both negative controls consisting of isotype-matched irrelevant antibody secondarily labeled with APC or FITC and positive fluorescence of reference beads; events to the right of these cursors are positive. The data shown represent one of three baboon marrows tested.
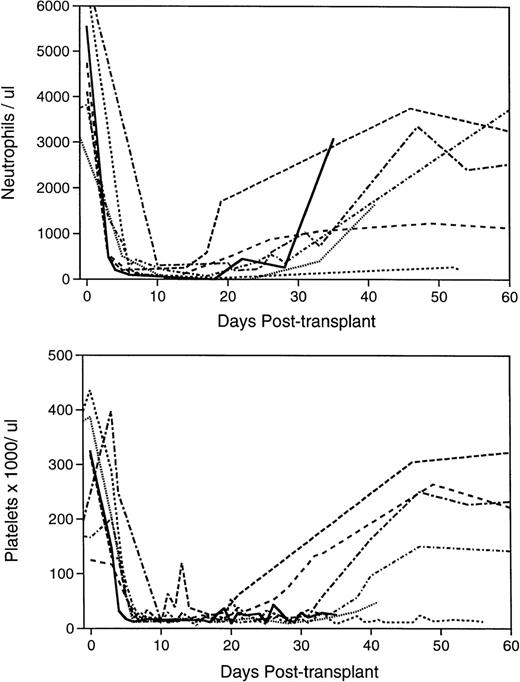
Both animals, which were infused with the expansion product derived from approximately 8 × 107 autologous CD34+ cells (Table 1), became transfusion independent within 23 days posttransplant (Table 2). The pattern of hematological reconstitution in the baboons is shown in Fig 4. ANC >500/μL were reached by days 24 and 26, and stably increasing platelet counts >20,000/μL were reached by 27 to 31 days posttransplant. These animals remained healthy with normal blood counts (Table 2).
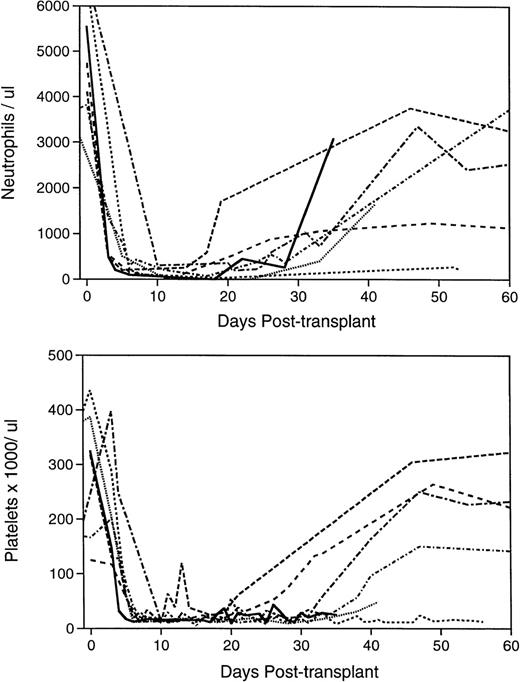
Pattern of hematological reconstitution in the lethally irradiated, transplanted baboons. Complete blood counts were performed on the animals before irradiation to establish baseline values at 24- to 72-hour intervals after completion of myeloablation, and biweekly after engraftment and removal from the tether system when applicable. Differential counts of 100 nucleated peripheral blood cells, where possible, were performed to determine the ANC. The initial 60-day course of the seven animals’ ANCs are depicted in the upper panel and platelet counts in the lower panel. PA6216 () received no graft; PA6126 (- - - - - ) and PA6594 (•••••) received a suboptimal number of mononuclear marrow cells; PA6592 (— — — ) received a sufficient mononuclear marrow cell graft; PA6245 (--------- ) was infused with a sufficient number of selected, unexpanded CD34+ marrow cells; PA6156 (-•-•-•-•- ) and PA6188 (—•—•— ) were given grafts composed of the cellular product of CD34+ marrow cells expanded for 10 days in PMVEC coculture.
Pattern of hematological reconstitution in the lethally irradiated, transplanted baboons. Complete blood counts were performed on the animals before irradiation to establish baseline values at 24- to 72-hour intervals after completion of myeloablation, and biweekly after engraftment and removal from the tether system when applicable. Differential counts of 100 nucleated peripheral blood cells, where possible, were performed to determine the ANC. The initial 60-day course of the seven animals’ ANCs are depicted in the upper panel and platelet counts in the lower panel. PA6216 () received no graft; PA6126 (- - - - - ) and PA6594 (•••••) received a suboptimal number of mononuclear marrow cells; PA6592 (— — — ) received a sufficient mononuclear marrow cell graft; PA6245 (--------- ) was infused with a sufficient number of selected, unexpanded CD34+ marrow cells; PA6156 (-•-•-•-•- ) and PA6188 (—•—•— ) were given grafts composed of the cellular product of CD34+ marrow cells expanded for 10 days in PMVEC coculture.
