We report the results of high-dose chemoradiotherapy and anti–B-cell monoclonal antibody-purged autologous bone marrow transplantation (ABMT) in patients with relapsed indolent follicular lymphoma. Between March 1985 and May 1995, 153 patients underwent ABMT using a uniform ablative regimen with cyclophosphamide and total body irradiation and bone marrow (BM) purging. All patients received multiple chemotherapy regimens before ABMT. At BM harvest, only 30% of patients were in complete remission, and overt BM infiltration was present in 47%. The disease-free survival (DFS) and overall survival (OS) are estimated to be 42% and 66% at 8 years, respectively. Patients whose BM was negative by polymerase chain reaction (PCR) for bcl2/IgH rearrangement after purging experienced longer freedom from recurrence than those whose BM remained PCR positive (P < .0001). Continued PCR negativity in follow-up BM samples was also strongly predictive of continued complete remission (CR). The 12-year survival from diagnosis for these 153 patients is 69%. Considering that the median survival from diagnosis and first recurrence of patients with advanced follicular lymphoma are 8 and 5 years, respectively, our results provide evidence that myeloablative therapy and ABMT may prolong overall survival.
PATIENTS WITH LOW-GRADE follicular non-Hodgkin’s lymphomas (NHL) are not cured with conventional treatment.1 Although the median survival for these patients is 8 to 10 years, the disease-free survival (DFS) for previously untreated patients receiving conventional therapy is generally between 18 and 36 months.1-4 After relapse, the duration of remission with each subsequent treatment becomes progressively shorter. In contrast to more aggressive histologies in which most relapses are within 2 years of completing initial treatment a significant number of follicular lymphoma patients will recur beyond 3 years. Therefore, long follow-up is critical to assessing the impact of treatment on remission duration and survival in these diseases.
The rationale for the use of high-dose ablative therapy in follicular lymphoma is based on the fact that relapsed patients can continue to respond to further conventional treatment and salvage regimens. However, a continuous rate of relapse continues to be observed with salvage regimens. As had been previously demonstrated for relapsed/refractory NHLs, resistant disease could be overcome with high-dose therapy, followed by allogeneic and autologous hematopoietic stem cell transplantation.5-7 This approach is being applied more frequently in patients with relapsed low-grade NHL, despite uncertain efficacy.7-15 One problem with these studies is that the length of follow-up has been relatively short considering the long natural history of these diseases. An alternative to clinical assessment of remission status may be molecular studies of minimal residual disease. After autologous bone marrow transplantation (ABMT) in patients with follicular lymphoma, the detection of minimal residual disease by polymerase chain reaction (PCR) has been shown to be a useful surrogate marker for relapse and may help obviate the need for extended follow-up in a disease in which late relapses are common.12 16-19
In the present study, we report the results in 153 patients with relapsed follicular NHL who underwent high-dose chemoradiotherapy and anti–B-cell monoclonal antibody (MoAb)-purged ABMT between 1985 and 1995. In the results to be reported below, we demonstrate that the DFS and overall survival (OS) are 42% and 66% at 8 years, respectively. Furthermore, the 12-year survival from diagnosis for these patients is 69%. These results suggest that a significant fraction of these patients with follicular NHL experience prolonged DFS and OS.
MATERIALS AND METHODS
Selection of patients and treatment protocol.
Patients were eligible for this study if they were less than 65 years of age, had follicular small cleaved cell or follicular mixed NHL as defined by the International Working Formulation (WF B or C) or follicular center cell lymphoma grade I or II in the REAL classification, and had relapsed after standard chemotherapeutic regimens.20,21 Patient’s lymphoma cells had to express the CD20 (B1) antigen as previously described.22 In addition, patients with sensitive disease but who had failed to enter complete remission after more than 1 standard chemotherapeutic regimen were eligible. Minimal disease status was defined as lymph nodal mass less than 2 cm in its greatest diameter and histologic evidence of bone marrow (BM) involvement of 20% or less of the intratrabecular space as determined by iliac crest biopsy. Patients with 1 to 3 masses greater than 2 cm could receive 3,000 cGy of involved field radiation therapy. Additional criteria for entry included the absence of comorbid disease of the heart, kidney, lung, and liver and a Karnofsky score greater than 80%. Informed consent was obtained from all patients.9 22
Preparative therapy consisted of cyclophosphamide at 60 mg/kg of body weight, infused on each of 2 consecutive days before radiotherapy. Total body irradiation (TBI) was administered in fractionated doses (200 cGy) twice daily on 3 consecutive days (total of 1,200 cGy) in 144 patients; 9 patients received 1,400 cGy. Supportive care was provided as previously described.12
Collection, processing, and infusion of marrow.
