Abstract
Primary effusion lymphomas (PELs), which are rare lymphomas associated with Kaposi's sarcoma-associated herpesvirus (or human herpesvirus-8) infection, present as malignant lymphomatous effusions in body cavities. Because PELs prefer liquid growth, we hypothesized that increased vascular permeability would be required for effusions to form. We found that the PEL cell lines BC-1, BCP-1, and BCBL-1 produce high levels of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF). Reverse transcriptase-polymerase chain reaction analysis of RNA from the PEL cell lines amplified the 3 VEGF-secreted isoforms: VEGF/VPF121, VEGF/VPF145, and VEGF/VPF165. Two of the PEL cell lines expressed the VEGF/VPF receptor Flt-1, but VEGF/VPF did not stimulate proliferation in these cells. Most (13/14) control SCID/beige mice inoculated intraperitoneally with BCBL-1 cells and subsequently observed or treated with control antibodies developed effusion lymphoma of human cell origin with prominent bloody ascites. In contrast, none (0/9) of the mice treated with a neutralizing antihuman VEGF/VPF antibody developed ascites and effusion lymphoma. These results demonstrate that VEGF/VPF is critical to BCBL-1 growth as effusion lymphoma in mice and suggest that VEGF/VPF stimulation of vascular permeability may be critical to the pathogenesis of PELs.
KAPOSI'S SARCOMA-associated herpesvirus (KSHV; also known as human herpesvirus-8 [HHV-8]) is a herpesvirus linked to the development of Kaposi's sarcoma (KS), primary effusion lymphoma (PEL), and a proportion of Castleman's disease.1-4 KSHV-associated malignancies arise predominantly, but not exclusively, in human immunodeficiency virus (HIV)-positive individuals.5 PEL, otherwise termed body cavity-based lymphoma, is a peculiar and infrequent type of non-Hodgkin's lymphoma that displays a marked preference for liquid growth in the serous cavities of the body, usually in the absence of an identifiable tumor mass.6 PEL consistently originates from B cells, but the vast majority of cases exhibit a non-B, non-T phenotype, lacking expression of surface Igs and B-cell–associated antigens. At the molecular level, PEL cells are characterized by clonal Ig gene rearrangement, without c-myc rearrangement or alterations of the bcl-2, ras, and p53 genes.6 Most cases of PEL are dually infected with Epstein-Barr virus (EBV) and KSHV,7,8 but occasional cases of EBV-negative/KSHV-positive PEL have been reported.9-11 Thus, KSHV is likely to be involved in the pathogenesis of PEL, but its role is currently unclear.
Prior studies of KS-derived cell lines have shown that these cells can produce several cytokines, including granulocyte-macrophage colony-stimulating factor, tumor necrosis factor, transforming growth factor-β, interleukin-1β (IL-1β), IL-6, oncostatin M, and KSHV-encoded chemokine homologues.12-14 Some of these have been reported to modulate KS cell growth in an autocrine/paracrine fashion. Of particular interest is vascular endothelial growth factor (VEGF), originally discovered as vascular permeability factor (VPF),15 and its tyrosine kinase receptors, which have been proposed to play an important role in KS pathogenesis.16-20VEGF/VPF is a homodimeric glycoprotein that promotes endothelial cell proliferation, angiogenesis, and increased vascular permeability.21-23 VEGF/VPF binds to 1 of 2 tyrosine kinase receptors: fms-like tyrosine kinase-1 (Flt-1) and fetal liver kinase-1 (Flk-1/KDR). These receptors are found predominantly on endothelial cells, and their activation leads not only to cell proliferation, but also to increased vascular permeability and vasodilatation. When compared with histamine, VEGF/VPF is approximately 50,000 times more potent on a molar basis at increasing the permeability of microvessels to plasma macromolecules.15 In addition to playing a central role in promoting tumor neovascularization,24,25VEGF/VPF is directly associated with hyperpermeability of tumor vessels and is commonly detected in rodent and human tumor effusions.15,26,27 Recent studies have suggested that VEGF/VPF may play a role in the pathogenesis of certain experimental ascites tumors.28 29 In this study, we investigated a potential role of VEGF/VPF in the pathogenesis of PELs.
MATERIALS AND METHODS
Cell lines.