A third baboon was transplanted with 13.7 × 106/kg unexpanded, cryopreserved, selected CD34+ marrow cells, which were thawed, washed, and immediately infused after completion of the TBI regimen (Table 1). This animal experienced a rapid recovery with ANC >500/μL reached at day 16 and transfusion independence and platelets >20,000/μL at 11 days after infusion of the graft (Table1 and Fig 4). Although the baboon transplanted with an enriched CD34+ cell graft received only 30% to 50% the number of CD34+ cells, 50% to 70% the number of CFU-GM, and 20% to 50% of CAFC as the two animals that received expanded grafts (Table2), its hematological recovery was more rapid and transfusion independence was reached in half the time of that of the expanded graft recipients. A control animal was infused with cryopreserved, unexpanded whole mononuclear marrow with a similar total cell (175 × 106/kg) and CD34+ cell (21 × 106/kg) content to that of the two ex vivo–expanded grafts (Table 1). This baboon also engrafted more rapidly than did the two animals that received the expanded grafts (Tables 1 and 2, Fig 4).
Three baboons were used as controls to determine the lethality of the fractionated TBI regimen, as well as the potential of grafts containing fewer than 4 × 106 CD34+ cells/kg to rescue the irradiated hosts. A baboon that underwent the 4-day irradiation schedule, but did not receive a graft, reached granulocyte and platelet nadir by 7 days post-TBI (Fig 4). At this time, no hematopoietic progenitor cells or CAFC were assayable from the severely hypocellular marrow (data not shown). The animal died 37 days after the myeloablative regimen (Table 2), by which time some granulocyte, but not platelet recovery had occurred. A postmortem marrow sample contained assayable CAFC, confirming that some degree of endogenous myeloid recovery had occurred (data not shown). Two animals received grafts composed of a suboptimal number of mononuclear marrow cells16 17 (Table 1), which contained 1.0 and 3.8 × 106 CD34+ cells per kg of body weight. Both remained pancytopenic with extremely hypocellular marrows until death at 43 and 59 days posttransplant (Table 2 and Fig 4), at which time insufficient marrow cells were obtainable for assay.
DISCUSSION
We have demonstrated the safety and effectiveness of rescuing myeloablated nonhuman primates with expanded grafts derived from CD34+ marrow cells. These studies show the ability of microvascular endothelial cells to maintain the marrow engraftment capacity of marrow-derived stem cells during cycling of the cells. It is highly unlikely that the engraftment of the expanded marrow cells was due to the persistence of quiescent HSC which remain in G0 and are thus more resistant to differentiation pressure than actively dividing cells. At least 48 hours before harvest of the PMVEC cocultures, greater than 99.5% of the CD34+ cells present in the expansion cultures had undergone multiple rounds of cell division as evidenced by membrane labeling; no subpopulation of cells refractory to cycle induction was evident. The number of CD34+ cells that remained quiescent in the cultures was thus at least one log below the 1 to 3.8 × 106/kg that were found inadequate for rescue of two myeloablated animals. Because far greater numbers of native, unstimulated HSC were delivered to the control animals, which received a suboptimal number of whole mononuclear marrow cells,16 it is highly improbable that the hematopoietic reconstitution observed in the animals receiving expanded grafts was due to small numbers of HSC that failed to divide over the 10 days of culture. PMVEC have been reported to drive the extensive proliferation of highly enriched human HSC, which retain their primitive CD34+CD38− phenotype after several rounds of cell division.21,22 In these experiments, the numerical expansion of baboon CD34+ and CD34+CD38− cells was lower than that reported with human marrow cells by about one log. This discrepancy is likely due to the administration of human recombinant SCF, IL-3, IL-6, and GM-CSF to the cultures rather than recombinant baboon cytokines, which are currently unavailable, resulting in suboptimal stimulation of the baboon marrow cells. It is also possible that the signal(s) produced by PMVEC that promote the expansion of the compartment of cells possessing an HSC phenotype are not as efficiently transmitted to the baboon cells, especially under culture conditions optimized for the expansion of human cells. Here, PMVEC coculture has been demonstrated to support the proliferation of baboon marrow cells expressing this early phenotype in stark contrast to coculture with allogeneic baboon marrow stroma. Such attenuation of the proliferation and generation of progenitor cells by marrow stromal cells in the presence of multiple cytokines has been reported.26
The expansions described herein were performed in the presence of multiple human recombinant cytokines, specifically IL-3, IL-6, SCF, and GM-CSF. Exposure of stem cell populations to certain cytokines, notably IL-3, has been implicated in the acquisition of a defect in engraftment capacity,10-15 especially in the absence of flt3 ligand.27,28 Recent studies of the engraftment of mobilized peripheral blood HSC in an autologous rhesus, as well as a human/nonobese diabetic (NOD)/SCID xenogeneic system indicate that the ex vivo expansion or even entry of mobilized peripheral blood (MPB) HSC into cell cycle severely impairs their ability to repopulate the host.29,30 Our group has reported the correction of an apparent loss of marrow repopulation capacity by adult human HSC expanded in the presence of IL-3, IL-6, and GM-CSF by coculture with PMVEC in the absence of SCF and flt3 ligand.21 Whether prolonged in vitro exposure of expanded grafts to the cytokines used in these experiments leads to an impairment of long-term in vivo hematopoietic activity, which is corrected by PMVEC coculture, remains to be tested.