BM was obtained, treated in vitro as previously described, and stored within 4 weeks of its use in transplantation in all patients except 1. No patients were excluded from the protocol after BM harvest. The BM cells from 18 patients were treated with anti-B1 (CD20). The BM cells from 135 patients were treated with more than 1 MoAb, including 101 with anti-B1, anti-B5, and J5 (anti-CD10); 29 with anti-B1, anti-B4 (CD19), anti-B5, and J5 (anti-CD10); and 5 with combinations of anti-B1 with either anti-B5, J5, or J2 (anti-CD9), depending on the tumor cell phenotype (2 with anti-B1, anti-B5, and J2; 3 with anti-B1 and J5), as previously described.9,12,22 Eleven of these patients with ≤20% BM involvement had their BM mononuclear cells treated with multiple MoAbs between 1986 and 1988. After 1988, all patients’ BM mononuclear cells were treated with anti-B1, anti-B5, and J5. After treatment, the cells were cryopreserved as previously described.12 Within 18 hours of the completion of radiotherapy, the cryopreserved marrow cells were rapidly thawed and diluted in medium containing DNAase as previously described.12
PCR analysis.
Nested PCR amplification at the major breakpoint region (MBR) and minor cluster region (mcr) of the bcl-2/IgH rearrangement of t(14;18) were performed as previously described.23 Analysis was performed initially on diagnostic material (lymph node biopsy, or BM aspirate if histologically involved). Samples were analyzed at the time of BM harvest. Assessment was also performed after ex vivo marrow purging. Serial BM samples at the time of restaging after ABMT were also analyzed.
Evaluation and statistical methods.
Failure was defined as relapse of disease or death in remission. DFS was calculated from the day of marrow transplantation (day 0) to the date of failure or to the date that the patient was last known to be alive and disease-free. Freedom from relapse (FFR) was calculated from the day of marrow transplantation to the date of relapse; deaths in remission were considered censored for this analysis. DFS curves and FFR curves were estimated by the method of Kaplan and Meier, with confidence intervals calculated using Greenwood’s formula, and compared by the log rank test.24 25 The Cox proportional hazards model was used to assess prognostic factors for DFS and to build multiple regression models. Comparison of the clinical characteristics of those patients who were PCR+ or PCR− after purging was performed with the Fisher exact test, and the criterion for statistical significance (.05) was not adjusted for multiple comparisons. Comparisons of patient age in this analysis used the Wilcoxon rank sum test.
RESULTS
Patient characteristics.
One hundred fifty-three consecutive patients (median age, 43 years) with follicular NHL in sensitive relapse or incomplete first remission underwent ABMT between March 1985 and May 1995. Before ABMT, 120 of the patients had follicular small cleaved cell (FSC) and 33 had follicular mixed (FM) histology. The characteristics of the 153 patients who underwent ABMT are detailed in Table 1. At diagnosis, 102 patients (67%) had stage IV disease, largely by virtue of BM involvement. At some time before consideration for ABMT, extranodal sites of involvement, exclusive of the marrow, including skin, peripheral blood, gastrointestinal tract, as well as pleural and peritoneal fluid, were present in 35% of patients.
Prior therapy.
All patients had previously received combination chemotherapy. The median number of regimens with which patients were treated was 3 (range, 2 to 7). At some time in their history, including before ABMT, 27% of patients received involved field radiotherapy and 1 patient had been previously treated with TBI (150 cGy). A complete remission (CR) at anytime during their disease course, including the time of BM harvest, was documented in only 78 (51%) of the patients. At BM harvest, only 46 (30%) of the patients were in CR. Moreover, of the 107 patients in partial remission (PR) at harvest, 72 had residual BM involvement. These results suggest that these patients who proceeded to ABMT did not have exquisitely sensitive and/or minimal amounts of disease.