The PEL cell lines BC-1 and BCP-1 were kind gifts from Dr Y. Chang (Columbia University, New York, NY), and the BCBL-1 cell line was a kind gift from Dr R. Yarchoan (National Cancer Institute, Bethesda, MD). All of these cell lines are positive for KSHV. BC-1 cells also harbor EBV, whereas BCBL-1 and BCP-1 cells are EBV-negative. The KSHV/EBV-negative effusion type lymphoma cell line DS-1 was a kind gift from Dr D. Nelson (National Cancer Institute). EW36, BL41, CA46, and JLP119 are EBV-negative Burkitt's lymphoma cell lines, whereas Eubanks, Raji, and Namalwa are EBV-positive Burkitt's lymphoma cell lines.30 These cells were maintained as suspension cultures in RPMI1640 (Biowhittaker, Walkersville, MD) supplemented with 10% or 20% (for BCP-1) heat-inactivated fetal bovine serum (FBS) at 37°C in 5% CO2. DS-1 was maintained in RPMI1640 with 10% FBS and 10 U/mL of recombinant human IL-6. Primary cultures of human umbilical vein endothelial cells (HUVECs) and the human cell lines Hs68 (skin fibroblast), DU145 (prostate carcinoma), SK-N-MC (neuroblastoma), A-375 (malignant melanoma), MDA-MB-468 (breast carcinoma), and SW-480 (colon carcinoma) were purchased from the American Type Culture Collection (Manassas, VA). Hs68 and A-375 cell lines were maintained in Dulbecco's modified Eagles medium (Biofluids, Rockville, MD) with 10% FBS. DU145 cell line was maintained in RPMI1640 with 10% FBS. HUVECs were maintained in RPMI1640 with 15% FBS, 20 U/mL porcine heparin (Sigma, St Louis, MO), and 100 μg/mL endothelial cell growth supplement (Calbiochem-Novabiochem, La Jolla, CA). SK-N-MC cell line was maintained in Eagle minimum essential medium and Earle's BSS (Biowhittaker) with 10% FBS. DMA-MB-468 and SW-480 cell lines were maintained in Leibovitz's L-15 medium (Life Technologies, Gaithersburg, MD) with 10% FBS.
Preparation of conditioned media.
Suspension cells were seeded in 12-well plates (Becton Dickinson, Franklin Lakes, NJ) at 1 × 106 cells/well in 2.5 mL of RPMI1640 medium supplemented with 10% FBS and cultured with or without 20 ng/mL phorbol 12-myristate 13-acetate (TPA; Sigma) for 72 hours. Adherent cells were seeded in 6-well plates at 1 × 106 cells/well in 2.5 mL culture media (described above) and cultured for 72 hours.
Quantification of VEGF/VPF.
VEGF/VPF was measured by enzyme-linked immunosorbent assay (ELISA) using a human VEGF Quantikine kit (R&D, Minneapolis, MN), following the manufacturer's instructions.
Cell proliferation assays.
PEL cells were seeded in 96-well plates (Costar, Cambridge, MA) at 1 × 104 cells/well in RPMI1640 with 1% or 10% FBS and cultured with or without 2 to 50 ng/mL human VEGF/VPF (PeproTech, Rocky Hill, NJ) for 48 hours. In some experiments, the same number of PEL cells were cultured in autologous conditioned medium that was preincubated with 10 μg/mL of mouse monoclonal antihuman VEGF/VPF antibody (Ab; A4.6.1; a generous gift from Genentec Inc, South San Francisco, CA) to neutralize endogenous VEGF/VPF.31Proliferation was measured by a 6-hour pulse with 1 μCi/well of [3H]-thymidine (Amersham, Arlington Heights, IL). [3H]-thymidine incorporation was determined after harvesting cells onto glass fiber filters.
Reverse transcriptase-polymerase chain reaction (RT-PCR).
Total RNA was isolated using the Tri-zol reagent (Life Technologies) according to the manufacturer's instructions. RNA (0.25 μg for each reaction) was treated with RNase-free DNase I (Promega, Madison, WI) at 37°C for 30 minutes, heated at 75°C for 5 minutes, reverse-transcribed using an RNase H-RT (SuperScript; Life Technologies), and suspended in 100 μL of Tris-Ethylenediaminetetraacetic acid (TE). The resultant cDNA was amplified by PCR using primers specific to human Flt-1, Flk-1/KDR, and VEGF/VPF.17,18 PCR amplification was performed in 50 μL containing 20 mmol/L Tris-HCl, 50 mmol/L KCl, 1.5 mmol/L MgCl2, 0.2 mmol/L dNTP mixture (Life Technologies), 0.4 μmol/L of each primer, and 2.5 U Taq DNA polymerase (Life Technologies). An initial denaturation step at 94°C was followed by 35 cycles of denaturation at 94°C for 1 minute, annealing at 59°C for 1 minute, and extension at 72°C for 1 minute, followed by a final extension step at 72°C for 5 minutes. Each amplified product was electrophoresed through a 1.8% agarose gel prestained with 1 μg/mL of ethidium bromide and was visualized under UV light. The quality of RNA was confirmed in all samples by parallel RT-PCR for GAPDH.32
Establishment of PEL tumors and treatment with anti-VEGF/VPF Ab.