The hematopoietic recovery of the two baboons transplanted with ex vivo–expanded grafts was delayed in comparison to that experienced by an animal that received a large number of unexpanded CD34+cells or by an animal infused with a large number of unfractionated marrow cells. The animal transplanted with selected, unexpanded CD34+ cells received only one half to one third the number of CFU-GM and one half to one fifth the number of CAFC as the two baboons receiving the expanded grafts. Likewise, the expanded grafts, although initiated with fewer CD34+ cells than were contained in the unexpanded grafts (8.1 to 8.4 × 106/kg v 13.7 to 21 × 106/kg), contained two to three times the number of CD34+ cells after expansion than did the selected, unexpanded or large mononuclear marrow graft. However, although cells expanded for 10 days in PMVEC coculture contained significant numbers of cells expressing a CD34+CD38− phenotype that were the product of cell proliferation, the total number of CD34+CD38− cells was diminished from input number, which may partially account for the observed delay in engraftment by these cells. Güenechea et al31 have recently reported the impaired short-term, but not long-term, engraftment of human cord blood CD34+ cells expanded in the presence of IL-3, IL-6, and SCF. Assayable progenitor cell content did not correlate with rapidity of engraftment in these studies, and the relevance of these in vitro data have been questioned of late in the literature.29,31-33 These data and those of other groups also suggest that the phenotypes of HSC and early progenitor cells may deviate after prolonged in vitro culture from those of their native counterparts, making their direct functional comparison to freshly isolated cells by surface markers less straightforward.21,34 35 Ongoing efforts to define phenotypic and functional changes in HSC resulting from long-term in vitro culture should prove useful in clarifying this controversy.
The level of TBI delivered to these animals has been generally accepted to be myeloablative and lethal.19 While the regimen used herein was certainly lethal, it is well recognized that no therapeutic dose of radiation is totally myeloablative.36-38 Whether the expanded grafts are capable of providing durable, long-term hematopoiesis or simply rescue of the host until endogenous marrow recovery occurs is not possible to determine in the autologous stem cell transplant setting without the complete and durable marking of HSC and their progeny. Alternatively, sex-mismatched, compatible donors have provided elegant tools for investigation of this question.17 Such studies will be pursued to definitively establish the long-term contribution of the ex vivo expanded graft.
The safety of the delivery of marrow grafts expanded in culture in the presence of xenogeneic stromal cells has been debated. The expanded grafts used in this report contained some porcine cells, which were not totally separated before infusion into the hosts. The engraftment of foreign stromal cells harboring xenotropic viruses remains a legitimate concern in the clinical setting. Indeed, porcine retroviruses and endogenous proviruses capable of infecting human cells have been identified in various porcine tissues.39 The persistence of porcine tissue in the host and potential activation of porcine retroviruses with tropism for human or primate cells will require further investigation.
The data reported herein demonstrate the use of an ex vivo expanded HSC product for hematopoietic reconstitution of a myeloablated primate host. The rapid and total cycling of the population of cells used to initiate these cultures suggests a pivotal role for PMVEC coculture in inducing cell division without terminal differentiation for retroviral gene marking and gene therapy protocols. Such approaches favoring stem cell self-renewal in vitro may prove clinically useful for genetic modification and the expansion of hematopoietic stem cell grafts.
ACKNOWLEDGMENT
The authors thank Christine Joy, Kimberly Gibbons, and Jeffrey Oswald, DVM of the UIC Biological Resources Laboratory, without whose diligent work this study would not have been possible.
This work was performed under an evaluation Cooperative Research and Development Agreement between the Naval Medical Research Institute and the University of Illinois dated July 12, 1996. Views presented in this manuscript are those of the authors and no endorsement by the Department of the Navy has been given or should be inferred.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Ronald Hoffman, MD, University of Illinois at Chicago, Section of Hematology/Oncology M/C 734, 900 S Ashland Ave, Chicago, IL 60607.