Treatment outcome.
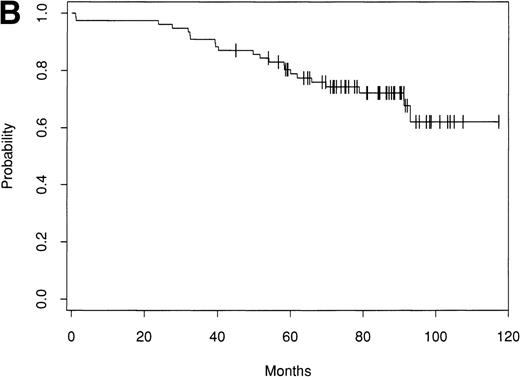
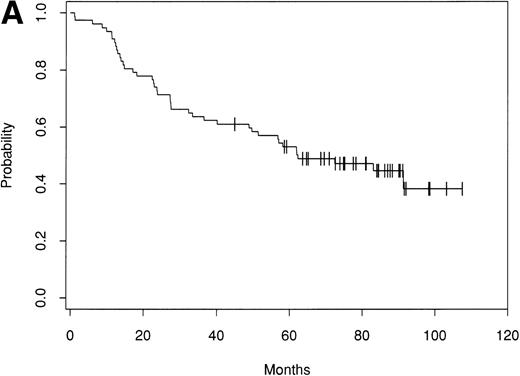
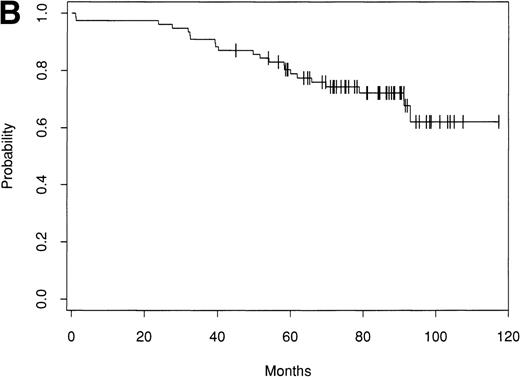
Of the 153 patients treated, only 1 early treatment related death due to venoocclusive disease was seen (day 20). An additional patient died of chronic liver disease without evidence of lymphoma at 46 months. Of the remaining 151 patients, as of September 1998 there have been 63 relapses. Seventy-nine patients remain alive and in CR with a median follow-up of 61 months (range, 24 to 156 months). The Kaplan-Meier estimate of the percentage of patients alive and disease-free after ABMT is 42% (90% confidence interval [CI], 30% to 51%) at 8 years (Fig 1A). The estimate of the OS at 8 years is 66% (90% CI, 57% to 74%; Fig 1B). The survival from diagnosis for the entire group of patients is 69% at 12 years (90% CI, 61% to 76%; Fig 2).
Kaplan-Meier estimate of probability of DFS (A) and OS (B) for 153 patients with indolent follicular lymphoma after ABMT.
Kaplan-Meier estimate of probability of DFS (A) and OS (B) for 153 patients with indolent follicular lymphoma after ABMT.
Kaplan-Meier estimate of probability of OS after ABMT from diagnosis for 153 patients with indolent follicular lymphoma.
Kaplan-Meier estimate of probability of OS after ABMT from diagnosis for 153 patients with indolent follicular lymphoma.
Of the 63 patients who relapsed, the overwhelming majority relapsed in sites of prior disease (Table 2). Entirely new sites of disease were observed in only 11 patients, and in 4 of these patients, new sites were the only sites of relapse. Nineteen of the 63 relapses involved the marrow, and 14 of these 19 patients had marrow involvement at the time of harvest. After relapse, 34 of 63 patients are alive at a median follow-up of 80 months post-ABMT.
Second malignancies.