All animal experiments were performed according to National Institute of Health guidelines for the care and handling of mice. Groups of 6-week-old male C.B-17 SCID/beige mice received 400 rad of total body irradiation. The following day, mice were injected intraperitoneally (IP) with BCBL-1 cells (1 × 107 cells/mouse suspended in 0.2 mL phosphate-buffered saline [PBS]). As controls, mice were injected IP with the same number of Eubanks, Raji, or Namalwa cells. The neutralizing antihuman VEGF/VPF Ab or an isotype control Ab (mouse IgG1; Cappel ICN, Aurora, OH) was injected IP at a dose of 100 μg twice weekly in a volume of 0.2 mL. Treatment was initiated 1 day after BCBL-1 cell injection.
Histopathology and immunohistochemistry.
Histochemical staining of ascites tumor samples, peripheral blood smears, and formalin-fixed paraffin-embedded tissues was performed using Diff-Quick (Baxter Scientific Products, McGaw Park, IL). Cytospin samples of ascites tumor cells were fixed in acetone at −20°C for 1 minute and stained for human CD38 and HLA-DR by the avidin-biotin-peroxidase method using Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA), mouse antihuman CD38 Ab (Becton Dickinson), or rat anti–HLA-DR Ab (Serotec Inc, Raleigh, NC), according to the manufacturer's instructions. Mouse IgG and rat IgG (Cappel ICN, Aurora, OH) were used as negative controls.
Flow cytometric analysis.
BCBL-1 cells that had been cultured in complete medium were stained with mouse antihuman CD38 Ab or rat anti–HLA-DR Ab, followed by fluorescein isothiocyanate-labeled antimouse IgG (Becton Dickinson) or phycoerythrin-labeled anti-rat IgG (Becton Dickinson). Single-color analysis was performed using FACScan and CELLQuest (Becton Dickinson). After gating on live cells by forward and side light scattering, the percentage of positive cells was determined by integrating profiles on the basis of 2 × 104 live cells.
RESULTS
VEGF/VPF production from PEL cells.
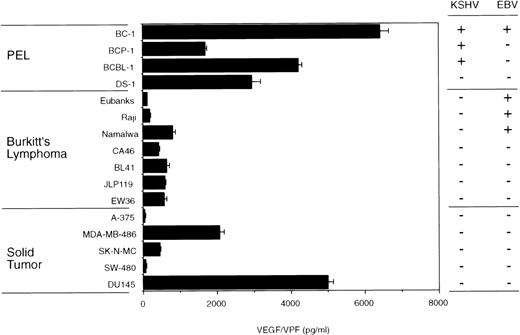
VEGF/VPF is commonly detected in solid tumor tissues, malignant ascites fluid, lymphoma, and KS tissues.15-20 Malignant and transformed cells are known to express VEGF/VPF.21-23 Using a specific ELISA, we found that 3 KSHV-positive PEL cell lines (BC-1, BCP-1, and BCBL-1) constitutively express high levels of VEGF/VPF (Fig 1). DS-1, an IL-6–dependent KSHV/EBV-negative primary effusion lymphoma cell line,33also produced large amounts of VEGF/VPF. Levels of VEGF/VPF in the culture supernatants of the KSHV-positive PEL cells and the DS-1 cells were comparable or higher than those in the culture supernatants of 7 Burkitt's lymphoma and 5 solid tumor-derived cell lines (Fig 1). In previous studies, we demonstrated that these solid tumor-derived cell lines consistently give rise to progressively growing subcutaneous tumors in athymic mice.34
VEGF/VPF production by PEL, Burkitt's lymphoma, and solid tumor cells. PEL cells, Burkitt's lymphoma cells, and solid tumor-derived cell lines were cultured (1 × 106cells/well) for 72 hours in 6-well plates (2.5 mL/well). A-375, melanoma; MDA-MB-486, breast cancer; SK-N-MC, neuroblastoma; SW-480, colon carcinoma; DU145, prostate carcinoma. VEGF/VPF levels in the culture supernatants were determined by ELISA. Results represent the mean (±SD) of 3 independent experiments.
VEGF/VPF production by PEL, Burkitt's lymphoma, and solid tumor cells. PEL cells, Burkitt's lymphoma cells, and solid tumor-derived cell lines were cultured (1 × 106cells/well) for 72 hours in 6-well plates (2.5 mL/well). A-375, melanoma; MDA-MB-486, breast cancer; SK-N-MC, neuroblastoma; SW-480, colon carcinoma; DU145, prostate carcinoma. VEGF/VPF levels in the culture supernatants were determined by ELISA. Results represent the mean (±SD) of 3 independent experiments.
VEGF/VPF isoforms produced by PEL cells.