After ABMT, second malignancies have developed in 18 patients. These include solid tumors in 5 patients, involving testis prostate, lung (small cell), breast (ductal carcinoma in situ), and melanoma. Four of these patients remain alive and in remission from both follicular lymphoma and the second solid tumor. The patient with small cell lung cancer died 1 month after diagnosis, with no evidence of recurrent NHL at autopsy. One patient developed acute lymphoblastic leukemia (ALL) 28 months post-ABMT and remains in remission from both ALL and NHL. Twelve patients have developed myelodysplastic syndrome (MDS) with 1 evolving into acute myeloid leukemia (AML) from 9 to 64 months post-ABMT. Ten of these patients have died, and 6 patients had no evidence of recurrent lymphoma. Four of these 10 patients died after allogeneic BMT (alloBMT). Of the remaining 2 patients who are alive after the development of MDS, both have relapsed NHL as well.
PCR analysis after ABMT.
PCR analysis of the MBR and mcr of the bcl-2/IgH rearrangement was performed on diagnostic samples obtained from 150 patients. In the remaining 3 patients, no diagnostic material was available for analysis. In the majority of patients, PCR was performed on BM aspirate obtained at the time of documented evidence of histologic infiltration of the BM. In cases in which no diagnostic BM sample was available, PCR was performed on DNA isolated from diagnostic lymph node biopsy. A bcl-2/IgH rearrangement could be PCR amplified in 116 patients. In 88 cases (76%), the breakpoint was at the MBR, and in 27 cases (23%), the breakpoint was at the mcr. One patient had markers indicating breakpoints at both the MBR and the mcr. These 116 patients were deemed informative for subsequent assessment of the clinical significance of PCR detectable disease at the time of and after ABMT. In the remaining 34 cases, DNA isolated from diagnostic tissue yielded no PCR product at the MBR or mcr. These cases therefore represent those patients in whom there was no t(14;18) or in whom the t(14;18) could not be amplified using this strategy.
BM samples were available from the BM harvest and post-immunologic purging in 113 of the informative patients. PCR detected residual disease at the time of BM harvest in all informative patients. After immunologic purging, PCR analysis showed no detectable disease in 48 of these patients (42%), whereas in the remaining 65 patients (58%), there was persistence of PCR-detectable disease after purging. We contrasted patient characteristics among those patients who were positive and negative after purging. There were no differences in stage, B symptoms, gender, masses greater than 10 cm, histology, histologic BM involvement at harvest, or remission status at harvest (CR v PR) between those patients who purged negative and those who did not.
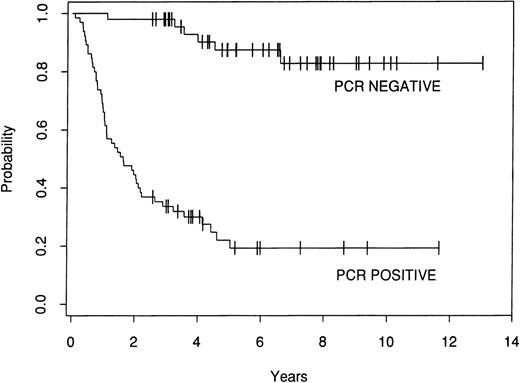
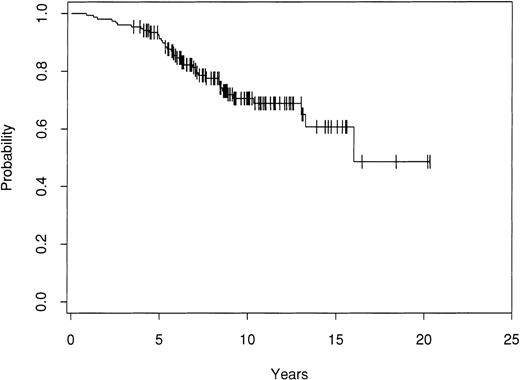
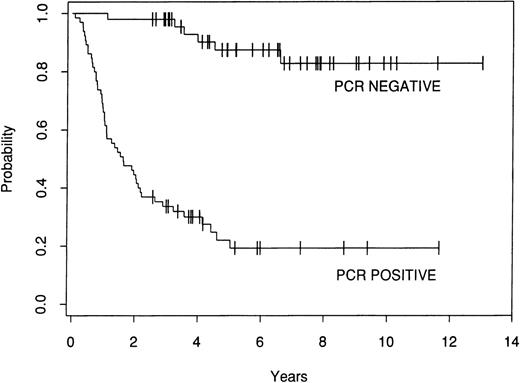
The effect of marrow purging was examined in the 113 informative patients. Among the 48 patients who were PCR− after purging, there have been 6 relapses. In contrast, there have been 49 relapses among the 65 patients who were PCR+ after purging. The 8-year FFR for the PCR− patients is 83%, whereas the FFR for the patients who were PCR+ after lysis is 19% (P = .0001; Fig 3).