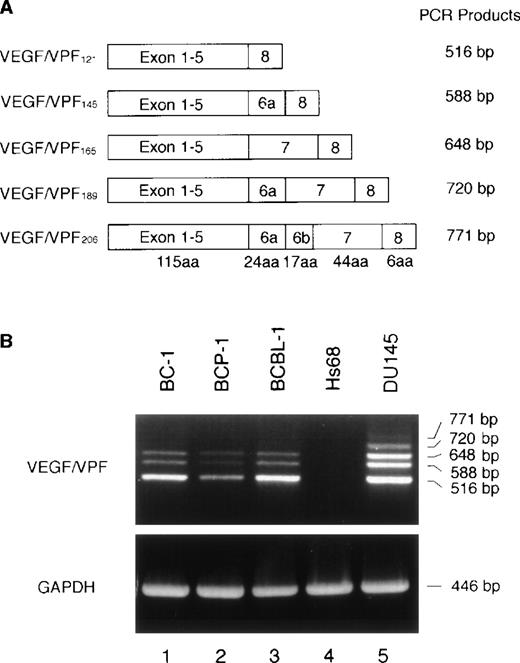
Genomic and cDNA analyses of the human VEGF/VPF gene showed the occurrence of at least 5 subtypes: VEGF/VPF121, VEGF/VPF145, VEGF/VPF165, VEGF/VPF189, and VEGF/VPF206(Fig 2A).17 These isoforms are generated by alternative splicing from a single gene. We performed RT-PCR analysis to identify the VEGF/VPF subtypes expressed in PEL cells. By this method, 5 bands were amplified from the control prostate carcinoma DU145 cell line (Fig 2B). By contrast, only 3 predominant bands corresponding to VEGF/VPF121, VEGF/VPF145, and VEGF/VPF165 were amplified from the PEL cell lines BC-1, BCP-1, and BCBL-1. These VEGF/VPF isoforms correspond to secreted forms of the protein.
Expression of VEGF/VPF isoforms by PEL cell lines. (A) Schematic representation of the VEGF/VPF mRNA splice variants together with the expected length of the respective RT-PCR amplification products. aa, amino acids. (B) Expression of VEGF/VPF isoforms in PEL (BC-1, BCP-1, and BCBL-1), skin fibroblast (Hs68), and prostate carcinoma (DU145) cells. Total RNA was translated into cDNA and amplified by PCR. The 3 splice variants coding for the secreted forms of VEGF/VPF were amplified from all 3 PEL cell lines. Signals for all 5 isoforms were amplified from DU145 cells and none from Hs68 cells.
Expression of VEGF/VPF isoforms by PEL cell lines. (A) Schematic representation of the VEGF/VPF mRNA splice variants together with the expected length of the respective RT-PCR amplification products. aa, amino acids. (B) Expression of VEGF/VPF isoforms in PEL (BC-1, BCP-1, and BCBL-1), skin fibroblast (Hs68), and prostate carcinoma (DU145) cells. Total RNA was translated into cDNA and amplified by PCR. The 3 splice variants coding for the secreted forms of VEGF/VPF were amplified from all 3 PEL cell lines. Signals for all 5 isoforms were amplified from DU145 cells and none from Hs68 cells.
Detection of the VEGF/VPF receptor Flt-1 on PEL cells.
The high-affinity VEGF/VPF receptors Flt-1 and Flk-1/KDR are expressed predominantly on vascular endothelial cells. Recent studies have also described expression of the VEGF/VPF receptor Flt-1 on a subset of primary hematopoietic cells,35 but not in most human tumor cell lines.21 We looked for expression of VEGF/VPF receptors on PEL cell lines. By RT-PCR, Flt-1 message was amplified from BCBL-1 and BC-1 cell lines, but not from the BCP-1 cell line (Fig 3). Flk-1/KDR was not detectable in these 3 PEL cell lines (Fig 3). Despite their expression of Flt-1 mRNA, BC-1 and BCBL-1 cell lines did not display enhanced proliferation in response to recombinant VEGF/VPF (2 to 50 ng/mL; Table 1). This result is consistent with the reported failure of VEGF/VPF to promote the proliferation in NIH3T3 cells transfected with the Flt-1 gene.21 We tested whether VEGF/VPF could contribute to the autocrine growth factor activity displayed by conditioned media from PEL cells. However, a neutralizing Ab directed at VEGF/VPF had minimal effect on the proliferation of PEL cells (Table 1). Based on these results, we conclude that VEGF/VPF does not act as an autocrine growth factor for PEL cells.
Expression of VEGF/VPF receptors in PEL cells detected by RT-PCR. cDNAs from HUVECs, PEL cells (BC-1, BCP-1, and BCBL-1), and skin fibroblast (Hs68) cells were subjected to PCR amplification for Flt-1 and Flk-1/KDR (498- and 709-bp products, respectively). The quality of RNA was confirmed by parallel RT-PCR amplification for GAPDH. MWM, molecular weight marker.