Kaplan-Meier estimate of FFR after ABMT for 113 informative patients who were either PCR− or PCR+ after ex vivo purging.
Kaplan-Meier estimate of FFR after ABMT for 113 informative patients who were either PCR− or PCR+ after ex vivo purging.
We analyzed time to relapse for those patients whose BM was purged with anti-B1 only (18 patients), compared with the remaining patients whose BM was purged with more than 1 antibody (2 with 2 antibodies, 104 with 3 antibodies, and 29 with 4 antibodies). We have found no difference in freedom from relapse between these groups (P = .33 by the log rank test).
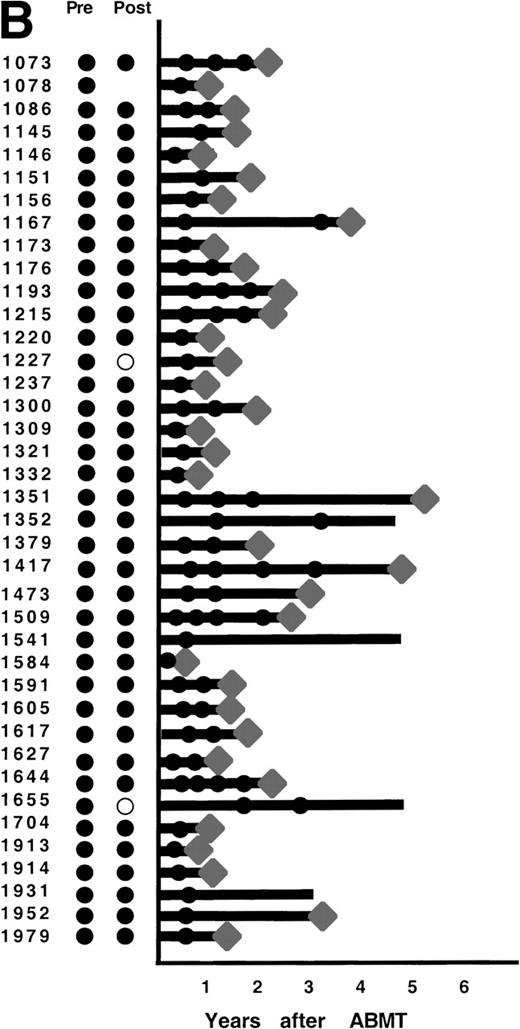
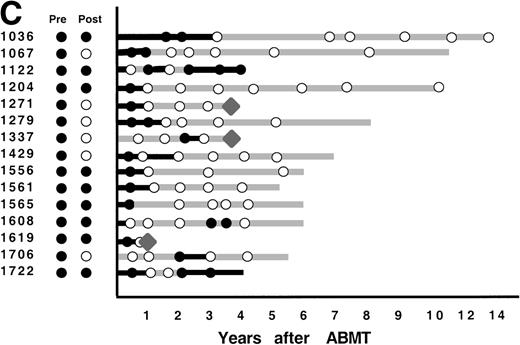
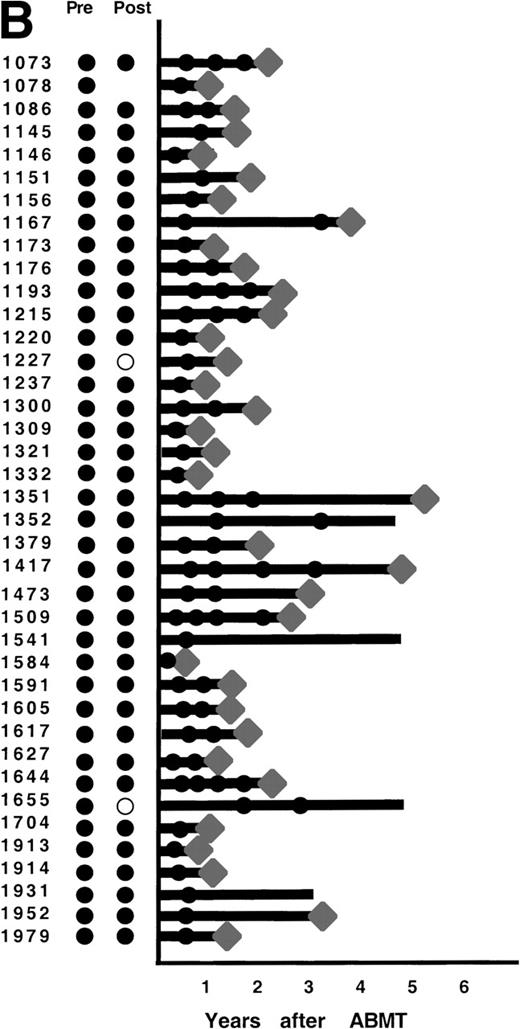
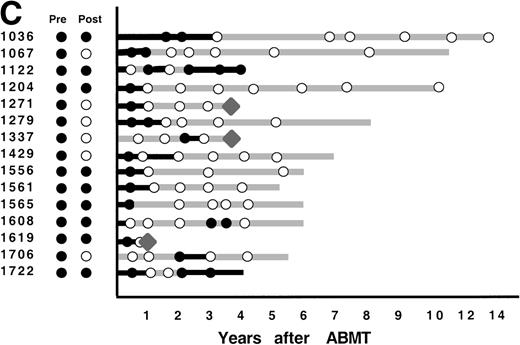
After ABMT, BM samples were obtained at 6-month intervals for 2 years and yearly thereafter for 101 of the 116 informative patients. In 2 informative patients, no samples were available either after lysis or after ABMT, and in 13 informative patients, no BM samples were submitted after ABMT. The results obtained on samples that were available for PCR analysis are shown in Fig 4. As previously described,12 3 patterns of results are seen. In 47 patients (47%), no BM samples analyzed had evidence of PCR-detectable lymphoma at any time point after ABMT (Fig 4A). It is noteworthy that in only 8 of these 47 patients were PCR-detectable lymphoma cells detectable after immunologic purging. Only 5 of these 46 patients have relapsed to date. PCR-detectable minimal residual disease was detected in every BM sample obtained