Expression of VEGF/VPF receptors in PEL cells detected by RT-PCR. cDNAs from HUVECs, PEL cells (BC-1, BCP-1, and BCBL-1), and skin fibroblast (Hs68) cells were subjected to PCR amplification for Flt-1 and Flk-1/KDR (498- and 709-bp products, respectively). The quality of RNA was confirmed by parallel RT-PCR amplification for GAPDH. MWM, molecular weight marker.
Effects of a neutralizing antihuman VEGF/VPF Ab on experimental effusion lymphoma.
Previous studies have shown that the PEL cell lines KS-1, BCP-1, HBL-6, and BCBL-1 are transplantable IP into certain immunodeficient mice, giving rise to lymphomatous effusions that resemble PEL occurring in humans.5,11 36 Using a specific neutralizing Ab, we looked for a potential role of VEGF/VPF in PEL cell growth in vivo. To this end, 23 SCID/beige mice were injected IP with BCBL-1 cells (1 × 107 cells/mouse) after γ-irradiation with 400 rad. Beginning 2 days after cell inoculation and continuing twice per week for 4 weeks, 8 mice received IP injections of PBS (0.2 mL), 9 mice received IP injections of a monoclonal antihuman VEGF/VPF Ab (A4.6.1, 0.1 mg in 0.2 mL PBS), and 6 mice received IP injections of murine IgG1 (0.1 mg in 0.2 mL PBS). After 24 or 25 days, 7 of 8 mice inoculated with PBS alone and 6 of 6 mice inoculated with control murine IgG1 developed abdominal distention attributable to the presence of ascites. A test puncture on day 29 after cell inoculation showed the presence of bloody ascites in 5 of 5 tested animals. By contrast, none of the mice (0/9) treated with anti-VEGF/VPF Ab developed abdominal distention (Table2). All mice were killed on day 30 after cell inoculation, at which time gross and histological examinations of the peritoneal cavity, liver, spleen, kidneys, heart, lung, and lymph nodes were performed.
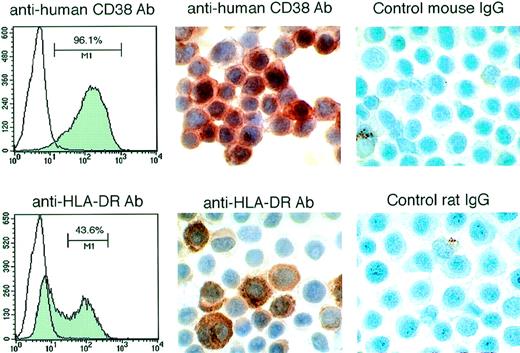
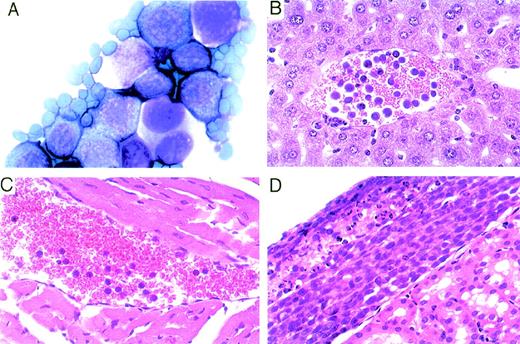
The PEL-derived BCBL-1 cell line injected IP into these mice expresses CD38 and HLA-DR detectable by fluorescence-activated cell sorting (FACS) analysis (Fig 4). By immunohistochemistry, most of the nucleated cells in the ascites fluids stained positively for human CD38 and HLA-DR (Fig 4). Morphologically, these lymphoid cells were similar to human PEL (Fig 5A). In most cases, the volume of maligant effusions was greater than 2 mL. Human VEGF/VPF was detected in ascites fluids from 5 of 5 tested animals at concentrations ranging between 5.7 and 15.1 ng/mL. Peripheral blood smears and organ sections showed the presence of large lymphoid cells in 6 of 7 control mice tested (4 of 5 mice treated with PBS and 2 of 2 treated with murine IgG), suggesting the presence of BCBL-1 cells in the circulation (Fig5B and C). In 8 of 9 control mice (4 of 5 treated with PBS and 4 of 4 treated with IgG), solid lymphoma-like tissue was observed under the renal capsule (Fig 5D) or in the mesenteric fat tissue proximal to the spleen. By immunohistochemistry, these lymphomatous lesions were positive for HLA-DR (not shown), suggesting that the mice had developed BCBL-1 lymphoma. Despite the absence of perceptible ascites in 9 mice treated with anti-VEGF/VPF Ab, microscopic analysis showed that 1 mouse had a similar lymphomatous nodule under the renal capsule and lymphoma cells in peripheral blood. Gross and microscopic examination of other organs showed no abnormalities, except for the presence of mild to moderate hepatosplenomegaly in mice with peritoneal effusions. These results demonstrate that, in mice, neutralizing Ab against VEGF/VPF are effective in preventing the development of malignant PEL ascites and reducing PEL dissemination to distant sites.
Expression of human CD38 and HLA-DR in lymphoid cells from the ascites fluid of SCID/beige mice inoculated IP with BCBL-1 cells. (Left panels) FACS analysis of CD-38 and HLA-DR expression by BCBL-1 cells cultured in vitro. (Middle panels) Immunohistochemical staining for human CD-38 and HLA-DR of cytospin preparations from ascites of mice injected IP with BCBL-1 cells. (Right panels) Control staining with mouse IgG and rat IgG of cytospin preparations from ascites of mice injected IP with BCBL-1 cells.
Expression of human CD38 and HLA-DR in lymphoid cells from the ascites fluid of SCID/beige mice inoculated IP with BCBL-1 cells. (Left panels) FACS analysis of CD-38 and HLA-DR expression by BCBL-1 cells cultured in vitro. (Middle panels) Immunohistochemical staining for human CD-38 and HLA-DR of cytospin preparations from ascites of mice injected IP with BCBL-1 cells. (Right panels) Control staining with mouse IgG and rat IgG of cytospin preparations from ascites of mice injected IP with BCBL-1 cells.
Identification of lymphoma-like cells in the peritoneal cavity, circulation, and kidney of mice inoculated IP with BCBL-1 cells. (A) Representative cytospin preparation of ascites fluid stained with Diff-Quick showing the presence of large lymphoid cells (original magnification × 1,000). (B and C) Representative peripheral blood smears depicting the presence of large lymphoid cells in the circulation (hematoxylin-eosin stain; original magnification × 400). (D) Representative lymphoma tissue localized under the renal capsule (hematoxylin-eosin stain; original magnification × 200).
Identification of lymphoma-like cells in the peritoneal cavity, circulation, and kidney of mice inoculated IP with BCBL-1 cells. (A) Representative cytospin preparation of ascites fluid stained with Diff-Quick showing the presence of large lymphoid cells (original magnification × 1,000). (B and C) Representative peripheral blood smears depicting the presence of large lymphoid cells in the circulation (hematoxylin-eosin stain; original magnification × 400). (D) Representative lymphoma tissue localized under the renal capsule (hematoxylin-eosin stain; original magnification × 200).
Association between VEGF/VPF production and effusion development.
To explore further the potential role of VEGF/VPF in the pathogenesis of effusion lymphoma, the Burkitt's lymphoma cell lines Eubanks, Raji, and Namalwa were tested for their ability to induce malignant ascites formation. As shown in Fig 1, Eubanks cells produced 108 pg/mL VEGF/VPF in the culture supernatant, Raji cells produced 178 pg/mL VEGF/VPF, and Namalwa cells produced 802 pg/mL VEGF/VPF. Groups of 8 SCID/beige mice were γ-irradiated with 400 rad and subsequently inoculated IP with 1 × 107 cells from each of the cell lines. Four mice of each group were killed on day 30, the remaining mice were killed on day 40 after cell injection, and gross pathology and tissue histology were obtained. Consistent with Eubanks cells failure to induce subcutaneous tumors in athymic mice,30 none of 4 SCID/beige mice inoculated IP with Eubanks cells presented any abnormality. In contrast, 2 of 4 mice inoculated with Raji cells and 3 of 4 mice inoculated with Namalwa cells showed the presence of multiple tumor nodules on the peritoneum, diaphragm, and mesentery, ranging in size between 3 and 20 mm in diameter (Table 3). Macroscopic and microscopic examination showed mild hepatosplenomegaly and the occurrence of infiltration with lymphoma cells. Histological examination of these nodules showed the presence of lymphoma consistent with Burkitt's lymphoma. During the initial 30 days of observation, none of the mice developed ascites. In the subsequent 10 days, abdominal distention appeared in 2 of 4 mice injected with Namalwa cells. The peritoneal cavities of these mice were occupied by large tumor nodules (35 mm in maximum diameter) associated with small amounts (<0.3 mL) of bloody ascites. Histological examination of the cells in the ascites fluid showed the presence of red blood cells and large cells consistent morphologically with Burkitt's lymphoma cells. By contrast, neither Raji nor Eubanks cells developed visible effusions. These results are consistent with the notion that VEGF/VPF is important to the development of lymphomatous effusions and suggest that threshold amounts of VEGF/VPF may be required for the formation of malignant lymphoma effusion. Many of the animals developed nodules of lymphoma in the absence of malignant effusion, suggesting that factors other than VEGF/VPF play a critical role in the formation of solid lymphoma.