after ABMT in 39 patients (39%), 36 of whom were infused with autologous BM that contained residual PCR-detectable lymphoma (Fig 4B). Thirty-six of these 39 patients have relapsed to date. The remaining 15 patients (15%) had different results obtained at different time points after ABMT (Fig 4C). PCR-detectable disease was observed at some, but not all time points after BMT. Of these, 9 were positive early after ABMT and became consistently negative and 6 remain with mixed results.
PCR analysis of BM samples before and after BM purging (left) and after ABMT (right) in 101 informative patients with t(14;18). Patients were grouped according to follow-up BM PCR results. (A) Patients in whom all post-ABMT follow-up BM samples were PCR−. (B) Patients in whom all post-ABMT follow-up BM samples were PCR+. (C) Patients in whom all post-ABMT follow-up BM samples were both PCR+ and PCR−. (•) PCR+ results; (○) PCR−results; (⧫) relapse. Solid lines indicate continuous PCR+ results and shaded lines indicate continuously PCR− results.
PCR analysis of BM samples before and after BM purging (left) and after ABMT (right) in 101 informative patients with t(14;18). Patients were grouped according to follow-up BM PCR results. (A) Patients in whom all post-ABMT follow-up BM samples were PCR−. (B) Patients in whom all post-ABMT follow-up BM samples were PCR+. (C) Patients in whom all post-ABMT follow-up BM samples were both PCR+ and PCR−. (•) PCR+ results; (○) PCR−results; (⧫) relapse. Solid lines indicate continuous PCR+ results and shaded lines indicate continuously PCR− results.