DISCUSSION
In this study, we found that 3 KSHV-associated PEL cell lines (BC-1, BCBL-1, and PCP-1) produce high levels of VEGF/VPF in the culture supernatant. RT-PCR analysis of RNA from these PEL cell lines amplified predominantly the message for the secreted VEGF/VPF isoforms VEGF/VPF121, VEGF/VPF145, and VEGF/VPF165. The cell lines BC-1 and BCBL-1, but not PCP-1, expressed the tyrosine kinase VEGF/VPF receptor Flt-1, but neither exogenous nor endogenous VEGF/VPF stimulated PEL cell proliferation in culture. When inoculated into the peritoneal cavity of SCID/beige mice, the PEL cell line BCBL-1 produced effusion lymphomas with bloody ascites in 7 of 8 animals. Administration of a neutralizing antihuman VEGF/VPF Ab, but not control IgG1, to these mice inhibited the formation of effusion lymphomas and the associated ascites in all 9 animals. Despite the absence of identifiable effusion tumor in the peritoneal cavity, 1 of 9 mice had evidence of PEL-derived lymphoma under the renal capsule. Other mice were inoculated IP with 1 of 3 Burkitt's lymphoma cell lines secreting varying amounts of VEGF/VPF. The Namalwa cell line that secreted 802 pg/mL VEGF/VPF gave rise to malignant ascites in 2 of 8 animals, whereas the cell lines Eubanks and Raji that secreted 109 and 178 pg/mL VEGF/VPF, respectively, did not produce ascites. A proportion of the animals injected with Raji (3/8) and Namalwa (5/8) developed solid lymphoma nodules on the peritoneum, diaphragm, and mesentery. In the peritoneal cavity of SCID/beige mice, Burkitt's lymphoma cells expanded primarily as neoplastic nodules, whereas PEL cells displayed suspension growth. These results demonstrate that VEGF/VPF is critical to the development of experimental PEL in mice and point to an association between VEGF/VPF production by lymphoma cells and the development of effusion lymphoma.
VEGF/VPF is a highly conserved, disulfide-bonded, dimeric glycoprotein of 34 to 42 kD that displays a limited structural similarity to platelet-derived growth factor.23 The domain encoded by exon 1, which is common to all VEGF/VPF isoforms, contains information required for the recognition of the known VEGF/VPF receptors Flk-1/KDR and Flt-1 (Fig 1A).21 The amino acids encoded by exon 8 are also present in all of the VEGF/VPF splice variants. Peptides encoded by exons 6 and 7 distinguish various VEGF/VPF isoforms. VEGF/VPF121, VEGF/VPF145, and VEGF/VPF165 are secreted forms of VEGF/VPF, whereas VEGF/VPF189 and VEGF/VPF206 are sequestered by cell surface heparan sulfates.21 The most abundant product of the VEGF/VPF gene appears to be VEGF/VPF165.21 Consistent with the detection of VEGF/VPF in the culture supernatant of PEL cells and in PEL ascites, we found that PEL cells express predominantly the secreted VEGF/VPF isoforms VEGF/VPF121, VEGF/VPF145, and VEGF/VPF165.
The actions of VEGF/VPF are mediated by 2 cell surface receptors: Flt-1 and Flk-1/KDR. In the current study, we found that the PEL cell lines BC-1 and BCBL-1 express Flt-1 mRNA but do not proliferate in response to exogenous or endogenous VEGF/VPF. Flt-1 shows at least a 10-fold higher affinity to but approximately 10-fold lower kinase activity than Flk-1/KDR.21 When overexpressed in NIH3T3 cells or bovine endothelial cells, Flt-1 did not support cell proliferation in the presence of VEGF/VPF, whereas Flk-1/KDR overexpressed in these cells did.21 Flt gene transcripts are present in vascular endothelial cells starting from early stages of embryogenesis.21 Flt-1–deficient homozygous mice showed a marked disorganization of blood vessels and died at embryonic day 8.5 to 9.37 However, Flt-1 tyrosine kinase-deficient homozygous mice developed normal vessels with normal vascular permeability and survived.38 In adult mice, Flt-1 mRNA is detectable in most normal tissues, particularly in placenta, lung, kidney, and brain.21 No cells or cell lines other than endothelial cells are known to express both Flt-1 and Flk-1/KDR receptors and to proliferate in response to VEGF/VPF, strongly suggesting the existence of endothelial-specific signaling mechanisms. The role of Flt-1 on PEL cells is currently unknown. Like PEL cells, monocytes can express Flt-1 in the absence of Flk-1/KDR and through Flt-1 VEGF/VPF can stimulate tissue factor production and chemotaxis in monocytes.35
Functionally, VEGF/VPF is a potent inducer of angiogenesis attributed to direct stimulation of endothelial cell growth and of vascular permeability attributed to stimulation of nitric oxide release.39 Angiogenesis is required for a wide variety of physiological and pathological processes, including tumor growth.40 The expression of VEGF/VPF is necessary for the formation of blood vessels in mouse and rat embryos.41,42Many tumor cell lines secrete VEGF/VPF, and a variety of solid tumor tissues express high levels of VEGF/VPF.40 Transfection of VEGF/VPF renders Chinese hamster ovary cells tumorigenic in nude mice,43 and treatment with a neutralizing monoclonal Ab directed against VEGF/VPF inhibited the growth of a variety of experimental tumors.25,31,44 Stimulation of vascular permeability by VEGF/VPF is believed to be critical to the pathogenesis of certain malignant effusions.15 It is well documented that the formation of malignant ascites results largely from increased permeability of peritoneal lining vessels and the accumulation of a plasma exudate in the peritoneal cavity.27,45,46 A temporal and spatial correlation was noted between the appearance of malignant ascites and the presence of VEGF/VPF in the peritoneal cavity.15 Recently, a neutralizing Ab against VEGF/VPF was reported to significantly reduce the accumulation of malignant ascites in mice bearing intraperitoneal human ovarian tumors or syngeneic breast adenocarcinoma tumors.29,44 However, levels of VEGF/VPF in malignant ascites were found to vary widely depending upon the nature of the malignancy; tumors of sarcoma- and carcinoma-origin produced substantially more VEGF/VPF than did tumors of hematological derivation.28 In addition, anti-VEGF/VPF Ab completely inhibited ascites production in mice bearing ovarian tumors, whereas it only partially inhibited intraperitoneal tumor growth.29Also, VEGF/VPF neutralization had a minimal effect on peritoneal fluid and tumor cell accumulation in mice bearing a syngeneic lymphoma.44
In the present study, PEL cell lines secreted VEGF/VPF at levels comparable to those produced by solid tumor-derived cell lines. In addition to secreting VEGF/VPF, PEL cells lack expression of several adhesion and homing molecules, including intercellular adhesion molecule-1, L- and E-selectin, CD19, CD31, CD44, CD11a, and CD11c.11 The absence of certain adhesion molecules could impair anchorage of PEL cells to the peritoneal wall. This would explain why PEL cells inoculated IP did not form tumor nodules on the peritoneal serous membrane. However, most mice inoculated IP with PEL cells, either alone or in conjunction with control IgG, developed a solid lymphoma under the renal capsule or proximal to the spleen, suggesting the occurrence of transmigration through the capillary vessel wall. Only one of the animals treated with anti-VEGF/VPF Ab displayed one of these lymphomas. The receptor CX3CR1 and its ligand fractalkine, a transmembrane molecule with a CX3C-motif, can promote cell adhesion in the absence of other adhesion molecules, raising the possibility that PEL cell trafficking to the endothelium could occur through this or other pathways.47
KSHV can promote VEGF/VPF secretion through the virally encoded G-coupled protein receptor and the viral cytokine vIL-6.48,49 It can also promote angiogenesis directly, through the expression of the chemokines viral macrophage inflammatory protein-I (vMIP-I) and vMIP-II.14 This redundancy of tools for induction of angiogenesis and VEGF/VPF secretion has suggested that neovascularization is important to KSHV survival and spread. However, it is unclear how these effects might be mediated.50 It was proposed that VEGF/VPF may stimulate KSHV replication. It was also proposed that, by inducing the expression of VEGF/VPF and other angiogenic proteins, KSHV may favor vascularization and nutrients supply of virally infected cells, ensuring their growth. Enhanced angiogenesis, vascular permeability, and the presence of VEGF/VPF are characteristic features of KS tissues.16-20 In Castleman's disease, lymphoid hyperplasia is often associated with evidence of excessive vascularization in the germinal centers, which has been attributed to local VEGF/VPF expression by nonlymphoid cells.51 The current study provides evidence that the VEGF/VPF that PEL cells secrete contributes to lymphoma growth essentially by accelerating vascular permeability rather than tumor vascularization. The study also shows that VEGF/VPF can play a critical role in the pathogenesis of PEL, because VEGF/VPF neutralization prevented PEL formation in mice. Patients with PEL usually have advanced acquired immunodeficiency syndrome (AIDS) and are poor candidates for aggressive chemotherapy, which has resulted in a median survival of only 3 months.2,52 53 Based on the results presented here, anti-VEGF/VPF neutralizing Ab represents a rational approach to the investigational treatment of PEL.
ACKNOWLEDGMENT
The authors thank Drs Y. Chang for providing us BC-1 and BCP-1 cells, R. Yarchoan for BCBL-1 cells, D. Nelson for DS-1 cells, L. Yao for control HUVEC RNA, and J. Teruya for help in reviewing sections.
The publication costs of this article were defrayed in part by page charge payment. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. section 1734 solely to indicate this fact.
REFERENCES
Author notes
Address reprint requests to Yoshiyasu Aoki, MD, PhD, Division of Hematologic Products, Center for Biologics Evaluation and Research, Food and Drug Administration, Bldg 29A, Room 2D06, HFM-535, 8800 Rockville Pike, Bethesda, MD 20892; e-mail: AOKI@CEBR.FDA.GOV.