Prognostic models.
In an attempt to identify prognostic variables for these patients, a number of factors available before and at ABMT were examined in a univariate comparison of DFS using the log rank test. These variables included age, sex, stage (I, II, III, v IV), follicular small cleaved versus follicular mixed histologies, B symptoms, extranodal (extramedullary) disease, mass greater than 10 cm, interval from diagnosis to harvest, histologic BM involvement at harvest, remission status at harvest (CR v PR), and postlysis PCR positivity. The only parameter that was associated with unfavorable FFR in univariate analysis was postlysis PCR positivity (P < .0001). When post-ABMT PCR positivity of the BM was considered, this was also strongly associated with unfavorable FFR (P < .0001). No other factors were associated with FFR at the .05 significance level.
A stepwise proportional hazards regression was performed to identify factors that affected FFR in the 113 informative patients with postlysis BM samples available. PCR positivity after purging was the strongest factor in the multiple regression model, increasing the risk of failure to 11.7 times that of patients who were PCR− after lysis. Other clinical factors associated with a poor prognosis include B symptoms (2.1 times) and histologic involvement of the marrow at harvest (1.8 times).
DISCUSSION
In this report, we present the results of high-dose chemoradiotherapy and ABMT in 153 consecutive patients with relapsed indolent follicular NHL treated between 1985 and 1995. Considering that the median DFS of second remission with conventional therapy for patients with this disease is 13 months, we have seen a significant prolongation of DFS with high-dose therapy.4 Moreover, the median survival after first relapse from conventional therapy for advanced stage patients is 5 years.2 The OS after ABMT in this series was 66% at 8 years, suggesting that we may be observing a prolonged survival with high-dose therapy. However, the patients who underwent ABMT in this study were younger than the median age of patients with follicluar NHL, had sensitive disease, and could achieve a minimal disease state with conventional chemotherapy. Despite the fact that these patients were treated with a median of 3 regimens and only 30% were in clinical CR at ABMT, the current results are encouraging.
The DFS and OS in the present series are comparable with those reported in other series of high-dose therapy and autologous stem cell transplantation for patients with relapsed follicular NHL.7,10,11,13,15,26,27 The clinical prognostic factors that significantly influenced failure-free survival include age greater than 50 years and the number of chemotherapy regimens in the Nebraska series13 and B symptoms at diagnosis in the recent series of Fouillard et al.15 In univariate analysis, we found no clinical parameters to be significantly associated with unfavorable DFS. However, in a multiple regression model, B symptoms and histologic involvement of the marrow at harvest were associated with increased risk of relapse. These differences between the present study and these others are most likely due to the heterogeneity of the patient populations and the selection criteria used.
As we have previously reported in patients undergoing ABMT for follicular NHL in second or greater remission as well as first remission,12,17 the presence of minimal residual disease in the reinfused marrow was the most significant prognostic factor for relapse. A similar association between the degree of purging as measured by residual number of colony-forming unit–granulocyte-macrophage (CFU-GM) reinfused after mafosfamide marrow purging and incidence of relapse has been recently reported.15 These studies suggest that only a subset of patients presently benefit from purging. Considering that 89% of the patients who relapsed were reinfused with a PCR+ BM, further improvements in techniques to yield a more tumor-free stem cell product are likely to have an impact on DFS.
One of the problems of demonstrating an impact on remission duration and survival in patients with follicular lymphoma is that exceedingly long follow-up is necessary. For this reason, we have restricted our analysis to patients who were at least 3 years from ABMT. The median follow-up of the patients in remission is more than 5 years. Because virtually all of the relapses have occured in the first 4 years after ABMT, a subset of these patients may be cured of follicular NHL. However, despite long clinical follow-up, one must remain cautious about these results considering the long natural history of follicular NHL and that late relaspes occur. The detection of minimal residual disease by PCR after ABMT may be a useful endpoint to examine the effect of a treatment modality such as ABMT.
The observation that minimal residual disease at ABMT and in follow-up is predictive for relapse has stimulated novel treatment approaches. In vitro studies have demonstrated a potential for generating endogenous immune responses against follicular lymphoma cells. Amplification of effector cells with cytokines such as interleukin-2 or interferon-α has been one such approach.28,29 Further ways to enhance and induce host immunity against the patients’ tumor cells include tumor cell vaccination using engineered cells that express costimulatory molecules cells or granulocyte-macrophage colony-stimulating factor (GM-CSF).30 Another approach that has been reported after conventional therapy is to use idiotypic peptides derived from the patients’ lymphoma cells with or without GM-CSF.31-33 One problem with this strategy after high-dose therapy is the marked suppressive effects on T-cell subsets and antigen presenting cells that occurs after autologous transplantation.34 MoAbs either unconjugated or conjugated to toxins are also being administered after ASCT to target tumor cells.35 The anti–B-cell immunotoxin anti-B4–blocked ricin has been used in the adjuvant setting after ABMT and was reported to be without benefit in phase III trial.36 Unconjugated MoAbs such as Rituximab that are cytotoxic in the absence of effector cells37 will continue to be investigated as an attractive alternative for minimal residual disease. These immunomodulatory and targeted therapies may be particularly well suited for patients who are at high risk for relapse after high-dose therapy, specifically those receiving a PCR+ stem cell product and those who are persistently PCR+ after transplant.
Although relapse is the principal cause of morbidity and mortality after ABMT, the development of second malignancies, particularly MDS/AML, is a major concern for patients longitudinally.38-48 In the present series, 12 patients (8%) have developed MDS or secondary AML, and to date, 10 have died, of whom 6 died with no evidence of recurrent NHL. We have also observed solid tumors develop in 5 patients. Second, nonhematologic malignancies have been reported to occur after alloBMT. Most of these are epithelial derived, with a significant number involving the skin, particularly malignant melanoma. A recent series of second tumors after autologous stem cell transplants for lymphoma reported an actuarial risk of second malignancy of 16.7% at 13 years.46 These studies as well as our own data suggest that patients undergoing autologous transplant are at increased risk of secondary solid tumors. This is particularly a concern in patients with follicular NHL who may experience prolonged DFS or OS after ABMT.
It is clear that other strategies are needed to improve on the results of autologous transplantation. One approach is the use of radioimmunoconjugates, which have been used in both myeloablative and nonmyeloablative doses with high response rates.49-53AlloBMT has been used in a limited fashion in patients with relapsed follicular NHL.6,7,54,55 The OS rates for patients undergoing conventional alloBMT are similar to ABMT because of a significantly higher treatment-related mortality but with a lower relapse rate. This lower relapse rate with alloBMT as well as anecdotal responses to donor lymphocyte infusions are evidence for a graft-versus-lymphoma effect.56,57 One strategy to reduce the high morbidity and mortality of alloBMT, while generating a graft-versus-lymphoma effect, would be to combine selective T-cell–depleted alloBMT58,59 with donor lymphocyte infusions or nonmyeloablative transplants.60 Ongoing and future studies in these areas as well as in the development of more effective ablative regimens, tumor cell purging, and treatment of minimal residual disease may provide more effective treatment than those that currently only benefit a minority of patients.
ACKNOWLEDGMENT
The authors are indebted to the nurses, adult oncology fellows, and social workers of the Dana-Farber Cancer Institute; and the housestaff of the Brigham and Women’s Hospital and Beth Israel Hospital for their excellent care of these patients. We also thank the technicians of the Connell-O’Reilly Cell Manipulation Laboratory and the Blood Component Laboratory of Dana-Farber Cancer Institute for processing of the BMs; the oncologic surgeons of the Brigham and Women’s Hospital and Beth Israel-Deaconess Hospital for their surgical assistance; and the staffs of the Departments of Radiology and Infectious Disease for their assistance in patient care. We also thank Lisa Warren for secretarial assistance in preparation of the manuscript.
Supported by National Institutes of Health Grant No. CA 66996.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Arnold S. Freedman, MD, Department of Hematologic Malignancies, Dana-Farber Cancer Institute, 44 Binney St, Boston, MA 02115.